Abstract
Purpose
To demonstrate drug/polymer nanoparticles can increase the rate and extent of oral absorption of a low-solubility, high-permeability drug.
Methods
Amorphous drug/polymer nanoparticles containing celecoxib were prepared using ethyl cellulose and either sodium caseinate or bile salt. Nanoparticles were characterized using dynamic light scattering, transmission and scanning electron microscopy, and differential scanning calorimetry. Drug release and resuspension studies were performed using high-performance liquid chromatography. Pharmacokinetic studies were performed in dogs and humans.
Results
A physical model is presented describing the nanoparticle state of matter and release performance. Nanoparticles dosed orally in aqueous suspensions provided higher systemic exposure and faster attainment of peak plasma concentrations than commercial capsules, with median time to maximum drug concentration (Tmax) of 0.75 h in humans for nanoparticles vs. 3 h for commercial capsules. Nanoparticles released celecoxib rapidly and provided higher dissolved-drug concentrations than micronized crystalline drug. Nanoparticle suspensions are stable for several days and can be spray-dried to form dry powders that resuspend in water.
Conclusions
Drug/polymer nanoparticles are well suited for providing rapid oral absorption and increased bioavailability of BCS Class II drugs.
KEY WORDS: bioavailability, celecoxib, ethyl cellulose, nanoparticles, rapid absorption
INTRODUCTION
Recent reports estimate that at least 40% of new drug candidates are poorly soluble in water, resulting in low bioavailability (1–3). The oral absorption of low-solubility, high-permeability BCS Class II compounds, in particular, is often limited (4,5). Many of these compounds require solubilization technologies to achieve the desired extent and rate of oral absorption.
A number of delivery systems have been pursued to increase the oral bioavailability of these compounds through increased dissolution rate and/or increased dissolved-drug levels. These approaches include nanocrystals (6,7); inclusion complexes, such as cyclodextrins (8); and solution- or emulsion-based formulations (2,9). While some of these technologies have shown promise for oral delivery of low-solubility compounds, each technology has its limitations.
For example, inclusion complexes can effectively solubilize drugs that fit well into the cavity of the carrier, which is typically hydrophobic. Binding is drug-specific, and the modest binding constants for most drugs limit the increase in solubilized drug levels relative to crystalline solubility (8). Liquid formulations can provide high dissolved-drug levels and more rapid release, but can pose greater challenges with respect to low drug loading and physical and chemical stability compared with solid forms (3). Nanocrystals have the potential to increase bioavailability of dissolution-limited drugs by increasing the surface area, and therefore the dissolution rate, of low-solubility drug crystals (7,10,11). In situations where increasing the dissolution rate is not sufficient to adequately increase bioavailability, amorphous drug forms that can provide dissolved-drug levels higher than crystalline solubility can be advantageous. The drug/polymer nanoparticles described here are complementary to other bioavailability-enhancing technologies and possess favorable characteristics that may position them to be enabling for applications where rapid dissolution and/or increased dissolved-drug levels are required.
In general, solid amorphous dispersions can provide high bioavailability for low-solubility drugs, while being readily incorporated into solid dosage forms (12–14). Although such dispersions can provide rapid absorption, the rate of absorption depends on a number of dispersion properties, including drug loading and the nature of the dispersion matrix material. High-surface-area dispersions, such as the drug/polymer nanoparticles described here, are particularly well suited to rapid-onset applications due to their rapid release rate (15). In addition, these nanoparticles, like other amorphous dispersions, can provide dissolved-drug concentrations higher than crystalline solubility, which contribute to faster absorption rates and higher total absorption (16).
This paper demonstrates the feasibility of using a drug/polymer nanoparticle dispersion to improve the oral bioavailability and achieve rapid absorption of a model BCS Class II compound, celecoxib. The preparation, characterization, and in vivo performance of the nanoparticles in canine and human clinical trials are described.
Celecoxib is a cyclooxygenase-2 (COX-2) inhibitor used in the treatment of osteoarthritis and rheumatoid arthritis (17) and has been used in anticancer therapy (18). Several solid-state forms of celecoxib have been investigated (19), and celecoxib is marketed commercially as capsules containing crystalline drug. While these capsules have shown acceptable systemic exposure in humans,1 solubilized formulations offer the potential to provide increased bioavailability, and therefore a reduced dose. In addition, such formulations may be absorbed rapidly, reaching effective blood levels more quickly and providing faster onset of pain relief. Rapid dissolution rates have been achieved using an emulsion-diffusion nanoparticle system (20). Celecoxib proniosomes have also been produced and provided an approximately 20% increase in bioavailability, but a significantly longer time to maximum plasma drug concentration (Tmax) relative to the commercial capsule in humans (21).
The data presented here support the use of amorphous drug/polymer nanoparticles as a promising formulation approach for oral delivery of celecoxib and other BCS Class II compounds, particularly when rapid absorption is desired.
MATERIALS AND METHODS
Materials
Bulk crystalline celecoxib and 200-mg commercial capsules of celecoxib (Celebrex®) were obtained from Pfizer Inc. (Groton, CT). Ethyl cellulose (Ethocel® Viscosity 4) was a generous gift from the Dow Chemical Co. (Midland MI). Sodium taurocholate (NaTC) was purchased from Sigma Aldrich (St. Louis, MO). 1-Palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) was purchased from Avanti Polar Lipids Inc. (Alabaster, AL). Sodium chloride (NaCl) was purchased from VWR (Radnor, PA). Disodium hydrogen phosphate (Na2HPO4) and potassium dihydrogen phosphate (KH2PO4) were purchased from Sigma Aldrich, and potassium chloride (KCl) was purchased from Fisher Scientific (Pittsburgh, PA). The –L grade of hydroxypropyl methyl cellulose acetate succinate (HPMCAS-L) was obtained from Shin-Etsu Chemical Co. Ltd. (Tokyo, Japan).
β-Casein was obtained from Sigma Chemicals (St. Louis, MO). Sodium β-caseinate was formed by adding 400 mg of β-casein to 80 mL of deionized water and then adding NaOH solution to achieve a pH of 7.0. The solution was lyophilized to obtain solid sodium β-caseinate, which is referred to as “casein” below for brevity.
The model fasted duodenal solution (MFDS) consisted of 7.3 mM NaTC, 1.4 mM POPC, 82.1 mM NaCl, 20 mM Na2HPO4, and 46.7 mM KH2PO4, which was adjusted to pH 6.5 with NaOH and then to 290 mOsm using 1:20.4 NaCl:KCl.
Syringe filters (1-μm glass microfiber and 0.45-μm Supor® polyethersulfone membrane filters) were purchased from Pall Corp. (Port Washington, NY). Molecular-weight-cutoff (MWCO) filters (100 kDa, Microcon Ultracel YM-100) were purchased from Millipore Corp. (Billerica, MA).
Preparation of Nanoparticle Formulations
Nanoparticle Suspensions for In Vitro Characterization
Nanoparticle suspensions were prepared for in vitro dissolution testing and free-drug (dissolved drug plus drug in bile-salt micelles) measurement using the following method. Bulk crystals of celecoxib and solid ethyl cellulose were dissolved in approximately 8 g of methylene chloride. Formulations were prepared at four drug/polymer ratios—1:9, 1:3, 1:1, and 3:1—each at a total solids concentration of 15 mg/g solids in methylene chloride. Approximately 14 mg of NaTC was dissolved in 20 mL of water. The methylene chloride solution containing dissolved polymer and drug was mixed with the NaTC solution using a Polytron 3100 rotor stator (Kinematica Inc., Bohemia, NY) at 10,000 rpm for 5 min. This coarse emulsion was then further emulsified using a Microfluidizer M110S fluids processor (Microfluidics, Newton, MA) fitted with Z-shaped interaction chamber with a 100-μm-diameter channel and operated at 12,500 psi for 5 min. The emulsion was then placed on a rotoevaporator, and the methylene chloride was removed under reduced pressure at approximately 25°C for approximately 20 min.
