Abstract
Recent studies have found that processing information according to an evolutionary relevant (i.e., survival) scenario improves its subsequent memorability, potentially as a result of fitness advantages gained in the ancestral past. So far, research has not revealed much about any proximate mechanisms that might underlie this so-called survival processing advantage in memory. Intriguingly, research has shown that the memorability of stressful situations is enhanced via the release of stress hormones acting on brain regions involved in memory. Since survival situations habitually involve some degree of stress, in the present study, we investigated whether stress serves as a proximate mechanism to promote survival processing. Participants rated words for their relevance to either a survival or a neutral (moving) scenario after they had been exposed to a psychosocial stressor or a no-stress control condition. Surprise retention tests immediately following the rating task revealed that survival processing and acute stress independently boosted memory performance. These results therefore suggest that stress does not serve as a proximate mechanism of the survival processing advantage in memory.
Keywords: Adaptive memory, Trier Social Stress Test (TSST), Cortisol
An accumulating body of research indicates that there is an important evolutionary dimension to memory—that is, that memory evolved so as to remember fitness-relevant information (e.g., Nairne & Pandeirada, 2008a, 2008b, 2010; Nairne, Pandeirada, & Thompson, 2008; Nairne, Thompson, & Pandeirada, 2007). Thus, it has been suggested that natural selection, via fitness benefits accrued in the ancestral past, “tuned” memory to promote the processing of survival-relevant information. In support of this suggestion, Nairne et al. (2007) showed that processing a list of words for its fitness value leads to superior retention performance as compared with processing those words in other contexts (e.g., embedded in a moving scenario). A similar survival processing advantage has been found under a variety of control conditions (e.g., when control contexts were matched in terms of novelty or arousal; see Kang, McDermott, & Cohen, 2008; Nairne, Pandeirada, Gregory, & Van Arsdall, 2009; Otgaar et al., 2011; Weinstein, Bugg, & Roediger, 2008) as well as when pictures instead of words were employed (Otgaar, Smeets, & van Bergen, 2010). Together, these results indicate that the survival processing advantage is a robust phenomenon, possibly reflecting an adaptive function. Nonetheless, to date not much is known about any proximate mechanisms that might trigger this survival processing advantage in memory.
One potential proximate mechanism relates to stressful events being remembered better than neutral ones, an effect driven by adrenal stress hormones that act on brain structures involved in regulating human memory performance (e.g., the amygdala and hippocampal formation; see Labar & Cabeza, 2006; McGaugh & Roozendaal, 2002; Phelps, 2004). Crucially, although stress and heightened glucocorticoid (i.e., cortisol in humans) stress hormone concentrations generally impair memory retrieval processes (e.g., de Quervain, Roozendaal, Nitsch, McGaugh, & Hock, 2000; Smeets, 2011; Smeets, Otgaar, Candel, & Wolf, 2008), they can enhance memory when released around the time of learning (i.e., during the encoding or consolidation phase; see, e.g., Cahill, Gorski, & Le, 2003; Joëls, Pu, Wiegert, Oitzl, & Kruger, 2006; Smeets, Giesbrecht, Jelicic, & Merckelbach, 2007; Smeets et al., 2008). Clearly, enhanced storage of emotionally arousing events in times of stress has an adaptive value since such a privileged processing guarantees that information most relevant to survival is given high priority (Roozendaal, McEwen, & Chattarji, 2009).
Situations in which survival is critical are often stressful (e.g., when robbed at gunpoint or attacked by a vicious animal). When encountering such a situation, physiological stress responses that include the fight-or-flight response (e.g., adrenalin and noradrenalin release) and activation of the hypothalamic–pituitary–adrenal axis (e.g., cortisol secretion) help to increase the probability of survival. In concert, these stress reactions namely not only result in supplementary energy becoming available for the individual to act in response to the stressor, but also facilitate the storage of memories related to the survival situation (see above). Nonetheless, research so far has consistently demonstrated the mnemonic benefits of survival processing in laboratory situations devoid of stress, and thus may have underestimated the actual magnitude of the survival processing advantage in memory.
