Abstract
This study was designed to determine the contents of total polyphenols, flavonoids, flavonols, flavanols, and anthocyanins of purple corn (Zea mays L.) extracts obtained with different methanol:water concentrations, acidified with 1% HCl (1 N). Another objective was to determine the antioxidant activity by 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), ferric reducing antioxidant power (FRAP), and deoxyribose assay, individual phenolic compounds by high-performance liquid chromatography (HPLC), and endogenous antioxidant enzyme (superoxide dismutase [SOD], catalase [CAT], and total peroxidase [TPX]) activity and lipid peroxidation activity (thiobarbituric acid–reactive substances [TBARS] assay) in isolated mouse organs. Overall, the highest total content of polyphenols, anthocyanins, flavonoids, flavonols, and flavanols was obtained with the 80:20 methanol:water extract, acidified with 1% HCl (1 N). The 50% inhibitory concentration values obtained by the DPPH and ABTS assays with this extract were 66.3 μg/mL and 250 μg/mL, respectively. The antioxidant activity by the FRAP assay was 26.1 μM Trolox equivalents/g, whereas the deoxyribose assay presented 93.6% inhibition. Because of these results, the 80:20 methanol:water extract, acidified with 1% HCl (1 N), was used for the remaining tests. Eight phenolic compounds were identified by HPLC: chlorogenic acid, caffeic acid, rutin, ferulic acid, morin, quercetin, naringenin, and kaempferol. Furthermore, it was observed that the purple corn extract was capable of significantly reducing lipid peroxidation (lower malondialdehyde [MDA] concentrations by the TBARS assay) and at the same time increasing endogenous antioxidant enzyme (CAT, TPX, and SOD) activities in isolated mouse kidney, liver, and brain. On the basis of the results, it was concluded that the purple corn extract contained various bioactive phenolic compounds that exhibited considerable in vitro antioxidant activity, which correlated well with the decreased MDA formation and increase in activity of endogenous antioxidant enzymes observed in the isolated mouse organs. This warrants further in vivo studies with purple corn extracts to assess its antioxidant activity and other bioactivities.
Key Words: antioxidant activity, endogenous antioxidant enzymes, isolated mouse organs, oxidative stress, phenolic compounds, purple corn, Zea mays L.
Introduction
The Peruvian Andes Mountains have a wide variety of maize such as purple corn (Zea mays L.) with potential for industrial use and export. This crop and its health effects have been represented in ceremonial ceramics in Peru and other Hispanic countries (Inca and Pre-Inca era). Currently, in Peru a purple corn drink and dessert such as chicha morada and mazamorra morada, respectively, are recognized as highly nutritious.
A poor diet is a contributing factor in the etiology of chronic diseases such as heart disease and cancer.1 However, it is important to note the beneficial properties of the constituents of the food we eat. There is also considerable evidence that an adequate intake of fruits, vegetables, grains, cereals, and other herbs has a beneficial effect on health and aid in prevention of various diseases.2,3 These preventive effects can be attributed to various bioactive compounds such as flavonols, flavones, catechins, flavanones, anthocyani(di)ns, procyanidin B, and dimers, which are widely distributed in plants and processed foods.4–8 Numerous in vitro investigations have demonstrated potent effects of flavonoids in mammalian systems that are potentially anticarcinogenic and anti-atherogenic. These include antioxidant DNA and protection against effects of low-density lipoprotein, modulation of inflammation, inhibition of platelet aggregation, estrogenic effects, and modulation of adhesion receptor expression.1
Purple corn is an important source of anthocyanins and other polyphenols that are distributed throughout the plant.9 Various phenolic phytochemicals have been characterized in purple corn, including anthocyanins, cyanidin-3-glucoside, pelargonidin-3-glucoside and peonidin-3-glucoside,9–11 cyanidin-3-(6′′-malonylglucoside), pelargonidin-3-(6′′-malonylglucoside), peonidin-3-(6′′-malonylglucoside), cyanidin-3-(6′′-ethylmalonylglucoside), pelargonidin-3-(6′′-ethylmalonylglucoside), and peonidin-3-(6′′-ethylmalonylglucoside).10 Flavanol-anthocyanins such as catechin-(4,8)-cyanidin-3-glucoside, catechin-(4,8)-cyanidin-3-malonylglucoside, epicatechin-(4,8)-cyanidin-3-malonylglucoside, catechin-(4,8)-peonidin-3-glucoside, epicatechin-(4,8)-peonidin-3-glucoside, catechin-(4,8)-pelargonidin-3-glucoside, catechin-(4,8)-cyanidin-3,5 diglucoside, catechin-(4,8)-cyanidin-3-malonylglucoside-5 glucoside, and epicatechin-(4,8)-cyanidin-3-malonylglucoside-5 glucoside have been identified.12 Phenolic acids such as p-coumaric acid, vanillic acid, protocatechuic acid, ferulic acid,9 p-hydroxybenzoic acid, p-hydroxyphenyl acetic acid, syringic acid, and caffeic acid have been found.13 Flavonoids such as derivatives of hesperitin and quercetin9 and kaempferol have also been reported in hydrolysates of the aleurone tissue of corn.14