Spray-Dried Nanoparticles for In Vitro Dissolution Experiments
Spray-dried nanoparticles were prepared from an aqueous nanoparticle suspension prepared using the emulsification technique described above. Two solutions were used to prepare the suspension: 14.4 g celecoxib and 14.4 g ethyl cellulose in 138.6 g ethyl acetate and 9.6 g casein in 461.5 g water using an Avestin C55 homogenizer (Avestin Inc., Ottawa, Ontario, Canada).
Spray-dried nanoparticles were prepared from this suspension using a Niro Mobile Minor™ spray-dryer (GEA Niro, Søborg Denmark). The suspension was pumped at about 20 g/min using a high-pressure pump to the spray-dryer, which was equipped with a Schlick No. 1.0 pressure nozzle (Dusen Schlick GmbH, Untersiemau, Germany) and a 9-inch chamber extension to increase the vertical height of the dryer. Nitrogen drying gas was introduced at 1,900 g/min and an inlet temperature of 90°C, and the evaporated solvent and drying gas exited the spray-dryer at an outlet temperature of 50°C. The solid powder was collected in a cyclone. The powder composition had a mass ratio of 37.5:37.5:25 celecoxib:ethyl cellulose:casein.
Nanoparticle Suspensions for Dog Studies
Nanoparticle suspensions were prepared for dog pharmacokinetic studies from two solutions: 1.375 g of celecoxib and 1.375 g ethyl cellulose dissolved in 50 mL methylene chloride and 917 mg casein dissolved in 200 mL of water. The two solutions were mixed using a rotor stator for 3 min at 10,000 rpm. The coarse emulsion was then emulsified further using an M-110S Microfluidizer at 12,500 psi for 20 min. Methylene chloride was then removed by rotoevaporation for 15 min. Two such batches were made and mixed. The potency of celecoxib was measured using high-performance liquid chromatography (HPLC), and the suspension was diluted to 5 mg active (mgA)/mL.
Nanoparticle Oral Powder for Constitution (OPC) for Dog Studies
A nanoparticle OPC was prepared for the same dog pharmacokinetic studies by preparing a similar emulsion at higher concentration (80 mg/mL solids). This emulsion was then spray-dried using a Mobile Minor spray-dryer using an inlet temperature of 80°C, an outlet temperature of 50°C, and a flow rate of 20 g/min. The resulting OPC was dried at ambient temperature under reduced pressure in a vacuum desiccator for approximately 18 h to remove residual solvent.
Spray-Dried Nanoparticles for Clinical Studies
For the clinical studies, nanoparticles were made by dissolving 8.62% celecoxib (w/w) and 8.62% (w/w) ethyl cellulose in ethyl acetate. An aqueous solution of 2% casein (w/w) in water was prepared. The solutions were mixed in a stainless-steel tank using a Bematek rotor stator at 3600 rpm for 20 min (Bematek Systems Inc., Salem, MA). The mixture was then emulsified by 20 passes through an Avestin C55 homogenizer at 12,500 psi. Solvent was removed under vacuum at 40°C.
The resulting aqueous nanoparticle suspension was pumped at about 24 g/min using a high-pressure pump to the Mobile Minor spray-dryer equipped as described above. A high-pressure pump was used to deliver liquid to the nozzle. Drying gas (i.e., nitrogen) at a flow rate of 1,850 g/min was circulated at an inlet temperature of 100°C, and the evaporated solvent and drying gas exited the spray-dryer at a temperature of 50°C. The resulting solid powder, which was collected in a cyclone, had a mass ratio of 37.5:37.5:25 celecoxib:ethyl cellulose:casein. This powder was used for imaging using scanning electron microscopy (SEM) analysis, powder x-ray diffraction (PXRD) analysis, and the resuspension studies described below.
Spray-Dried Dispersion (SDD) for Clinical Studies
A 50:50 celecoxib:HPMCAS-L SDD was prepared for clinical studies using the Mobile Minor spray-dryer described above. Celecoxib and HPMCAS-L were dissolved at 5 wt% solids each in methanol, yielding a solution with a total solids content of 10 wt%. The solution was prepared in a stainless-steel tank equipped with a top-mounted mixer. In a representative batch, 36 kg (44.4 L) of room-temperature methanol was added to the tank, and 2 kg of celecoxib was then added. The mixture was stirred for 30 min at room temperature until the drug was dissolved. Two kilograms of HPMCAS-L was added and stirred for 1 h.
The resulting methanol solution was pumped at about 60 g/min using a high-pressure pump to the Mobile Minor spray-dryer. Drying nitrogen gas was introduced at 1,900 g/min and an inlet temperature of 110°C, and the evaporated solvent and drying gas exited the spray-dryer at 55°C. The resulting SDD powder, which was collected in a cyclone, had a mass ratio of 50:50 celecoxib:HPMCAS-L.
The SDD was placed in a tray-dryer for secondary drying at controlled temperature and humidity to remove residual methanol to a target level of < 0.05 wt%. The SDD was spread out in a tray to a bed depth of no more than 1 in. and held at 40°C/15% relative humidity (RH) for a minimum of 3 h.
Particle Sizing
Nanoparticle size was measured by dynamic light scattering (DLS) using a BI-200SM particle size analyzer with a BI-9000AT correlator (Brookhaven Instruments Inc., Holtsville, NY). Particle size is reported as the diameter determined using the cumulant cubic fitting algorithm.
Nanoparticle morphology and size heterogeneity was assessed using cryogenic transmission electron microscopy (cryo-TEM). The suspensions were sampled and prepared for cryo-TEM analysis on an FEI Vitrobot™ (FEI Company, Hillsboro, OR) using the following method. A 10-μL sample of suspension was applied to a Lacey Carbon TEM grid in the Vitrobot chamber equilibrated to 90% RH. The grid was blotted once for 2 s and then immediately plunged into freezing liquid ethane. The grid was transferred to a Taylor-Wharton liquid-nitrogen storage dewar and stored until imaging could be performed. Imaging was carried out at 200 kV (using a thermionic LaB6 electron source) in an FEI Tecnai20 Sphera TEM instrument using a Gatan Model 626 cryogenic holder (Gatan Inc., Pleasanton, CA) and low-dose imaging mode facilitated by the FEI Tecnai User Interface. The TEM was equipped with a Gatan Multiscan CCD Model 794 camera, and images were captured using a Gatan digital micrograph.
For SEM imaging, the spray-dried nanoparticle powders were spread on a post using double-sided tape. They were then sputter-coated with Au/Pd using an Anatech Hummer 6.2 sputter system for approximately 8 min at 7 V and 15 to 20 mA. They were then imaged on a Hitachi S-3400 N SEM instrument (Hitachi High Technologies America Inc., Schaumburg, IL).
