Abstract
For the first time in more than 50 years, the US Food and Drug Administration has approved a drug specifically for the treatment of systemic lupus erythematosus (SLE). This drug, belimumab (Benlysta), is a human monoclonal antibody that neutralizes the B-cell survival factor, B-lymphocyte stimulator (BLyS). The approval of belimumab combined a pioneering approach to genomics-based gene discovery, an astute appreciation of translational medicine, a disciplined clinical strategy, a willingness to take calculated risks, a devoted cadre of patients and physicians and a healthy dose of serendipity. Collectively, these efforts have provided a model for the development of a new generation of drugs to treat the broad manifestations of SLE. However, as a substantial percentage of SLE patients do not respond to belimumab, further research is needed to better characterize the pathogenetic mechanisms of SLE, identify additional therapeutic targets, and develop effective and nontoxic novel agents against these targets.
On March 9, 2011, the US Food and Drug Administration (FDA) did something it had not done in more than 50 years—it approved a drug specifically for the treatment of SLE. The drug, belimumab, is a human monoclonal antibody (mAb) that binds and neutralizes B-lymphocyte stimulator (BLyS, also commonly known as BAFF). The milestone is all the more remarkable in that as recently as 1998, the target of the approved therapeutic agent (BLyS) was itself an unknown entity to the scientific community. We review the sometimes bumpy journey from identification of BLyS to approval by the FDA of belimumab, focusing on the scientific and clinical strategies used to transform a genomics-based discovery into an approved product for the treatment of SLE. We also comment on the discovery path for this drug in the context of an FDA-approved agent that targets B cells and other agents in development against BLyS.
Identification of BLyS
The identification of BLyS and, ultimately, its antagonist belimumab is inextricably linked to the convergence of a technological advance in automated DNA sequencing and a vision for the creation of new medicines from the millions of gene fragments that emerged from the DNA sequencers. These pioneering concepts were brought together in 1992 by the formation of Human Genome Sciences (HGS; Rockville, MD, USA) and its nonprofit sister company, The Institute for Genomics Research (TIGR; Rockville, MD, USA; Fig. 1). Within 3 years of their founding, the companies had amassed almost 175,000 expressed sequence tags (ESTs) derived from hundreds of tissue-specific human cDNA libraries1. Extensive bioinformatics analyses revealed ~77,000 new partial gene sequences, a number that more than tripled the worldwide number of disclosed ESTs. This output created the first genome-wide estimate of human gene diversity and provided the foundation for HGS’s emerging genomics-based drug discovery efforts.
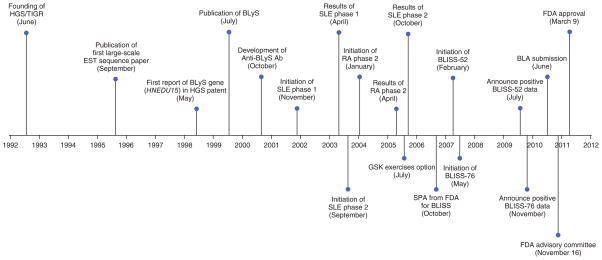
Figure 1.
Important milestones in belimumab (Benlysta) achieving FDA approval in SLE. RA, rheumatoid arthritis; SPA, special protocol assessment; BLA, biologics license application.
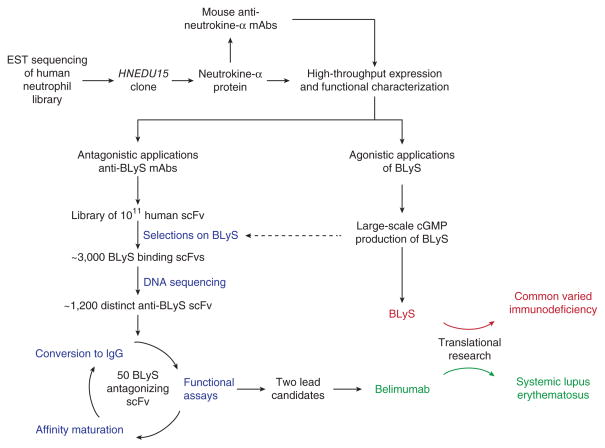
Among the many libraries sequenced at HGS was one derived from primary human neutrophils. It was from this library that a single clone (HNEDU15), encoding a new member of the tumor necrosis factor (TNF) ligand family, was identified (Fig. 2). The protein product of HNEDU15 (now known as TNFSF13B) was designated ‘Neutrokine alpha’2, a name that was subsequently changed to ‘BLyS’, based on its agonist properties for B cells as defined through a series of high-throughput biological screening assays3. As the importance of BLyS in normal B-cell development and homeostasis unfolded, HGS redirected many of its high-throughput processes for protein expression, biological function and mAb discovery to explore BLyS and antagonistic mAbs thereof as potential therapies in diseases associated with aberrant B-cell activity and/or function.
Figure 2.
Coordinated development of BLyS and anti-BLyS (belimumab) for the treatment of aberrant B-cell function in CVI and SLE, respectively.
It was clear that HGS was not alone in the pursuit of BLyS. The competitive landscape was flooded with five reports between May and December of 1999, each describing the same novel TNF ligand family member—TALL-1 (ref. 4), THANK5, BAFF6, BLyS3 and TNFSF20 (ref. 7; Fig. 1). Interestingly, there was no consensus biological activity among the five reports. Nevertheless, the investigative teams from Biogen (Cambridge, MA, USA; now Biogen Idec) and HGS each recognized that BLyS has agonist properties for B cells3,6. Indeed, it was soon realized that BLyS-deficient mice display considerable reductions in mature B cells leading to marked reductions in baseline total serum IgM, IgG and IgA levels as well as attenuated antigen-specific humoral responses to T cell–dependent and T cell–independent antigens8,9. Along the same lines, researchers found that administration of a BLyS antagonist to BLyS-sufficient mice blunts their antigen-specific IgM and IgG responses10–12. Conversely, overexpression of BLyS in transgenic mice results in significant increases in all serum immunoglobulin isotypes (IgM, IgG, IgA, IgE), with particularly robust increases in IgA levels13. Direct administration of exogenous BLyS to mice at the time of antigen challenge enhances in vivo antigen-specific IgM and IgG antibody production (D.M.H. and collaborators14). Moreover, repeated administration of BLyS to mice without specific antigenic immunization results in B-cell expansion and polyclonal hypergammaglobulinemia (D.M.H. and collaborators3). It was clear that BLyS was a biologically important molecule.
Establishment of a connection between BLyS and SLE
Before establishing the link between BLyS and SLE, HGS explored BLyS as a means of restoring endogenous immunoglobulin production to individuals with humoral immunodeficiencies, such as common varied immunodeficiency (CVI) and selective IgA deficiency. Although HGS demonstrated that B cells obtained from some CVI patients proliferated and secreted IgM in response to BLyS (D.M.H. and collaborators15), the overall frequency and magnitude of the responses were low and not supportive of continued development. In retrospect, this decision proved fortunate for HGS because it was subsequently demonstrated in 2007 that BLyS expression is actually greater in patients with CVI than in healthy controls16. That is, the elevated circulating levels of BLyS in these patients notwithstanding, their deficiencies in immunoglobulin were not corrected. Accordingly, it is highly unlikely that replacement therapy with BLyS would have promoted a clinically meaningful normalization of immunoglobulin levels in these immunodeficient individuals, given their refractoriness to increased endogenous BLyS levels. Abandoning CVI as a disease target at an early stage permitted HGS to redirect its dwindling resources from applying BLyS itself as a therapeutic agent to focusing on potential therapeutic applications of BLyS antagonists.