In the present study, we examined whether the memory advantage associated with survival processing would be even greater in times of stress than under no-stress control conditions. Specifically, we exposed participants to a psychosocial stressor or no-stress control condition after which they had to rate words for their relevance to a survival or a moving scenario. As is typical in the survival processing paradigm, recall was tested after a short (2–3 min) retention interval. Survival processing following stress exposure was expected to yield higher levels of retention than survival processing in the absence of stress, whereas information processing according to the moving scenario in the no-stress control condition was expected to yield the lowest levels of retention performance.
Method
Participants
Eighty healthy young male undergraduates (mean age: 22.47 years; SE = 0.51) participated in the study. Eligibility was assessed using a structured interview, with cardiovascular diseases, severe physical illnesses (e.g., fibromyalgia), endocrine disorders, current or lifetime psychopathology, substance abuse, smoking > 10 cigarettes/day, or being on medication serving as exclusion criteria. Test protocols were approved by the standing ethics committee of the Faculty of Psychology and Neuroscience, Maastricht University. Participants provided informed consent and received a financial reimbursement (12.5€) for participating.
Procedure
Participants were tested in individual sessions between 09 hr and 12 hr. To allow for controlled saliva collection, participants were asked not to brush their teeth and were deprived of food, drinks, and heavy exercise at least 1 hr prior to the test phase. Participants were randomly allocated to a stress or a no-stress control condition and received either survival processing instructions or a moving scenario (see below) so that four groups were formed: survival–control (n = 20); moving–control (n = 20); survival–stress (n = 20); and moving–stress (n = 20). Figure 1 depicts the sequence of the experimental events.
Fig. 1.
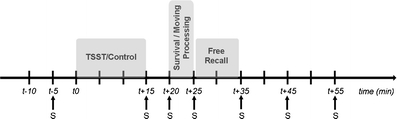
Sequence of the experimental events, with t0 referring to onset of the TSST or control condition. Ss denote times when saliva was sampled
Stress versus no-stress control procedure
Stress was induced using the Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, 1993), a psychosocial challenge test that basically consists of a preparation period, a 5-min mental arithmetic task, and a 5-min speech on any topic of participants’ choice. In keeping with our previous work (e.g., Smeets et al., 2009), the TSST was rendered even more demanding by asking participants to critically describe their own personality in English (i.e., a non-native language) while standing in front of a live audience and being audio- and video-taped.
In the no-stress control condition, a control version of the TSST was used in which participants were instructed to give a speech on any topic of their choice and to perform a simple mental arithmetic task without the uncontrollability and social-evaluative threat of the TSST (i.e., in the absence of an experimenter, live audience, and recordings; see Het, Rohleder, Schoofs, Kirschbaum, & Wolf, 2009).
Manipulation check
Cortisol was measured in response to the TSST to evaluate the stress responsiveness of the hypothalamic–pituitary–adrenal axis. Cortisol data were obtained with synthetic Salivette (Sarstedt®, Etten-Leur, the Netherlands) devices over a 60-min period at seven assessment points: t−5, t+15, t+20, t+25, t+35, t+45, and t+55 min with reference to the start of the stressor (t0). Saliva samples were stored at −20°C immediately on collection. Cortisol levels were determined by a luminescence immuno assay with high sensitivity (IBL, Hamburg, Germany). Mean intra- and interassay coefficients of variation are typically less than 8% and 12%, respectively.