A phenol is any compound that contains a hydroxyl (–OH) group attached to a benzene ring. Plants contain a huge range of phenols, including the tocopherols and tocotrienols. Most phenols found in purple corn exert antioxidant effects in vitro,15,16 inhibiting lipid peroxidation by acting as chain-breaking peroxyl radical scavengers. In addition, phenols often scavenge other reactive species such as OH⋅, NO2⋅, N2O3, ONOOH, and HOCl.16,17 Recently, it has been proposed that the other mechanism by which phenolic phytochemicals function in counteracting the oxidative stress could be by stimulating the synthesis and/or replenishment of cellular antioxidant status or by inducing and improving the host cellular antioxidant enzyme response through the superoxide dismutase (SOD) and catalase (CAT) systems.18
The aims of this study were to determine (1) the content of polyphenols, flavonoids, flavonols, flavanols, and anthocyanins and the spectral analysis, (2) the antioxidant activity by the 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), ferric reducing antioxidant power (FRAP), and deoxyribose assays, and (3) the individual polyphenols by high-performance liquid chromatography (HPLC) and (4) to evaluate changes in cellular antioxidant response in isolated mice organs using markers of oxidative stress such as SOD, CAT, and total peroxidase (TPX).
Materials And Methods
Sample
The genetic material of purple corn (Z. mays L.) was acquired from the Estación Experimental Agraria Baños del Inca (Cajamarca, Peru), located in the Chonta River Valley, between 7°9′56′′ South latitude and 78°27′07′′ West longitude. The sample corresponds to an improved variety of purple corn (INIA-601) basic. For the processing of the sample, 10 kg of purple corn (approximately 2,000 grains) was processed in 10 different batches (about 200 grains or 1 kg per batch) on different days within a 2-week period. Each batch was processed as described under Sample preparation below, and then the 10 different batches were combined into one large batch from which material was collected to perform the extractions using the different extraction systems.
Chemicals
The specific chemicals used were purchased from Sigma Chemical Co. (St. Louis, MO, USA): ABTS, 2,2′-azobis(2-amidinopropane) HCl (ABAP), DPPH, Folin–Ciocalteu reagent, p-dimethylaminocinnamaldehyde, phenolic acids (caffeic, chlorogenic, ferulic, and gallic acids), and flavonoids [rutin (quercetin-3-O-rutinoside), (+)-catechin, (–)-epicatechin, quercetin, and kaempferol]. HPLC-grade acetonitrile, methanol, and acetic acid were purchased from Merck KGaA (Darmstadt, Germany).
Instruments
The absorbance measurements were collected in a model UV/VIS 2550 spectrophotometer (Shimadzu Co., Columbia, SC, USA). Chromatography was carried out using a model LaChrom D-7000 HPLC apparatus (Merck-Hitachi, Tokyo, Japan). The data runs were collected using the D-7000 HPLC System Manager software version 4.0 (Merck and Hitachi Instruments [San Jose, CA, USA]).
Sample preparation
The purple corn (grains) underwent reduction in size using two different procedures: the first by using a mortar and pestle and the second using a Mini chopper (Moulinex Co., Berkshire, United Kingdom) with an exposure time of 8 seconds. Then, the particles were filtered through a mesh with pore diameter of 600 μm. The extraction was performed through six extraction systems. Approximately 5 g of sample was extracted with 50 mL of different methanol:water concentrations (100:0, 80:20, 60:40, 40:60, 20:80, and 0:100), all of them acidified with 1% HCl (1 N). The extraction was performed on a water bath at 40°C using a magnetic stirrer for 1 hour in complete darkness. The aqueous fraction was placed in plastic tubes, and 15 mL was centrifuged at 2,000 g and stored under refrigeration (2–8°C) until analysis. For the remaining in vitro assays, all the extraction systems using the different methanol:water concentrations were used. As stated below, the in vitro assay (cellular antioxidant response in isolated mouse organs) was performed with the 80:20 (methanol:water) extract acidified with 1% HCl (1 N).
Content of total polyphenols
Total polyphenols were determined by the Folin–Ciocalteu method.5,6,19 A sample aliquot (100 μL of 100 μg/mL) or standard was reacted with 750 μL of 0.2 N Folin–Ciocalteu reagent; after 5 minutes of reaction 750 μL of sodium carbonate (7.5%) was added. The calibration curve consisted of the following concentrations of gallic acid: 5, 10, 40, 70, and 100 μg/mL. The reaction was carried out at 25°C for 30 minutes in darkness. The absorbance was read at 725 nm.