The scientific rationale for the development of BLyS antagonists emerged in late 1999 and early 2000 as investigative groups studied the biological consequences of BLyS overexpression in BLyS-transgenic mice. Remarkably, SLE-like features, including high titers of circulating anti-double-stranded (ds) DNA autoantibodies, immune-complex glomerulonephritis and proteinuria, developed in these otherwise nonautoimmune-prone mice that constitutively overexpressed BLyS13,17,18. Importantly, treatment of either of two genetically disparate, widely studied and well-established strains of SLE model mice (MRL/lpr; and (NZB × NZW)F1, also known as BWF1) with a recombinant fusion protein (TACI-Ig), comprising one of the BLyS receptors (transmembrane activator and calcium-modulating ligand (CAML) interactor; TACI) and the Fc portion of IgG, ameliorated the bona fide SLE disease that spontaneously develops in these mice18. By 2001, investigators at the University of Alabama at Birmingham (UAB) and our group at the University of Southern California (USC; Los Angeles) had documented the frequent elevation in circulating BLyS levels among human SLE patients19,20. At this point, the link between BLyS and human SLE was sufficiently intriguing to pursue the development of BLyS antagonists for testing in human clinical trials.
Rationale for therapeutic targeting of BLyS
Although the manifestations of SLE are highly protean and heterogeneous, a common thread across all varieties of SLE is B-cell hyperactivity. Given the central role for B cells in the pathogenesis of SLE, B cells emerged as a logical therapeutic target. Because the function of BLyS as a vital B-cell survival factor was already well established14,21,22, BLyS emerged as a logical candidate target. Although it was not appreciated at the time, indirect targeting of B cells by focusing on BLyS, rather than direct targeting of B cells through, for example, a CD20-based approach, turned out to be a most auspicious decision for several reasons.
First, there is now compelling evidence in mice that certain (but not all) autoreactive B cells have a greater dependency on BLyS for their survival than do nonautoreactive B cells23–26. Thus, antagonism of BLyS could preferentially eliminate at least some pathogenic B cells while sparing those B cells that play a protective role (e.g., B cells with specificities for microbial pathogens). In contrast, there is no evidence that any autoreactive B cells are more sensitive to CD20-based depletion than are nonautoreactive B cells. This distinction is not simply of academic interest because serious safety concerns associated with chronic profound B-cell depletion have emerged during the past few years27.
Second, it is now known that a subset of B cells has an important regulatory role in autoimmune disease. Using experimental autoimmune encephalomyelitis in C57BL/6 mice as a model system, the downregulatory capacity of interleukin-10–producing B cells (B10 cells) was documented as early as 2002 (ref. 28), although the unique phenotype of these cells was not delineated until 2008 (ref. 29). The protective capacity of B10 cells in murine SLE was not reported until 2010 when a direct correlation was documented between anti-CD20–mediated depletion of B cells (including B10 cells) in 4-week-old BWF1 mice with development of accelerated SLE disease30,31. Very recently, circulating B10 cells were identified in humans32, raising the possibility that these cells play a similar downregulatory role in human autoimmune conditions (e.g., SLE) as they do in murine autoimmunity. The effects on B10 cells of anti-CD20–mediated B-cell depletion in humans and of BLyS neutralization in either mice or humans remain unknown at present.
Third, not only might BLyS neutralization and direct B cell–depleting approaches (e.g., a CD20-based one) differ in their effects on B10 cells, but evidence is increasing that the direct effects of BLyS extend beyond B cells to include T cells as well. In vitro studies have demonstrated that BLyS can stimulate proliferation of, and cytokine production by, T cells33,34. In mice, BLyS overexpression leads to skewing of in vivo inflammatory responses toward a T helper 1 (Th1) cell profile and away from a T helper 2 (Th2) cell profile35. Of note, complete B-cell deficiency does not alter this Th1/Th2 polarizing effect of BLyS overexpression, demonstrating a potent BLyS-driven effect on T cells that is independent of B cells. Moreover, BLyS promotes generation of T helper 17 (Th17) cells, at least in part, through direct effects on T cells (W.S. and collaborators36). Given the likely role for Th17 cells in SLE37,38, it is certainly plausible that therapeutic targeting of BLyS in SLE not only targets pathogenic B cells but targets pathogenic Th1 and/or Th17 cells as well.
Early development of belimumab
In collaboration with Cambridge Antibody Technology (Cambridge, UK; subsequently acquired by AstraZeneca in 2006), a human single-chain antibody (scFv) phage library was screened to detect scFvs that bind to human BLyS (Fig. 2). The initial screening resulted in ~3,000 scFvs with BLyS-binding activity, of which ~1,200 displayed significant BLyS inhibitory activity. HGS then leveraged its high-throughput sequencing capabilities to determine the heavy- and light-chain variable sequences used by each clone. Based on inhibitory activity and VH/VJ family homology, a select number (~50) of scFvs were cloned into expression vectors containing IgG1 heavy and lambda light chain constant region sequences (that is, converted to full-length human immunoglobulins). Subsequent rounds of affinity maturation, conversion and functional analyses yielded two fully human mAbs, each of which bound BLyS with high affinity and antagonized its ability to interact with its cognate cellular receptors (D.M.H. and collaborators39). One of these clones was selected for further development and initially called ‘LymphoStat B’, a name that was eventually replaced by belimumab through negotiations with the US Adopted Names Council of the American Medical Association (Chicago).
Pharmacokinetic analyses in cynomolgus monkeys revealed a terminal serum half-life for belimumab of 11.2–14.6 days in monkeys treated with either single or multiple intravenous doses of belimumab, with clearance ranging from 5.5 to 7.2 ml/day/kg and the volume of distribution at steady state ranging from 85 to 126 ml/kg. Pharmacodynamic studies in these monkeys consistently documented B-cell reductions. Reductions in tissue B-cell populations were evident as early as 4 weeks into treatment, whereas reduction of peripheral blood B cells was not evident until after 2–3 months of treatment. Recovery of B cells began as early as 13 weeks after cessation of treatment and was complete by 32 weeks. Serum immunoglobulin levels generally were unchanged, although infants born to belimumab-treated pregnant monkeys did harbor decreased IgM levels at birth (which returned to normal by 6 months of age). Although no effects on natural killer cell or monocyte populations were observed in monkeys receiving belimumab, some increases in the percentage of T cells were appreciated in lymphoid tissue, likely secondary to the belimumab-induced decreases in B cells. Toxicology studies documented the absence of unusual cage-side observations as well as any unusual changes in food consumption, body weight, electrocardiographic findings or ophthalmic findings. Transient sneezing and red nasal discharge were noted in some monkeys, but these monkeys came from both control and belimumab-treated groups40.
