Celiac disease (CD) is the most common food intolerance in Western populations. However, recent studies have shown that the prevalence of CD in individuals of non-European descent is similar to that of Western populations. Currently, this disorder represents a major health issue that is globally underdiagnosed. CD has a broad clinical spectrum, with signs and symptoms including iron-deficiency anemia, constipation, diarrhea, malabsorption, and weight loss. Extraintestinal manifestations have also been described, including dermatologic and neurologic disorders. To our knowledge, this case is the first report of a Middle Eastern woman with celiac enteropathy who presented with skin rash and gait ataxia. This case highlights the prevalence of CD in non-Western populations, and it illustrates the importance of considering CD in the differential diagnosis of patients with atypical gastrointestinal and neurologic symptoms.
Case Report
A 37-year-old woman from the United Arab Emirates presented to our institution's Movement Disorder Center with a 5-year history of undiagnosed gait disturbance, imbalance, and dysarthria. She described a slowly progressive course over several years; for the last 3 years, she had used a wheelchair when traveling longer distances due to concerns about frequent falls. The patient reported moderate impairment in fine motor skills and some visual disturbances, but she denied numbness and tingling in her extremities. She admitted to increased urinary frequency and urge incontinence beginning after the onset of her gait disturbance. Initial blood laboratory analysis showed profound iron-deficiency anemia, and the patient was referred to the gastroenterology clinic for further evaluation. Except for chronic constipation and weight gain, she had no other gastrointestinal symptoms. She also reported an undiagnosed pruritic skin rash that had appeared 3 years prior to presentation. She had no other significant past medical history and no family history of any neurologic disorder. However, her parents were first cousins.
On physical examination, the patient appeared weak and unsteady on her feet. She was alert and oriented to person, place, and time. Her vitals were stable, and there was no evidence of orthostatic hypotension. She was not jaundiced. A healing, symmetric, papulovesicular rash was noted on her trunk and extremities (Figures 1 and 2). Cardiac and pulmonary examination results were normal. Her abdomen was benign, with no hepatosplenomegaly or other mass found on palpation.
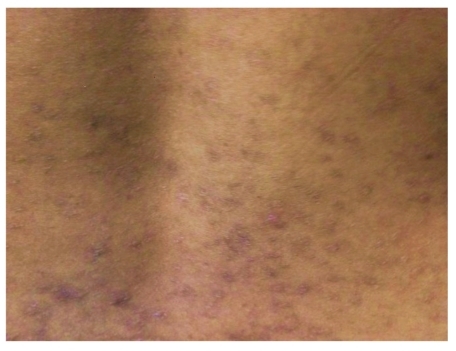
Figure 1.
Papulovesicular eruptions with symmetrical distribution noted, on the patient's back with areas of hyper-pigmentation suggestive of dermatitis herpetiformis.
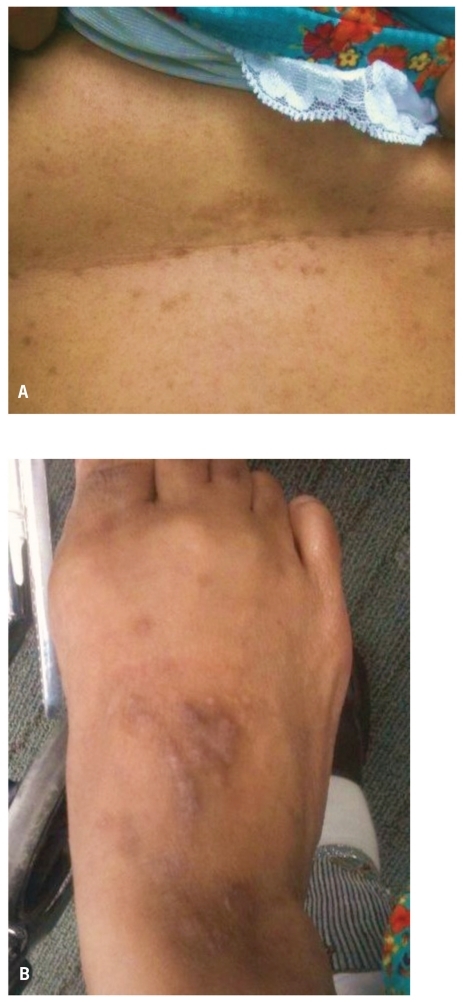
Figure 2.
Papulovesicular eruptions noted on the abdomen (A) and the dorsum of the foot (B), which are suggestive of dermatitis herpetiformis.
