Abstract
The cephalostatin and ritterazine natural products comprise a potent family of bis-steroidal pyrazines that display potent single-digit nanomolar inhibition of tumor cell growth. An active fluorescent ritterazine-cephalostatin hybrid probe was developed using detailed SAR data derived through total synthetic efforts. A combination of time course and confocal imaging studies indicate that this natural product family is rapidly up taken in tumor cells and localizes subcellularly within ER and surrounding the nuclear-ER interface.
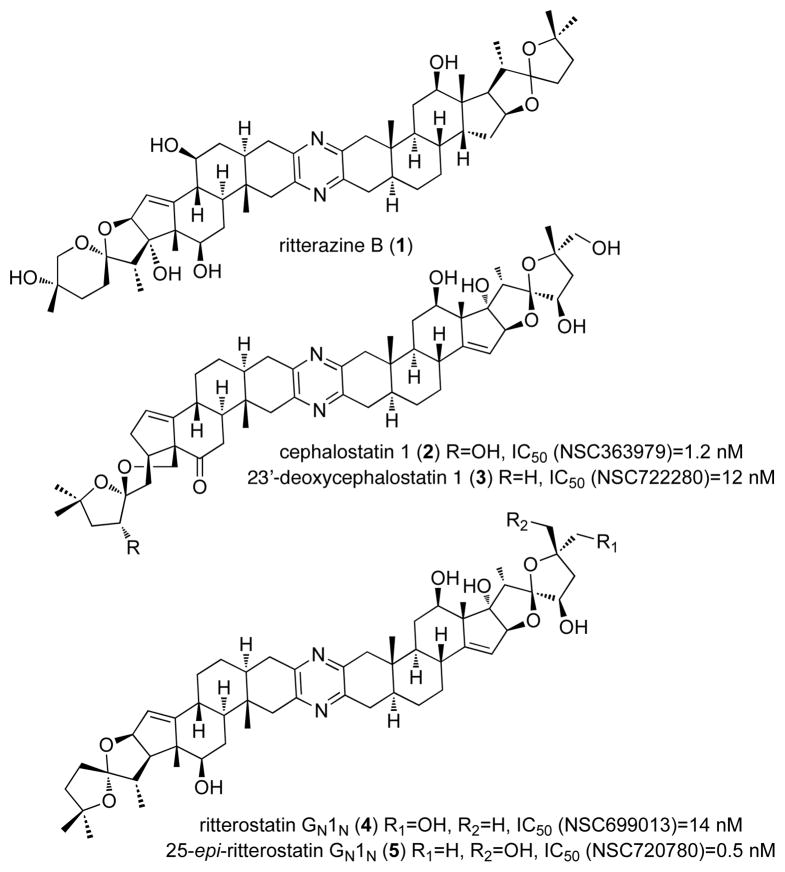
Ritterazine B (1) is a member of a large family of natural products (over 45 cephalostatin and ritterazine congeners) that were isolated from marine organisms by the laboratories of Pettit1a and Fusetani.1b The unique structure of these bis-steroidal compounds (see Fig. 1) along with their potent single-digit nanomolar inhibition of tumor cell growth is highlighted by an average GI50 value of 1.2 nM for cephalostatin 1 (2) in the NCI-60 cell line screen. This potent activity along with their unique cell selectivity and apoptotic response has drawn attention to their potential as clinical leads for cancer.2
Figure 1.
Structures of ritterazine B (1), cephalostatin 1 (2), 23′-deoxycephalostatin 1 (3), ritterostatin GN1N (4) and 25-epi-ritterostatin hybrid GN1N (5).