Because belimumab binds to murine BLyS with much lower affinity than it binds to human BLyS and, like other fully human antibodies, is highly immunogenic in mice, efficacy studies of belimumab in murine SLE models were not tractable. Thus, the first in vivo trial of belimumab in any disease state was in human SLE. A randomized, double-blind, placebo-controlled, dose-ranging phase 1 trial of belimumab in individuals with mild-to-moderate SLE disease activity was initiated in late 2001 (Fig. 1). Belimumab was shown to be safe, in that the prevalence of adverse events was no different in patients treated with the study drug at any tested dose (1, 4, 10 or 20 mg/kg) than in placebo-treated patients (W.S. and collaborators41). Moreover, belimumab was demonstrated to be biologically active by virtue of a (subtotal) reduction in peripheral blood B cells across all cohorts treated with one or two doses of belimumab along with significant declines in circulating anti-dsDNA antibody levels across all dose levels in patients whose baseline anti-dsDNA antibody levels were ≥10 IU/ml. In contrast, no changes in either peripheral blood B cells or in circulating anti-dsDNA levels occurred in placebo-treated patients. No clinical efficacy was demonstrated, but the small number of patients (n = 70) and brief treatment schedules (single infusion or two infusions 3 weeks apart) and follow-up period (12 weeks after final infusion) precluded demonstration of clinical benefit. The absence of overt clinical benefit notwithstanding, the phase 1 trial was viewed as a success and enabled initiation of phase 2 trials in both SLE and rheumatoid arthritis.
Concurrent with the phase 1 trial, HGS presciently organized a longitudinal study of 245 adult SLE patients from four medical centers (Johns Hopkins (Baltimore), USC, UAB and University of Michigan (Ann Arbor)). These individuals were highly diverse with regard to age, racial and/or ethnic backgrounds, affected organ systems and medications taken. The results from this study as well as from additional smaller studies showed significant associations between BLyS expression and clinical disease activity42–45. Although the relationship between BLyS expression and disease activity was not ascertained until after the phase 2 trial had been initiated, the positive results did support the decision to pursue large phase 3 trials, despite being considered by many at the time to be risky due to the failure of the phase 2 trial to meet its primary endpoints.
Early development of alternative BLyS antagonists
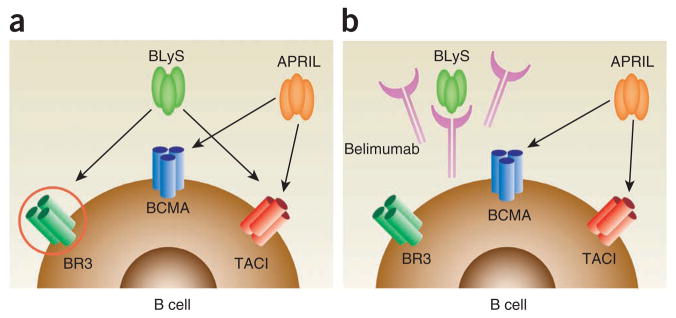
Concurrent with development of belimumab by HGS was the development of TACI-Ig (subsequently known as atacicept) by ZymoGenetics (Seattle; Table 1). Treatment with TACI-Ig of mice with established SLE had already been shown to ameliorate disease severity and improve survival18, so it logically followed to assess the efficacy of this agent in human SLE. Of note, atacicept neutralizes both BLyS and the related APRIL (a proliferation-inducing ligand; member of the TNF superfamily), in contrast to belimumab, which neutralizes only BLyS. (BLyS and APRIL share two receptors, TACI and B-cell maturation antigen (BCMA)10,46–48. In addition, BLyS binds to a third receptor, BLyS receptor 3 (BR3), which does not bind APRIL49,50 (Fig. 3)). Although APRIL has little, if any, effect on the size of the mature B-cell pool51,52, APRIL does importantly contribute to survival of plasmablasts and plasma cells53,54. Moreover, it was known that BLyS and APRIL could form heterotrimers, and circulating levels of such heterotrimers were elevated in at least some SLE patients (W.S. and collaborators55). Thus, neutralization of BLyS plus APRIL could, in principle, have a greater neutralizing effect on autoantibodies, particularly pathogenic ones, than would neutralization of BLyS alone.
Table 1.
BLyS antagonists in clinical development for SLE
| BLyS antagonist | Agent type | Specificity | Earliest testing in SLE | Current status |
|---|---|---|---|---|
| Belimumab | Human mAb | BLyS | 2001 | Approved by FDA |
| Atacicept | Receptor-Fc fusion protein | BLyS + APRIL | ~2005 | Phase 2/3 |
| Blisbimod (AMG 623; A-623) | Peptibody | BLyS | 2004 | Phase 2 |
| Tabalumab (LY2127399) | Human mAb | BLyS | 2011 | Phase 3 |
Figure 3.
The effect of belimumab on the binding of BLyS to its receptors. (a) BLyS can bind to three receptors (BCMA, TACI, BR3 (also known as BAFF-R)), each of which being a distinct member of the TNF superfamily and each being expressed to different degrees on B cells, T cells and plasma cells. APRIL can bind to two of these receptors (BCMA, TACI) but not to the third (BR3). (b) Belimumab binds BLyS and blocks engagement of BLyS with BCMA, TACI and BR3. Belimumab does not bind APRIL, so engagement of APRIL with BCMA or TACI is not affected.
In collaboration with Merck Serono (Geneva), preclinical studies of atacicept in mice and cynomolgus monkeys documented reversible subtotal depletion of B cells and reduction in circulating immunoglobulin (especially IgM) levels, with the only apparent systemic toxicity being transient elevations in liver-derived transaminases (without any histologic changes in the livers)56. Moreover, favorable safety and tolerability were subsequently demonstrated in a randomized, double-blind, placebo-controlled phase 1b trial of atacicept in human SLE57. SLE patients (n = 49) received a single dose of atacicept (0.3, 1, 3 or 9 mg/kg) or placebo or four weekly doses of atacicept (1 or 3 mg/kg) or placebo. Dose-dependent reductions in peripheral blood B cells and in circulating immunoglobulin levels were noted, but, as in the case with the phase 1 trial of belimumab in SLE, clinical efficacy could not be demonstrated due to the limited treatment and limited follow-up period. Nevertheless, this phase 1 trial with atacicept, as the phase 1 trial with belimumab, was viewed as a success.
A different BLyS antagonist, AMG 623, was developed by Amgen (Thousand Oaks, CA, USA). AMG 623 (blisbimod) is a so-called ‘peptibody’—a fusion protein between IgG Fc and a peptide sequence selected for its ability to bind with high affinity to BLyS (but not to APRIL). In a double-blind, placebo-controlled phase 1 trial launched in 2004, SLE patients (n = ~60) received four weekly doses of AMG 623 (0.3, 1 or 3 mg/kg subcutaneously or 6 mg/kg intravenously) or matching placebo58,59. A dose-independent decrease in naive and total peripheral blood B cells was accompanied by an actual increase in memory B cells. Clinical responses were not indicated, and a full report of this trial still has not been published. In any case, Amgen did not continue to pursue development of AMG 623 for human SLE (and ultimately licensed the rights to AMG 623 to Anthera Pharmaceuticals (Hayward, CA, USA) in 2008).