HPLC Analysis
Aqueous drug-containing samples were analyzed using a Hewlett Packard Series 1100 HPLC instrument (Hewlett Packard Development Corp., Palo Alto, CA) with an Agilent Zorbax SB-C8 column (3.5 μm, 75 mm × 4.6 mm) with an injection volume of 10 μL, column temperature of 25°C, and flow rate of 1.5 mL/min with ultraviolet (UV) detection at 254 nm. Celecoxib had a retention time of approximately 4 min using an isocratic method with 55:44 acetonitrile:10 mM ammonium acetate.
Thermal Analysis
The glass-transition temperature (Tg) of casein, ethyl cellulose, celecoxib, and the drug/polymer nanoparticles was obtained by modulated differential scanning calorimetry (mDSC) using a TA Q-1000 differential scanning calorimeter (TA Instruments, New Castle, DE). Thermograms were analyzed using TA Instruments software.
Polarized Light Microscopy (PLM)
The nanoparticle suspensions were examined for the presence of crystals using PLM. A Nikon E600 microscope was used at 200- to 400-fold magnification.
PXRD
Spray-dried nanoparticle powders were analyzed by PXRD using a Bruker AXS diffractometer. Approximately 10 to 20 mg of powder was placed in a zero-background-holder sample cup with single-crystal silicon sample surfaces. The sample cup was spun in the horizontal plane while the goniometer was stepped through a 2Θ angle of 4° to 40° with a 0.01° step size at a rate of 1 s/step.
Solubility Testing
Celecoxib solubility was measured in water and in MFDS by placing excess bulk crystalline drug in an aqueous medium and stirring overnight at room temperature. A portion of the supernatant was placed in a microcentrifuge tube and centrifuged at 12,000 rpm for 5 min before it was analyzed by HPLC.
Release Testing
Nonsink release testing of suspensions containing the drug/polymer nanoparticles or bulk crystalline drug was performed as a function of drug loading in the particle. Drug/polymer nanoparticles were manufactured as described above at solids contents of approximately 6 mg/mL. Each suspension was then diluted to a concentration of 40 μg active (μgA)/mL in MFDS, placed in a glass scintillation vial, and stirred at 60 rpm using a magnetic stir bar. At each time point, duplicate 500-μL aliquots were removed from the vial, placed into microcentrifuge tubes fitted with 100-kDa MWCO filters, and spun at 12,000 rpm for 5 min. After spinning, 100 μL of the filtrate was mixed with 500 μL of dimethylsulfoxide (DMSO) and analyzed by HPLC.
Resuspension Testing of Spray-Dried Nanoparticle Powders
The resuspendability of spray-dried nanoparticle powders was assessed using a “gastric transfer” release test. For this test, 267 mg of spray-dried 37.5:37.5:25 celecoxib:ethyl cellulose: casein nanoparticle powder was placed in 5 mL of water. The resulting suspension was then added to 45 mL of 0.1 N HCl to form a suspension containing 2 mgA/mL celecoxib. This simulated gastric suspension (pH ~1.2) was stirred at 100 rpm. Aliquots of 0.75 mL were removed 5, 15, and 35 min after the suspension was prepared.
At 35 min, the suspension was diluted with 50 mL of a pH 6.5 phosphate-buffered saline (PBS) solution. The pH of the diluted suspension was 6.5. After dilution with the PBS solution, additional aliquots were taken at 1, 3, 5, 10, 30, and 60 min. All aliquots were assayed for celecoxib in three different ways: with no filtering, after filtering through a 1-μm syringe filter, and after filtering through a 1-μm syringe filter and a 0.45-μm syringe filter. The goal was to assess if the spray-dried powder was likely to resuspend to re-form nanoparticles without aggregation when dosed orally as a solid.
Pharmacokinetic Studies in Dogs
The pharmacokinetics of celecoxib nanoparticles and commercial celecoxib capsules were evaluated in a fasted dog model. In the nanoparticle study, performed at Covance Labs Inc. (Princeton, NJ), six male beagle dogs were each given a 20-mgA/kg dose of celecoxib nanoparticles. The animals were fasted overnight (at least 8 h before dosing through approximately 12 h after dosing). Individual doses were calculated based on body weights taken on the day each dose was administered.
To determine if drying and resuspending the nanoparticles affected in vivo performance, the nanoparticles were dosed as an OPC and as a suspension. In the first leg of the study, the OPC was tested. Spray-dried celecoxib nanoparticles (described above) were resuspended on the day of dosing. A stir bar was added to a bottle containing the spray-dried formulation (2000 mg), and approximately 400 mL of deionized water was added to the bottle. This produced a milky white suspension having a final concentration of 5 mg/mL. The final dosing suspension was administered to dogs via oral gavage with a dosing volume of 4 mL/kg.
In the second leg of the study, the same 5-mg/mL formulation was prepared as described above and refrigerated as a liquid suspension until dosing. The formulation was a uniform white suspension. On the day of dosing, the formulation was removed from refrigeration and administered to dogs at a dosing volume of 4 mL/kg.
Blood (approximately 2 mL) was collected via jugular venipuncture into tubes containing tripotassium ethylenediaminetetraacetic acid (EDTA) anticoagulant before dosing and at 2, 5, 10, 15, 30, 60, and 90 min and 2, 4, 6, 8, 12, and 24 h after dosing. Blood samples were maintained on wet ice before centrifugation to obtain plasma. Centrifugation began within 1 h of sample collection. Plasma was placed on dry ice before storage at approximately –70°C.
Plasma samples were analyzed for celecoxib concentrations using a liquid chromatography method with tandem mass spectrometry (LC/MS/MS). In this method, celecoxib was extracted from dog plasma following protein precipitation caused by addition of acetonitrile. The drug peak was isolated by HPLC using a Shimadzu SIL/HT HPLC instrument (Shimadzu Scientific Instruments, Columbia, MD) equipped with a Keystone Scientific BDS Hypersil C8 column (50 mm by 2.0 mm) at 25°C. A gradient elution using a mixture of 0.02% acetic acid in water and 0.02% acetic acid in acetonitrile was employed, with a flow rate of 0.8 mL/min. Multiple-reaction-monitoring mass spectrometry was used to quantify celecoxib content using a Sciex 3000 instrument (AB Sciex, Foster City, CA). The instrument was operated in negative electrospray mode with an ionization potential of –4500 V, analyzing for a precursor ion with m/z of 380 and a product ion with m/z of 316.
In a separate study, performed at Pfizer Inc., 16 beagle dogs were each given a commercial Celebrex™ capsule containing 200 mg of celecoxib, for a mean dose of 26.9 mg/kg. The animals were fasted overnight (approximately 14 h before dosing through approximately 4 h after dosing). Each dog was weighed on the day of dosing, and the mean dose was corrected, based on the body weight, to 26.9 mg/kg.
Blood (approximately 2 mL) was collected from the dogs via jugular venipuncture into tubes containing potassium EDTA anticoagulant before dosing and at 10, 20, 60, and 90 min and 2, 3, 4, 5, 6, 8, 12, and 24 h after dosing. Blood samples were maintained on wet ice before centrifugation to obtain plasma. The resulting plasma samples were transferred to polypropylene vials for storage at approximately –20°C until analysis.
