Abstract
Aims and Methods: Several serum biomarkers such as FibroTest, aspartate transaminase-platelet ratio index (APRI), FIB-4, and liver stiffness measurement by FibroScan have been validated as alternatives to biopsy for the diagnosis of fibrosis in patients with chronic liver disease. This paper aims to assess the 5-year prognostic values of these biomarkers. A meta-analysis combined all published prognostic studies. Baseline biopsy and APRI data were used as references. Results: Only 3 biomarkers had several prognostic validations: FibroTest (4 studies; 2,396 patients), APRI (5 studies; 2,422 patients), and FIB-4 (3 studies; 1,184 patients). For the prediction of survival without liver-related death, the areas under the receiver operating characteristic curves (AUROCs) were 0.86 for biopsy (95% confidence interval [CI], 0.77–0.95), 0.88 for FibroTest (95% CI, 0.79–0.98), 0.73 for FIB-4 (95% CI, 0.62–0.85), and 0.66 for APRI (95% CI, 0.57–0.75). APRI had a significantly lower prognostic value versus biopsy, with a mean difference between AUROCs of –0.21 (95% CI, –0.33 to –0.10; P<.001); FIB-4 had a significantly lower prognostic value versus biopsy, with a mean difference between AUROCs of –0.21 (95% CI, –0.20 to –0.02; P=.02). Only FibroTest did not show a significant difference in prognostic value versus biopsy, with a mean difference in AUROCs of +0.02 (95% CI, –0.05 to +0.09; P=.85). Conclusion: FibroTest is a validated biomarker for the prognosis of patients with chronic liver disease.
Keywords: Fibrosis biomarkers, meta-analysis, prognostic value, FibroTest, FibroScan, aspartate transaminase-platelet ratio index, HepaScore, FIB-4, biopsy, elastography
Complications of cirrhosis are the main causes of mortality related to chronic liver disease. Progressive hepatic fibrosis with the development of cirrhosis is a feature in the majority of chronic liver disease cases.1 Therefore, liver fibrosis stage can be a significant predictive factor for mortality related to liver complications.
Liver biopsy is traditionally recommended to assess fibrosis stage in most patients with chronic liver disease. Due to the 3 main limitations of biopsy—severe complications, sampling error, and interobserver variability—several biomarkers have been validated as noninvasive alternatives, and these biomarkers are increasingly being used in practice.2 In France, FibroTest and FibroScan were recommended for first-line assessment of fibrosis in 2006.3
In the absence of a true gold standard, most stakeholders agree that the validation of these biomarkers by strong clinical endpoints will be the most convincing proof of their utility.3,4 The aim of the current study was to perform a meta-analysis of the prognostic value of bio-markers recognized as being validated for the diagnosis of liver fibrosis.
Methods
This study was conducted according to the principles expressed in the Declaration of Helsinki. The primary outcome measure was difference in overall survival at the end of the follow-up period. To select published studies, we used the Standards for Reporting of Diagnostic Accuracy (STARD) criteria and the Cochrane Database of Systematic Reviews methods.5 Key STARD criteria included: whether the study population was relevant to the clinical question being addressed; whether there was a careful description of the population from which the patients were drawn, as well as actual inclusions and exclusions; whether recruitment and the mode of sampling were carefully described; whether researchers interpreting the noninvasive test were blinded to the reference test result; and whether sufficient data were provided to complete a 2 × 2 table of true- and false-positive and -negative diagnoses. Studies that were published with only an abstract provided insufficient data and were excluded.
Search Strategy
We searched MEDLINE with the following keywords: prognosis, liver disease, biomarkers, and fibrosis. We manually searched key journals (Gastroenterology, Hepatology, Journal of Hepatology, Gut, Journal of Viral Hepatitis, and American Journal of Gastroenterology) from February 2001 to March 2011 to validate the search. We also manually searched the references of publications identified by previous searches.