Soon after their isolation, synthetic programs were launched, with the first total synthesis reported in 1995.3 While these synthetic tools now provide access to analogs improved activity and ease of access,4 a detailed understanding of the mechanisms by which the ritterazines and cephalostatins induce tumor cell apoptosis remains unclear.5 Recent studies from a team led by Shair suggest that 1 and 2 target an oxysterol binding protein (OSBP), however, a link between this target and the apoptotic activity of 1 and 2 has yet to be established.6
Our ability to synthesize cephalostatin, ritterazine and hybrid analogs has been used to supply sufficient quantities of materials for detailed biological and in vivo studies. For instance, the first-generation synthesis of 110 mg of 23′-deoxycephalostatin 1 (3) was used to characterize their apoptotic activity and evaluate the accompanying mitochondrial damage and cytosolic Ca2+ increase.7 These studies have also shown that 3 engenders a 50–60% increase in mouse lifespan in U87MG brain cancer xenografts.8 Synthetic samples (from 245 mg total) of 25-epi-ritterostatin GN1N (5) were further screened for in vitro activity against multiple cancer cells lines of different tissue origins.3a,4a
Given our synthetic access, application of these materials to prepare and evaluate fluorescent probes was a logical next step. Our unsymmetrical pyrazine synthetic route operates through a coupling of a α–azidoketone and a α–aminomethoxime.4a Since both steroidal precursors pass through the α-azidoketone stage, there are two coupling modes to the same product. The bottom line is that this unsymmetrical pyrazine synthesis provides dependable late-stage coupling of an exceptionally valuable pair of 3-ketosteroids with the expectation of excellent overall yield and preservation of acid-labile spiroketal stereochemistry. The average yield for the initial 59 cases examined was 72%.
Using SAR studies as a guide,9 the C-25 position was targeted, as it has shown tolerance to modification. Our studies focused on use of an immunoaffinity fluorescent (IAF) label,10 as this label has been shown to provide effective probes without phenotypic modification and modest loss in cell viability and activity.
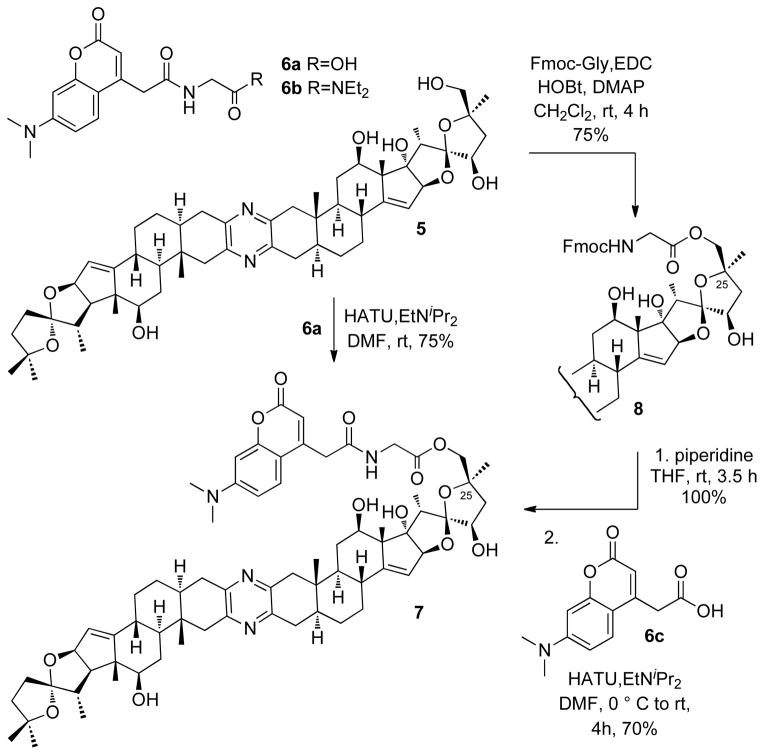
Glycine IAF label 6a11 was coupled with 25-epi-ritterostatin GN1N (5) at the C-25 position to deliver probe 7 (Scheme 1). The same probe 7 was also obtained through a stepwise procedure involving an Fmoc-protected intermediate 8.
Scheme 1.
Synthesis of IAF-labeled fluorescent probe 7.
Using the MTT assay, probe 7 demonstrated an activity of IC50 value of 79±4 nM against HCT-116 cells.12 With active probe and access to panel of IAF-labeled natural products and controls,11b our studies shifted to develop a detailed description of the uptake and subcellular localization of 7.