Plasma samples were analyzed for celecoxib concentrations using LC/MS/MS. In this method, celecoxib was extracted from dog plasma following protein precipitation caused by addition of buffer and acetonitrile. The drug peak was isolated by HPLC using a PE Series 200 HPLC micropump system and autosampler (PerkinElmer, Waltham, MA) equipped with a Phenomenex phenyl hexyl 5-μm column (50 mm × 2.0 mm) at room temperature. A gradient elution using a mixture of methanol:1.5 mM ammonium formate and 3.5 mM ammonium hydroxide (30:70, v/v, Mobile Phase A) and 5 mM ammonium hydroxide in methanol (Mobile Phase B) was employed at a flow rate of 0.4 mL/min. Multiple-reaction-monitoring mass spectrometry was used to quantify celecoxib content using a Sciex 3000 instrument configured with an atmospheric-pressure chemical ionization (APCI) source. The instrument was operated in negative ionization mode with a turbo ion spray voltage of –5000 V, analyzing for a precursor ion with m/z of 380, and a product ion with m/z of 316.
Clinical Pharmacokinetic Study
In the human clinical study, three celecoxib formulations were assessed: a single 400-mg dose of the marketed celecoxib capsule (two 200-mg capsules), a single 400-mg celecoxib dose given as an OPC containing spray-dried celecoxib nanoparticles, and a single 400-mg celecoxib dose given as an OPC containing an enteric SDD. Trial treatments were separated by a washout of at least 7 days.
The Phase 1 open-label, randomized, single-dose crossover study in 12 healthy adult subjects in the fasted state was managed by Pfizer Global Research and Development and conducted by investigators contracted by and under the direction of Pfizer (Study Protocol A3191216). The study was conducted in compliance with the ethical principles originating in or derived from the Declaration of Helsinki and in compliance with all International Conference on Harmonization (ICH) Good Clinical Practice (GCP) guidelines. In addition, all local regulatory requirements were followed, in particular, those affording greater protection to the safety of trial participants.
Clinical study subjects fasted for at least 8 h before dosing and 4 h after dosing for each treatment period. Water intake was restricted for 1 h before and after dosing, except for the volume of water (240 mL/8 fluid ounces) administered with the celecoxib capsules. The total volume for subjects receiving suspensions plus water was 240 mL. Subjects received standardized meals scheduled at the same time in each period of the study.
Blood samples were collected for analysis of plasma celecoxib concentrations before dosing and at 15, 30, 45, 60, and 90 min and 2, 3, 4, 6, 8, 12, 24, and 48 h after dosing. Samples were drawn into tubes containing tripotassium EDTA. The plasma was separated from the whole blood within approximately 30 min of collection and stored frozen at approximately –20°C within 60 min of sample collection. Samples were analyzed using a validated HPLC-MS/MS method.
Relative Bioavailability
The relative bioavailability—i.e., maximum plasma drug concentration (Cmax) and area under curve (AUC)—of the test formulations was assessed and compared with that of the capsule (control formulation). The log-transformed Cmax and AUC values were analyzed with a crossover analysis of variance model that consisted of sequence, subject within sequence, period, and treatment effects. The subject within sequence effect was considered random. Least-squares means and model-based 90% confidence intervals (CIs) were generated.
RESULTS
Celecoxib:ethyl cellulose:casein nanoparticles were characterized to determine their size, state of matter, release performance, and resuspendability. Pharmacokinetic tests were then performed in canine tests and a human clinical study.
Nanoparticle Size and Shape
The typical nanoparticle diameters measured by DLS were 100 to 150 nm and were unchanged after spray-drying and resuspension in aqueous media. Nanoparticles were uniformly spherical in shape as determined by cryo-TEM imaging. Figure 1 is a representative cryo-TEM image for 37.5:37.5:25 celecoxib:ethyl cellulose:casein nanoparticles. Based on DLS analysis of this sample, the nanoparticles were 128 nm in diameter.
Fig. 1.
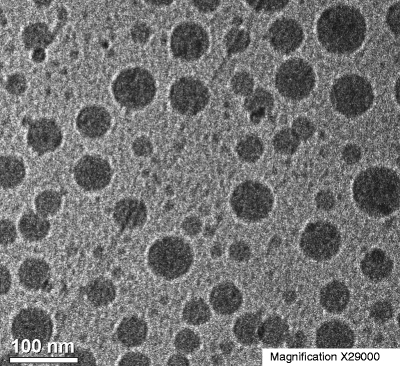
Representative cryo-TEM image of a 37.5:37.5:25 celecoxib:ethyl cellulose:casein nanoparticle suspension at 29,000-fold magnification.
Nanoparticle State of Matter
To assess whether the drug was molecularly dispersed within the nanoparticle polymer matrix, 37.5:37.5:25 celecoxib:ethyl cellulose:casein nanoparticles were analyzed by mDSC. Figure 2 is a representative mDSC scan showing that the nanoparticles have a single distinct Tg of 68°C. This Tg lies between that of celecoxib (54°C) and that of the polymer matrix components (ethyl cellulose 126°C, casein 198°C). No crystals were observed in the nanoparticle suspensions viewed by PLM out to several weeks after manufacture. PXRD showed no evidence of celecoxib crystallinity in the spray-dried powders of nanoparticle suspensions of the composition reported here. The PXRD pattern in Fig. 3 was taken after storage for 5 years at ambient conditions.
Fig. 2.
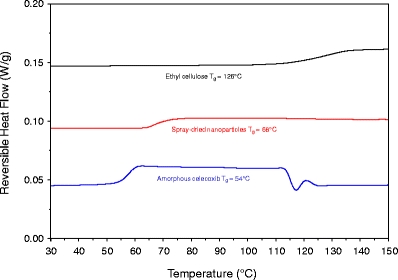
Representative mDSC results for amorphous celecoxib (bottom), spray-dried nanoparticles (middle), and ethyl cellulose (top).
Fig. 3.
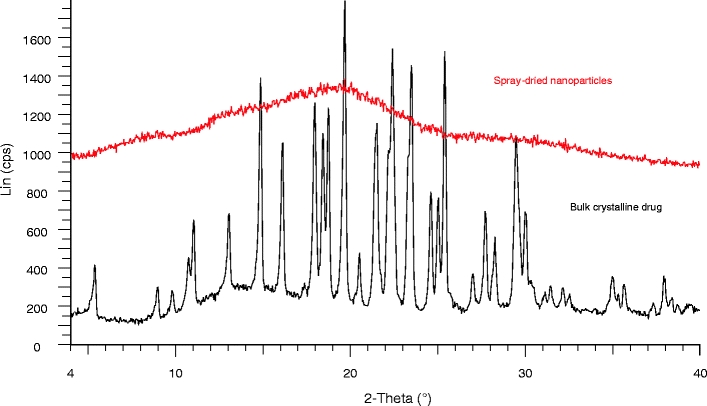
PXRD pattern for celecoxib:ethyl cellulose:casein spray-dried particles after storage for 5 years.
Release Testing
A nonsink release test was performed to determine the release rate and the drug activity of the celecoxib nanoparticles. Figure 4 shows the release profiles of nanoparticles with drug loadings of 10%A, 25%A, and 50%A in MFDS at 40 μg/mL (the NaTC is disregarded when reporting the loading, since it is believed to be largely in solution or at the surface of the nanoparticle and not in the core). The dissolution profile of bulk celecoxib crystals is shown for comparison. As Fig. 4 shows, the extent of release from the nanoparticles is a monotonic function of celecoxib loading, with a higher proportion of the drug releasing at higher loadings. Release of celecoxib from the nanoparticles is quite rapid, reaching the terminal value within the first minute. In contrast, the bulk celecoxib crystals take at least 1 h to reach their maximum concentration in MFDS (i.e., 16 μg/mL). This maximum concentration is the sum of dissolved celecoxib plus celecoxib in bile-salt micelles. As the equilibrium partition coefficient of celecoxib into the bile–salt micelles is approximately 1,000 and the volume of bile–salt micelles in MFDS is about 0.5%, the dissolved celecoxib concentration at equilibrium is approximately 2.5 μg/mL, whereas the concentration of celecoxib in bile–salt micelles is about 13.5 μg/mL.