A final BLyS antagonist, BR3-Fc (a fusion protein between BR3 and IgG Fc; subsequently known as briobacept), was developed by Genentech (South San Francisco, CA, USA; now Roche) and was shown to be efficacious in murine SLE60. Briobacept, which binds and neutralizes BLyS but not APRIL, was shown in preclinical studies to effect subtotal B-cell depletion in the blood and secondary lymphoid tissues of cynomolgus monkeys61. Despite the similarity in preclinical results between briobacept and the other BLyS antagonists, briobacept was not pursued in human SLE.
Other B cell–directed therapies for SLE
BLyS antagonism (with or without concurrent APRIL antagonism) was not the only B cell–directed therapy being evaluated for SLE. The anti-CD20 mAb, rituximab (Rituxan), was approved by the FDA in 1997 for use in patients with non-Hodgkin’s B-cell lymphomas. Rituximab triggers B-cell lysis by at least three mechanisms: antibody-dependent, cell-mediated cytotoxicity; complement-dependent cytotoxicity; and direct ability to induce apoptosis62. Although the precise contribution of each individual mechanism in vivo is a matter of debate, the net result is rapid depletion of B cells.
Among rheumatic diseases, rheumatoid arthritis was the first to be studied for clinical responsiveness to rituximab. The striking clinical efficacy of rituximab in rheumatoid arthritis patients63 led the FDA to ultimately approve this agent in 2006 for use in rheumatoid arthritis patients with an inadequate response to at least one TNFα antagonist.
Given the response in rheumatoid arthritis patients to rituximab, investigators turned to SLE as a target disease. Open-label studies as early as 2002 with rituximab in SLE were spectacularly encouraging64,65. In comparison to BLyS antagonists, B-cell depletion effected by rituximab was much more profound. Indeed, the paradigm ‘the more B-cell depletion, the better’ was quite prevalent, and many leaders in the field were convinced that rituximab would ultimately be proven to be clinically far superior to any BLyS antagonist.
Phase 2 experience with belimumab
Undaunted by the potency of rituximab in depleting B cells and buoyed by the impressive preclinical studies of BLyS antagonists in SLE mice as well as the very favorable safety and tolerability profiles that emerged from the human phase 1 trial with belimumab, HGS initiated a 52-week, randomized, double-blind, placebo-controlled phase 2 trial of belimumab in human SLE (n = 449) in late 2003 (Fig. 1). In this trial, subjects received the standard of care (e.g., corticosteroids, anti-malarials and/or immunosuppressants other than cyclophosphamide) plus belimumab (1, 4 or 10 mg/kg) or the standard of care plus placebo at weeks 0, 2 and 4, and then every 4 weeks through week 52.
Around the same time, HGS initiated a 24-week, randomized, double-blind, placebo-controlled phase 2 trial in rheumatoid arthritis (Fig. 1). The rationale for this trial was based on favorable results with a BLyS antagonist in murine collagen-induced arthritis (a model for rheumatoid arthritis)8,66 along with the presumption on the part of regulators and many rheumatology opinion leaders that any drug safe in SLE with its wide range of autoimmune manifestations and attendant immunosuppression (from the disease itself and/or from medications) would likely also be safe in rheumatoid arthritis.
In this phase 2 trial, patients with active rheumatoid arthritis (n = 283) were treated with one of three doses of belimumab (1, 4 or 10 mg/kg) or placebo at weeks 0, 2, 4 and every 4 weeks thereafter through 24 weeks. Belimumab or placebo was added to standard-of-care therapy (e.g., disease-modifying, anti-rheumatic drug treatments, such as methotrexate), with a major restriction being that the subjects could not have been concurrently receiving biologic therapy (e.g., TNFα antagonists or rituximab). Among all belimumab-treated patients, 29% experienced a clinical response (defined as an ACR20 response, which indicates a reduction of at least 20% in the number of swollen and tender joints along with at least 20% reduction in specific parameters defined by the American College of Rheumatology that measure systemic inflammation and overall well-being of the patient), whereas only 16% of the placebo-treated patients had an ACR20 response67. Among the belimumab-treated patients, peripheral blood B cells declined by ~20%, and serum rheumatoid factor levels declined by ~30% (compared with no change in placebo-treated patients for either; W.S. et al.68). Although the response rate among belimumab plus standard-of-care–treated patients was significantly greater than that among placebo plus standard-of-care–treated patients, the low absolute response rate among the former along with the slower onset of action as compared with the TNFα antagonists curbed the enthusiasm in pursuing rheumatoid arthritis as a target disease, and no additional clinical trials of belimumab in rheumatoid arthritis have been initiated to date.
Although the phase 2 trial of belimumab in SLE was initiated several months before initiation of the phase 2 belimumab trial in rheumatoid arthritis, the longer duration of the former resulted in the outcomes not becoming available until after the rheumatoid arthritis trial results were known. During this period of time, GlaxoSmithKline (Brentford, UK) exercised its option for development of belimumab (Fig. 1). This turned out to be a critical event because great disappointment awaited the sponsors, investigators and SLE advocacy groups at the conclusion of the phase 2 belimumab trial in SLE. Neither of the primary endpoints (disease activity at 24 weeks and time to first flare during the 52 weeks) were met (W.S. and collaborators69). These ostensibly negative results prompted some opinion leaders to dismiss belimumab (and BLyS antagonists in general) as a viable therapeutic option in SLE. Had GlaxoSmithKline not been committed to belimumab at this time with its considerable financial resources, it is reasonable to speculate that further development of belimumab would have been halted.
Fortunately, post hoc analysis of the results identified a likely deficiency in the design of the phase 2 trial. The entry criteria to the phase 2 trial required only a history of a positive anti-nuclear antibody (ANA) test; it did not require a positive ANA at the time of entry. In retrospect, testing a B cell–directed therapy (belimumab) in subjects who at the time of treatment did not manifest objective evidence of B-cell dysfunction (circulating autoantibodies) may not have been prudent. Indeed, when only seropositive subjects (ANA titer ≥1:80 and/or a positive anti-dsDNA test at entry) were considered and a novel composite index of clinical response was used (SLE responder index or SRI), patients treated with the standard of care plus belimumab (all doses combined) had a significantly better response than did patients treated with the standard of care plus placebo. (To be considered an SRI responder, three conditions must be met that, in aggregate, demonstrate meaningful improvement in overall disease activity without meaningful deterioration in any individual organ system. These conditions are the following: first, reduction in Safety of Estrogens in Lupus Erythematosus National Assessment–SLE Disease Activity Index (SELENA-SLEDAI) of ≥4 points; second, no new British Isles Lupus Assessment Group (BILAG) A organ domain score or no more than one B organ domain score; and third, no worsening (increase <0.3) in Physician’s Global Assessment (PGA) score versus baseline (W.S. and collaborators70).)