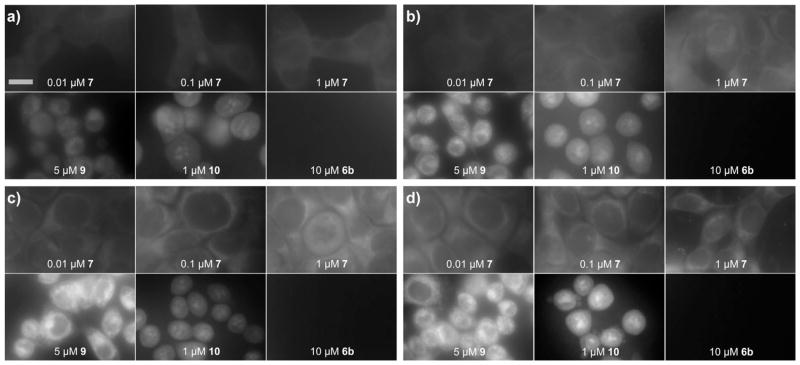
Using inexpensive components, a live cell imaging system was built containing a polycarbonate CO2 incubator mounted on the head of computer-driven fiberoptic microscope (see Supporting Information). The instrument was designed to rapidly capture images from 24 experiments in parallel at 30 min intervals over a 24 h period. On this system, the uptake of three concentrations of probe 7 was measured in triplicate along the respective positive and negative controls (Fig. 2).
Figure 2.
Time-course imaging depicting the uptake and subcelluar localization of probe 7 in live HCT-116 cells. Images were collected using a custom built fiberoptic microscope (see Supporting Information) that was mounted on an computer-driven XYZ micron adjustable stage and was equipped to excite with an LED at λex= 380 nm and collect emission at λem= 448 ± 20 nm. Images were collected at 30 min intervals over 24 h period using an identical optical parameters. A concentration gradient of each probe was conducted and compared to cells treated with dragmacidin D (9), an ER stain, and IAF-labeled nogalamycin (10), a nuclear stain and an IAF control, compound 6b. Images are provided at: a) 1 h, b) 6 h, c) 12 h and d) 24 h. Bar denotes 10 μm.
An identical outcome was observed after multiple of repetitions. After 6 h, probe 7 appeared in the nuclear membrane and extending into the endoplasmic reticulum (ER). The intensity of this stain increased until 12 h where it remained consistent thereafter (imaging was conducted up to 24 h). This localization was confirmed by comparison with a complementary blue-fluorescent nuclear stain, IAF-labeled nogalamycin (10),8b and blue-fluorescent ER stain dragmacidin D (9) (structures of 9 and 10 are provided in the Supporting Information).13 Further controls indicated that the label alone did not stain cells under identical conditions.14 Even at concentrations up to 250 μM, the lack of fluorescence from controls such as 6b (Scheme 1) in live cells indicated that the uptake and localization was indeed due to the natural product’s activity.
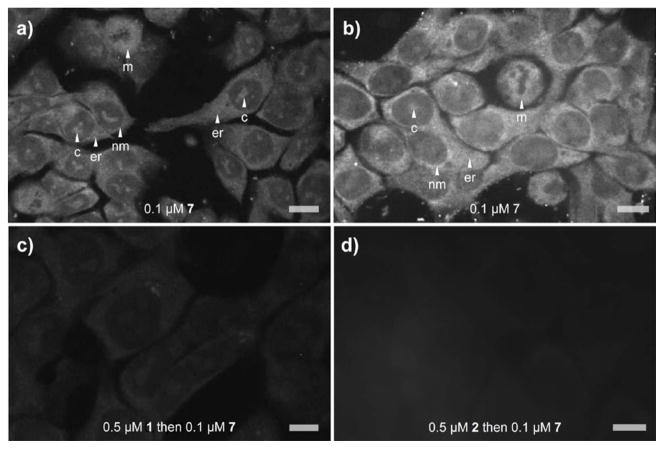
While time course imaging served for initial analyses, confocal microscopy provided improved resolution. Using the data from the time course studies, HCT-116 cells were treated with 0.1 μM 7 for 12 h, washed with media and imaged. These studies provided further support for the localization in the endoplasmic reticulum (er, Fig. 3a) and around the nuclear membrane (nm, Fig. 3a). However, probe 7 was also observed on chromatin (label c, Fig. 3a) within the nucleus of HCT-116 cells. By evaluating other cell lines, this observation was linked to a unique selectivity in HCT-116 cells. For example, probe 7 was not localized on chromatin in HeLa cells (label c, Fig. 3b).
Figure 3.