Content of total flavonoids
The content of flavonoids was determined by the method described by Miliauskas et al.20 Rutin (quercetin-3-O-rutinoside) was used as the reference standard at the corresponding concentrations for the calibration curve: 2, 5, 10, 25, 50, and 100 μg/mL. To a 600-μL standard or sample (100 μg/mL) aliquot, 600 μL of AlCl3 at 40 g/L was added. The mixture was allowed to react at 25°C for 40 minutes; after this time the absorbance was read at 415 nm.
Content of total flavonols
The content of flavonols was determined by the method described by Djeridane et al.21 Rutin (quercetin-3-O-rutinoside) was used as the reference standard, and the concentration range used was the same as in the case of total flavonoids. To a 500-μL extract (100 μg/mL) aliquot, 500 μL of AlCl3 at 20 g/L and 1.5 mL of sodium acetate at 50 g/L were added. The reaction was developed for 2 hours at 25°C, and the absorbance was read at 440 nm.
Content of total flavanols
The content of flavanols was estimated using 4-(dimethylamino)cinnamaldehyde, as described by Arnous et al.22 (+)-Catechin was used as a reference standard at the following concentrations: 1, 2, 4, 8, 12, and 16 mg/L. The reaction was developed using 200 μL of sample (100 μg/mL) or standard and 1 mL of 4-(dimethylamino)cinnamaldehyde solution (0.1% in 1 N HCl in methanol). The mixture was stirred strongly for 10 minutes, and the absorbance was read at 640 nm.
Content of total anthocyanins
The content of anthocyanins was determined by the pH differential method described by Giusti and Wrolstad.23 To a 300-μL aliquot of extract (100 μg/mL), 2.7 mL of buffer was added. The buffer consisted of two solutions: the first at pH 1 (0.2 M KCl and 0.2 M HCl) and the second at pH 4.5 (1 M sodium acetate, 1 M HCl, and H2O). Spectral scanning was performed from 250 to 750 nm. The maximum absorbance was recorded at 510 nm, corresponding to cyanidin-3-O-glucoside with a molecular weight of 449.2 and ɛ=26,800. To calculate the absorbance of the diluted sample (A) the following formula was used:
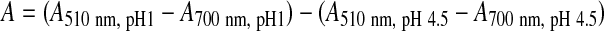 |
The concentration (C) of anthocyanins (in milligrams of cyanidin-3-O-glucoside/L) was calculated from the formula:
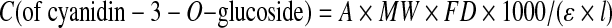 |
where ɛ is the molar absorptivity, l is the width of the cuvette, A is the absorbance, MW is the molecular weight of reference standard, and FD is the dilution factor.
Measurement of antioxidant activity
DPPH assay
The method used for the DPPH radical scavenging was adapted from that of de Campos et al.24 The reaction occurred between 50 μL of sample and 950 μL of 100 μM DPPH. The sample concentrations were in the range of 5–500 μg/mL. The absorbance values were recorded every 30 seconds for 10 minutes. The antioxidant activity was expressed as the 50% inhibitory concentration (IC50) value,7,8 and the values were adjusted to the following models: reciprocal-Y model of Y=1/(a+[b×X]) and the logarithmic-X model of Y=a+[b×ln(X)].
ABTS assay
The total radical trapping antioxidant potential assay was developed using the ABAP/ABTS reagent.25 The sample concentrations ranged between 125 and 1000 μg/mL. A sample aliquot of 10 μL was allowed to react with 990 μL of ABTS/ABAP reagent. The ABTS/ABAP reagent consisted of a mixture of 2.25 mM ABTS, 20 mM ABAP, and phosphate-buffered saline buffer (50 mM K2HPO4 and 0.9% NaCl, pH 7.4). The reaction was incubated at 70°C for 20 minutes. The absorbance was recorded every 20 seconds for 10 minutes at 734 nm. The antioxidant activity was expressed as the IC50 value. The absorbances were adjusted for the following models: multiplicative model of ln(Y)=a – [b×ln(X)] and reciprocal-X model of Y=a+(b/X).
FRAP assay
The total antioxidant potential was determined using the method developed by Thaipong et al.26 and Benzie and Strain27 with some modifications. The sample concentrations ranged between 250 to 2500 μg/mL. A 50-μL sample aliquot and 50 μL of FeCl3 (3 mM in 5 mM citric acid) were incubated at 37°C for 30 minutes, then 900 μL of 2,4,6-tripyridyl-s-triazine (1 mM in 0.05 M HCl) was added, the mixture was stirred strongly, and after 10 minutes of reaction the absorbance was read at 620 nm. The results were expressed in micromolar Trolox equivalents (TE)/g.