Phase 3 experience with belimumab
Some opinion leaders remained unconvinced, but the FDA accepted this novel evidence-based SRI as the primary endpoint of the subsequent phase 3 trials. In early 2007, the pivotal BLISS-52 (n = 865) and BLISS-76 (n = 819) trials were initiated71,72 (Fig. 1). Each of these trials studied only SLE patients who were seropositive at entry and who manifested at least moderate disease activity (SELENA-SLEDAI ≥ 6) at entry. Subjects in each trial were randomized in a 1:1:1 ratio, receiving in addition to the standard of care either placebo, belimumab 1 mg/kg, or belimumab 10 mg/kg intravenously on days 0, 14, 28 and then every 28 days until 48 weeks (BLISS-52) or 72 weeks (BLISS-76). The primary endpoint in each trial was the response rate at 52 weeks, as assessed with the SRI.
The BLISS-52 trial recruited its subjects from Latin America, the Asia-Pacific area and Eastern Europe. The primary endpoint was met with either dose of belimumab (SRI response rate: placebo, 44%; belimumab 1 mg/kg, 51% (P = 0.0129); belimumab 10 mg/kg, 58% (P = 0.0006)). That is, relative to the response rate among patients who received placebo, the response rates among patients who received the lower and higher belimumab doses, respectively, were 16% and 32% greater. In fact, consistently greater response rates were noted by week 28 for patients treated with the lower belimumab dose and by week 24 for patients treated with the higher belimumab dose. When the three components of the SRI were individually analyzed at 52 weeks, the higher dose of belimumab remained significantly superior to placebo for each, and the lower dose of belimumab remained significantly superior to placebo both in terms of reducing the SELENA-SLEDAI by ≥4 points and in terms of no incremental worsening of the PGA. Moreover, the time to first flare during the course of the 52-week trial was substantially prolonged among patients treated with either dose of belimumab in comparison to those that received placebo. Importantly, there were no differences in total adverse events, serious adverse events, or severe adverse events among the treatment groups.
The concurrent BLISS-76 trial recruited its subjects from North America, Central America and Europe. Not surprisingly, the demographic composition of the subjects in this trial substantially differed from that in the BLISS-52 trial. In the latter, only 3–4% of the patients were Black Americans, whereas 14–15% of the patients were Black Americans in BLISS-76. On the other hand, only 12–13% of the patients in BLISS-76 were indigenous Americans (Alaska Natives or American Indians from North, South or Central America), whereas 31–34% of the patients in BLISS-52 were indigenous Americans.
Another important difference between the subjects in BLISS-76 and BLISS-52 was the medication profile. Whereas the vast majority (96%) of patients in BLISS-52 were taking corticosteroids at the time of entry (with 67–71% of the subjects taking prednisone >7.5 mg/d), only 73–78% of patients in BLISS-76 were taking corticosteroids at baseline (with 44–48% taking prednisone >7.5 mg/d). In contrast, only 42–43% of the subjects were taking any immunosuppressive medication at entry in BLISS-52 (with only 6–7% taking the potent mycophenolate mofetil), whereas 54–56% of the subjects in BLISS-76 were taking some immunosuppressive medication at baseline (with 15–18% taking mycophenolate mofetil). Importantly, patients that had taken cyclophosphamide within 6 months were excluded from both BLISS-52 and BLISS-76 trials by protocol, as were patients with “severe active lupus nephritis or central nervous system lupus.” These patients were felt to be at undue risk for infectious complications that could arise after any incremental immunosuppression affected by belimumab. This admirable concern notwithstanding, the sickest SLE patients (that is, those who a priori would have the greatest need for more efficacious/less toxic therapy) were not studied, so, ironically, neither BLISS trial is informative with regard to efficacy or safety of belimumab among this most needy subset of patients.
The results in BLISS-76 were favorable, albeit not as impressive as those in BLISS-52. Whereas the primary endpoint in BLISS-76 was met with the higher dose of belimumab, it was not met with the lower dose (SRI response rate: placebo, 33%; belimumab 1 mg/kg, 41% (P = 0.089); belimumab 10 mg/kg, 43% (P = 0.017)). Moreover, when the three components of the SRI were individually analyzed at 52 weeks, the ≥4-point reduction in SELENA-SLEDAI was significant only among patients treated with the higher dose of belimumab, whereas the BILAG and PGA components were (paradoxically) significantly better only among patients treated with the lower dose. Causing considerable consternation among many in the rheumatology community, SRI response rates at 76 weeks among patients treated with either dose of belimumab were no longer significantly different from those among patients treated with placebo (SRI response rate: placebo, 32%; belimumab 1 mg/kg, 39% (P = 0.11); belimumab 10 mg/kg, 38% (P = 0.13)). Whether differences in demographics and/or medication profiles between the patients in BLISS-52 and those in BLISS-76 contributed to the somewhat disparate outcomes is uncertain, but in any case, it is clear that >40% of belimumab-treated patients in either trial (42% in BLISS-52; 57% in BLISS-76) did not achieve an SRI response at 52 weeks.
All the caveats above notwithstanding, the net result was that both the BLISS-52 and BLISS-76 trials met their primary endpoints with regard to belimumab at a dose of 10 mg/kg. Thus, for the first time ever, positive results for a drug specifically targeting SLE emerged, not just from one, but from two placebo-controlled phase 3 trials. This was especially remarkable because clinical trials in SLE with atacicept were having difficulties, including the discontinuation in 2007 of a trial of atacicept in combination with mycophenolate mofetil in SLE nephritis patients due to increased serious infections (ClinicalTrials.gov no. NCT00573157).
Moreover, by 2008, two controlled phase 2/3 trials of rituximab in SLE had each failed to reach their primary and secondary endpoints73,74. In the EXPLORER trial, which focused on patients with moderately to severely active extra-renal SLE, neither primary endpoint (“major clinical response” or “partial clinical response” at 52 weeks as assessed using each of the eight BILAG index organ system scores) was met (12.4% major clinical response and 17.2% partial clinical response for rituximab; 15.9% major clinical response and 12.5% partial clinical response for placebo)73. Given the virtually identical combined response rates for the rituximab and placebo groups (29.6% versus 28.4%), it is unlikely that the relatively small size of this trial (n = 257 total patients) lay at the heart of the failure. The LUNAR trial, which focused on patients with proliferative SLE nephritis, also did not meet either primary endpoint (“complete renal response” or “partial renal response” at 52 weeks), with 26.4% and 30.6% of patients treated with rituximab (plus mycophenolate mofetil and corticosteroids) achieving complete or partial responses, respectively, compared with corresponding rates of 30.6% and 15.3% among patients treated with placebo (plus mycophenolate mofetil and corticosteroids). That is, the combined response rates for the rituximab and placebo groups were 56.9% and 45.8%, respectively74. For this trial, the small number of total patients studied (n = 144) as well as other design flaws may have contributed to its failure75,76. Nevertheless, the hard reality remains that the trials with belimumab achieved their primary endpoints, whereas the trials with rituximab did not.
The home stretch to FDA approval
One might have thought that with positive results from two very large phase 3 trials belimumab approval by the FDA would have been a slam dunk. In fact, several issues remained that required resolution.