Confocal fluorescent images detailing the subcellular localization of probe 7 and corresponding natural products ritterazine B (1) or celphalostatin 1 (2). a) Live HCT-116 cells that were incubated with probe 7 for 12 h. b) Comparable uptake arising from treating HeLa cells with probes 7 for 12 h. c) HCT-116 cells that were treated with ritterazine B (1) for 12 h followed by probe 7 for an additional 12 h. d) HCT-116 cells that were treated with ritterazine B (1) for 12 h followed by probe 7 for an additional 12 h. Labels denote (c) chromatin, (er) endoplasmic reticulum, (nm) nuclear membrane and (m) mitotic cell. Images were collected with excitation at λex= 405 nm and emission at λem= 448 ± 20 nm. Bars denote 10 μm.
While control experiments indicated the label did not alter the subcellular localization, further studies were conducted to confirm the localization of probe 7 was comparable to ritterazine B (1) or celphalostatin 1 (2). Both 1 and 2 were shown to effectively block the uptake of probe 7 (as shown in Fig. 3c–3d).
This observation suggests two key points. First, ritterazine B (1) as well as probe 7 bind to their target in a strong and irreversible manner (if this was not the case treatment of cells exposed to 1 with probe 7, as in Fig. 3c or 3d, would have led to comparable localization as in Fig. 3a). Second and perhaps most importantly, probe 7 shares a common target with ritterazine 1 and cephalostatin 2. While suggested by related hybrid natural products,15 this study provides direct evidence that the cephalostatins, ritterazines and hybrids of both natural products share a common mode of action.
Further insight into the cellular targeting was obtained from examining mitotic cells treated with 7 (m, Fig. 3b). While localizing on chromatin in mature cells, probe 7 was not observed binding to the chromosomes during mitosis. As illustrated in Fig. 3b, fluorescence from 7 was observed in the cytosolic space prophase to telophase. This observation was consistent with the transitioning of the nuclear envelope and ER membranes during mitosis.16
In summary, this study describes the preparation of a fluorescent probe based on a cephalostatin-ritterazine hybrid. Time course and confocal microscopy indicate that a ritterazine-cephalostatin hybrid probe shares a common uptake and subcellular localization in the ER, nuclear membrane and chromatin with ritterazine B (1) and cephalostain 1 (2). The observed uptake and subcellular localization was consistent with the transitioning during mitosis.13
The study further illustrates how scarce natural materials made available through complex chemical synthesis play a pivotal role in evaluating natural product activity. Our program is now focused on applying these materials to identify the molecular targets and pathways modulated by this unique class of bis-steroidal alkaloid, with the goal of identify the pathways regulated by probe7 during cell growth and division.
Supplementary Material
Acknowledgments
This work was supported by funding from the NIH (5U01CA060548-17 and CA 60548) and internal support from XRI. We sincerely thank Dr. Douglas Lantrip for laboratory assistance and Dr. Karl Wood and Arlene Rothwell of Purdue University for assistance with collection of the mass spectral data.
Footnotes
Supporting Information Available Synthetic procedures, copies of NMR spectra on the probes as well as detailed procedures for the cellular imaging studies and time course imaging system are available. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Pettit GR, Inoue M, Kamano Y, Herald DL, Arm C, Dufresne C, Christie ND, Schmidt JM, Doubek DL, Krupa TS. J Am Chem Soc. 1988;110:2006–2007. [Google Scholar]; (b) Fukuzawa S, Matsunaga S, Fusetani N. J Org Chem. 1994;59:6164–6166. doi: 10.1021/jo970091r. [DOI] [PubMed] [Google Scholar]