Pertinent neurologic findings included prominent cerebellar oculomotor dysfunction as evidenced by sac-cadic pursuit, hypermetric saccades, impaired suppression of the vestibulo-ocular reflex, and mild gaze-evoked nystagmus. Her speech was consistent with cerebellar dysarthria. She had normal muscle tone with no weakness or tremor. She had 2+ symmetric reflexes and downgoing toes. Her sensation was intact to light touch, propriocep-tion, and vibration. Rapid alternating movements were slow and dysrhythmic. There was moderate dysmetria on finger-to-nose and heel-to-shin testing. Her walk was wide-based and unsteady. Her score on the scale for the assessment and rating of ataxia was 15/40.
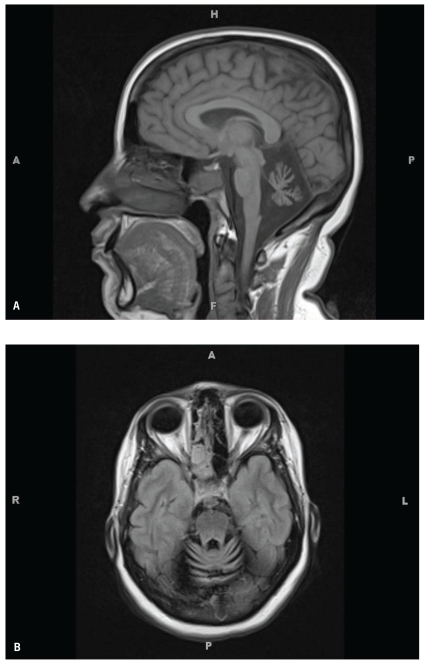
Neurologic work-up for sporadic ataxia, including a paraneoplastic antibody panel, was negative. Vitamin E, vitamin B12, thyroid-stimulating hormone, rapid plasma reagin, methylmalonic acid, coenzyme Q10, anti—glutamic acid decarboxylase 65 antibodies, and hexosaminidase levels were all within normal limits. A lumbar puncture was performed; cerebrospinal fluid revealed a normal cell count, protein content, and negative cytopathology. The patient refused genetic testing. Brain magnetic resonance imaging revealed significant cerebellar and borderline pontine atrophy (Figure 3).
Figure 3.
Cerebellopontine atrophy seen on a sagittal view (A) and axial view (B) of a rna.gnetic resonance imaging scan of the brain.
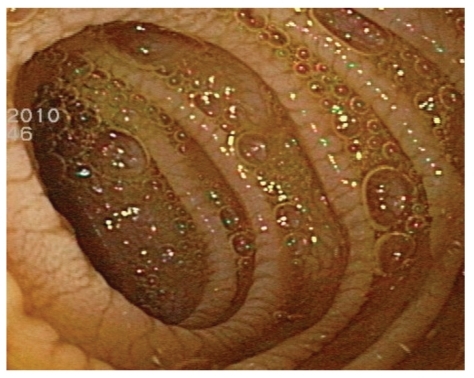
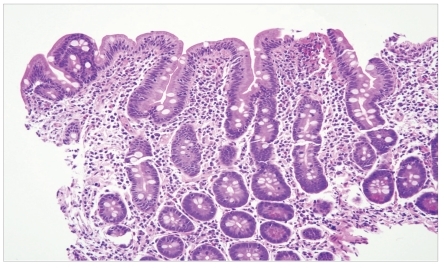
Blood analysis was notable for profound iron-deficiency anemia, with a hemoglobin level of 9 g/dL, mean corpuscular volume of 68 fL, and ferritin level less than 3 ng/mL. A celiac serology panel was positive: Her gliadin antibody immunoglobulin (Ig)G level was 34 mg/dL (normal, <20 mg/dL), IgA level was 59 mg/dL (normal, <20 mg/dL), endomysial IgA antibody was positive at 1:80 units (negative, <10 units), and tissue transglutaminase IgA was positive at 189 units (normal, <20 units). The patient's vitamin D level was low (16 ng/mL). Upper endoscopy revealed diffuse scalloping of the duodenal mucosa with marked flattening and mosaic appearance of the bulbar mucosa that was suggestive of CD (Figures 4 and 5). Marsh type III lesions were confirmed on histopathology and were marked by villous atrophy, prominent intraepithelial lymphocytes, and crypt hyperplasia (Figure 6). The distribution of the intermittent, pruritic, pustulovesicular rash was very suggestive of dermatitis herpetiformis. The lesions were not biopsied.