First, although the results from each trial were positive, the magnitude of the clinical response in each trial was ostensibly only modest. This was in striking contrast to the dramatic improvement observed in mice treated with BLyS antagonists18,60,77. In fairness, the difference between responses in humans and responses in mice is likely more apparent than real. Whereas human subjects received the standard of care in addition to belimumab, murine subjects received no treatment other than the BLyS antagonist. Patients treated with belimumab alone would likely do much better than untreated patients, but this hypothesis has never been, and will never be, formally tested because such human trials would be unethical. Moreover, the clinical response (SRI) in the human trials was based on a composite of several instruments each rooted in multiple organ systems, whereas clinical response in the murine trials was simply the absence of premoribund proteinuria and death. Belimumab may be very effective in preventing premoribund proteinuria and death, but it has not yet been empirically tested in SLE patients with severe nephritis. In truth, substantial BLyS-independent autoimmunity and (modest) renal immunopathology develop over time in SLE-prone NZM 2328 mice that are genetically deficient in BLyS (W.S. and collaborators78). Furthermore, BLyS-antagonist treatment of BWF1 mice with established SLE nephritis, despite ameliorating renal disease and enhancing survival, has at most only partial inhibitory effects on circulating anti-dsDNA autoantibody levels18,77. Thus, the persistence of SLE features among belimumab-treated human SLE patients may, in fact, not be much different from that in mice in which BLyS is either eliminated or neutralized.
Second, the absence of a significant difference between the standard-of-care-plus-belimumab and the standard-of-care-plus-placebo groups in the BLISS-76 trial at 76 weeks became an issue. Whether this loss of significance truly represents a loss of staying power of belimumab or whether changes in concurrent medications obscured true differences between the groups remains uncertain. Of note, post hoc analysis of SRI with higher thresholds of disease activity (change in the SELENA-SLEDAI of 5–10 points rather than only the prescribed 4 points) did demonstrate significant improvement, even at 76 weeks, among patients treated with the higher belimumab dose (W.S. and collaborators67). In any case, no one disputes the benefit of 52 weeks of belimumab treatment.
Third, subgroup analysis did not reveal a positive effect for belimumab among Black American subjects. Whether this reflects an underpowered cohort of subjects or whether Black Americans truly are insensitive to the beneficial effects of belimumab as a consequence of genetic and/or environmental factors is uncertain. Of interest, post hoc analysis of the 321 seropositive SLE patients in the phase 2 belimumab trial documented the efficacy of belimumab even among Black Americans (W. Freimuth, HGS, personal communication). In any case, no one disputes the benefit of belimumab in the non-Black American subjects.
FDA approval
HGS spent great effort in addressing the remaining issues, and in November 2010, an FDA advisory panel voted 13–2 in favor of approving belimumab for the treatment of SLE (Fig. 1). Although the FDA did not render its final verdict on belimumab in December 2010 as it had originally promised, approval for Benlysta came on March 9, 2011, for “the treatment of adult patients with active, autoantibody-positive, systemic lupus erythematosus who are receiving standard therapy.” There is no question that SLE advocacy groups had been strongly aligned in favor of approval, which, in light of the unexpected failure of rituximab in its late-phase trials, crescendoed once it became publicly known that two independent SLE phase 3 trials had met their primary endpoints for the first time in history. Whether political pressure from these groups had any (subliminally) positive effect on the FDA decision is not known, but it certainly did not hurt. In any case, additional studies will be done to prove (or disprove) efficacy among Black Americans with SLE, and studies that address the efficacy of subcutaneous (rather than intravenous) belimumab (NCT00732940) are already in progress. In addition, subjects from the phase 2 and phase 3 SLE trials continue to be treated in an open-label fashion with belimumab (NCT00583362 and NCT00724867) and followed for safety and efficacy. Moreover, a trial will be undertaken to evaluate belimumab in SLE patients with severe active nephritis. Finally, another anti-BLyS antibody, the human mAb tabalumab (LY2127399), developed by Eli Lilly (Indianapolis), is starting to undergo clinical evaluation in a phase 3 trial in SLE. (This mAb has not undergone phase 1 or phase 2 evaluation in SLE.)
Conclusions
Several lessons can be learned from the belimumab-SLE saga. First, conventional wisdom does not invariably lead to correct predictions. Contrary to the expectations of many key opinion leaders, less may be more with regard to therapeutic depletion of B cells. Rituximab is a very potent depleter of B cells; its trials in SLE failed. Belimumab is a relatively modest depleter of B cells; its (phase 3) trials in SLE succeeded. Keeping an open mind and heeding the clinical data rather than being wed to any preconceived notions ultimately carried the day for belimumab.
Second, ‘less may be more’ is also applicable to the target patient population. It is naive to believe that a heterogeneous and complex disease such as SLE can be meaningfully ameliorated by any single agent targeting any single biological pathway in all patients. Although murine studies have unequivocally documented the indispensability of B cells in the initiation of SLE79,80, there is no a priori reason to insist that B cells are indispensable in the maintenance of SLE. There likely are many SLE patients in whom ongoing disease activity is driven through B cell–independent mechanisms. Through a meticulous post hoc analysis of the phase 2 belimumab trial results in SLE (W.S. and collaborators69), it became apparent that the appropriate target subjects for belimumab were not all SLE patients but rather those who manifested objective evidence of ongoing B-cell dysfunction (seropositivity). By restricting the target population, patients who likely would not have responded to belimumab were excluded from the pivotal phase 3 trials, thereby permitting detection of a beneficial effect of belimumab among patients who had a high a priori likelihood of responding.
Third, a chain is only as strong as its weakest link. For SLE trials, the weakest link has repeatedly been the instrument used to measure clinical outcomes. The careful and painstaking development of the SRI (W.S. and collaborators70) represented a major breakthrough for SLE trials. The success of the SRI as a primary endpoint in the BLISS-52 and BLISS-76 trials has already led to the adoption of the SRI as the primary endpoint in trials of other agents (NCT01262365, NCT01261793, NCT01405196).
Finally, mice are not merely small humans with long tails. Expectations for human clinical trials based on mouse studies can be unrealistic. In addition to inherent biological differences between mouse and man, ethical considerations often preclude exact replication of mouse studies in humans. Whereas the clinical efficacy of belimumab in the human SLE trials was nowhere near as dramatic as the efficacy of BLyS antagonists had been in murine SLE studies, the fact remains that belimumab did deliver a measurable amount of clinical benefit. As the full impact of belimumab on SLE continues to unfold, the lessons learned in the development of belimumab will hopefully inspire patients, physicians and scientists to once again coalesce new technologies and visionary ideas in the search for medicines that address the full complexity of SLE.
Acknowledgments
The authors thank T.-S. Migone and W. Freimuth of HGS for helpful discussions and assistance with the time line. The work was supported in part by NIH grant R01 AR050193 to W.S.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare competing financial interests: details accompany the full-text HTML version of the paper at http://www.nature.com/naturebiotechnology.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Adams MD, et al. Initial assessment of human gene diversity and expression patterns based upon 83 million nucleotides of cDNA sequence. Nature. 1995;377 (suppl):3–174. [PubMed] [Google Scholar]
- 2.International Publication Number WO 98/18921 published on May 7, 1998, corresponding to International Application Number PCT/US96/17957, filed October 25, 1996.