Fig. 4.
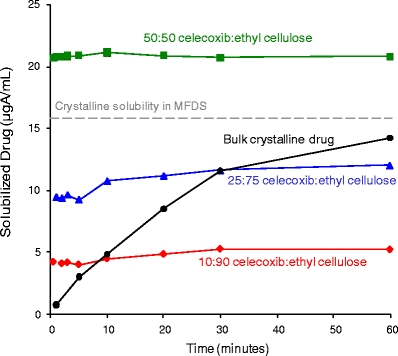
Nonsink dissolution performance of 37.5:37.5:25 celecoxib:ethyl cellulose:casein nanoparticles in MFDS, compared with bulk crystalline drug. The dose for all suspensions was 40 μgA/mL. Solubilized crystalline drug in MFDS is 16 μg/mL.
Figure 5 shows drug activity as a function of drug loading in the nanoparticle expressed as the ratio of free–drug concentration provided by the nanoparticle suspension to crystalline drug solubility. As the figure shows, depending on the drug loading, the nanoparticles provide an equilibrium free–drug level that can be either higher or lower than the crystalline drug solubility.
Fig. 5.
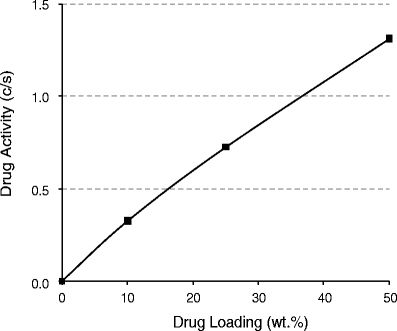
Drug activity as a function of celecoxib loading in nanoparticles. Activity is defined as the ratio of solubilized drug provided by the nanoparticle (C) to the solubility of bulk crystalline drug (S). Solubilized drug is the sum of dissolved drug and drug present in bile-salt micelles.
As shown in Fig. 6, the partition coefficient can be calculated by fitting a line to the plot of the ratio of dissolved drug to drug in nanoparticles versus the volume fraction of ethyl cellulose. This gives an ethyl cellulose/water partition coefficient for celecoxib of approximately 21,500.
Fig. 6.
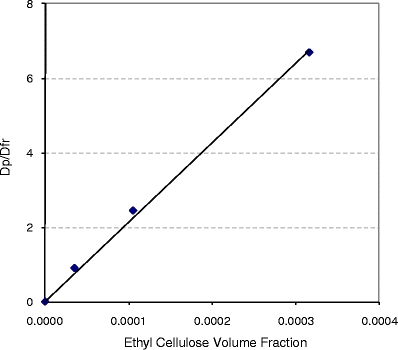
The ratio of drug in the polymeric particle over drug dissolved in aqueous medium plotted as a function of ethyl cellulose volume fraction. The slope (~21,500) gives the partition coefficient of celecoxib in ethyl cellulose (Eq. 1).
Resuspendability
When spray-dried, the nanoparticles form powders consisting of larger particles 5 to 20 μm in size, each of which is comprised of numerous nanoparticles. Figure 7 is a SEM of spray-dried nanoparticles. DLS measurements showed the particle size of the spray-dried particles when redispersed in water were similar to the particle size in the initial suspension (data not shown). To assess whether the spray-dried particles are likely to resuspend to recover the original nanoparticles in the gastrointestinal (GI) tract, a resuspension test was performed with solutions that mimic gastric and intestinal pH. After transfer of the spray-dried nanoparticles from the simulated gastric to simulated intestinal medium, the nanoparticles rapidly redispersed.
Fig. 7.

SEM of spray-dried 37.5:37.5:25 celecoxib:ethyl cellulose:casein nanoparticles.
Figure 8 shows the percentage of drug from resuspended spray-dried nanoparticles passing through a single 1-μm filter or sequentially through 1- and 0.45-μm filters. As the data show, at gastric pH, nearly 100% of the drug from the resuspended powder (OPC) passes through the 1-μm filter, whereas little of the drug passes through the 0.45-μm filter. Nearly all of the drug passing through the filter (>99%) is present as nanoparticles, as the dissolved-drug levels in the simulated gastric medium are <10 μgA/mL (see, for example, the dissolved-drug levels implied by Fig. 5). At intestinal pH, nearly 100% of the drug from the resuspended powder (OPC) passes through the 1-μm filter and ~80% passes through the 0.45-μm filter.
Fig. 8.
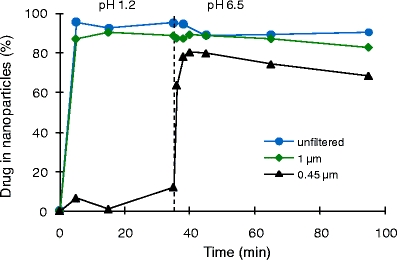
Filter potency as a function of time for resuspended 37.5:37.5:25 celecoxib:ethyl cellulose:casein nanoparticles.
Pharmacokinetic Studies in Dogs
The pharmacokinetic performance of spray-dried celecoxib nanoparticles and the commercial celecoxib capsules were evaluated in a dog model. In the nanoparticle study, six fasted beagle dogs were each given either a 20-mgA/kg dose of celecoxib nanoparticles that was prepared and maintained in a liquid suspension or celecoxib nanoparticles that were spray-dried as an OPC and resuspended before dosing. In a separate study, 16 dogs were each given a 200-mg commercial celecoxib capsule, resulting in a mean dose of 26.9 mgA/kg.
Since the doses in the two dog studies were slightly different, the systemic exposure data (i.e., Cmax and AUC data) were normalized to the dose and dog’s body weight. The results are summarized in Table I, and profiles of mean plasma concentration versus time are presented in Fig. 9.
Table I.
Data from Pharmacokinetic Testing Using a Dog Model
| Formulation | Dose (mg/kg) | Dose-Normalized AUC (hr*ng/ml)/(mg/kg) | Dose-Normalized Cmax (ng*ml)/(mg/kg) | Tmax (hr) | Dogs (n) |
|---|---|---|---|---|---|
| 200-mgA commercial capsule | 26.9 | 698.3 (413.9) | 81.7 (34.3) | 3.40 (7.87) | 16 |
| Nanoparticle OPC | 20.0 | 1963 (1219) | 312.5 (109.7) | 1.63 (1.30) | 6 |
| Nanoparticle suspension | 20.0 | 2031 (1250) | 366.1 (108.1) | 1.04 (0.64) | 6 |
Fig. 9.
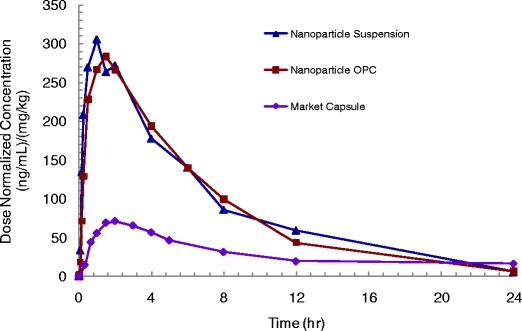
Dog pharmacokinetic data for the nanoparticle suspension, resuspended nanoparticles (OPC), and commercial capsule (dose is 200 mgA).
