Abstract
Background and Purpose
Cell therapy with bone marrow stromal cells (BMSCs) improves functional recovery after stroke in nondiabetic rats. However, its effect on diabetics with stroke is unknown. This study investigated the effect of BMSCs on stroke outcome in Type 1 diabetic (T1DM) rats.
Methods
T1DM was induced in adult male Wistar rats by injecting streptozotocin. Nondiabetic and T1DM rats were subjected to 2 hours of middle cerebral artery occlusion (MCAO), treated with or without BMSCs (3×106) at 24 hours after MCAO, and monitored for 14 days.
Results
Functional benefit was not detected in T1DM-MCAO treated with BMSC rats compared with corresponding T1DM-MCAO controls. BMSC treatment in T1DM-MCAO rats had increased mortality, blood–brain barrier leakage, brain hemorrhage, and angiogenesis. Internal carotid artery neointimal formation and cerebral arteriole narrowing/occlusion were also observed in T1DM-MCAO+BMSCs rats compared with T1DM-MCAO controls (P<0.05), but not in nondiabetic stroke rats. We further studied the underlying mechanisms responsible for BMSC-induced blood–brain barrier leakage and accelerated vascular damage in T1DM-MCAO rats. We found that the expression of angiogenin (an angiogenic factor) and ED1 (a marker for macrophages) was significantly increased in the T1DM-MCAO+BMSC rats in the ischemic brain and internal carotid artery compared with nontreated T1DM-MCAO rats, but not in nondiabetic stroke rats.
Conclusions
BMSC therapy in T1DM-MCAO rats does not improve functional outcome. On the contrary, it increases blood–brain barrier leakage and cerebral artery neointimal formation, and arteriosclerosis, which possibly is due to increased expression of angiogenin. Thus, BMSC treatment starting 24 hours after MCAO may not be beneficial for diabetic subjects with stroke.
Keywords: angiogenin, arteriosclerosis, bone marrow stromal cell, stroke, Type 1 diabetic
Diabetes instigates a cascade of events leading to vascular endothelial cell dysfunction, increased vascular permeability,1 and poor recovery after ischemic stroke.2 Tissue plasminogen activator treatment of stroke attenuates infarct growth in nondiabetics but not in diabetics3 and induces an increased incidence of intracerebral hemorrhage and unfavorable outcomes.4 Therefore, response to treatment of stroke in patients with diabetes may differ from that in the nondiabetic population.
Bone marrow stromal cell (BMSC) therapy is a promising approach to facilitate regeneration and functional recovery after stroke in nondiabetics. BMSC treatment stimulates angiogenesis, vascular stabilization, and promotes functional outcome after stroke in nondiabetic rat.5,6 BMSCs exhibit a strong adhesive capacity and contribute to vascular remodeling of diseased vessels, but also induce intimal hyperplasia.7 Diabetic animals have vascular injury and are prone to arteriosclerosis. The effect of BMSC on stroke outcome and vascular lesions in diabetic subjects has not been investigated.
Angiogenin is a member of the ribonuclease superfamily and promotes degradation of basement membrane and extra-cellular matrix, allowing endothelial cells to proliferate, penetrate, and migrate into the perivascular tissue.8 Plasma angiogenin levels are related to heart failure severity9 and have been implicated in the pathogenesis myocardial ischemia10 and associated with inflammation and atherosclerosis. However, the role of angiogenin is not clear in angiogenic response to ischemic stroke, particularly in animals with diabetes subjected to stroke. We address the clinically relevant question whether BMSC treatment of stroke in streptozotocin-induced Type 1 diabetes (T1DM) rats affects functional outcome and angiogenin expression.
Materials and Methods
Adult male Wistar rats (225–250 g; Jackson Laboratory, Wilmington, MA) were used to induce T1DM by a single intraperitoneal injection of streptozotocin (Sigma Chemical Co, St Louis, MO; 60 mg/kg).11 The fasting blood glucose was tested by using a glucose analyzer (Accu-Chek Compact System; Roche Diagnostics, Indianapolis, IN). Diabetes was defined by fasting blood glucose >300 mg/dL. Animals were used 2 weeks after diabetes induction.
BMSC Culture
Normal male Wistar rat (n=6/group) bone marrow was isolated and cultured in α-Dulbecco’s modified Eagle’s medium media with 20% fetal bovine serum and 1% penicillin streptomycin. Cells were maintained at 37°C in 5% CO2 and nonadherent cells were removed. BMSCs were used within passage 4.
Animal Middle Cerebral Artery Occlusion Model and Experiment Group
Nondiabetic wild-type (WT; Jackson Laboratory, Wilmington, MA) rats and T1DM rats were initially anesthetized with 3.5% halothane and maintained with 1.0% to 2.0% halothane. Right temporal (2 hours) middle cerebral artery occlusion (MCAO) was induced using the filament model, as previously described.5 After 2 hours of MCAO, restoration of blood flow was performed by withdrawal of the filament. Sham-operated rats underwent the same surgical procedure without suture insertion.