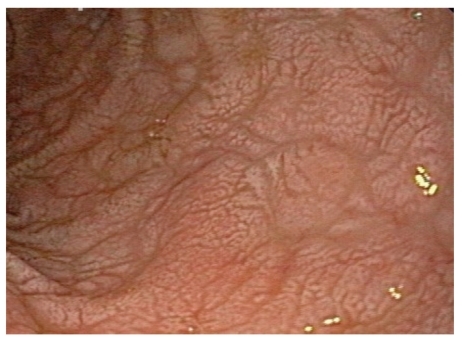
Figure 4.
Mosaic pattern and flattening of a duodenal bulb seen on upper endoscopy.
Figure 5.
Scalloping of duodenal folds seen on upper endoscopy.
Figure 6.
At high-power magnification, marked villous blunting, prominent intraepithelial lymphocytes, and crypt hyperplasia consistent with Marsh type III lesions were seen (hematoxylin and eosin stain).
After an extensive neurologic work-up and in the absence of any pertinent family history that could suggest hereditary ataxia, the patient was diagnosed with gluten ataxia as a manifestation of biopsy-proven celiac enteropa-thy. The patient received nutritional education regarding a strict gluten-free diet. She was also given vitamin D and iron supplements.
On follow-up examination 6 months later, the patient had remained adherent to a gluten-free diet. She reported regular bowel movements, her anemia had improved, and she had regained her strength. She also reported resolution of her skin rash without the use of any topical agents. Although she noted improvement in her speech, she continues physical therapy for persistent but stable gait ataxia.
Discussion
CD is caused by a chronic, immune-mediated response to ingested gluten and related proteins found in wheat, rye, and barley. Gluten sensitivity is strongly heritable, with 40% of the genetic load coming from major histocom-patibility complex class II associations, notably haplotype HLA DQ2 (present in 90% of whites) and HLA DQ8.1 In genetically susceptible individuals, this disorder leads to an inflammatory process in the proximal small bowel, which results in lymphocytic infiltration, crypt hyper-plasia, and villous atrophy. Malabsorption also develops, which results in diarrhea, micronutrient deficiency, and weight loss.
CD was previously thought to be most common in individuals of European descent and rare in other populations. The Middle East is known for wheat and barley cultivation, and these grains are a major dietary staple for more than 90% of the population. It was thought that this diet conferred immune tolerance to gluten in this population. Recent studies have shown that the prevalence of CD among low-risk patients in the Middle East and North Africa is similar to the prevalence in Western populations, ranging from 0.14% to 1.17%, and possibly higher in at-risk populations, ranging from 2.4% to 44%.2,3 The Sahawari population of Arab Berber origin in Algeria has the highest world prevalence of CD, at 5.6%.4 This high prevalence may be explained by a high frequency of HLA DQ2 and consanguinity. The exact prevalence of CD in the United Arab Emirates remains unknown.
Dermatitis herpetiformis has been widely accepted as an extraintestinal manifestation of CD for many decades. Although numerous cases of neurologic manifestations in patients with gluten enteropathy have been described since 1966, this association remains highly debated, as a mechanistic pathway has not been clearly elucidated.5 Neurologic manifestations in patients with established gluten enteropathy have been estimated to occur in 6–10% of patients with CD. Common neurologic manifestations include gluten ataxia and peripheral neuropathy. Encephalopathy, myelopathy, epilepsy, dementia, and myopathy have also been reported.6,7
Gluten ataxia is described as sporadic cerebellar ataxia that occurs in the absence of other etiology, is associated with gluten sensitivity, and is demonstrated by the presence of positive serologic markers (ie, circulating antiglia-din antibodies [AGAs]).8 AGAs are antibodies to a food component, and they are known to be less sensitive and specific than anti-endomysial and anti—tissue transgluta-minase antibodies for the detection of enteropathy. The prevalence of biopsy-proven CD in patients with ataxia of unknown origin ranges from 12% to 15%, whereas the prevalence of a positive AGA result in patients with ataxia of unknown origin ranges between 0% and 41%.9–12 Some studies have failed to demonstrate a difference in the prevalence of AGAs in sporadic versus hereditary ataxia.13–15
Gluten ataxia has been described as having an insidious onset of predominantly gait ataxia, and it is often associated with peripheral neuropathy. Gluten ataxia affects both genders equally and has a mean age at onset of 53 years. Evidence of limb ataxia is seen in up to 90% of patients, with lower limbs affected more often than upper limbs. Gaze-evoked nystagmus and other ocular signs of cerebellar dysfunction are seen in up to 80% of cases. Bladder dysfunction is seen in up to one third of patients. Although our patient presented at a relatively young age, all of the previously mentioned symptoms were noted on presentation.