As shown in Table I, the pharmacokinetic performance of the two celecoxib nanoparticle formulations is similar. Thus, the effect of spray-drying and resuspension of the nanoparticles on in vivo performance is minimal for this formulation. The bioavailability of the two nanoparticle formulations—as measured by AUC—was roughly three-fold higher than that for the commercial capsules. Given that the bioavailability for the commercial capsules in dogs is estimated at 25% (17), this translates to roughly 75% bioavailability for nanoparticles in dogs at this dose.
The Tmax for the nanoparticles is 1.0 to 1.6 h, which is much shorter than the 3.4 h for the commercial capsules. The Cmax for nanoparticles is also substantially higher, with a roughly four-fold increase relative to the commercial capsules. Even more importantly, it took only 10 min for the plasma concentration of the spray-dried nanoparticle formulation to reach the Cmax of the commercial capsules. Based on these results, the spray-dried nanoparticles are expected to provide faster onset of action.
Clinical Pharmacokinetic Study
We describe three formulations used in the clinical pharmacokinetic study: the same nanoparticle OPC formulation that was used in the dog studies, an OPC formulation containing a solid amorphous SDD consisting of 50% celecoxib and 50% HPMCAS-L (described in the Materials and Methods section), and the commercial capsule.
Data from the clinical study are presented in Table II and Fig. 10. In this study, the SDD and nanoparticles had substantially higher mean Cmax values and somewhat higher AUC values than the commercial capsules. Specifically, the Cmax values for the nanoparticles and SDD were roughly 3.1-fold and 2.4-fold those of the commercial capsules, respectively. The AUC values for the nanoparticle and SDD were 36% and 26% higher than that of the commercial capsule, respectively. The rate of absorption for the nanoparticle formulation was particularly rapid, with a mean Tmax of 0.75 h, compared with 3.0 h for the commercial capsules and 2.0 h for the SDD.
Table II.
Pharmacokinetic Data and Relative Bioavailability Comparisons in Humans
| Formulation | Dose (mg) | Adjusted Geometric Means (90% CI in parentheses)a | Ratiob (90% CI in parentheses) | |||
|---|---|---|---|---|---|---|
| AUC (hr*ng/ml) | Cmax (ng/ml) | Tmax (hr) | AUC0-tlqc c | Cmax | ||
| Commercial capsule (2 × 200 mg) (n = 11) | 400 | 10,076 (8,965 to 11,371) | 799 (667 to 969) | 3.0 (1.5 to 4.0) | NAd | NA |
| Nanoparticle OPC (n = 10) | 400 | 13,714 (12,091 to 15,512) | 2,487 (2,029 to 3,052) | 0.75 (0.75 to 1.0) | 136 (120 to 154) | 311 (254 to 382) |
| HPMCAS SDD OPC (n = 12) | 400 | 12,734 (11,285 to 14,308) | 1,947 (1,606 to 2,365) | 2.0 (1.0 to 4.0) | 126 (112 to 142) | 244 (201 to 296) |
aCI = confidence interval
bRatio of treatment mean values compared to commercial capsule, expressed as a percentage (100% × test/reference)
cAUC0-tlqc = area under the curve from 0 to time of last quantifiable concentration
dNA = not applicable
Fig. 10.
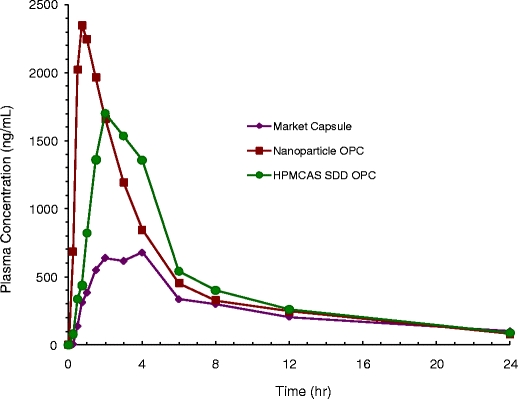
Human pharmacokinetic data for the commercial capsule, resuspended nanoparticles (OPC), and resuspended HPMCAS SDD OPC.
DISCUSSION
Physical Characterization of Nanoparticles
As shown by the cryo-TEM image in Fig. 1, the nanoparticles were spherical, which is typical for solid particles formed using either emulsion or precipitation methods, due to the favorable energetics associated with minimization of surface area. The mDSC data, which shows a single Tg between that of the pure drug and pure ethyl cellulose, strongly indicates a single phase consisting of celecoxib molecularly dispersed in ethyl cellulose.
The nonsink release studies were performed in MFDS to increase the signal-to-noise ratio in the HPLC analysis. MFDS is also a model for the fasted intestinal environment. When the nanoparticle suspensions were diluted in MFDS, the concentration of free drug essentially reached its steady-state value by the first time point at 1 min. This rapid dissolution is due to the short diffusion distance of drug from inside the nanoparticle core to its surface. The rapid release relative to the bulk crystalline drug (shown in Fig. 4) is a large part of the reason why the nanoparticles lead to more rapid absorption of celecoxib.
The release profile, showing that the nanoparticles release celecoxib rapidly until the concentration of celecoxib reaches the equilibrium value, demonstrates that the celecoxib that remains in the nanoparticles is not kinetically trapped within the polymeric matrix of the nanoparticle, but resides within the nanoparticle due to a high thermodynamic solubility. The release data suggest thermodynamic partitioning of the drug between the core of the nanoparticle and the aqueous medium. The partition coefficient can be calculated by comparing the ratio of drug in the nanoparticle to drug in the aqueous media at steady state (reached within 1 min):
 |
1 |
where D p is drug in the nanoparticle, D fr is the sum of dissolved drug and drug in bile-salt micelles, K p is the polymer/aqueous partition coefficient, and V p is the volume fraction of ethyl cellulose in the suspension. Fig. 6 plots the ratio calculated using Eq. 1 as a function of ethyl cellulose volume fraction for different drug loadings at equal total drug concentration. The linearity of the plot provides strong validation of the partitioning model. The partition coefficient of 21,500 calculated from the slope of the plot suggests a solubility of celecoxib in ethyl cellulose of approximately 0.30 g/g polymer (assuming an ethyl cellulose density of 1.14 g/mL).
When diluted in an aqueous suspension, celecoxib will release from the nanoparticle until the drug activities inside the polymeric nanoparticle and in the aqueous phase are equal. Physical stability with respect to crystallization of drug from solution for such nanoparticle aqueous suspensions is a function of the degree of supersaturation of the aqueous medium, which in turn is a function of drug activity or loading in the nanoparticles. (Drug activity is defined as the ratio of solubilized drug provided by the nanoparticle [C] to the solubility of bulk crystalline drug [S].) If the drug activity is higher than crystalline, indefinite physical stability in the liquid state is not to be expected, due to the driving force for crystallization of dissolved drug. However, kinetic stability in suspension of many days is typically observed for formulations with activities 3- to 10-fold that of crystalline drug. In this case, activity is less than 2, so the nanoparticles could be expected to have good kinetic stability. Note that the drug/polymer nanoparticles described here also have excellent physical stability in suspension with respect to aggregation, due to the use of the charged surface-active agents.
To produce a solid dosage form such as a tablet or capsule, drying the nanoparticle suspensions to form a resuspendable powder is desirable. In addition to making processing simpler, this step also typically increases the storage stability with regard to crystallization. To achieve rapid absorption due to quick release of drug, the solid should ideally resuspend quickly and completely into the original unaggregated, high-surface-area nanoparticles when exposed to aqueous medium.