- 3.Moore PA, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 4.Shu HB, Hu WH, Johnson H. TALL-1 is a novel member of the TNF family that is down-regulated by mitogens. J Leukoc Biol. 1999;65:680–683. [PubMed] [Google Scholar]
- 5.Mukhopadhyay A, Ni J, Zhai Y, Yu GL, Aggarwal BB. Identification and characterization of a novel cytokine, THANK, a TNF homologue that activates apoptosis, nuclear factor-κB, and c-Jun NH2-terminal kinase. J Biol Chem. 1999;274:15978–15981. doi: 10.1074/jbc.274.23.15978. [DOI] [PubMed] [Google Scholar]
- 6.Schneider P, et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tribouley C, et al. Characterization of a new member of the TNF family expressed on antigen presenting cells. Biol Chem. 1999;380:1443–1447. doi: 10.1515/BC.1999.186. [DOI] [PubMed] [Google Scholar]
- 8.Gross JA, et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease: impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 9.Schiemann B, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 10.Yu G, et al. APRIL and TALL-1 and receptors BCMA and TACI: system for regulating humoral immunity. Nat Immunol. 2000;1:252–256. doi: 10.1038/79802. [DOI] [PubMed] [Google Scholar]
- 11.Yan M, et al. Identification of a receptor for BLyS demonstrates a crucial role in humoral immunity. Nat Immunol. 2000;1:37–41. doi: 10.1038/76889. [DOI] [PubMed] [Google Scholar]
- 12.Xia XZ, et al. TACI is a TRAF-interacting receptor for TALL-1, a tumor necrosis factor family member involved in B cell regulation. J Exp Med. 2000;192:137–144. doi: 10.1084/jem.192.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khare SD, et al. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc Natl Acad Sci USA. 2000;97:3370–3375. doi: 10.1073/pnas.050580697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Do RKG, et al. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J Exp Med. 2000;192:953–964. doi: 10.1084/jem.192.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart DM, McAvoy MJ, Hilbert DM, Nelson DL. B lymphocytes from individuals with common variable immunodeficiency respond to B lymphocyte stimulator (BLyS protein) in vitro. Clin Immunol. 2003;109:137–143. doi: 10.1016/s1521-6616(03)00215-8. [DOI] [PubMed] [Google Scholar]
- 16.Knight AK, et al. High serum levels of BAFF, APRIL, and TACI in common variable immunodeficiency. Clin Immunol. 2007;124:182–189. doi: 10.1016/j.clim.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackay F, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gross JA, et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. 2001;166:6–10. doi: 10.4049/jimmunol.166.1.6. [DOI] [PubMed] [Google Scholar]
- 20.Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001;44:1313–1319. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 21.Thompson JS, et al. BAFF binds to the tumor necrosis factor receptor-like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. J Exp Med. 2000;192:129–136. doi: 10.1084/jem.192.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batten M, et al. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192:1453–1466. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lesley R, et al. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 24.Thien M, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Ota M, et al. Regulation of the B cell receptor repertoire and self-reactivity by BAFF. J Immunol. 2010;185:4128–4136. doi: 10.4049/jimmunol.1002176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikbakht N, Migone TS, Ward CP, Manser T. Cellular competition independent of BAFF/B lymphocyte stimulator results in low frequency of an autoreactive clonotype in mature polyclonal B cell compartments. J Immunol. 2011;187:37–46. doi: 10.4049/jimmunol.1003924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carson KR, et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood. 2009;113:4834–4840. doi: 10.1182/blood-2008-10-186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 29.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haas KM, et al. Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. J Immunol. 2010;184:4789–4800. doi: 10.4049/jimmunol.0902391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watanabe R, et al. Regulatory B cells (B10 cells) have a suppressive role in murine lupus: CD19 and B10 cell deficiency exacerbates systemic autoimmunity. J Immunol. 2010;184:4801–4809. doi: 10.4049/jimmunol.0902385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwata Y, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117:530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huard B, Schneider P, Mauri D, Tschopp J, French LE. T cell costimulation by the TNF ligand BAFF. J Immunol. 2001;167:6225–6231. doi: 10.4049/jimmunol.167.11.6225. [DOI] [PubMed] [Google Scholar]
- 34.Ng LG, et al. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol. 2004;173:807–817. doi: 10.4049/jimmunol.173.2.807. [DOI] [PubMed] [Google Scholar]
- 35.Sutherland APR, et al. BAFF augments certain Th1-associated inflammatory responses. J Immunol. 2005;174:5537–5544. doi: 10.4049/jimmunol.174.9.5537. [DOI] [PubMed] [Google Scholar]
- 36.Zhou X, et al. BAFF promotes Th17 cells and aggravates experimental autoimmune encephalomyelitis. PLoS ONE. 2011;6:e23629. doi: 10.1371/journal.pone.0023629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacob N, et al. Accelerated pathological and clinical nephritis in systemic lupus erythematosus-prone New Zealand Mixed 2328 mice doubly deficient in TNF receptor 1 and TNF receptor 2 via a Th17-associated pathway. J Immunol. 2009;182:2532–2541. doi: 10.4049/jimmunol.0802948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, Kyttaris VC, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. J Immunol. 2009;183:3160–3169. doi: 10.4049/jimmunol.0900385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker KP, et al. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum. 2003;48:3253–3265. doi: 10.1002/art.11299. [DOI] [PubMed] [Google Scholar]
- 40.Halpern W, et al. Chronic administration of belimumab, a BLyS antagonist, decreases tissue and peripheral blood B-lymphocyte populations in cynomolgus monkeys: pharmacokinetic, pharmacodynamic and toxicologic effects. Toxicol Sci. 2006;91:586–599. doi: 10.1093/toxsci/kfj148. [DOI] [PubMed] [Google Scholar]
- 41.Furie R, et al. Biologic activity and safety of belimumab, a neutralizing anti- B-lymphocyte stimulator (BLyS) monoclonal antibody: a phase I trial in patients with systemic lupus erythematosus. Arthritis Res Ther. 2008;10:R109. doi: 10.1186/ar2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petri M, et al. Association of plasma B lymphocyte stimulator levels and disease activity in systemic lupus erythematosus. Arthritis Rheum. 2008;58:2453–2459. doi: 10.1002/art.23678. [DOI] [PubMed] [Google Scholar]
- 43.Collins CE, et al. B lymphocyte stimulator (BLyS) isoforms in systemic lupus erythematosus: disease activity correlates better with blood leukocyte BLyS mRNA levels than with plasma BLyS protein levels. Arthritis Res Ther. 2006;8:R6. doi: 10.1186/ar1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Becker-Merok A, Nikolaisen C, Nossent HC. B-lymphocyte activating factor in systemic lupus erythematosus and rheumatoid arthritis in relation to autoantibody levels, disease measures and time. Lupus. 2006;15:570–576. doi: 10.1177/0961203306071871. [DOI] [PubMed] [Google Scholar]