The absence of additional autonomic dysfunction and Parkinsonian findings distinguishes patients with gluten ataxia from those with a cerebellar variant of multiple system atrophy. Up to 60% of patients with gluten ataxia have evidence of cerebellar atrophy on magnetic resonance imaging, as was seen in our patient.16 Gluten ataxia resembles dermatitis herpetiformis when gastrointestinal symptoms are not prominent, despite the presence of enteropathy. Less than 10% of patients with gluten ataxia will have gastrointestinal symptoms, and only one third will have evidence of enteropathy on biopsy.16
It has been suggested that the neurologic manifestations of this condition may be the result of micronutrient malabsorption. Deficiencies in vitamin B1 vitamin B6, vitamin B12, vitamin E, niacin, and riboflavin can result in neurologic symptoms. More recently, the discovery of the homologous nature of certain members of the trans-glutaminase family—transglutaminase 2 (TG2), which is predominant in the gut; transglutaminase 3 (TG3), which is predominant in the skin; and transglutaminase 6 (TG6), which is predominant in the brain—strongly suggests an immune-mediated etiopathogenesis. The ability of TG2 to de-amidate and crosslink gluten peptides is important for gluten-dependent production of TG2 autoantibodies and development of gluten enteropathy. TG3 in skin appears to be the target autoantigen in dermatitis herpetiformis. In gluten ataxia, autoantibodies reactive to TG6 are present. Antibodies to TG6 have been found in cerebrospinal fluid, and deposits have been seen in Purkinje cells. Postmortem data from patients with neurologic symptoms have shown inflammation in the cerebellum, other parts of the central nervous system, and the peripheral nervous system.10,17,18
The response to a gluten-free diet has been inconsistent in terms of neurologic changes. In contrast, a significant response is seen in patients with dermatitis herpetiformis, as noted. Restriction of gluten alone has not been consistently shown to be effective in patients with gluten ataxia, nor has the efficacy of empiric vitamin replacement been proven. Intravenous steroids and immunoglobulin treatments have also been tried with varying results.19–22
To our knowledge, this case is the first report of gluten ataxia in a Middle Eastern patient with CD that was confirmed by serology and histology. The spectrum of gastrointestinal symptoms in CD, ranging from asymptomatic disease to significant malnutrition, renders clinical suspicion of this condition challenging. Furthermore, establishing a diagnosis in the setting of extraintestinal manifestations is difficult, as a majority of these patients will not test positive for currently available celiac serologic markers. A high index of suspicion for CD should be maintained in Western and non-Western patients who present with typical or atypical gastrointestinal symptoms, iron-deficiency anemia, and neurologic symptoms.
References
- 1.Hunt KA, Zhernakova A, Turner G, et al. Newly identified genetic risk variants for celiac disease related immune response. Nat Genet. 2008;40:395–402. doi: 10.1038/ng.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shahbazkhani B, Malekzadeh R, Sotoudeh M, et al. High prevalence of celiac disease in apparently healthy Iranian blood donors. Eur J Gastroenterol Hepatol. 2003;15:475–478. doi: 10.1097/01.meg.0000059118.41030.96. [DOI] [PubMed] [Google Scholar]
- 3.Ashabani A, Abushofa U, Abusrewill S, Abdelazez M, Tucková L, Tlaskalová-Hogenová H. The prevalence of celiac disease in Libyan children with type 1 diabetes mellitus. Diabetes Metab Res Rev. 2003;19:69–75. doi: 10.1002/dmrr.333. [DOI] [PubMed] [Google Scholar]
- 4.Catassi C, Rätsch IM, Gandolfi L, et al. Why is celiac disease endemic in the people of the Sahara? Lancet. 1999;354:647–648. doi: 10.1016/s0140-6736(99)02609-4. [DOI] [PubMed] [Google Scholar]