Inclusion and Exclusion Criteria
The following inclusion criteria were used: patients with chronic liver disease; previously validated biomarkers of fibrosis assessed at baseline, with or without liver biopsy; and survival data for true positives and negatives, false positives and negatives, and area under the receiver operating characteristic curves (AUROCs) for prognosis (either overall survival, survival without liver disease-related death, or complications without death). The following fibrosis biomarkers, which had several studies validating their diagnostic values, were included in the meta-analysis: FibroTest (α2-macroglobulin, apolipoprotein A1, haptoglobin, gamma glutamyl transpeptidase, and total bilirubin), aspartate transaminase-platelet ratio index (APRI; aspartate transaminase [AST] and platelets), FibroMeter (according to the version of the test and the patient's liver disease: platelets, hyaluronic acid or gamma glutamyl transpeptidase, prothrombin index, AST, and α2-macroglobulin), FIB-4 (platelets, AST, alanine aminotransferase [ALT], and age), Hepa-Score (α2-macroglobulin, hyaluronic acid, and gamma glutamyl transpeptidase), ELF (hyaluronic acid, tissue inhibitor of metalloproteinase-1, and procollagen 3 peptide N-terminal), and FibroScan (measuring stiffness by elastography).6
Forns score was excluded, as it had no significant diagnostic value in alcoholic liver disease (ALD) patients.7 Isolated hyaluronic acid was a pioneer among fibrosis biomarkers, with an early study being the first to demonstrate a significant prognostic value.8 However, hyaluronic acid was excluded as its isolated diagnostic performance was lower than its performance in combination with other biomarkers, as in composite scores such as ELF, HepaScore, and FibroMeter.7 We also excluded biomarkers and scores directly related to hepatic insufficiency (such as Child-Pugh or Model for End-Stage Liver Disease scores), as they were not designed for diagnosing fibrosis. Finally, we were careful to avoid including data from duplicate publications.
Statistical Methods
Three meta-analyses were performed: 1 assessing the significance of prognostic values; 1 using direct comparisons versus biopsy as the reference; and 1 using direct comparisons versus APRI, which is a nonpatented fibrosis biomarker.
The significance of each biomarker's prognostic value was assessed versus random; in other words, the mean AUROC of the biomarker was compared with 0.50 (the “random” value, indicating the absence of any diagnostic value). The direct comparison of biomarker versus biopsy or biomarker versus APRI used direct comparisons when they had been performed in at least 2 studies.
The AUROC was estimated by the empirical (non-parametric) method of DeLong and colleagues, which is equivalent to the Mann-Whitney statistic and was compared using the paired method of Zhou and coworkers.9,10 The analysis used a random effect model, and the heterogeneity between effects according to biomarkers and according to studies (if at least 2 studies were identified) has been tested using Cochran's Q heterogeneity test (Q). Analyses were performed on Number Cruncher Statistical System software.11
Results
Included Studies
The search initially retrieved 253 references. Among these 253 references, only 8 were original prognostic studies and were pre-included. Two studies were then excluded, as no specific fibrosis biomarker was assessed.12,13 The 6 included studies (listed in Table 1) allowed us to compare the prognostic performances of 7 fibrosis biomarkers in a total of 16 populations: APRI in 5; FibroTest in 4; FIB-4 in 3; and HepaScore, FibroMeter, ELF, and FibroScan in 1 population each.7,14–18
Table 1.
Description of Prognostic Studies: 6 Publications and 16 Assessments
| Reference | Disease | Biomarker assessed with area under the ROC curve |
|---|---|---|
| Ngo Y, Munteanu M, Messous D, et al14 | HCV | FibroTest, APRI, biopsy |
| Ngo Y, Benhamou Y, Thibault V, et al15 | HBV | FibroTest, APRI, biopsy |
| Naveau S, Gaude G, Asnacios A, et al7 | ALD | FibroTest, APRI, FIB-4, HepaScore, FibroMeter, biopsy |
| Nunes D, Fleming C, Offner G, et al17 | HCV | APRI, FIB-4 |
| Parkes J, Roderick P, Harris S, et al18 | Mixed liver disease | ELF, biopsy |
| Vergniol J, Foucher J, Terrebonne E, et al16 | HCV | FibroTest, APRI, FibroScan, FIB-4, biopsy |
- ALD
alcoholic liver disease
- APRI
aspartate transaminase-platelet ratio index
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- ROC
receiver operating characteristic
Characteristics of these studies are detailed in Table 2. APRI and FibroTest were assessed in patients with chronic hepatitis C virus (HCV) infection, chronic hepatitis B virus (HBV) infection, and ALD; FIB-4 in patients with HCV infection and ALD; FibroMeter in patients with ALD; FibroScan in patients with HCV infection; and ELF in a population with miscellaneous chronic liver diseases.
Table 2.