Deoxyribose assay
This assay is based on the quantification of the hydroxyl radical (⋅OH) generated by the reaction of Fe-EDTA complex with hydrogen peroxide (H2O2) (Fenton reaction) in the presence of ascorbic acid to attack the pentose sugar deoxyribose to form product reactants of thiobarbituric acid. The method used was described by Sandoval et al.28 An amount of 200 μL of deoxyribose (2 mM), 200 μL of KH2PO4 (20 mM), 100 μL of FeCl3 (100 μM), 100 μL of EDTA (100 μM), 100 μL of H2O2 (1 mM), 100 μL of ascorbic acid (100 μM), and 50 μL of extract (100 μg/mL) were mixed and incubated at 37°C for 2 hours. Then, thiobarbituric acid (1%) was added, and the pink color produced by the reaction with thiobarbituric acid was measured at 540 nm.
Phenolic compounds analyzed by HPLC
The analysis was performed with a LaChrom D-7000HPLC apparatus. The HPLC method was adapted from previous published reports29–31 with some modifications. In brief, the separation was carried out at room temperature with a LiChrospher RP-18 (250×4 mm; particle size, 5 μm) column (Merck KGaA). The chromatographic run was performed at a flow rate of 1 mL/minute, and the mobile phase was a mixture of eluent A (water–phosphoric acid, pH 2.5) and eluent B (acetonitrile) under the following gradient conditions: 0 minute (100% A), 2 minutes (80% A), 15 minutes (70% A), 16 minutes (40% A), 22 minutes (60% A), 26 minutes (100% A), and 28 minutes (100% A). The injection volume was 40 μL, and the data runs were collected by D-7000 HPLC System Manager software version 4.0. Calibration curves for each phenolic compound were prepared by dilution of their stock solutions (0.1 mg/mL) to yield a series of concentrations: 0.25, 0.5, 1, 5, 10, and 50 μg/mL. The limit of quantification of this assay for all the phenolic compounds was 0.25 μg/mL.
Cellular antioxidant response in isolated mouse organs
Experimental animals
Twenty-four albino male mice 2–4 months old and weighing 25–40 g, bred in the rodent vivarium of the Faculty of Veterinary Medicine, National University of San Marcos, Lima, Peru, were used. They were then transferred to the vivarium of the Faculty of Human Medicine, University of San Martin de Porres, Lima, where they were maintained in accordance with international regulations for environment moisture, food, circadian cycles, and temperature. Animals were fed with standard rodent balanced diet (food pellets) and had free access to food and water throughout the duration of the study. The animals were maintained for 48 hours with no treatment besides ad libitum access to food and water because the objective was to extract different organs for measurement of in vitro cellular antioxidant response in isolated mouse organs. For this, the experimental animals were euthanized by placing them in a CO2 chamber; the organs (kidney, liver, and brain) were immediately isolated and placed in phosphate-buffered saline.
Preparation of homogenates
The organs were homogenized using a Potter–Elvehjem glass–Teflon® (Dupont, Wilmington, DE, USA) homogenizer (Omni International, Kennesaw, GA, USA). The homogenate was clarified by centrifugation at 4,200 g for 10 minutes, and the supernatant and pellet were separated and stored at −10°C.
Experimental design
The sample was prepared at a concentration of 100 mg/mL, using the 80:20 methanol:water extract containing 1% HCl (1 N). This system was used because it exhibited on average the highest antioxidant activity in the DPPH, FRAP and deoxyribose assays and also exhibited on average a higher total content of polyphenols, flavonoids, flavonols, flavanols, and anthocyanins. The 80:20 methanol:water extract containing 1% HCl (1 N) was evaporated to dryness under a constant flow of compressed nitrogen gas. The residue was reconstituted with sufficient water volume to render a final purple corn extract (PCEx) of 100 mg/mL, vortex-mixed for 1 minute, and centrifuged at 3,000 g for 5 minutes. The soluble supernatant was collected and used for the treatment. The tissue homogenates were treated using H2O, H2O2 (100 μM), PCEx (100 mg/mL), and PCEx+H2O2 (Table 1). For this, 490 μL of tissue homogenate was mixed with 210 μL of either H2O, H2O2, PCEx, or PCEx+H2O2 depending on the treatment. All samples were incubated at 37°C for 2 hours and then maintained at 0°C for a brief period of time (maximum of 30 minutes) until analysis of the different endogenous enzymes. For the different endogenous enzyme activity assays, PTX was measured by the method described by Laloue et al.,32 SOD by that of Marklund and Marklund,33 CAT by that of Beers and Sizer,34 and lipid peroxidation (thiobarbituric acid–reactive substances [TBARS] production) by that of Tamagnone et al.35
Table 1.