- 5.Cooke WT, Smith WT. Neurological disorders associated with adult coeliac disease. Brain. 1966;89:683–722. doi: 10.1093/brain/89.4.683. [DOI] [PubMed] [Google Scholar]
- 6.Holmes GKT. Neurological and psychiatric complications in celiac disease. In: Gobbi G, Anderman F, Naccarato S, Banchini G, et al., editors. Epilepsy and Other Neurological Disorders in Celiac Disease. London, England: John Libbey; 1997. pp. 251–264. [Google Scholar]
- 7.Trier JS. Celiac sprue. NEngl J Med. 1991;325:1709–1719. doi: 10.1056/NEJM199112123252406. [DOI] [PubMed] [Google Scholar]
- 8.Hadjivassiliou M, Grünewald RA, Chattopadhyay AK, et al. Clinical, radiological, neurophysiological, and neuropathological characteristics of gluten ataxia. Lancet. 1998;352:1582–1585. doi: 10.1016/s0140-6736(98)05342-2. [DOI] [PubMed] [Google Scholar]
- 9.Hadjivassiliou M, Grünewald R, Sharrack B, et al. Gluten ataxia in perspective: epidemiology, genetic susceptibility and clinical characteristics. Brain. 2003;126(pt3):685–691. doi: 10.1093/brain/awg050. [DOI] [PubMed] [Google Scholar]
- 10.Hadjivassiliou M, Gibson A, Davies-Jones GA, Lobo AJ, Stephenson TJ, Milford-Ward A. Does cryptic gluten sensitivity play a part in neurological illness? Lancet. 1996;347:369–371. doi: 10.1016/s0140-6736(96)90540-1. [DOI] [PubMed] [Google Scholar]
- 11.Combarros O, Infante J, López-Hoyos M, et al. Celiac disease and idiopathic cerebellar ataxia. Neurology. 2000;54:2346. doi: 10.1212/wnl.54.12.2346. [DOI] [PubMed] [Google Scholar]
- 12.Bushara KO, Goebel SU, Shill H, Goldfarb LG, Hallett M. Gluten sensitivity in sporadic and hereditary cerebellar ataxia. Ann Neurol. 2001;49:540–543. [PubMed] [Google Scholar]
- 13.Abele M, Schöls L, Schwartz S, Klockgether T. Prevalence of antigliadin antibodies in ataxia patients. Neurology. 2003;60:1674–1675. doi: 10.1212/01.wnl.0000069606.82919.04. [DOI] [PubMed] [Google Scholar]
- 14.Wong D, Dwinnel M, Schulzer M, Nimmo M, Leavitt BR, Spacey SD. Ataxia and the role of antigliadin antibodies. Can J Neurol Sci. 2007;34:193–196.. doi: 10.1017/s031716710000603x. [DOI] [PubMed] [Google Scholar]
- 15.Bushara KO, Nance M, Gomez CM. Antigliadin antibodies in Huntingtons disease. Neurology. 2004;62:132–133. doi: 10.1212/wnl.62.1.132. [DOI] [PubMed] [Google Scholar]
- 16.Hadjivassiliou M, Sanders DS, Woodroofe N, Williamson C, Grünewald RA. Gluten ataxia. Cerebellum. 2008;7:494–498. doi: 10.1007/s12311-008-0052-x. [DOI] [PubMed] [Google Scholar]
- 17.Hadjivassiliou M, Aeschlimann P, Strigun A, Sanders DS, Woodroofe N, Aeschlimann D. Autoantibodies in gluten ataxia recognize a novel neuronal transglutaminase. Ann Neurol. 2008;64:332–343. doi: 10.1002/ana.21450. [DOI] [PubMed] [Google Scholar]
- 18.Stamnaes J, Dorum S, Fleckenstein B, Aeschlimann D, Sollid LM. Gluten T cell epitope targeting by TG3 and TG6; implications for dermatitis herpetiformis and gluten alaxia. Amino Acids. 2010;39:1183–1191. doi: 10.1007/s00726-010-0554-y. [DOI] [PubMed] [Google Scholar]
- 19.Bushara KO, Shill H, Hallett M. Open label trial of gluten free diet in sporadic and hereditary cerebellar ataxia with gluten sensitivity. Mov Disord. 2002;17:S325. [PubMed] [Google Scholar]
- 20.Hadjivassiliou M, Davies Jones GA, Sanders DS, Grünewald RA. Dietary treatment of gluten ataxia. J Neurol Neurosurg Psychiatry. 2003;74:1221–1224. doi: 10.1136/jnnp.74.9.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mauro A, Orsi L, Mortara P, Costa P, Schiffer D. Cerebellar syndrome in adult celiac disease with vitamin E deficiency. Acta Neurol Scand. 1991;84:167–170. doi: 10.1111/j.1600-0404.1991.tb04927.x. [DOI] [PubMed] [Google Scholar]
- 22.Bürk K, Melms A, Schulz JB, Dichgans J. Effectiveness of intravenous immu-noglobin therapy in cerebellar ataxia associated with gluten sensitivity. Ann Neurol. 2001;50:827–828. doi: 10.1002/ana.1281. [DOI] [PubMed] [Google Scholar]