Characteristics of the 16 Populations Included in the Prognostic Studies
| Biomarker | Disease | Reference | Number of patients | Age (years) | Male % | Cirrhosis % | White % | Alcohol drinker % | HIV % | Treated % |
|---|---|---|---|---|---|---|---|---|---|---|
| FibroTest | HCV | Ngo Y, Munteanu M, Messous D, et al14 | 537 | 46 | 60 | 11 | 85 | 14 | 4 | 26 |
| HCV | Vergniol J, Foucher J, Terrebonne E, et al16 | 663 | 51 | 53 | 18 | 80 | 0 | 10 | 52 | |
| HBV | Ngo Y, Benhamou Y, Thibault V, et al15 | 978 | 41 | 69 | 19 | 26 | 3 | 6 | 60 | |
| ALD | Naveau S, Gaude G, Asnacios A, et al7 | 218 | 47 | 78 | 31 | 80 | 100 | 0 | 21* | |
| APRI | HCV | Ngo Y, Munteanu M, Messous D, et al14 | 260 | 46 | 60 | 11 | 85 | 14 | 4 | 26 |
| HCV | Nunes D, Fleming C, Offner G, et al17 | 303 | 44 | 64 | NA | 49 | 25 | 68 | NA | |
| HCV | Vergniol J, Foucher J, Terrebonne E, et al16 | 663 | 51 | 53 | 18 | 80 | 0 | 10 | 52 | |
| HBV | Ngo Y, Benhamou Y, Thibault V, et al15 | 978 | 41 | 69 | 19 | 26 | 3 | 6 | 60 | |
| ALD | Naveau S, Gaude G, Asnacios A, et al7 | 218 | 47 | 78 | 31 | 80 | 100 | 0 | 21* | |
| FIB-4 | HCV | Nunes D, Fleming C, Offner G, et al17 | 303 | 44 | 64 | NA | 49 | 25 | 68 | NA |
| HCV | Vergniol J, Foucher J, Terrebonne E, et al16 | 663 | 51 | 53 | 18 | 80 | 0 | 10 | 52 | |
| ALD | Naveau S, Gaude G, Asnacios A, et al7 | 218 | 47 | 78 | 31 | 80 | 100 | 0 | 21* | |
| HepaScore | ALD | Naveau S, Gaude G, Asnacios A, et al7 | 218 | 47 | 78 | 31 | 80 | 100 | 0 | 21* |
| FibroMeter | ALD | Naveau S, Gaude G, Asnacios A, et al7 | 218 | 47 | 78 | 31 | 80 | 100 | 0 | 21* |
| ELF | Mixed liver disease | Parkes J, Roderick P, Harris S, et al18 | 457 | 42 | 67 | 17 | 95 | 10 | NA | NA |
| FibroScan | HCV | Vergniol J, Foucher J, Terrebonne E, et al16 | 663 | 51 | 53 | 18 | 80 | 0 | 10 | 52 |
Abstinent.
- ALD
alcoholic liver disease
- APRI
aspartate transaminase-platelet ratio index
- HBV
hepatitis B virus
- HCV
hepatitis C virus
Prognostic Value of Biomarkers
The number of events observed and the prognostic values (AUROCs) of biomarkers are detailed in Table 3. Survival estimates were available for a total of 3,156 patients. The total number of deaths by population (whether related to liver disease or not) varied from 20 to 93. The most frequent endpoint used was survival without liver-related deaths; this endpoint was used in 19 comparisons. Overall survival was used in 17 comparisons. For 2 studies, no details were given regarding the prognostic value of biomarkers (AUROC) in an untreated group of patients, including APRI (in the study by Nunes and colleagues) and ELF (in the study by Parkes and coworkers).17,18
Table 3.
Number of Events Observed and Prognostic Values of Biomarkers
| Number of events | Prognostic value (AUROC) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Disease* | Reference | Biomarker | Number of patients | Follow-up (years) | Death: liver-related | Death: not liver-related | Complications | All | Liver-related | Complications |
| HCV | Ngo Y, Munteanu M, Messous D, et al14 | FibroTest | 537 | 5 | 9 | 11 | 20 | 0.76 | 0.96 | 0.96 |
| APRI | 260 | 0.67 | 0.76 | 0.82 | ||||||
| Biopsy | 537 | 0.66 | 0.87 | 0.85 | ||||||
| Nunes D, Fleming C, Offner G, et al17 | APRI | 303 | 5 | 31 | 44 | NA | NA | 0.85 | NA | |
| FIB-4 | 303 | NA | 0.85 | NA | ||||||
| Vergniol J, Foucher J, Terrebonne E, et al16 | FibroTest | 663 | 5 | 55 | 38 | NA | 0.80 | 0.81 | NA | |
| FibroScan | 663 | 0.82 | 0.87 | NA | ||||||
| APRI | 663 | 0.66 | 0.69 | NA | ||||||
| FIB-4 | 663 | 0.75 | 0.76 | NA | ||||||
| Biopsy | 663 | 0.76 | 0.84 | NA | ||||||
| HBV | Ngo Y, Benhamou Y, Thibault V, et al15 | FibroTest | 978 | 4 | 27 | 9 | 14 | 0.94 | 0.95 | 0.89 |
| APRI | 978 | 0.57 | 0.58 | 0.55 | ||||||
| Biopsy | 98 | 0.97 | 0.96 | 0.97 | ||||||
| ALD | Naveau S, Gaude G, Asnacios A, et al7 | FibroTest | 218 | 10 | 42 | 43 | NA | 0.69 | 0.79 | NA |
| HepaScore | 218 | 0.69 | 0.80 | NA | ||||||
| FibroMeter | 218 | 0.69 | 0.77 | NA | ||||||
| APRI | 218 | 0.56 | 0.59 | NA | ||||||
| FIB-4 | 218 | 0.64 | 0.65 | NA | ||||||
| Biopsy | 218 | 0.69 | 0.77 | NA | ||||||
| Mixed liver disease | Parkes J, Roderick P, Harris S, et al18 | ELF | 457 | 7 | 39 | 26 | 22 | NA | NA | 0.87 |
| Biopsy | 457 | NA | NA | 0.82 | ||||||
Patients with chronic hepatitis C virus (HCV) infection, chronic hepatitis B virus (HBV) infection, and alcoholic liver disease (ALD).