Different Treatments Used in the Homogenates of Isolated Mouse Organs
| |
Treatmenta |
|||
|---|---|---|---|---|
| Organ | H2O | H2O2 | PCEx | PCEx+H2O2 |
| Kidney | 490:210 (vol/vol) | 490:210 (vol/vol) | 490:210 (vol/vol) | 490:105:105 (by volume) |
| Liver | 490:210 (vol/vol) | 490:210 (vol/vol) | 490:210 (vol/vol) | 490:105:105 (by volume) |
| Brain | 490:210 (vol/vol) | 490:210 (vol/vol) | 490:210 (vol/vol) | 490:105:105 (by volume) |
The volume unit is microliters.
H2O2, hydrogen peroxide (100 μM); PCEx, purple corn extract (100 mg/mL).
Statistical analysis
The analysis of variance was developed by a completely randomized design using Tukey's test (Honestly Significant Difference) for variables. The data were processed by SAS software (1996 version) (SAS Institute, Inc., Cary, NC, USA). The significance level established was P<.001 when possible; if not, it was established at P<.05.
Results and Discussion
These results include measurements of total polyphenols, flavonoids, flavonols, flavanols, and anthocyanins. The results obtained are due to a particular characteristic of the sample; these values are estimates of the methods used for certain phenols with different chemical structures.
Content of polyphenols, flavonoids, flavonols, flavanols, and anthocyanins
The content of polyphenols, flavonoids, flavonols, and flavanols of different extraction systems are presented in Table 2. The results shown in Table 2 correspond to the assays conducted using different extraction systems. It is important to note that each extract showed a different total content of bioactive compounds, which correlated with the expected different logP values and affinities for the various extraction systems used. The mean polyphenol content across all the extraction systems was 6.99 g of gallic acid equivalents/kg; however, the more efficient extraction for polyphenols occurred with the 80:20 methanol:water system acidified with 1% HCl (1 N), which appears to indicate the relationship between the polarity of the extraction system and the affinity of the bioactive components. This same relationship occurs for flavonoids and flavanols; however, there is a variation for flavonols that exhibit higher concentration with the extraction system of 100% methanol acidified with 1% HCl (1 N), which was recorded as 0.47 g of rutin equivalents/kg. Similarly, Kuskoski et al.36 reported that the best method for flavonol extraction is with ethanol acidified with 0.1% HCl, recording values between 6.61 to 13.3 g/kg in baguaçu (Eugenia umbelliflora Berg). Furthermore, Sellappan et al.37 reported values of 2.61–9.29 g/kg fresh weight in several species of blueberries and blackberries: rabbiteye blueberry (Vaccinium ashei Reade), highbush blueberry (Vaccinium corymbosum L.), and blackberry (Rubus L.).
Table 2.
Total Content of Bioactive Compounds in Purple Corn (Z. mays L.) Extracts Using Different Extraction Systems
|
Extraction system |
|
|
|
|
|
|
|---|---|---|---|---|---|---|
| Methanol (%) | Water (%) | Polyphenols (g of GAE/kg) | Flavonoids (g of RE/kg) | Flavonols (g of RE/kg) | Flavanols (g of CE/kg) | Anthocyanins (g of C3G/kg) |
| 100 | 0 | 8.67±0.12b | 2.61±0.02a | 0.47±0.03a | 0.20±0.01ab | 2.87±0.04a |
| 80 | 20 | 9.06±0.07a | 2.66±0.04a | 0.41±0.02b | 0.23±0.01a | 2.76±0.05b |
| 60 | 40 | 8.14±0.03c | 2.24±0.06b | 0.39±0.01b | 0.22±0.01a | 2.51±0.02c |
| 40 | 60 | 6.36±0.10d | 1.67±0.04c | 0.42±0.02b | 0.18±0.01bc | 1.93±0.03d |
| 20 | 80 | 5.08±0.09e | 1.15±0.06d | 0.38±0.01b | 0.15±0.01cd | 1.09±0.03e |
| 0 | 100 | 4.63±0.06f | 1.18±0.05d | 0.38±0.02b | 0.15±0.01d | 0.88±0.02f |
| Mean | 6.99 | 1.92 | 0.41 | 0.19 | 2.01 | |
The data for polyphenols were calculated from a standard curve of gallic acid (y=0.0071x+0.0021, R2=0.9985), those for flavonoids were calculated from a standard curve of rutin (y=0.0124x+0.0013, R2=0.9938), those for flavonols were calculated from a standard curve of rutin (y=0.0061x – 0.0214, R2=0.9994), those for flavanols were calculated from a standard curve of catechin (y=0.0589x – 0.0095, R2=0.996), and those for anthocyanins were calculated from a standard curve of cyanidin-3-glucose. Data are mean values from three repetitions.
abcdef: Significant difference at P<.001.