- 45.Ju S, et al. Correlation of the expression levels of BLyS and its receptors mRNA in patients with systemic lupus erythematosus. Clin Biochem. 2006;39:1131–1137. doi: 10.1016/j.clinbiochem.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 46.Marsters SA, et al. Interaction of the TNF homologues BLyS and APRIL with the receptor homologues BCMA and TACI. Curr Biol. 2000;10:785–788. doi: 10.1016/s0960-9822(00)00566-2. [DOI] [PubMed] [Google Scholar]
- 47.Wu Y, et al. Tumor necrosis factor (TNF) receptor superfamily member TACI is a high affinity receptor for TNF family members APRIL and BLyS. J Biol Chem. 2000;275:35478–35485. doi: 10.1074/jbc.M005224200. [DOI] [PubMed] [Google Scholar]
- 48.Rennert P, et al. A soluble form of B cell maturation antigen, a receptor for the tumor necrosis factor family member APRIL, inhibits tumor cell growth. J Exp Med. 2000;192:1677–1684. doi: 10.1084/jem.192.11.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thompson JS, et al. BAFF-R, a novel TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 50.Yan M, et al. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001;11:1547–1552. doi: 10.1016/s0960-9822(01)00481-x. [DOI] [PubMed] [Google Scholar]
- 51.Varfolomeev E, et al. APRIL-deficient mice have normal immune system development. Mol Cell Biol. 2004;24:997–1006. doi: 10.1128/MCB.24.3.997-1006.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Castigli E, et al. Impaired IgA class switching in APRIL-deficient mice. Proc Natl Acad Sci USA. 2004;101:3903–3908. doi: 10.1073/pnas.0307348101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benson MJ, et al. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol. 2008;180:3655–3659. doi: 10.4049/jimmunol.180.6.3655. [DOI] [PubMed] [Google Scholar]
- 54.Belnoue E, et al. APRIL is critical for plasmablast survival in the bone marrow and poorly expressed by early-life bone marrow stromal cells. Blood. 2008;111:2755–2764. doi: 10.1182/blood-2007-09-110858. [DOI] [PubMed] [Google Scholar]
- 55.Roschke V, et al. BLyS and APRIL form biologically active heterotrimers that are expressed in patients with systemic immune-based rheumatic diseases. J Immunol. 2002;169:4314–4321. doi: 10.4049/jimmunol.169.8.4314. [DOI] [PubMed] [Google Scholar]
- 56.Carbonatto M, et al. Nonclinical safety, pharmacokinetics, and phamcodynamics of atacicept. Toxicol Sci. 2008;105:200–210. doi: 10.1093/toxsci/kfn105. [DOI] [PubMed] [Google Scholar]
- 57.Dall’Era M, et al. Reduced B lymphocyte and immunoglobulin levels after atacicept treatment in patients with systemic lupus erythematosus: results of a multicenter, phase Ib, double-blind, placebo-controlled, dose-escalating trial. Arthritis Rheum. 2007;56:4142–4150. doi: 10.1002/art.23047. [DOI] [PubMed] [Google Scholar]
- 58.Belouski SS, Rasmussen HE, Thomas JK, Ferbas J, Zack DJ. Changes in B cells and B cell subsets induced by BAFF neutralization in vivo. Arthritis Rheum. 2007;56:S565. [Google Scholar]
- 59.Sabahi R, et al. Immunologic effects of BAFF antagonism in the treatment of human SLE. Arthritis Rheum. 2007;56:S566. [Google Scholar]
- 60.Kayagaki N, et al. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-κB2. Immunity. 2002;17:515–524. doi: 10.1016/s1074-7613(02)00425-9. [DOI] [PubMed] [Google Scholar]
- 61.Vugmeyster Y, et al. A soluble BAFF antagonist, BR3-Fc, decreases peripheral blood B cells and lymphoid tissue marginal zone and follicular B cells in cynomolgus monkeys. Am J Pathol. 2006;168:476–489. doi: 10.2353/ajpath.2006.050600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cragg MS, Walshe CA, Ivanov AO, Glennie MJ. The biology of CD20 and its potential as a target for mAb therapy. Curr Dir Autoimmun. 2005;8:140–174. doi: 10.1159/000082102. [DOI] [PubMed] [Google Scholar]
- 63.Edwards JCW, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 64.Leandro MJ, Edwards JC, Cambridge G, Ehrenstein MR, Isenberg DA. An open study of B lymphocyte depletion in systemic lupus erythematosus. Arthritis Rheum. 2002;46:2673–2677. doi: 10.1002/art.10541. [DOI] [PubMed] [Google Scholar]
- 65.Looney RJ, et al. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum. 2004;50:2580–2589. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 66.Wang H, et al. TACI-ligand interactions are required for T cell activation and collagen-induced arthritis in mice. Nat Immunol. 2001;2:632–637. doi: 10.1038/89782. [DOI] [PubMed] [Google Scholar]
- 67.McKay J, et al. Belimumab (BmAb), a fully human monoclonal antibody to B-lymphocyte stimulator (BLyS), combined with standard of care therapy reduces the signs and symptoms of rheumatoid arthritis in a heterogeneous subject population. Arthritis Rheum. 2005;52:S710–S711. [Google Scholar]
- 68.Stohl W, et al. Belimumab (BmAb), a novel fully human monoclonal antibody to B-lymphocyte stimulator (BLyS), selectively modulates B-cell sub-populations and immunoglobulins in a heterogeneous rheumatoid arthritis subject population. Arthritis Rheum. 2005;52:S444. [Google Scholar]
- 69.Wallace DJ, et al. A phase II, randomized, double-blind, placebo-controlled, dose-ranging study of belimumab in patients with active systemic lupus erythematosus. Arthritis Rheum. 2009;61:1168–1178. doi: 10.1002/art.24699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Furie RA, et al. Novel evidence-based systemic lupus erythematosus responder index. Arthritis Rheum. 2009;61:1143–1151. doi: 10.1002/art.24698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Navarra SV, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 72.Furie R, et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum. 2011;63:3918–3930. doi: 10.1002/art.30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Merrill JT, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62:222–233. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Furie R, et al. Efficacy and safety of rituximab in subjects with active proliferative lupus nephritis (LN): results from the randomized, double-blind phase III LUNAR study. Arthritis Rheum. 2009;60:S429. doi: 10.1002/art.38037. [DOI] [PubMed] [Google Scholar]
- 75.Ramos-Casals M, Díaz-Lagares C, Khamashta MA. Rituximab and lupus: good in real life, bad in controlled trials. Comment on the article by Lu et al. Arthritis Rheum. 2009;61:1281–1282. doi: 10.1002/art.24726. [DOI] [PubMed] [Google Scholar]
- 76.Looney RJ. B cell-targeted therapies for systemic lupus erythematosus: an update on clinical trial data. Drugs. 2010;70:529–540. doi: 10.2165/11535420-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 77.Ramanujam M, et al. Similarities and differences between selective and nonselective BAFF blockade in murine SLE. J Clin Invest. 2006;116:724–734. doi: 10.1172/JCI26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jacob CO, et al. Paucity of clinical disease despite serological autoimmunity and kidney pathology in lupus-prone New Zealand Mixed 2328 mice deficient in BAFF. J Immunol. 2006;177:2671–2680. doi: 10.4049/jimmunol.177.4.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shlomchik MJ, Madaio MP, Ni D, Trounstein M, Huszar D. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med. 1994;180:1295–1306. doi: 10.1084/jem.180.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jacob N, et al. B cell and BAFF dependence of IFN-α-exaggerated disease in systemic lupus erythematosus-prone NZM 2328 mice. J Immunol. 2011;186:4984–4993. doi: 10.4049/jimmunol.1000466. [DOI] [PMC free article] [PubMed] [Google Scholar]