- APRI
aspartate transaminase-platelet ratio index
- AUROC
area under the receiver operating characteristic curve
Meta-Analysis of Performances
No meta-analyses were possible for ELF, HepaScore, and FibroMeter, as only 1 study has been performed for each of these biomarkers. A meta-analysis of survival without complications was only possible for FibroTest, which has shown a significant prognostic value for survival without liver-related complications in more than 1 study.
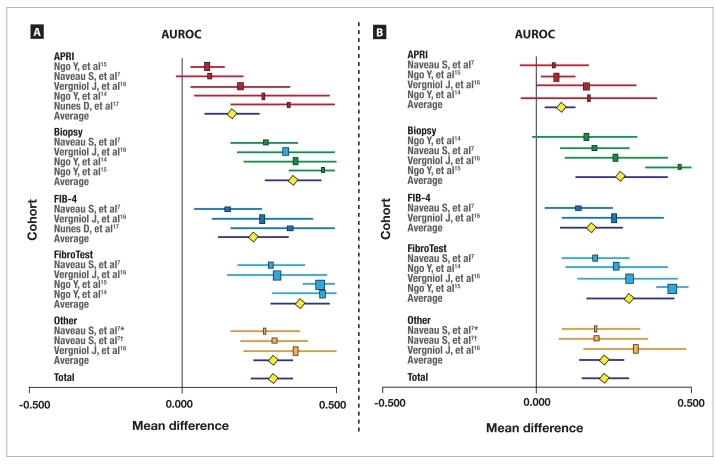
Biomarkers Versus Random The meta-analysis of biomarkers' prognostic value in terms of survival without liver-related deaths (19 comparisons) is described in Figure 1A. All biomarkers had significant (P<.001) prognostic value versus random (AUROC=0.50), with a significant heterogeneity between biomarkers (Cochran Q=123; P<.001). The mean AUROCs were 0.66 for APRI (95% CI, 0.57–0.75; lower than biopsy and FibroTest [P<.01 for both comparisons]), 0.86 for biopsy (95% CI, 0.77–0.95), 0.73 for FIB-4 (95% CI, 0.62–0.85), and 0.88 for FibroTest (95% CI, 0.79–0.98).
Figure 1.
Meta-analysis of the prognostic value of fibrosis biomarkers versus random for survival without liver-related deaths (A). Meta-analysis of the prognostic value of fibrosis biomarkers versus random for overall survival (B).
The horizontal lines indicate the 95% confidence interval for the mean difference between biomarkers and random (0.500) or between biomarkers' area under the receiver operating characteristic curves (AUROCs) and biopsy's AUROCs. The vertical lines indicate the equivalence line (0% difference). Positive differences indicate a difference in favor of the reference test (biopsy or aspartate transaminase-platelet ratio index [APRI] or FIB-4 or FibroTest or Other). When the horizontal line crosses the vertical line, there is no significant difference.
*HepaScore; †FibroMeter.
The meta-analysis of the prognostic value of bio-markers for overall survival (17 comparisons) is described in Figure 1B. All biomarkers had significant (P<.001) prognostic value versus random (AUROC=0.50), with significant heterogeneity between biomarkers (Cochran Q=121; P<.001). The mean AUROCs were 0.58 for APRI (95% CI, 0.53–0.63; lower than biopsy [P<.05] and FibroTest [P<.01]), 0.77 for biopsy (95% CI, 0.62–0.93), 0.68 for FIB-4 (95% CI, 0.58–0.78; lower than FibroTest [P<.05]), and 0.80 for FibroTest (95% CI, 0.76–0.95).