Rats were randomized and assigned to different groups and were treated starting 24 hours after surgery and intravenous injection through the tail vein with: (1) WT-MCAO control: phosphate-buffered saline (n=8); (2) WT-MCAO-BMSC treatment: BMSCs (3×106) in WT-MCAO rats (n=8); (3) T1DM-MCAO control: phosphate-buffered saline in T1DM-MCAO rats (n=13); (4) T1DM-MCAO+BMSC treatment: BMSCs (3×106) in T1DM-MCAO rats (n=12); (5) WT-sham control: phosphate-buffered saline (n=4); and (6) T1DM-sham control: phosphate-buffered saline (n=4).
Our previous studies have found that BMSC (3×106) treatment improves functional outcome after stroke in WT-MCAO rats.5 Therefore, a dose of 3×106 BMSCs was used. A battery of functional outcome tests was performed before MCAO and at 1, 7, and 14 days after MCAO. The animals were euthanized at 14 days after MCAO for immunostaining.
Neurological Functional Tests
Adhesive removal test, Foot-fault test, and a modified neurological severity score evaluation were performed by an investigator who was blinded to the experimental groups.5 Modified neurological severity score is a composite of motor, sensory, balance, and reflex tests. Rats were excluded if modified neurological severity score was <6 or >13 before treatment.
Mortality Rate
The number of dead animals in each group was counted and the mortality rate is presented as a percentage of rats that died between 24 hours and 14 days after MCAO to the total animals in each group.
Brain Hemorrhage Measurement
Brain hemorrhage were measured by using hematoxylin and eosin staining under light microscopy including the animals that died in the early stage after stroke. The percentage areas of petechial and gross hemorrhage were measured in each histological section and summed.
Blood–Brain Barrier Leakage Measurement
WT-MCAO rats and T1DM-MCAO rats (n=5/group) were treated with or without BMSCs (3×106). Five days after MCAO, 2% Evans blue dye was injected intravenously at 2 hours before euthanasia. Evans blue dye fluorescence intensity was measured using a microplate fluorescence reader (excitation 620 nm and emission 680 nm).12
Lesion Volume and Histological Assessment
All brains were fixed by transcardial perfusion with saline followed by perfusion and immersion in 4% paraformaldehyde and then embedded in paraffin. Seven coronal sections of tissue were processed and stained with hematoxylin and eosin for calculation of lesion volume and presented as a percentage of the lesion compared with the contralateral hemisphere.
A standard block was obtained from the center of the lesion (bregma −1 mm to +1 mm). A series of 6-μm thick sections was cut from the block. Every tenth coronal section for a total 5 sections was used for immunohistochemical staining. Antibody against von Willebrand factor (a endothelial cell marker, 1:400; Dako, Carpinteria, CA), α-smooth muscle actin (a SMC marker, mouse monoclonal IgG 1:800; Dako), angiogenin (monoclonal, 1:500; Abcam, Cambridge, MA), a mouse mAb against rat microglia/macrophages (ED1, monoclonal, 1:30; AbD Serotec Raleigh, NC). Control experiments consisted of staining brain coronal tissue sections as outlined previously, but nonimmune serum was substituted for the primary antibody. The immunostaining analysis was performed by an investigator blinded to the experimental groups.
Angiogenin and ED1 Expression Quantification
Each slide containing 8 fields of view from the ischemic border zone were digitized (Olympus BX40) interfaced with an MCID image analysis system (Imaging Research, St Catharines, Canada). The ischemic border zone is defined as the area surrounding the lesion. The data from 5 sections and 8 regions within each section were averaged to obtain a single value for 1 animal and presented as percentage of positive area of immunoreactive cells, respectively. Angiogenin and ED1 expression in the internal carotid artery was measured and presented as percent of total artery wall area.
Vascular Density Measurement
The vascular density of the von Willebrand factor-immunostained coronal section was digitized and counted in the ischemic border zone through the MCID computer imaging analysis system.
Alpha-Smooth Muscle Actin-Positive Coated Vessel Density, Artery Wall Thickness, and Occlusion Measurement
The density of α-smooth muscle actin-stained vessels was analyzed with regard to small and large vessels (≥10 μm diameter) in the ischemic border zone. The 10 largest arterial wall thicknesses were measured. The numbers of α-smooth muscle actin-immunoreactive vessels were counted. The total number of α-smooth muscle actin-positive vessels per millimeter squared is presented. In addition, the total number of occluded arteries in the ipsilateral hemisphere was counted.