C3G, cyanidin-3-glucose equivalents; CE, catechin equivalents; GAE, gallic acid equivalents; RE, rutin equivalents.
The anthocyanin content of purple corn using different extraction systems is also shown in Table 2. It can be observed that the anthocyanin content using different extraction systems ranged between 0.88 and 2.87 g of cyanidin-3-glucose equivalents/kg. This falls within the range of previous reports with other plant species; for instance, Andersen38 reported 1.74 g/kg fresh weight for lingonberry (Vaccinium viteas-idea L.) using ethanol acidified with 1% HCl, whereas Benvenuti et al.39 reported an anthocyanin content between 0.67 and 1.27 g/kg for blackberry (Rubus fruticosus L.), 0.29 and 0.35 g/kg for raspberry (Rubus idaeus L.), 1.52 and 2.61 g/kg for black currant (Ribes nigrum L.), 0.22 and 0.40 g/kg for red currant (Ribes rubrum L.), and 4.60 g/kg for black chokeberry (Aronia melanocarpa Elliot) using an extraction system of methanol/HCl 2% (95:5 vol/vol). Furthermore, Zheng and Wang40 reported anthocyanin content of fresh weight for blueberry (cv. Serra) (1.20 g/kg), cranberry (cv. Ben Lear) (0.32 g/kg), lingonberry (cv. Amberland) (0.45 g/kg), and chokeberry (wild) (4.28 g/kg) using an extraction solvent of acetone containing 0.2% formic acid.
Antioxidant activity
The scavenger activity of free radicals by DPPH and ABTS was determined by the capacity of the purple corn extracts to scavenge 50% of radicals. The FRAP method is based on the reducing power of the sample. The deoxyribose test is based on the measurement of the hydroxyl radical (⋅OH) generated by the reaction of the Fe-EDTA complex with H2O2 (Fenton reaction). Table 3 shows the antioxidant activity of purple corn extracts using different extraction systems measured by DPPH, ABTS, FRAP, and deoxyribose. The results shown in Table 3 indicate that the bioactive compounds present in purple corn extracts have good activity as free radical scavengers. The measurement of the antioxidant activity using different extraction systems obtained using the DPPH, ABTS, FRAP, and deoxyribose assays exhibited significant differences. It was observed that the addition of methanol at different ratios has a large influence on the antioxidant activity. The best response, taking into account the extraction system applied for the DPPH and FRAP tests, was 80:20 methanol:water acidified with 1% HCl (1 N), which showed a better response with an IC50 of 66.3 μg/mL and 26.1 μM TE/g, respectively. The DPPH IC50 values observed with purple corn extracts are significantly lower compared with those of other plant species. For instance, Benvenuti et al.39 reported DPPH IC50 values for blackberry (R. fruticosus L.) between 4.6 and 9.5 mg/mL, raspberry (R. idaeus L.) between 5.5 and 10.9 mg/mL, black currant (R. nigrum L.) between 1.0 and 4.2 mg/mL, red currant (R. rubrum L.) between 4.3 and 5.9 mg/mL, and black chokeberry (A. melanocarpa Elliot) of 1.8 mg/mL.
Table 3.
Antioxidant Activity of Purple Corn (Z. mays L.) Extracts Using Different Extraction Systems, Measured by 1,1-Diphenyl-2-Picrylhydrazyl, 2,2′-Azinobis(3-Ethylbenzothiazoline-6-Sulfonic Acid), Ferric Reducing Antioxidant Power, and Deoxyribose Assays
|
Extraction system |
Antioxidant activity |
||||
|---|---|---|---|---|---|
| Methanol (%) | Water (%) | DPPH IC50 (μg/mL) | ABTS IC50 (μg/mL) | FRAP (μM TE/g) | Deoxyribose protection (%) |
| 100 | 0 | 76.6±0.80bc | 297±0.50d | 20.5±0.05d | 93.8±0.60a |
| 80 | 20 | 66.3±0.80a | 250±0.40c | 26.1±0.04a | 93.6±0.60a |
| 60 | 40 | 75.9±0.40b | 225±0.30b | 23.5±0.07b | 93.2±0.20a |
| 40 | 60 | 78.4±0.30cd | 219±0.10a | 22.2±0.03c | 90.5±0.10b |
| 20 | 80 | 78.0±0.50de | 366±0.40e | 17.6±0.03e | 87.6±0.50c |
| 0 | 100 | 79.8±0.30e | 799±0.30f | 13.1±0.03f | 67.7±1.0d |
Results of both the 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assays are given as 50% inhibitory concentration (IC50) values. Results for the ferric reducing antioxidant power (FRAP) assay was calculated from the following equation: Absorbance=2.1824×[Trolox]+0.1234 (R2=0.9994). Data are mean values from three repetitions.
abcdef: Significant difference at P<.001.