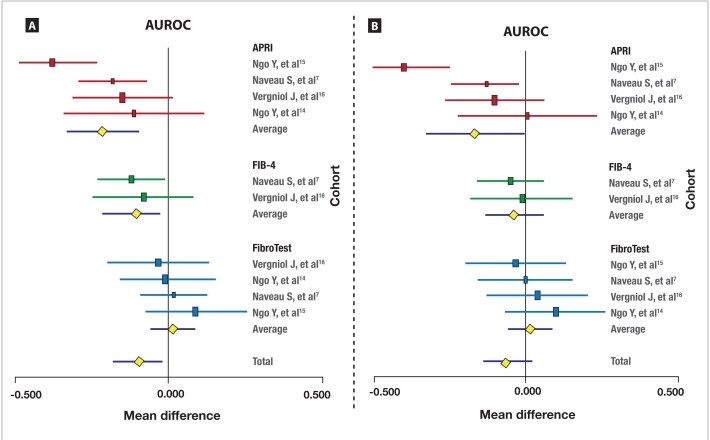
Biomarkers Versus Biopsy The meta-analysis of bio-markers' prognostic value versus biopsy for survival without liver-related deaths (10 direct comparisons) is described in Figure 2A. APRI had a significantly lower prognostic value versus biopsy, with a mean difference between AUROCs of –0.21 (95% CI, –0.33 to –0.10; P<.001); no significant heterogeneity between studies was observed (Cochran Q=7; P=.08). FIB-4 also had a significantly lower prognostic value versus biopsy, with a mean difference between AUROCs of –0.21 (95% CI, –0.20 to –0.02; P=.02); again, no significant heterogeneity between studies was observed (Cochran Q=0.2; P=.70). Only FibroTest showed no significant difference in prognostic value versus biopsy, with a mean difference in AUROCs of +0.02 (95% CI, –0.05 to +0.09; P=.85); no significant heterogeneity between studies was observed (Cochran Q=1.2; P=.76).
Figure 2.
Meta-analysis of the prognostic value of fibrosis biomarkers versus biopsy for survival without liver-related deaths (A). Meta-analysis of the prognostic value of fibrosis biomarkers versus biopsy for overall survival (B).
The horizontal lines indicate the 95% confidence interval for the mean difference between biomarkers and random (0.500) or between biomarkers' area under the receiver operating characteristic curves (AUROCs) and biopsy's AUROCs. The vertical lines indicate the equivalence line (0% difference). Negative differences indicate a difference in favor of the reference test (biopsy). When the horizontal line crosses the vertical line, there is no significant difference.
APRI=aspartate transaminase–platelet ratio index.
The meta-analysis of biomarkers' prognostic value versus biopsy for overall survival (10 direct comparisons) is described in Figure 2B. APRI had a significantly lower prognostic value than biopsy, with a mean difference between AUROCs of –0.17 (95% CI, –0.33 to –0.10; P<.001); however, significant heterogeneity between studies was observed for this comparison (Cochran Q=13; P=.005). In contrast, FIB-4 did not show a significant difference in prognostic value versus biopsy, with a mean difference between AUROCs of –0.04 (95% CI, –0.20 to –0.02; P=.43); no significant heterogeneity between studies was found (Cochran Q=0.2; P=.70). FibroTest also did not show a significant difference in prognostic value versus biopsy, with a mean difference in AUROCs of +0.02 (95% CI, –0.05 to +0.09; P=.61); no significant heterogeneity between studies was observed (Cochran Q=1.5; P=.68).
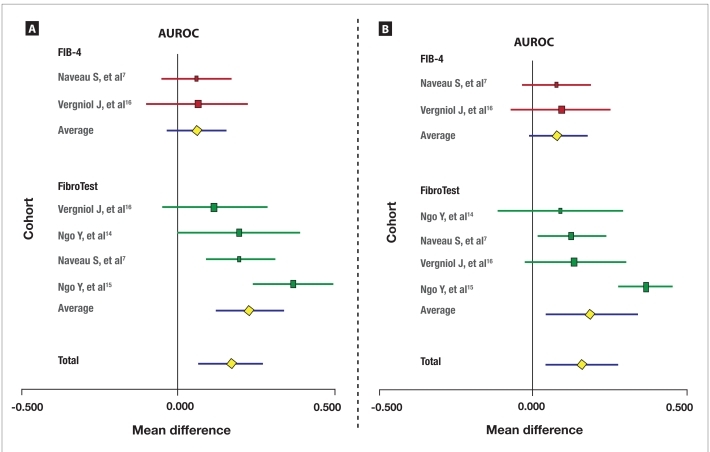
Biomarkers Versus APRI The meta-analysis of the prognostic values of FibroTest and FIB-4 versus APRI for survival without liver-related deaths (6 direct comparisons) is described in Figure 3A. FibroTest had a significantly higher prognostic value versus APRI, with a mean difference between AUROCs of +0.23 (95% CI, +0.12 to +0.34; P<.001); heterogeneity between studies was not significant (Cochran Q=6.5; P=.09). In contrast, FIB-4 did not have a significantly different prognostic value versus APRI, with a mean difference in AUROCs of +0.06 (95% CI, –0.03 to +0.16; P=.18); again, no significant heterogeneity between studies was observed (Cochran Q=0.1; P=.92).
Figure 3.