Trichrome Immunostaining and Measurement
Using Gomori One-Step Trichrome Stain (Sigma, St Louis, MO), brain sections were postfixed in Bouin fixative. Nuclei are stained with Weigert hematoxylin and then stained in Gomori trichrome stain followed by a 0.5% acetic water rinse. Connective tissue and collagen are stained blue, nuclei are stained dark red/purple, and cytoplasm is stained red/pink. Artery intimae, media, and artery diameter (minimum diameter) were measured in the ipsilateral and contralateral internal carotid artery.
Collagen Deposition
Using a modified picrosirius red staining,13 sections were postfixed in Bouins fluid, followed by iron hematoxylin stain, then stained with 0.1% picrosirius red. Collagen deposition was expressed as ratio of interstitial and perivascular collagen area to luminal area of the internal carotid artery.
Double Immunohistochemical Staining
Fluorescein isothiocyanate (Calbiochem) and cyanine-5.18 (Jackson Immunoresearch) were used for double-label immunoreactivity. Each coronal section was first treated with the primary antiangiogenin antibody with cyanine-5.18 and then followed by ED1 with fluorescein isothiocyanate. Control experiments consisted of staining brain coronal tissue sections as outlined previously but use nonimmune serum for the primary antibody.
Statistical Analysis
One-way analysis of variance was used for the evaluation of functional outcome and histology, respectively. A “contract/estimate” statement was used to test the group difference. Spearman partial correlation coefficient analysis was used for the correlation between functional tests and histology evaluations at Day 14 after MCAO. If an overall treatment group effect was detected at P<0.05, pairwise comparisons were made. All data are presented as mean±SE.
Results
BMSC Increased Early Mortality Without Improvement in Lesion Volume and Functional Outcome in T1DM-MCAO Rats
There were no significant differences in blood glucose level between T1DM-MCAO control (before MCAO: 409.1±46.9 mg/dL; before euthanasia: 413.3±51.4 mg/dL) and the BMSC treatment group (before MCAO: 441.7±34.5 mg/dL; before euthanasia: 417.7±41.2 mg/dL; P>0.05).
The mortality rate was increased in (1 WT-MCAO control, 1 WT-MCAO+BMSC-treatment, 4 T1DM-MCAO control, and 6 T1DM-MCAO+BMSC treatment) the T1DM-MCAO+BMSC treatment group (6 of 12 [50%]) compared with T1DM-MCAO controls (4 of 13 [31%]). Previous studies have found that BMSC treatment of stroke significantly improved functional outcome in nondiabetic rats.5 However, BMSC treatment of stroke in T1DM rats did not improve functional outcome (Figure 1A–C) and lesion volume (Figure 1D).
Figure 1.
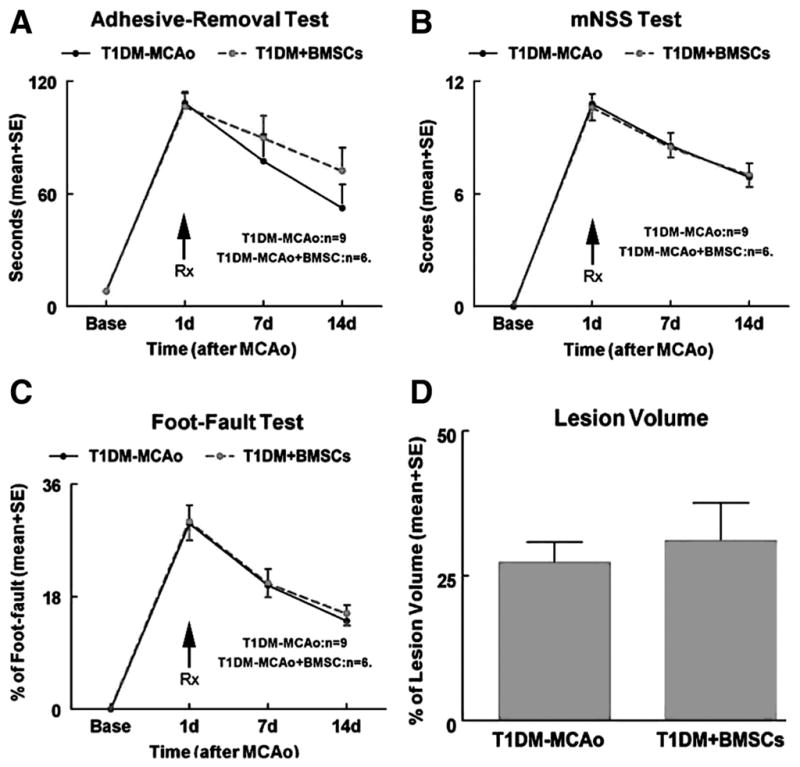
BMSC treatment of stroke in T1DM rats does not improve functional outcome and lesion volume. A Adhesive removal test. B, mNSS test. C, Foot-fault test. D, Lesion volume measurement. BMSC indicates bone marrow stromal cell; T1DM, Type 1 diabetes mellitus; mNSS, modified neurological severity score.