Survival processing paradigm
A Dutch translation of the stimulus materials employed in previous studies (e.g., Nairne et al., 2007; Otgaar et al., 2010) was used, consisting of 30 words of typical members selected from 30 unique categories (Van Overschelde, Rawson, & Dunlosky, 2004). Two additional words that were selected according to the same criteria were used as practice words. Words were presented in the same random order for a duration of 5 s on a 17-in. computer screen using PowerPoint (Microsoft Corporation) in capitals with font type Arial, font size 44. Participants were instructed to rate the words for their relevance to a predescribed scenario (survival vs. moving) using a 5-point scale (anchors: 1 = totally irrelevant; 5 = extremely relevant). The instructions were as follows:
Survival
“In this task, we would like you to imagine that you are stranded in the grasslands of a foreign land, without any basic materials. Over the next few months, you’ll need to find steady supplies of food and water and protect yourself from predators. We are going to show you some words, and we would like you to rate how relevant each word would be in this survival condition. Some of the words may be relevant and others not-it’s up to you to decide.”
Moving
“In this task, we would like you to imagine that you are planning to move to a new home in a foreign land. Over the next few months, you’ll need to locate and purchase a new home and transport your belongings. We are going to show you some words, and we would like you to rate how relevant each word would be in this moving condition. Some of the words may be relevant and others not-it’s up to you to decide.”
In keeping with previous work (e.g., Nairne et al., 2009; Nairne et al., 2008, and Nairne et al., 2007), a short (2–3 min) retention interval was implemented after the rating task. Next, memory for the presented words was assessed using a surprise free recall task in which participants were instructed to write down all the words they had seen during the rating task. The recall task lasted approximately 10 min.
Results
Manipulation check
To normalize the data, cortisol measures were log-transformed before analyses. Figure 2 shows the cortisol responses to the (control version of the) TSST for each of the groups. An ANOVA showed a significant Time × TSST Condition interaction, F(6, 450) = 15.17; p < .001; 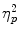 = 0.17, as well as main effects of time F(6, 450) = 27.35; p < .001;
= 0.17, as well as main effects of time F(6, 450) = 27.35; p < .001; 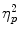 = 0.27, and TSST condition, F(1, 75) = 16.66; p < .001;
= 0.27, and TSST condition, F(1, 75) = 16.66; p < .001;  = 0.18, but no main or interactive effects involving scenario (all Fs < 1; all ps > .69). Bonferroni corrected post hoc tests showed that although cortisol levels did not differ between the stress and control groups prior to the TSST/control condition (t−5; p = .50), the stress groups displayed higher cortisol levels at t+15 (p = .004), t+20, t+25, t+35, and t+45 (all ps < .001), and t+55 (p = .001). Notice from Fig. 2 that survival processing instructions in the absence of stress (i.e., Survival-Control) did not elevate cortisol levels.
= 0.18, but no main or interactive effects involving scenario (all Fs < 1; all ps > .69). Bonferroni corrected post hoc tests showed that although cortisol levels did not differ between the stress and control groups prior to the TSST/control condition (t−5; p = .50), the stress groups displayed higher cortisol levels at t+15 (p = .004), t+20, t+25, t+35, and t+45 (all ps < .001), and t+55 (p = .001). Notice from Fig. 2 that survival processing instructions in the absence of stress (i.e., Survival-Control) did not elevate cortisol levels.
Fig. 2.
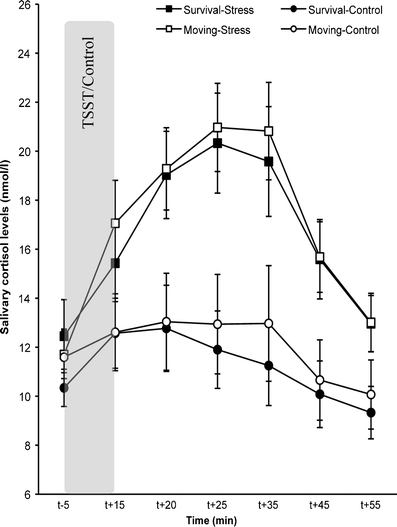
Salivary cortisol responses to the TSST or no-stress control condition for the survival processing and moving condition. Data represent the untransformed means ± standard errors
Stress and survival processing
As can be seen in Fig. 3, survival processing led to higher surprise free recall rates than did processing words according to the moving scenario. Similarly, exposure to the TSST elicited enhanced subsequent recall relative to the control condition. This finding was confirmed by an ANOVA revealing significant effects of scenario, F(1, 76) = 15.96; p < .001; 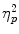 = 0.17, and TSST condition, F(1, 76) = 9.61; p = .003;
= 0.17, and TSST condition, F(1, 76) = 9.61; p = .003; 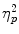 = 0.11, in the absence of a Scenario × TSST Condition interaction [F(1,76) < 1; p = 0.58]. An ANOVA on the amount of commission errors showed a significant effect of scenario, F(1, 76) = 4.34; p = .040;
= 0.11, in the absence of a Scenario × TSST Condition interaction [F(1,76) < 1; p = 0.58]. An ANOVA on the amount of commission errors showed a significant effect of scenario, F(1, 76) = 4.34; p = .040; 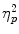 = 0.05, but no effect of TSST condition, F(1, 76) < 1; p = .42, or a Scenario × TSST Condition interaction, F(1, 76) < 1; p = .87 (see also Fig. 3).