- 2.(a) Moser BR. J Nat Prod. 2008;71:487–491. doi: 10.1021/np070536z. [DOI] [PubMed] [Google Scholar]; (b) Flessner T, Jautelat R, Scholz U, Winterfeldt E. Fortschr Chem Org Naturst. 2004;87:1–80. doi: 10.1007/978-3-7091-0581-8_1. [DOI] [PubMed] [Google Scholar]; (c) Lee S, LaCour TG, Fuchs PL. Chem Rev. 2009;109:2275–2314. doi: 10.1021/cr800365m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Jeong JU, Sutton SC, Kim S, Fuchs PL. J Am Chem Soc. 1995;117:10157–10158. [Google Scholar]; (b) Fortner KC, Kato D, Tanaka Y, Shair MD. J Am Chem Soc. 2010;132:275–280. doi: 10.1021/ja906996c. [DOI] [PubMed] [Google Scholar]
- 4.(a) Lee S, LaCour TG, Fuchs PL. Chem Rev. 2009;109:2275–314. doi: 10.1021/cr800365m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Phillips ST, Shair MD. J Am Chem Soc. 2007;129:6589–6598. doi: 10.1021/ja0705487. [DOI] [PubMed] [Google Scholar]
- 5.(a) Rudy A, López-Antón N, Dirsch VM, Vollmar AMJ. Nat Prod. 2008;71:482–486. doi: 10.1021/np070534e. [DOI] [PubMed] [Google Scholar]; (b) Komiya T, Fusetani T, Matsunaga S, Kubo A, Kaye FJ, Kelley JM, Tamura K, Yoshida M, Fukuoka M, Nakagawa K. Cancer Chemother Pharmacol. 2003;51:202–208. doi: 10.1007/s00280-002-0558-8. [DOI] [PubMed] [Google Scholar]
- 6.Burgett AW, Poulsen TB, Wangkanont K, Anderson DR, Kikuchi C, Shimada K, Okubo S, Fortner KC, Mimaki Y, Kuroda M, Murphy JP, Schwalb DJ, Petrella EC, Cornella-Taracido I, Schirle M, Tallarico JA, Shair MD. Nat Chem Biol. 2011;7:639–647. doi: 10.1038/nchembio.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unpublished results with Prof. Peng Huang (MD Anderson).
- 8.(a) Unpublished studies with Dr. John Beutler (National Cancer Institute). (b) Synthesis of 25-epi-ritterostatin GN1N will be reported in due course.
- 9.(a) LaCour TG, Guo C, Ma S, Jeong JU, Boyd MR, Matsunaga S, Fusetani N, Fuchs PL. Bioorg Med Chem Lett. 1999;9:2587–2592. doi: 10.1016/s0960-894x(99)00430-8. [DOI] [PubMed] [Google Scholar]; (b) Guo C, LaCour TG, Fuchs PL. Bioorg Med Chem Lett. 1999;9:419–424. doi: 10.1016/s0960-894x(98)00743-4. [DOI] [PubMed] [Google Scholar]
- 10.Yu WL, Guizzunti G, Foley TL, Burkart MD, La Clair JJ. J Nat Prod. 2010;73:1659–1666. doi: 10.1021/np100371k. [DOI] [PubMed] [Google Scholar]
- 11.(a) Hughes CC, MacMillan JB, Gaudêncio SP, Fenical W, La Clair JJ. Angew Chem Int Ed Engl. 2009;48:728–732. doi: 10.1002/anie.200804107. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Alexander MD, Burkart MD, Leonard MS, Portonovo P, Liang B, Ding X, Joullié MM, Gulledge BM, Aggen JB, Chamberlin AR, Sandler J, Fenical W, Cui J, Gharpure SJ, Polosukhin A, Zhang HR, Evans PA, Richardson AD, Harper MK, Ireland CM, Vong BG, Brady TP, Theodorakis EA, La Clair JJ. Chembiochem. 2006;7:409–416. doi: 10.1002/cbic.200500466. [DOI] [PubMed] [Google Scholar]
- 12.Activities were measured using the protocols described in: Kang M, Jones BD, Mandel AL, Hammons JC, DiPasquale AG, Rheingold AL, La Clair JJ, Burkart MD. J Org Chem. 2009;74:9054–9061. doi: 10.1021/jo901826d.
- 13.Forsyth CJ, Ying L, Chen J, La Clair JJ. J Am Chem Soc. 2006;128:3858–3859. doi: 10.1021/ja057087e. [DOI] [PubMed] [Google Scholar]
- 14.See examples in: La Clair JJ. Nat Prod Rep. 2010;27:969–995. doi: 10.1039/b909989c.
- 15.Suzuki K. Chem Rec. 2010;10:291–307. doi: 10.1002/tcr.201000030. [DOI] [PubMed] [Google Scholar]; (b) Meunier B. Acc Chem Res. 2008;41:69–77. doi: 10.1021/ar7000843. [DOI] [PubMed] [Google Scholar]
- 16.Ellenberg J, Siggia ED, Moreira JE, Smith CL, Presley JF, Worman HJ, Lippincott-Schwartz J. J Cell Biol. 1997;138:1193–1206. doi: 10.1083/jcb.138.6.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.