TE, Trolox equivalents.
For the ABTS assay the best extraction system was 40:60 methanol:water acidified with 1% HCl (1 N), giving an IC50 of 219 μg/mL, and for the deoxyribose assay there were no statistically differences between the methanol:water extraction systems at 100:0, 80:20, and 60:40, all acidified with 1% HCl (1 N), giving values of 93.8%, 93.6%, and 93.2% deoxyribose protection, respectively. This is in accordance with the polarity of the solvent used for the extraction and the solubility of phenolic compounds present in the extract.41 For samples assayed for the presence of anthocyanins, the ionization state can be important when assessing the antioxidant activity.22 For instance, the quinoidal pseudo-base and base of malvidin-3-glucoside generated at pH 4.0 and 7.0, respectively, exhibit differences in antioxidant activity.42 Furthermore, Traipong et al.26 reported differences in antioxidant activity using extraction systems with methanol and dichloromethane in different genotypes (Psidium guajava L.). In that study, it was observed that the ABTS, DPPH, FRAP, and oxygen radical absorbance capacity assays reported average values of 31.1, 25.2, 26.1, and 21.3 μM TE/g using methanol for the extraction, and with average values of 0.44, 0.27, and 0.16 μM TE/g, respectively, with dichloromethane extraction.
Correlation coefficients for the antioxidant activity assays (DPPH, ABTS, FRAP, and deoxyribose) with respect to the total content of bioactive compounds (phenolic, flavonoids, flavonols, flavanols, and anthocyanins) by different extraction systems were calculated by the Pearson coefficient.43,44 These results shown in Table 4 indicate that correlations between antioxidant activity and total content of bioactive compounds assays exhibit differences. It was observed that the correlation coefficient (r=0.87) between the FRAP and deoxyribose antioxidant assays was significantly different (P<.001) and similarly for the FRAP and ABTS assays (r=0.9105, P<.001) and for deoxyribose and ABTS assays (r=0.873, P<.001). However, the correlation coefficients between FRAP and DPPH, deoxyribose and DPPH, and DPPH and ABTS exhibited no statistically significant differences (Table 4).
Table 4.
Correlation Coefficients of Four Antioxidant Activity Assays with Respect to Total Content of Bioactive Compounds (Polyphenols, Flavonoids, Flavonols, Flavanols, and Anthocyanins) of Purple Corn (Z. mays L.) Extracts Using Different Extraction Systems
| Characteristic | DPPH IC50 | ABTS IC50 | FRAP | Deoxyribose |
|---|---|---|---|---|
| Polyphenols | −0.722 | −0.686 | 0.846* | 0.776 |
| Flavonoids | −0.705 | −0.610 | 0.789 | 0.711 |
| Flavonols | −0.135 | −0.415 | 0.338 | 0.512 |
| Flavanols | −0.768 | −0.669 | 0.898* | 0.714 |
| Anthocyanins | −0.626 | −0.729 | 0.841* | 0.805 |
| DPPH IC50 | 1 | |||
| ABTS IC50 | 0.390 | 1 | ||
| FRAP | 0.731 | 0.910* | 1 | |
| Deoxyribose | 0.470 | 0.873* | 0.870* | 1 |
Significant at the P<.05 level.
It has been observed in previous reports that antioxidant assays such as FRAP, DPPH, ABTS, and oxygen radical absorbance capacity exhibit a high correlation with polyphenols.26 For instance, Gil et al.45 reported a correlation coefficient (r>0.9, P≤.05) between the antioxidant activity (DPPH or ABTS) and total polyphenols in nectarines, peaches, and plums. Gardner et al.46 also reported a high correlation between total polyphenols and antioxidant activity measured by FRAP. On our study, it was observed that there is a high correlation between the antioxidant activity assays (DPPH, ABTS, FRAP, and deoxyribose) and the content of polyphenols, flavonoids, flavanols, and anthocyanins, which indicates that these classes of bioactive compounds contribute the most to the antioxidant activity of purple corn. However, there was a low correlation between the antioxidant activity assays and the flavonol content (Table 4).