Meta-analysis of the prognostic value of FibroTest and FIB-4 versus aspartate transaminase-platelet ratio index (APRI) for survival without liver-related deaths (A). Meta-analysis of the prognostic value of FibroTest and FIB-4 versus APRI for overall survival (B).
The horizontal lines indicate the 95% confidence interval for the mean difference between the area under the receiver operating characteristic curves (AUROCs) of the biomarkers (FIB-4 and FibroTest) and APRI. The vertical lines indicate the equivalence line (0% difference). Positive differences indicate a difference in favor of FibroTest. When the horizontal line crosses the vertical line, there is no significant difference (FIB-4).
The meta-analysis of the prognostic value of FibroTest and FIB-4 versus APRI for overall survival (6 direct comparisons) is described in Figure 3B. FibroTest had a significantly higher prognostic value versus APRI, with a mean difference between AUROCs of +0.20 (95% CI, +0.03 to +0.36; P<.001); however, significant heterogeneity between studies was observed (Cochran Q=22; P<.001). FIB-4 did not have a significantly different prognostic value versus biopsy, with a mean difference in AUROCs of +0.08 (95% CI, –0.01 to +0.18; P=.08); heterogeneity between studies was not significant (Cochran Q=0.1; P=.92).
Discussion
This first overview and meta-analysis of prognostic studies allowed us to validate the performances of liver fibrosis biomarkers using simultaneous baseline biopsy as a reference in patients with chronic HCV infection, chronic HBV infection, and ALD. At least 2 validations of prognostic significance were available for 3 biomarkers: FibroTest, APRI, and FIB-4.
When fibrosis staging using liver biopsy was taken as the reference, only FibroTest had a similar prognostic value for both overall survival and survival without liver-related deaths. The prognostic value of FibroTest was similar in patients with chronic HCV infection, chronic HBV infection, and ALD. FibroTest was also clearly validated using APRI as a common comparator in 5 studies, which showed significantly higher performances for FibroTest versus APRI.
APRI and FIB-4 had prognostic values that were significantly higher than random but significantly lower than the prognostic value of FibroTest. These results were expected, as they were similar to the diagnostic performance previously observed in diagnostic overviews.6,19 FibroTest had higher diagnostic performance than APRI and FIB-4.7,16,20–22 Besides their lower performance, another disadvantage of APRI and FIB-4 is that they include transaminases (ALT or AST) in their scores, which induces an interaction with necroinflammatory activity grades. Therefore, a variation in APRI or FIB-4 results can be related to a variation in necrosis grade rather than a variation in fibrosis stage, which raises a risk of false positives during flare-ups in patients with chronic HBV infection. In patients with chronic HCV or HBV infection, ALT or specific biomarkers of activity (such as ActiTest) are positively associated with virologic response to treatment, while biomarkers of fibrosis are negatively associated with treatment response.23–25 Additionally, the performance of APRI and FIB-4 was found to be weaker in patients with ALD compared with FibroTest and biopsy, and FIB-4 had no prognostic validation in patients with chronic HBV infection.7 However, the advantage of APRI and FIB-4 is their lower cost in comparison with patented biomarkers.
Only 1 study each was identified for FibroScan, ELF, FibroMeter, and HepaScore; while these tests may have significant prognostic value, no meta-analyses were possible. More prognostic studies are needed in various liver diseases, but these tests appear promising based on their performances for the diagnosis of fibrosis stage. In comparison with FibroTest, the weakness of FibroScan is its relatively low applicability rate: around 80% for FibroScan versus 98% for FibroTest.4,26,27 FibroMeter has 2 disadvantages: It has been assessed using different algorithms since its first validation, and it includes transaminases (as with APRI and FIB-4).28
The advantages of the present meta-analysis are that it included a total of 3,156 patients with a mean follow-up duration of 4–10 years and that it was able to directly compare biomarkers with 2 references. This meta-analysis could therefore assess the differences between biomarkers' performance using liver biopsy as the standard reference in 10 direct comparisons and APRI as a reference in 6 direct comparisons. Furthermore, strong endpoints—such as survival without liver-related deaths and overall survival—permitted the validation of fibrosis biomarkers' diagnostic performance in the absence of a perfect gold standard.4 Biopsy (even 25 mm in length) is an imperfect gold standard, with at least 20% false positives or false negatives for the diagnosis of advanced fibrosis, and therefore the true accuracies of biomarkers are unknown. If a biomarker had the same prognostic value as biopsy, this finding suggests that half of the discordant cases at baseline could be due to failure of the biomarker and half to failure of the biopsy. If all the misclassified cases had been due to the failure of the biomarker, then the prognostic value of biopsy should be higher than for the biomarker.