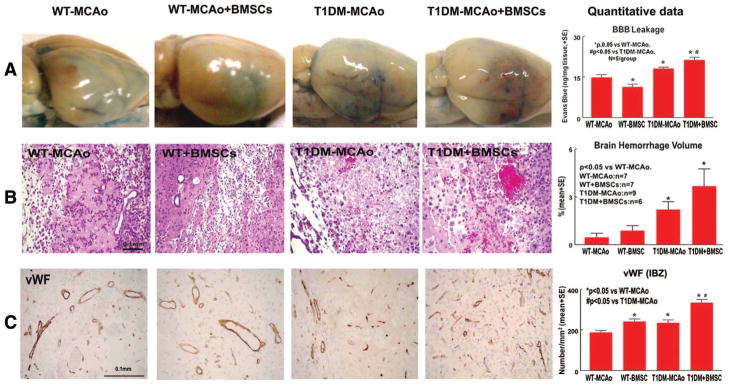
BMSC Treatment Increases Blood–Brain Barrier Leakage, Brain Hemorrhage, and Vascular Density in T1DM Rats
T1DM-MCAO significantly increased blood–brain barrier (BBB) leakage (Figure 2A), brain hemorrhage (Figure 2B), and vascular density (Figure 2C) compared with WT-MCAO rats (P<0.05). BMSC treatment in WT-MCAO rats did not increase brain hemorrhage but significantly decreased BBB leakage (Figure 2A) and increased vascular density (Figure 2C, P<0.05) compared with WT-MCAO rats. However, BMSC treatment in T1DM-MCAO rats significantly increased BBB leakage (Figure 2A), brain hemorrhage (Figure 2B), and increased vascular density (Figure 2C, P<0.05) compared with T1DM-MCAO rats.
Figure 2.
BMSC treatment in T1DM-MCAO rats significantly increases BBB leakage, brain hemorrhage volume, and vascular density in the ischemic brain. A, Evans blue dye assay for BBB leakage (n=5/group). B–C, Brain hemorrhage (B) and vascular density (C) measurement. Scale bar in B and C=0.1 mm. BMSC indicates bone marrow stromal cell; T1DM, Type 1 diabetes mellitus; MCAO, middle cerebral artery occlusion; BBB, blood–brain barrier.
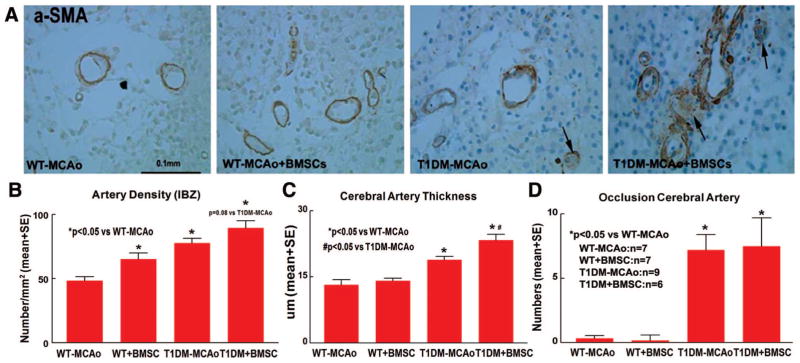
BMSC Treatment Increases Cerebral Artery Wall Thickening and Occlusion in T1DM-MCAO Rats
Both of T1DM-MCAO and T1DM-MCAO+BMSCs groups significantly increased arterial density (Figure 3B), cerebral artery wall thickening (Figure 3C), and arterial occlusion (Figure 3D) compared with WT-MCAO rats (P<0.05). BMSC treatment in T1DM-MCAO rats also significantly increased cerebral artery wall thickening (Figure 3C) compared with T1DM-MCAO controls.
Figure 3.
BMSC treatment in T1DM-MCAO rats significantly increases cerebral artery wall thickness and occlusion artery number. A, α-SMA staining. B–D, Quantitative data for artery density (B), cerebral artery wall thickness (C), and cerebral artery occlusion (D) in the ischemic brain. Scale bar in A=0.1 mm. BMSC indicates bone marrow stromal cell; T1DM, Type 1 diabetes mellitus; MCAO, middle cerebral artery occlusion; α-SMA, α-smooth muscle actin.