To promote this, a water-soluble matrix material, such as a sugar or hydrophilic polymer, is typically used to prevent close approach of the nanoparticles, which can lead to aggregation upon rewetting (22). We have found that water-soluble amphiphilic polymeric matrix materials are especially efficient for this purpose, including enteric polymers in nanoparticles with casein (23) and neutral polymers in nanoparticles with casein (24). For the celecoxib nanoparticles described here, a casein matrix comprising only 20% to 30% of the dry powder resulted in almost quantitative recovery of the original (unaggregated) nanoparticles upon resuspension at pH 6.5. The improved resuspension observed at the typical intestinal pH of 6.5, relative to typical gastric pH of 2, is likely due to the carboxy groups on the casein, which become charged at the higher pH, aiding resuspension through charge-charge repulsion and increased water solubility. Other amphiphilic synthetic polymers, such as certain cellulosics (e.g., carboxymethyl ethyl cellulose [CMEC] and HPMCAS-L), likewise perform well as resuspension matrix materials (25).
The powders in this study are made by spray-drying an aqueous suspension of nanoparticles and dissolved casein. Spray-drying is a convenient and effective process to prepare solid powders of nanoparticles. In particular, the rapid drying kinetics associated with the process helps prevent irreversible aggregation during drying.
Note that SDDs are not discussed at length in this paper; they have been described in detail elsewhere (26,27). In general, SDDs prepared with the enteric polymer HPMCAS provide excellent total exposure for low-solubility, lipophilic compounds such as celecoxib (14,28). The formulation described in this paper has not been optimized for rapid onset and is referenced only as a commonly used comparator for this paper.
Pharmacokinetics in Dog and Human
The polymeric nanoparticles described in this study were studied in the dog and human. In the dog study, the nanoparticles had a 1:1 celecoxib:ethyl cellulose ratio, which would be expected to provide free-drug concentrations of approximately 1.5 times higher than crystalline drug (as shown in release curve in Fig. 4). All else being equal, the extent of absorption is proportional to free-drug concentration. Therefore, part of the increase in AUC for the nanoparticles relative to the crystalline drug is likely due to higher dissolved-drug levels for the nanoparticles. The remainder of the AUC improvement was likely due to the increased rate of celecoxib release from the nanoparticles relative to the rate of dissolution of bulk crystalline celecoxib (Fig. 4). In vivo and in vitro data show that drying of the nanoparticles followed by resuspension before dosing had no significant effect on absorption rate in vivo.
In the human clinical study, administration of 400 mg of celecoxib as the nanoparticle and the HPMCAS-based SDD formulations produced higher AUC values than seen with an equivalent dose of the commercial capsules. The increase in AUC in humans with the nanoparticles relative to the commercial capsule was less than that seen in the dog study. In the studies presented here, absorption was likely higher in humans than in dogs when celecoxib was dosed as the capsule, thus limiting the magnitude of absorption enhancement possible when a nanoparticle formulation is given. The more complete absorption, particularly with the nanoparticle formulation, in humans may be related to the smaller relative dose in humans (3 to 6 mg/kg) versus that in dogs (20 to 30 mg/kg), as the relative advantage of a solubilized form increases with dose for low-solubility drugs. This hypothesis of complete absorption for the nanoparticle formulation is supported by the fact that the plasma levels, and therefore absorption, decreases sharply approximately 1 h after the nanoparticles are dosed, whereas absorption continues for at least 4 h with administration of the commercial capsules.
The rate of absorption for the nanoparticle formulation in the clinical study was the fastest of the three formulations, with a Tmax of 0.75 h. This rapid rate of absorption for the nanoparticle formulation relative to the other formulations tested would likely result in a commensurate decrease in the time to onset of pharmacological action for the nanoparticle formulation. In fact, in this study the nanoparticle formulation achieved plasma concentrations approximately equal to that of the Cmax for the commercial capsule in only 15 min. This result can be attributed to the rapid release from the nanoparticles in the GI tract, allowing rapid free-drug resupply as drug is absorbed.
The SDD OPC formulation provided similar AUC to the nanoparticles, but absorption was slower, with a Tmax of 2.0 h. The longer Tmax may be due to several factors, including a slower transit time through the gastric, slower release from the SDD particle, and lower free-drug levels. The longer Tmax is most likely partially due to slower drug release from HPMCAS-based SDDs. Drug release from enteric SDDs in gastric is often slow, since HPMCAS does not dissolve at gastric pH. Upon transiting to the higher-pH duodenum, HPMCAS SDDs dissolve to provide high levels of dissolved drug and drug/polymer colloids (14). Nanoparticles have higher surface areas and shorter required drug diffusion distances for release and, thus, readily source high levels of dissolved drug beginning in the gastric, and continuing into the duodenum. Note that the release from the nanoparticles is expected to be faster than for the SDD, even though the SDD can disintegrate/dissolve in the higher pH environment of the intestine. The release rate of the SDD would have likely been slower if an insoluble polymer, such as the ethyl cellulose used for the nanoparticles, had been used rather than an enteric polymer. The differing release characteristics likely account, in part, for the similar AUC but a longer Tmax and lower Cmax for the SDD. More work is required to determine the extent to which differing dissolution rates, GI transit times, and levels of dissolved drug contribute to the relatively slower absorption rate for the SDD compared to the nanoparticles.
The nanoparticles described here were dosed as a suspension rather than as a solid dosage form, which may have provided a release advantage because no disintegration of a dosage form was necessary, as it was for the capsule. In addition, the transit time of small nanoparticles through the stomach may have been shorter than for the capsule, which may have led to faster absorption. Based on previous experience, however, it is likely that a rapidly disintegrating tablet or capsule can be devised that maintains the performance advantage that the nanoparticles demonstrated with the OPC formulation. Demonstration of this would be part of future work.
CONCLUSIONS
The feasibility of using drug/polymer nanoparticles to improve oral bioavailability and achieve rapid absorption was demonstrated by incorporating celecoxib as a model compound within ethyl cellulose/casein nanoparticles. These nanoparticles, which had diameters of 100 to 150 nm, were prepared using an emulsion process. The nanoparticles consisted of a single amorphous phase of drug dispersed within the ethyl cellulose matrix, in which the drug has appreciable solubility (0.30 g/g polymer). Drug release from the particles was rapid, reaching its equilibrium value in the dissolution medium within 1 min. At high drug loading, the nanoparticles provided dissolved-drug levels higher than crystalline solubility. The nanoparticle suspensions were stable for several days and can be spray-dried to form a dry powder that resuspends in water to yield the original unaggregated nanoparticles.
The nanoparticles provided higher total celecoxib exposure and faster absorption in dogs and humans than commercial capsules. In humans, the celecoxib Tmax was 0.75 h for the nanoparticle formulation versus 3 h for the commercial capsules.
Based on the results presented here, drug/polymer nanoparticles can provide higher bioavailability than micronized crystals of BCS Class II compounds. In addition, they can provide more rapid absorption and, therefore, potentially more rapid onset of action, than either micronized crystals or slower dissolving amorphous drug forms of BCS Class II compounds. The nanoparticles described here are also amenable to preparation as a solid dosage form.