The present meta-analysis also has several limitations. First, we have not analyzed an integrated database with data from all the studies, which would have permitted us to adjust the analysis of prognostic factors by time-dependent multivariate analyses. Second, while the overall number of events was reasonable for patients with chronic HCV and HBV infection, it was small for patients with ALD, even if the frequency of events is higher in these patients. No prognostic studies have been identified to date in patients with nonalcoholic fatty liver disease (NAFLD). Also, prognostic studies comparing biomarkers with biopsy are difficult to perform for these 2 chronic liver diseases (ALD and NAFLD), due to the low acceptance of baseline biopsy. Furthermore, the risk of being lost to follow-up is also greater for patients with ALD than for patients with chronic HCV infection. Third, few studies reported liver-related complications that might help to strengthen the case for a liver-specific prognostic marker. It will also be interesting to compare the prognostic value of FibroTest to those assessed in the HALT-C studies.29,30
One final limitation is the possible conflict of interest of 3 coauthors, including the inventor of the FibroTest (TP) and 2 employees of the company marketing FibroTest (MM and YN). However, the 4 studies concerning FibroTest have been accepted for publication after undergoing peer review. Furthermore, among the 4 studies concerning FibroTest, the prognostic performance of this test was similar in the study conducted by an independent principal investigator (Naveau and associates) and in the independent study conducted without possible “dependent” coauthors (Vergniol and coworkers).7,16
Conclusion
This meta-analysis allowed for validation of the prognostic value of FibroTest for the prediction of 5-year survival in patients with chronic HCV infection, chronic HBV infection, and ALD. The performance of FibroTest was similar to the performance of fibrosis staging using liver biopsy, which is the standard reference. FIB-4 and APRI also had significant prognostic values in patients with chronic HCV infection, but these values were lower than for FibroTest. More studies are needed to confirm promising results concerning other patented biomarkers, including FibroScan. Prognostic validation of all fibrosis biomarkers is needed in patients with NAFLD.
Biography
Dr. Poynard is a consultant and has a capital interest in Biopredictive, the company marketing FibroTest; the patents belong to Assistance Publique Hôpitaux de Paris. Drs. Ngo and Munteanu are full-time employees of Biopredictive. Finally, the authors would like to acknowledge “Association pour la Recherche sur les Maladies Hépatiques et Virales” for support in the collection of data.
References
- 1.Poynard T, Mathurin P, Lai CL, et al. A comparison of fibrosis progression in chronic liver diseases. J Hepatol. 2003;38:257–265. doi: 10.1016/s0168-8278(02)00413-0. [DOI] [PubMed] [Google Scholar]
- 2.Castera L, Pinzani M. Non-invasive assessment of liver fibrosis: are we ready? Lancet. 2010;375:1419–1420. doi: 10.1016/S0140-6736(09)62195-4. [DOI] [PubMed] [Google Scholar]
- 3.Fontaine H, Petitprez K, Roudot-Thoraval F, Trinchet JC. Guidelines for the diagnosis of uncomplicated cirrhosis. Gastroenterol Clin Biol. 2007;31:504–509. doi: 10.1016/S0399-8320(07)89420-6. [DOI] [PubMed] [Google Scholar]
- 4.Poynard T, Ingiliz P, Elkrief L, et al. Concordance in a world without a gold standard: a new non-invasive methodology for improving accuracy of fibrosis markers. PLoS One. 2008;3:e3857. doi: 10.1371/journal.pone.0003857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. The Standards for Reporting of Diagnostic Accuracy Group. Croat Med J. 2003;44:635–638. [PubMed] [Google Scholar]
- 6.Poynard T, Morra R, Ingiliz P, et al. Biomarkers of liver fibrosis. Adv Clin Chem. 2008;46:131–160. doi: 10.1016/s0065-2423(08)00404-6. [DOI] [PubMed] [Google Scholar]
- 7.Naveau S, Gaude G, Asnacios A, et al. Diagnostic and prognostic values of noninvasive biomarkers of fibrosis in patients with alcoholic liver disease. Hepatology. 2009;49:97–105. doi: 10.1002/hep.22576. [DOI] [PubMed] [Google Scholar]
- 8.Körner T, Kropf J, Gressner AM. Serum laminin and hyaluronan in liver cirrhosis: markers of progression with high prognostic value. J Hepatol. 1996;25:684–688. doi: 10.1016/s0168-8278(96)80239-x. [DOI] [PubMed] [Google Scholar]
- 9.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 10.Zhou X, Obuchowski N, McClish D. Statistical Methods in Diagnostic Medicine. New York, NY: John Wiley & Sons; 2002. [Google Scholar]