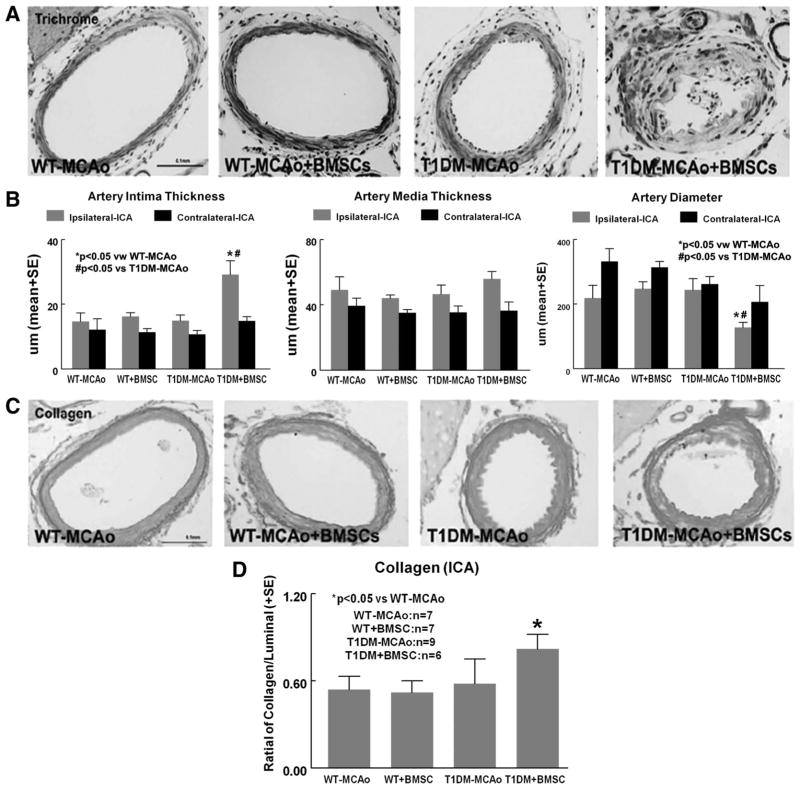
BMSC Treatment Accelerates Intracranial Artery Intimae Thickness and Collagen Deposition in T1DM-MCAO Rats
To test whether BMSC treatment induces arteriosclerosis in T1DM rats, trichrome staining was used. BMSC treatment in T1DM-MCAO rats significantly increased intimae thickness (Figure 4A) and collagen deposition (Figure 4C, P=0.05) and decreased artery diameter (Figure 4B) compared with T1DM-MCAO or WT-MCAO rats (P<0.05) in the ipsilateral internal carotid artery (ICA; Figure 4A–B).
Figure 4.
BMSC treatment in T1DM-MCAO rats significantly accelerates arteriosclerosis in the ICA. A, Trichrome immunostaining; (B) quantitative data for ICA artery intima thickness, artery media thickness, and artery diameter in ipsilateral ICA. C–D, Collagen staining and measurement in ICA. Scale bar in A and C=0.1 mm. BMSC indicates bone marrow stromal cell; T1DM, Type 1 diabetes mellitus; MCAO, middle cerebral artery occlusion; ICA, internal carotid artery.
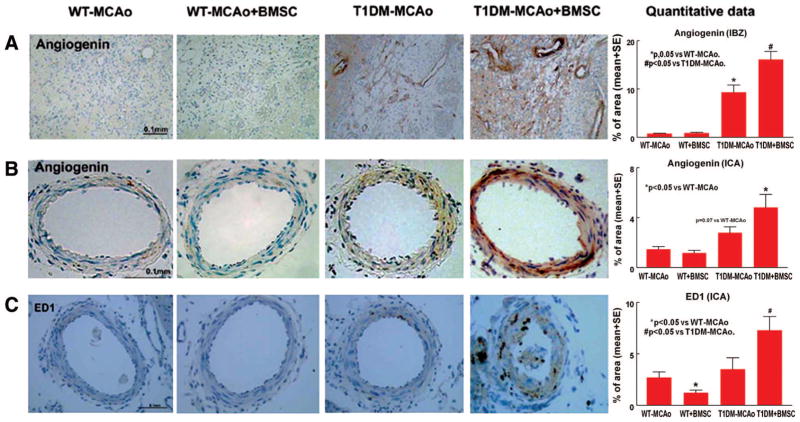
BMSC Treatment Increases Angiogenin and ED1 Expression in the T1DM-MCAO Rats
To obtain insight into the possible underlying mechanisms of BMSC-induced BBB leakage, brain hemorrhage and intimae thickness in T1DM-MCAO rats, angiogenin, and ED1 expression was measured. T1DM-MCAO+BMSCs significantly increased angiogenin expression in the ischemic border zone (P<0.05, Figure 5A) and was marginally increased angiogenin (P=0.07, Figure 5B) and ED1 (P<0.05, Figure 5C) expression in the ICA compared with T1DM-MCAO controls.
Figure 5.
T1DM-MCAO rats increases angiogenin expression and BMSC treatment in T1DM-MCAO rats significantly enhances angiogenin and ED1 expression. A–B, Angiogenin immunostaining and quantitative data in IBZ (A) and in the ICA (B). C, ED1 immunostaining and quantitative data in ICA. Scale bar in A–C=0.1 mm. T1DM indicates Type 1 diabetes mellitus; MCAO, middle cerebral artery occlusion; BMSC, bone marrow stromal cell; IBZ, ischemic border zone; ICA, internal carotid artery.