= 0.05, but no effect of TSST condition, F(1, 76) < 1; p = .42, or a Scenario × TSST Condition interaction, F(1, 76) < 1; p = .87 (see also Fig. 3).
Fig. 3.
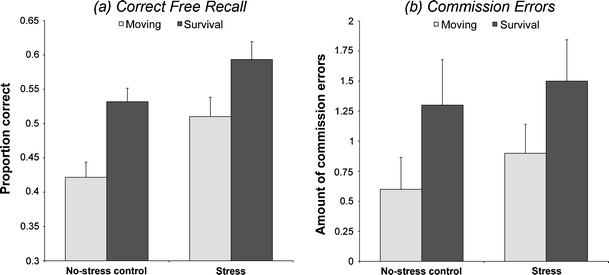
Proportion correct free recall performance (a) and the amount of commission errors (b) as a function of scenario (survival vs. moving) and TSST condition (TSST vs. control). Error bars represent standard errors
Mean relevance ratings are displayed in Table 1. An ANOVA on the rating data showed no Scenario × TSST Condition interaction, F(1, 76) = 2.63; p = .11, nor significant main effects of scenario, F(1, 76) = 1.60; p = .21, or TSST condition, F(1, 76) = 2.35; p = .13. These data imply that it is quite implausible that the relevance ratings can account for the observed differences in recall performance.
Table 1.
Average relevance ratings as a function of scenario (survival vs. moving) and TSST condition (TSST vs. control). Data represent means ± SEMs
| Stress M SEM | Control M SEM | |
|---|---|---|
| Survival | 2.74 ± 0.09 | 2.44 ± 0.10 |
| Moving | 2.47 ± 0.07 | 2.48 ± 0.11 |
Discussion
Evolutionary psychologists have argued that human memory systems evolved throughout time as a result of natural selection. By this view, mnemonic advantages such as the enhanced memorability of survival-relevant information may have been shaped because the ability to use past experiences properly in the present clearly signifies an adaptive advantage. Evidence for this line of argumentation comes from a host of studies (e.g., Nairne et al., 2007; for review, see Nairne & Pandeirada, 2008b, but see Howe & Derbish, 2010; Kroneisen & Erdfelder, 2011; Otgaar & Smeets, 2010; or Soderstrom & McCabe, 2011, for critical notes) that showed that processing information in a survival mode (i.e., for its fitness value) results in this information being recalled more efficiently afterward, even when compared with various control conditions that are geared toward maximal retention performance (e.g., Kang et al., 2008; Nairne et al., 2009; Otgaar et al., 2011; Weinstein et al., 2008). From an evolutionary point of view, this can be explained by assuming that memory likely evolved to enhance fitness and therefore may be tuned to remember fitness-relevant information well. Still, not much is known about the proximate mechanisms that may underlie the survival processing advantage in memory (but see Burns, Burns, & Hwang, 2011, who recently argued that a combination of relational and item-specific processing may drive the survival processing advantage).