ACKNOWLEDGMENTS & DISCLOSURES
The authors would like to thank Dr. Bill Curatolo, Dr. Dwayne Friesen, Dr. David Lyon, and Dan Dobry for valuable discussions; Dr. Jeremy Bartlett for contributions to the proof-of-concept formulations used in clinical studies; and Brice Murri, Franz Lembke, Dr. David Vodak, John Baumann, Ryan Holcomb, Tom Whitehead, Eric Sieber, Leah King, Jim Mullin, and Bob Barss for their contributions to this work.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
To our knowledge, no absolute bioavailability has been reported for the commercial capsule in humans, but the bioavailability of celecoxib has been reported to be 22% to 40% in dogs (17).
REFERENCES
- 1.Lipinski CA. Drug-Like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods. 2000;44:235–249. doi: 10.1016/S1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 2.Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58:173–182. doi: 10.1016/j.biopha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Hauss DJ. Oral lipid based formulations. Adv. Drug Deliv. Rev. 2007;59:667–676. doi: 10.1016/j.addr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Amidon GL, Lennernas H, Shaw VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995;12:413–420. doi: 10.1023/A:1016212804288. [DOI] [PubMed] [Google Scholar]
- 5.Butler JM, Dressman JM. The developability classification system: application of biopharmaceutics concepts to formulation development. J. Pharm Sci. 2010;99:4940–4954. doi: 10.1002/jps.22217. [DOI] [PubMed] [Google Scholar]
- 6.Muller RH, Keck CM. Challenges and solutions for the delivery of biotech drugs—a review of drug nanocrystal technology and lipid nanoparticles. J. Biotech. 2004;113:151–170. doi: 10.1016/j.jbiotec.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Van Eerdenbrugh B, Van den Mooter G, Augustijns P. Top-down production of drug nanocrystals: nanosuspension stabilization, miniaturization and transformation into solid products. Int. J. Pharm. 2008;364:64–75. doi: 10.1016/j.ijpharm.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Connors KA. Population characteristics of cyclodextrin complex stabilities in aqueous solution. J. Pharm. Sci. 1995;84(7):843–848. doi: 10.1002/jps.2600840712. [DOI] [PubMed] [Google Scholar]
- 9.Porter CJH, Pouton CW, Cuine JF, Charman WN. Enhancing intestinal drug solubilisation using lipid-based delivery systems. Adv. Drug Deliv. Rev. 2008;60:673–691. doi: 10.1016/j.addr.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Wu Y, Loper A, Landis E, Hettrick L, Novak L, Lynn K, Chen C, Thompson K, Higgins R, Batra U, Shelukar S, Kwei G, Storey D. The role of biopharmaceutics in the development of a clinical nanoparticle formulation of MK-0869: a beagle dog model predicts improved bioavailability and diminished food effect on absorption in human. Int.l J. Pharm. 2004;285:135–146. doi: 10.1016/j.ijpharm.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Hu J, Johnson KP, Williams RO III. Nanoparticle engineering processes for enhancing the dissolution rates of poorly water soluble drugs. Drug Dev Ind Pharm. 2004;30:233–45. [DOI] [PubMed]
- 12.Leuner C, Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000;50:47–60. doi: 10.1016/S0939-6411(00)00076-X. [DOI] [PubMed] [Google Scholar]
- 13.Yu L. Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv. Drug Deliv. Rev. 2001;48:27–42. doi: 10.1016/S0169-409X(01)00098-9. [DOI] [PubMed] [Google Scholar]
- 14.Friesen DT, Shanker RM, Crew MD, Smithey DT, Curatolo WJ, Nightingale JAS. Hydroxypropyl methyl cellulose acetate succinate-based spray-dried dispersions: An overview. Mol. Pharm. 2008;5:1003–1019. doi: 10.1021/mp8000793. [DOI] [PubMed] [Google Scholar]
- 15.Yang W, Tam J, Miller DA, Zhou J, McConville JT, Johnston KP, Williams RO. High bioavailability from nebulized itraconazole nanoparticle dispersions with biocompatible stabilizers. Int. J. Pharm. 2008;361:177–188. doi: 10.1016/j.ijpharm.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Hancock BC, Parks M. What is the true solubility advantage for amorphous pharmaceuticals? Pharm. Res. 2000;17:397–404. doi: 10.1023/A:1007516718048. [DOI] [PubMed] [Google Scholar]
- 17.Paulson SK, Vaughn MB, Jessen SM, Lawal Y, Gresk CJ, Yan B, Maziasz TJ, Cook CS, Karim A. Pharmacokinetics of celecoxib after oral administration in dogs and humans: effect of food and site of absorption. J. Pharmacol. Exp. Ther. 2001;297:638–645. [PubMed] [Google Scholar]
- 18.van Wijngaarden J, van Beek E, van Rossum G, van der Bent C, Hoekman K, van der Pluijm G, van der Pol MA, Broxterman HJ, van Hinsbergh VWM, Lőwik CWGM. Celecoxib enhances doxorubicin-induced cytotoxicity in MDA-MB231 cells by NF-κB-mediated increase of intracellular doxorubicin accumulation. Eur. J. Cancer. 2007;43:433–442. doi: 10.1016/j.ejca.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Chawla G, Gupta P, Thilagavathi R, Chakraborti AK, Bansal AK. Characterization of solid-state forms of celecoxib. Eur. J. Pharm. Sci. 2003;20:305–317. doi: 10.1016/S0928-0987(03)00201-X. [DOI] [PubMed] [Google Scholar]
- 20.Dolenc A, Kristl J, Baumgartner S, Planinšek O. Advantages of celecoxib nanosuspension formulation and transformation into tablets. Int. J. Pharm. 2009;376:204–212. doi: 10.1016/j.ijpharm.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 21.Nasr M. In vitro and in vivo evaluation of proniosomes containing Celecoxib for oral administration. AAPS Pharm. Sci. Tech. 2010;11:85–89. doi: 10.1208/s12249-009-9364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdelwahed W, Degobert G, Stainmesse S, Fessi H. Freeze-drying of nanoparticles: formulation, process and storage considerations. Adv. Drug Del. Rev. 2006;58:1688–1713. doi: 10.1016/j.addr.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Beyerinck RA, Bloom CJ, Crew MD, Friesen DT, Morgen MM, Smithey DT. Pharmaceutical compositions based on a) nanoparticles comprising enteric polymers and b) casein. World International Patent Office Application WO/2008/065502. 2008a.
- 24.Beyerinck RA, Smithey DT, Miller WK, Morgen MM, Bloom CJ. Pharmaceutical compositions comprising nanoparticles and casein. World International Patent Office Application WO/2008/135852. 2008b.
- 25.Miller WK, Smithey DT, Tadday R, Frankamp BL. Pharmaceutical compositions comprising nanoparticles and a resuspending material. World International Patent Office Application WO/2009/073215. 2009.
- 26.Vehring R. Pharmaceutical particle engineering via spray-drying. Pharm Res. 2007;25:999–1022. doi: 10.1007/s11095-007-9475-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dobry DE, Settell DM, Baumann JM, Ray RJ, Graham LJ, Beyerinck RA. A model-based methodology for spray-drying process development. J. Pharm. Innov. 2009;4:133–142. doi: 10.1007/s12247-009-9064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curatolo WJ, Nightingale JAS, Herbig SM. Utility of hydroxypropylmethyl cellulose acetate succinate (HPMCAS) for initiation and maintenance of drug supersaturation in the GI milieu. Pharm. Res. 2009;26:1419–1431. doi: 10.1007/s11095-009-9852-z. [DOI] [PubMed] [Google Scholar]