However, WT-MCAO+BMSC rats did not increase intimae thickness, artery diameter, collagen deposition, and ED1 and angiogenin expression in the ischemic brain and in the ICA compared with WT-MCAO control (P>0.05).
There were no significant differences between WT sham and T1DM sham controls in vascular density, arterial density, angiogenin, and ED1 expression and ICA intimae thickness (Supplemental Figure I; http://stroke.ahajournals.org). However, the numbers of occluded cerebral arterioles were marginally increased (P=0.06) in T1DM sham rats compared with WT sham rats.
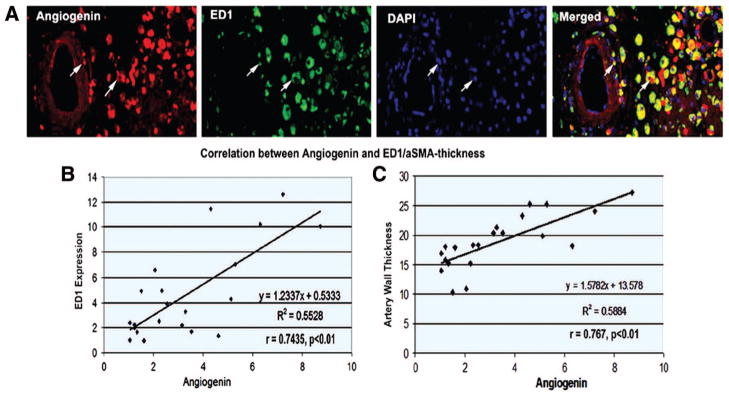
Angiogenin Is Correlated With ED1 and Arteriosclerosis in the T1DM-MCAO Rats
The expression of angiogenin colocalized with ED1 expression in the ischemic brain (Figure 6A). The increased angiogenin was significantly correlated with ED1 expression (Figure 6B) and cerebral artery thickness (Figure 6C).
Figure 6.
Angiogenin expression is colocalized with ED1. Angiogenin expression significantly correlated with ED1 expression and cerebral artery thickness in the ischemic brain. A, Angiogenin and ED1 double immunostaining in the ischemic brain. B, Correlation analysis of angiogenin and ED1. C, Correlation analysis of angiogenin and cerebral artery thickness.
Discussion
BMSC Treatment Increases BBB Leakage, Brain Hemorrhage, and Fails to Improve Functional Outcome After Stroke in T1DM-MCAO Rats
The vascular changes after stroke in nondiabetic and T1DM-MCAO rats may differ. Diabetic stroke animals exhibit increased angiogenic responses and BBB leakage and decreased tight-junction protein expression.14 Early BBB disruption contributes to intracerebral hemorrhage. Intracerebral hemorrhagic transformation is a multifactorial phenomenon in which ischemic brain tissue converts into a hemorrhagic lesion with blood-vessel leakage and extravasation, thereby exacerbating brain injury. These characteristics of vascular response to stroke may be responsible for the adverse effect of BMSC treatment observed in T1DM-MCAO rats. We found that BMSC treatment of stroke in T1DM-MCAO upregulated vascular and artery density and also increased brain hemorrhage, BBB leakage, but did not improve functional outcome. Increased BBB leakage and brain hemorrhage may contribute to the early and increased mortality in T1DM-MCAO animals. T1DM-MCAO+BMSCs also induced arteriosclerosis, marked by cerebral arterial intimae thickening and arteriole occlusion, which may negatively impact the functional recovery after stroke. Whereas in nondiabetic rats, treatment of stroke with BMSCs not only increased vascular density and vascular stabilization and decreased BBB leakage, but also improved functional outcome after stroke.5,15 This is the first report demonstrating that BMSC therapy has an adverse effect on vascular remodeling and functional outcome in T1DM-MCAO rats. Therefore, the beneficial effect of BMSCs administered at 24 hours after stroke in the nondiabetic population does not translate to the diabetic population.
BMSC Treatment Increases Cerebral Arteriosclerosis After Stroke in T1DM-MCAO Rats
The mechanism responsible for the adverse vascular response or arteriosclerosis to BMSC therapy in T1DM-MCAO is not known. Carotid intima-media thickness is the early sign of atherosclerosis and macrovascular diseases.16 BMSC treatment in T1DM-MCAO rats significantly increased intima-media thickness and intracerebral arteriole occlusion and decreased the diameter of the ICA compared with T1DM-MCAO control. ED1 expression significantly increased in the ICA in T1DM-MCAO rats treated with BMSCs. Subendothelial invasion by leukocytes is a characteristic of neointimal thickening in arteriosclerosis and in the response of a vessel to mechanical damage, and neutrophils also contribute to neointimal thickening in an arterial autograft model.17 Thus, the enhanced inflammatory response to stroke may promote the development of arteriosclerosis in T1DM-MCAO+ BMSC treatment.