In the present study, we investigated whether stress may serve as a proximate mechanism in enhancing memory following survival processing, since it is well established that the human stress response is adapted to increase the likelihood of survival by initiating physiological mechanisms to deal effectively with stressful situations and to improve responses to subsequent stressors. Specifically, when encountering a stressor, the individual almost immediately reacts with the activation of the fight-or-flight response that includes the release of adrenalin and noradrenalin, which in turn produce increases in heart rate, blood pressure, and respiration frequency. A second, slower response is then orchestrated by the hypothalamic–pituitary–adrenal axis in which a cascade of events eventually leads to the adrenal cortex releasing glucocorticoids into the bloodstream. Together, these physiological alterations generate more energy for the body to react to the stressor and for the brain to lay down the memory traces related to the event. Indeed, individuals who were exposed to stress before the word processing task displayed enhanced memory in a subsequent recall task—a finding that is well in line with previous studies demonstrating the mnemonic benefit of storing memories of emotionally arousing events in times of stress (e.g., Cahill et al., 2003; Smeets et al., 2007; Smeets et al., 2008). This is especially relevant given that circumstances in which survival is of primordial importance (as discussed above) are mostly situations that also generate physiological stress responses.
The present results provide further compelling evidence that survival processing and learning under stress are both powerful means to enhance retention, yet we failed to find evidence for the idea that survival processing while being stressed produces larger mnemonic benefits than survival processing or learning under stress alone. This suggests that stress does not serve as a proximate mechanism to enhance retention performance following survival processing. Of course, not all ancestral survival-related cognitive challenges are proximally stressful. For example, remembering the location of food sources or establishing effective hunting strategies are challenges that are relevant to survival, but are unlikely to elicit effective stress reactions. Given that the stressor used in the present study was unrelated to the to-be-remembered information of the survival processing task, the present findings also suggest that generalized stress reactivity is not driving the survival processing advantage. All in all, the present findings fit well with previous work showing that emotional arousal of survival processing does not mediate the survival processing effect (e.g., Kang et al., 2008; Nairne et al., 2007; Otgaar et al., 2010). Otgaar et al. (2010), for example, had participants rate the arousal and valence of the processed stimuli and found that although these individual ratings were associated with overall memory performance, neither measure was associated with the magnitude of the survival processing effect.
It is worth noting that in line with several previous studies (e.g., Howe & Derbish, 2010; Nairne et al., 2007, Experiment 1; Nairne & Pandeirada, 2008a, Experiment 1; Otgaar et al., 2010, Experiment 1; Otgaar & Smeets, 2010), more errors were made when processing information according to the survival scenario than according to the moving scenario. The finding that heightened error rates were specific to the survival processing groups challenges the adaptive value of survival processing. Indeed, from an adaptive memory view, processing fitness-relevant information would be expected to not only increase correct recall but also simultaneously decrease (or at least not influence) error rates (but see Howe & Derbish, 2010, who argued that high levels of commission errors are not necessarily maladaptive by themselves). Note that the present study also revealed that stress did not affect false recall, which is reminiscent of previous work that was specifically designed to investigate the effect of stress and stress hormones on false recollections (e.g., Diekelmann, Wilhelm, Wagner, & Born, 2011; Smeets, Jelicic, & Merckelbach, 2006; Smeets et al., 2008). In none of these studies did stress or the associated cortisol elevations lead to higher false recall rates than no-stress control conditions.
A limitation of the present study is that, in accordance with previous work (e.g., Nairne et al., 2009; Nairne et al.; 2008; and Nairne et al., 2007), the recall test was administered after a brief distraction task. In the present study, recall was thus assessed while the initial stress responses had subsided, but when glucocorticoid (i.e., cortisol) levels were still elevated. Thus, one cannot rule out that the stress-induced cortisol responses not only affected the processing of the stimuli during the rating task, but also the retrieval phase that succeeded the rating and distraction task. To be sure, in contrast with the memory-enhancing effects of stress when administered around the time of learning (e.g., Smeets et al., 2008), stress and associated glucocorticoid responses are known to bring about retrieval deficits (e.g., Smeets, 2011). This suggests that the positive effects of stress on memory obtained in the present study offset any negative effects that may have adversely influenced retrieval performance. A second obvious limitation is that the present study’s design does not allow for any firm conclusion regarding the nonsignificant interaction that accompanied both main effects, since nonordinal interactions (or lack thereof) are not definitive without precise information regarding how the observed variables map onto the underlying constructs (e.g., Loftus, 1978).