- 11.Hintze J. NCSS User's Guide I. Quick Start & Self Help, Introduction, Data, Toools, and Graphics. Kaysville, Utah: 2007. http://www.ncss.com/pdf/manuals/NCSSUG1.pdf [Google Scholar]
- 12.Wong VW, Wong GL, Chim AM, et al. Surrogate end points and long-term outcome in patients with chronic hepatitis B. Clin Gastroenterol Hepatol. 2009;7:1113–1120. doi: 10.1016/j.cgh.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 13.Su CW, Chan CC, Hung HH, et al. Predictive value of aspartate aminotransferase to alanine aminotransferase ratio for hepatic fibrosis and clinical adverse outcomes in patients with primary biliary cirrhosis. J Clin Gastroenterol. 2009;43:876–883. doi: 10.1097/MCG.0b013e31818980ac. [DOI] [PubMed] [Google Scholar]
- 14.Ngo Y, Munteanu M, Messous D, et al. A prospective analysis of the prognostic value of biomarkers (FibroTest) in patients with chronic hepatitis C. Clin Chem. 2006;52:1887–1896. doi: 10.1373/clinchem.2006.070961. [DOI] [PubMed] [Google Scholar]
- 15.Ngo Y, Benhamou Y, Thibault V, et al. An accurate definition of the status of inactive hepatitis B virus carrier by a combination of biomarkers (Fibrotest-Actitest) and viral load. PLoS One. 2008;3:e2573. doi: 10.1371/journal.pone.0002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vergniol J, Foucher J, Terrebonne E, et al. Non-invasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology. 2011;140:1970.e3–1979.e3. doi: 10.1053/j.gastro.2011.02.058. [DOI] [PubMed] [Google Scholar]
- 17.Nunes D, Fleming C, Offner G, et al. Noninvasive markers of liver fibrosis are highly predictive of liver-related death in a cohort of HCV-infected individuals with and without HIV infection. Am J Gastroenterol. 2010;105:1346–1353. doi: 10.1038/ajg.2009.746. [DOI] [PubMed] [Google Scholar]
- 18.Parkes J, Roderick P, Harris S, et al. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut. 2010;59:1245–1251. doi: 10.1136/gut.2009.203166. [DOI] [PubMed] [Google Scholar]
- 19.Poynard T, Ngo Y, Munteanu M, Thabut D, Ratziu V. Noninvasive markers of hepatic fibrosis in chronic hepatitis B. Curr Hepat Rep. 2011;10:87–97. doi: 10.1007/s11901-011-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Calvez S, Thabut D, Messous D, et al. Fibrotest has higher predictive values than APRI for fibrosis diagnosis in patients with chronic hepatitis C. Hepatology. 2004;39:862–863. doi: 10.1002/hep.20099. [DOI] [PubMed] [Google Scholar]
- 21.Castéra L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, Fibrotest, APRI and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Cacoub P, Carrat F, Bédossa P, et al. Comparison of non-invasive liver fibrosis biomarkers in HIV/HCV co-infected patients: the Fibrovic study—ANRS HC02. J Hepatol. 2008;48:765–773. doi: 10.1016/j.jhep.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Poynard T, Munteanu M, Ngo Y, et al. ActiTest accuracy for the assessment of histological activity grades in patients with chronic hepatitis C, an overview using Obuchowski measure. Gastroenterol Clin Biol. 2010;34:388–396. doi: 10.1016/j.gcb.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Poynard T, Ngo Y, Munteanu M, et al. Biomarkers of liver injury for hepatitis clinical trials: a meta-analysis of longitudinal studies. Antivir Ther. 2010;15:617–631. doi: 10.3851/IMP1570. [DOI] [PubMed] [Google Scholar]
- 25.Costa JM, Telehin D, Munteanu M, et al. HCV-GenoFibrotest: a combination of viral, liver and genomic (IL28b, ITPA, UGT1A1) biomarkers for predicting treatment response in patients with chronic hepatitis C. Clin Res Hepatol Gastroenterol. 2011;35:204–213. doi: 10.1016/j.clinre.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Castéra L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828–835. doi: 10.1002/hep.23425. [DOI] [PubMed] [Google Scholar]
- 27.Poynard T, Munteanu M, Deckmyn O, et al. Applicability and precautions of use of liver injury biomarker FibroTest. A reappraisal at 7 years of age. BMC Gastroenterol. 2011;11:39. doi: 10.1186/1471-230X-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cales P, Oberti F, Michalak S, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology. 2005;42:1373–1381. doi: 10.1002/hep.20935. [DOI] [PubMed] [Google Scholar]
- 29.Fontana RJ, Dienstag JL, Bonkovsky HL, et al. Serum fibrosis markers are associated with liver disease progression in non-responder patients with chronic hepatitis C. Gut. 2010;59:1401–1409. doi: 10.1136/gut.2010.207423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghany MG, Lok AS, Everhart JE, et al. Predicting clinical and histologic outcomes based on standard laboratory tests in advanced chronic hepatitis C. Gastroenterology. 2010;138:136–146. doi: 10.1053/j.gastro.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]