Arteriosclerosis and arterial stiffness are both risk factors associated with the occurrence of stroke.18 Abnormal collagen turnover and increased fibrosis are the common pathophysiological link with increased arterial stiffness. Diabetes induces artery stiffness and is associated with the changes in collagen biochemistry of the blood vessel.19 Collagen deposition was marginally increased in T1DM-MCAO+BMSC rats. BMSC-enhanced fibrotic response may accelerate arteriosclerosis and intracerebral artery damage in T1DM-MCAO rats.
Angiogenin May Contribute to BMSC-Induced Angiogenesis, BBB Leakage, and Arteriosclerosis in T1DM-MCAO Rats
Angiogenin is a potent angiogenic growth factor, which degrades the basement membrane, thereby facilitating cell invasion and migration.20 Severity of microvascular complications is associated with markedly increased serum of angiogenin levels.21 Angiogenin levels are increased in patients with peripheral artery occlusion22 and among diabetic youngsters.23 BMSCs behave as small biochemical and molecular “factories,” producing many cytokines and large amounts of angiogenic, antiapoptotic, and mitogenic factors.24 T1DM-MCAO rats had significantly increased angiogenin levels compared with WT-MCAO control and T1DM-MCAO+BMSC rats significantly enhanced angiogenin expression in the ischemic brain. The increased angiogenin expression is significantly correlated with ED1 expression and cerebral artery thickness, which is in agreement with data that angiogenin expression is significantly correlated with microvessel counts and focal macrophage infiltration.25
Limitations and Caveats
There are a number of limitations and caveats in the present study. In this initial study, we treated rats 2 weeks postinduction of diabetes with BMSC. For enhanced clinical relevance, additional studies are warranted in rats with long-term diabetes with control and regulation of blood glucose and with BMSCs administered at later times poststroke than 24 hours. In addition, many factors, and not only angiogenin, may contribute to the adverse effect of BMSC treatment of stroke in T1DM rats. Matrix metalloproteinase-9, vascular endothelial growth factor, and immunoresponse may also, at least partially, be responsible for the BMSC-induced adverse effects in T1DM rats after stroke. Further investigation of the mechanism underlying BMSC-induced adverse effects in T1DM rats is warranted.
Conclusions
BMSC treatment initiated 24 hours after MCAO in T1DM increases brain hemorrhage, BBB leakage, and accelerates cerebral arteriosclerosis and does not improve functional outcome. Increased angiogenin expression may contribute to the adverse effects of BMSC treatment in T1DM-MCAO rats. These data suggest that caution must be exercised in translating the therapeutic benefit of BMSC therapy in nondiabetics to diabetics, with diabetes potentially an exclusion factor for early BMSC therapy after stroke.
Supplementary Material
Acknowledgments
We thank Qinge Lu and Sutapa Santra for technical assistance.
Sources of Funding
This work was supported by National Institute on Aging RO1-AG031811 (J.C.), R01-AG037506 (M.C.) and 1R41NS064708 (J.C.), and American Heart Association grant 09GRNT2300151 (J.C.).
Footnotes
Disclosures
None.
References
- 1.Li W, Prakash R, Kelly-Cobbs AI, Ogbi S, Kozak A, El-Remessy AB, et al. Adaptive cerebral neovascularization in a model of type 2 diabetes: relevance to focal cerebral ischemia. Diabetes. 2010;59:228–235. doi: 10.2337/db09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- 3.De Silva DA, Ebinger M, Christensen S, Parsons MW, Levi C, Butcher K, et al. Baseline diabetic status and admission blood glucose were poor prognostic factors in the EPITHET trial. Cerebrovasc Dis. 2010;29:14–21. doi: 10.1159/000255969. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Sabin J, Molina CA, Montaner J, Arenillas JF, Huertas R, Ribo M, et al. Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator-treated patients. Stroke. 2003;34:1235–1241. doi: 10.1161/01.STR.0000068406.30514.31. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 6.Zacharek A, Chen J, Cui X, Li A, Li Y, Roberts C, et al. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 2007;27:1684–1691. doi: 10.1038/sj.jcbfm.9600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang CH, Cherng WJ, Yang NI, Kuo LT, Hsu CM, Yeh HI, et al. Late-outgrowth endothelial cells attenuate intimal hyperplasia contributed by mesenchymal stem cells after vascular injury. Arterioscler Thromb Vasc Biol. 2008;28:54–60. doi: 10.1161/ATVBAHA.107.147256. [DOI] [PubMed] [Google Scholar]