In sum, although the present results confirm that the mnemonic benefits of survival processing and those of information processing in times of stress are robust, they also indicate that stress is not the proximate mechanism underlying the survival processing advantage in memory. A promising avenue for future research to pursue would be to examine whether the extent to which one can accurately describe in detail the foreign grasslands, which is assumed to be a crucial section in the survival processing scenario (e.g., Nairne & Pandeirada, 2010), relates to the size of the survival processing advantage. This is all the more interesting given that the prototypical (i.e., undergraduate) participant in this type of study generally lacks knowledge about grassland-based survival situations (Nairne, 2010). The search for proximate mechanisms that may help us understand why processing information according to its fitness relevance is beneficial to memory up until now remains open to further empirical testing.
Author Note
We thank Sandra Hogenboom for assisting in the data collection. The present study was supported in part by Grant 451-08-005 from the Netherlands Organization for Scientific Research (NWO) to T.S. NWO had no further role in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; and in the decision to submit the manuscript for publication.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Burns DJ, Burns SA, Hwang AJ. Adaptive memory: Determining the proximate mechanisms responsible for the memorial advantages of survival processing. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2011;37:206–218. doi: 10.1037/a0021325. [DOI] [PubMed] [Google Scholar]
- Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: Interaction with the degree of arousal at encoding. Learning & Memory. 2003;10:270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Quervain DJF, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nature Neuroscience. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Wilhelm I, Wagner U, Born J. Elevated cortisol at retrieval suppresses false memories in parallel with correct correct memories. Journal of Cognitive Neuroscience. 2011;23:772–781. doi: 10.1162/jocn.2010.21493. [DOI] [PubMed] [Google Scholar]
- Het S, Rohleder N, Schoofs D, Kirschbaum C, Wolf OT. Neuroendocrine and psychometric evaluation of a placebo version of the “Trier Social Stress Test.”. Psychoneuroendocrinology. 2009;34:1075–1086. doi: 10.1016/j.psyneuen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- Howe ML, Derbish MH. On the susceptibility of adaptive memory to false memory illusions. Cognition. 2010;115:252–267. doi: 10.1016/j.cognition.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: How does it work? Trends in Cognitive Sciences. 2006;10:152–158. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kang SHK, McDermott KB, Cohen SM. The mnemonic advantage of processing fitness-relevant information. Memory & Cognition. 2008;36:1151–1156. doi: 10.3758/MC.36.6.1151. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K-M, Hellhammer DH. The “Trier Social Stress Test”: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kroneisen, M., & Erdfelder, E. (2011). On the plasticity of the survival processing effect. Journal of Experimental Psychology: Learning, Memory, and Cognition (in press). [DOI] [PubMed]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Loftus G. On the interpretation of interactions. Memory & Cognition. 1978;6:312–319. doi: 10.3758/BF03197461. [DOI] [Google Scholar]
- McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Current Opinion in Neurobiology. 2002;12:205–210. doi: 10.1016/S0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- Nairne JS. Adaptive memory: Evolutionary constraints on remembering. In: Ross BH, editor. The psychology of learning and motivation. Burlington, Vermont: Academic Press; 2010. pp. 1–32. [Google Scholar]
- Nairne JS, Pandeirada JNS. Adaptive memory: Is survival processing special? Journal of Memory and Language. 2008;59:377–385. doi: 10.1016/j.jml.2008.06.001. [DOI] [Google Scholar]