- 8.Hu GF, Riordan JF, Vallee BL. A putative angiogenin receptor in angiogenin-responsive human endothelial cells. Proc Natl Acad Sci U S A. 1997;94:2204–2209. doi: 10.1073/pnas.94.6.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel JV, Sosin M, Gunarathne A, Hussain I, Davis RC, Hughes EA, et al. Elevated angiogenin levels in chronic heart failure. Ann Med. 2008;40:474–479. doi: 10.1080/07853890802001419. [DOI] [PubMed] [Google Scholar]
- 10.Tello-Montoliu A, Marin F, Patel J, Roldan V, Mainar L, Vicente V, et al. Plasma angiogenin levels in acute coronary syndromes: implications for prognosis. Eur Heart J. 2007;28:3006–3011. doi: 10.1093/eurheartj/ehm488. [DOI] [PubMed] [Google Scholar]
- 11.Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976;193:415–417. doi: 10.1126/science.180605. [DOI] [PubMed] [Google Scholar]
- 12.Zhang ZG, Zhang L, Croll SD, Chopp M. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience. 2002;113:683–687. doi: 10.1016/s0306-4522(02)00175-6. [DOI] [PubMed] [Google Scholar]
- 13.Peng H, Carretero OA, Vuljaj N, Liao TD, Motivala A, Peterson EL, et al. Angiotensin-converting enzyme inhibitors: a new mechanism of action. Circulation. 2005;112:2436–2445. doi: 10.1161/CIRCULATIONAHA.104.528695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui X, Chopp M, Zacharek A, Ye X, Roberts C, Chen J. Angiopoietin/Tie2 pathway mediates type 2 diabetes induced vascular damage after cerebral stroke. Neurobiol Dis. 2011;43:285–292. doi: 10.1016/j.nbd.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borlongan CV, Lind JG, Dillon-Carter O, Yu G, Hadman M, Cheng C, et al. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain Res. 2004;1010:108–116. doi: 10.1016/j.brainres.2004.02.072. [DOI] [PubMed] [Google Scholar]
- 16.Gul K, Ustun I, Aydin Y, Berker D, Erol K, Unal M, et al. Carotid intima-media thickness and its relations with the complications in patients with type 1 diabetes mellitus. Anadolu Kardiyol Derg. 2010;10:52–58. doi: 10.5152/akd.2010.012. [DOI] [PubMed] [Google Scholar]
- 17.Jurado F, Bellon JM, Rodriguez M, Corrales C, Bujan J. Inflammatory cells induce neointimal growth in a rat arterial autograft model. Histol Histopathol. 2002;17:817–826. doi: 10.14670/HH-17.817. [DOI] [PubMed] [Google Scholar]
- 18.Agabiti-Rosei E, Muiesan ML. Carotid atherosclerosis, arterial stiffness and stroke events. Adv Cardiol. 2007;44:173–186. doi: 10.1159/000096729. [DOI] [PubMed] [Google Scholar]
- 19.Reddy GK. Age-related cross-linking of collagen is associated with aortic wall matrix stiffness in the pathogenesis of drug-induced diabetes in rats. Microvasc Res. 2004;68:132–142. doi: 10.1016/j.mvr.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Hu G, Riordan JF, Vallee BL. Angiogenin promotes invasiveness of cultured endothelial cells by stimulation of cell-associated proteolytic activities. Proc Natl Acad Sci U S A. 1994;91:12096–12100. doi: 10.1073/pnas.91.25.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiarelli F, Pomilio M, Mohn A, Tumini S, Verrotti A, Mezzetti A, et al. Serum angiogenin concentrations in young patients with diabetes mellitus. Eur J Clin Invest. 2002;32:110–114. doi: 10.1046/j.0014-2972.2001.00958.x. [DOI] [PubMed] [Google Scholar]
- 22.Burgmann H, Hollenstein U, Maca T, Zedwitz-Liebenstein K, Thalhammer F, Koppensteiner R, et al. Increased serum laminin and angiogenin concentrations in patients with peripheral arterial occlusive disease. J Clin Pathol. 1996;49:508–510. doi: 10.1136/jcp.49.6.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malamitsi-Puchner A, Sarandakou A, Dafogianni C, Tziotis J, Bartsocas CS. Serum angiogenin levels in children and adolescents with insulin-dependent diabetes mellitus. Pediatr Res. 1998;43:798–800. doi: 10.1203/00006450-199806000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Nagaya N, Kangawa K, Itoh T, Iwase T, Murakami S, Miyahara Y, et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;112:1128–1135. doi: 10.1161/CIRCULATIONAHA.104.500447. [DOI] [PubMed] [Google Scholar]
- 25.Etoh T, Shibuta K, Barnard GF, Kitano S, Mori M. Angiogenin expression in human colorectal cancer: the role of focal macrophage infiltration. Clin Cancer Res. 2000;6:3545–3551. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.