- Nairne JS, Pandeirada JNS. Adaptive memory: Remembering with a stone-age brain. Current Directions in Psychological Science. 2008;17:239–243. doi: 10.1111/j.1467-8721.2008.00582.x. [DOI] [Google Scholar]
- Nairne JS, Pandeirada JNS. Adaptive memory: Ancestral priorities and the mnemonic value of survival processing. Cognitive Psychology. 2010;61:1–22. doi: 10.1016/j.cogpsych.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Nairne JS, Pandeirada JNS, Gregory KJ, Van Arsdall JE. Adaptive memory: Fitness-relevance and the hunter-gatherer mind. Psychological Science. 2009;20:740–746. doi: 10.1111/j.1467-9280.2009.02356.x. [DOI] [PubMed] [Google Scholar]
- Nairne JS, Pandeirada JNS, Thompson SR. Adaptive memory: The comparative value of survival processing. Psychological Science. 2008;19:176–180. doi: 10.1111/j.1467-9280.2008.02064.x. [DOI] [PubMed] [Google Scholar]
- Nairne JS, Thompson SR, Pandeirada JNS. Adaptive memory: Survival processing enhances retention. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2007;33:263–273. doi: 10.1037/0278-7393.33.2.263. [DOI] [PubMed] [Google Scholar]
- Otgaar H, Smeets T. Adaptive memory: Survival processing increases both true and false memory in adults and children. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2010;36:1010–1016. doi: 10.1037/a0019402. [DOI] [PubMed] [Google Scholar]
- Otgaar H, Smeets T, Merckelbach H, Jelicic M, Verschuere B, Galliot A, van Riel L. Adaptive memory: Stereotype activation is not enough. Memory & Cognition. 2011;39:1033–1041. doi: 10.3758/s13421-011-0091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otgaar H, Smeets T, van Bergen S. Picturing survival memories: Enhanced memory for fitness-relevant processing occurs for verbal and visual stimuli. Memory & Cognition. 2010;38:23–28. doi: 10.3758/MC.38.1.23. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Current Opinion in Neurobiology. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nature Reviews Neuroscience. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Smeets T. Acute stress impairs memory retrieval independent of time of day. Psychoneuroendocrinology. 2011;36:495–501. doi: 10.1016/j.psyneuen.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Smeets T, Giesbrecht T, Jelicic M, Merckelbach H. Context-dependent enhancement of declarative memory performance following acute psychosocial stress. Biological Psychology. 2007;76:116–123. doi: 10.1016/j.biopsycho.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Smeets T, Jelicic M, Merckelbach H. Stress-induced cortisol responses, sex differences, and false recollections in a DRM-paradigm. Biological Psychology. 2006;72:164–172. doi: 10.1016/j.biopsycho.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Smeets T, Otgaar H, Candel I, Wolf OT. True or false? Memory is differentially affected by stress-induced cortisol elevations and sympathetic activity at consolidation and retrieval. Psychoneuroendocrinology. 2008;33:1378–1386. doi: 10.1016/j.psyneuen.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Smeets T, Wolf OT, Giesbrecht T, Sijstermans K, Telgen S, Joëls M. Stress selectively and lastingly promotes learning of context-related high arousing information. Psychoneuroendocrinology. 2009;34:1152–1161. doi: 10.1016/j.psyneuen.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Soderstrom NC, McCabe DP. Are survival processing memory advantages based on ancestral priorities? Psychonomic Bulletin & Review. 2011;18:564–569. doi: 10.3758/s13423-011-0060-6. [DOI] [PubMed] [Google Scholar]
- Van Overschelde JP, Rawson KA, Dunlosky J. Category norms: An updated and expanded version of the Battig and Montague (1969) norms. Journal of Memory and Language. 2004;50:289–335. doi: 10.1016/j.jml.2003.10.003. [DOI] [Google Scholar]
- Weinstein Y, Bugg JM, Roediger HL., III Can the survival recall advantage be explained by basic memory processes? Memory & Cognition. 2008;36:913–919. doi: 10.3758/MC.36.5.913. [DOI] [PubMed] [Google Scholar]