Phenolic compounds by HPLC
As presented in the previous two sections, overall, the highest antioxidant activity and total content of polyphenols, anthocyanins, flavonoids, flavonols, and flavanols were obtained with the 80:20 methanol:water extract acidified with 1% HCl (1 N). Thus, the 80:20 methanol:water extract acidified with 1% HCl (1 N) was used for the remaining studies. The phenolic compounds profile from purple corn (Z. mays L.) is shown in Table 5. It was observed that eight phenolic compounds were identified by HPLC: chlorogenic acid, caffeic acid, rutin, ferulic acid, morin, quercetin, naringenin, and kaempferol. It was also observed that gallic acid, catechin, and hesperidin were not detected (limit of quantification=0.25 μg/mL). It should be mentioned that previous reports indicate that purple corn is rich in other compounds such as anthocyanins and condensed pigments (flavanol-anthocyanin), which are produced during ripening. For instance, Pedreschi and Cisneros-Zevallos9 found protocatechuic acid, vanillic acid, and p-coumaric acid, which were derived from quercetin and hydroxycinnamic acid in fractions of ethyl acetate. Other reported anthocyanins include cyanidin-3-glucoside, pelargonidin-3-glucoside, and peonidin-3-glucoside.47 Furthermore, Pascual-Teresa et al.10 and Aoki et al.48 also found cyanidin-3-(6′′-malonylglucoside), pelargonidin-3-(6′′-malonylglucoside), peonidin-3-(6′′-malonylglucoside), cyanidin-3-(6′′-ethylmalonylglucoside), pelargonidin-3-(6′′-ethylmalonylglucoside), and peonidin-3-(6′′-ethylmalonylglucoside).
Table 5.
Individual Phenolic Compounds Detected in the 80:20 Methanol:Water Extract Acidified with 1% HCl (1 N) of Purple Corn (Zea mays L.)
| Peak | Retention time (minutes) | Compound | Concentration (mg/kg of sample)a |
|---|---|---|---|
| 1 | 2.06 | Gallic acid | Not detected |
| 2 | 5.82 | Catechin | Not detected |
| 3 | 8.69 | Chlorogenic acid | 10.5 |
| 4 | 10.4 | Caffeic acid | 38.1 |
| 5 | 11.3 | Rutin | 27.4 |
| 6 | 14.4 | Ferulic acid | 55.2 |
| 7 | 14.8 | Hesperidin | Not detected |
| 8 | 20.1 | Morin | 2020 |
| 9 | 20.5 | Quercetin | 15.8 |
| 10 | 21.4 | Naringenin | 148 |
| 11 | 21.6 | Kaempferol | 2240 |
Mean concentration of two runs; no SD reported.
Cellular antioxidant response in isolated mouse organs
The beneficial effects of polyphenols to decrease reactive oxygen species are well documented.49–51 Table 6 shows the activity of endogenous enzymes and TBARS production for the different treatments in isolated mouse kidney, liver, and brain. The results suggest that treatments with purple corn extract had a protective effect in maintaining cellular homeostasis through stimulation of antioxidant enzymes in isolated mouse organs. Oxidative stress in isolated mouse organs was induced by H2O2 as a source of reactive oxygen species. Oxidation of membrane lipids had been reported to change the membrane plasticity and flexibility, which can cause an increase in membrane permeability. This increase in membrane permeability could have resulted in increased uptake of partially hydrophobic phenols in the presence of H2O2.52 The results shown in Table 6 indicate that the PCEx+H2O2 and the PCEx treatments were more effective in stimulating the production of the antioxidant enzymes SOD, CAT, and TPX than the control (H2O2) and the negative control (H2O), which indicates the functionality of the bioactive compounds present in purple corn. The H2O2 treatment showed a high content of malondialdehyde (MDA) formed in all the tested organs (kidney, liver, and brain), indicating its pro-oxidative effect. This correlates with the fact that lipid oxidation in biological systems is carried by the action of reactive oxygen species, resulting in the formation of MDA, which it is also a metabolite of hydroperoxides53 and serves as a good marker of oxidation and injury to the cell membrane.54 However, this system is closely regulated by several antioxidant enzymes such as SOD and CAT,55 which help to remove reactive oxygen species. As shown in Table 6, the MDA (measured by the TBARS assay) value reaches its highest levels in organs treated with H2O2; this is probably due to a rapid progression of secondary lipid oxidation induced by H2O2, which decreases the cellular antioxidant response. However, when organs are treated with purple corn extract low MDA values are observed, most likely due to the presence of polyphenols, which have the ability to scavenge free radicals and to increase levels of endogenous antioxidant enzymes. For instance, when calculating the SOD ratio between treatment and the negative control (H2O2), it was observed that this ratio ranged between 1.81 and 3.42 for kidney, liver, and brain, indicating an increase in SOD levels after treatment with purple corn. Similarly, the ratio for CAT ranged between 1.64 and 25.66, whereas the ratio for TPX ranged between 1.07 and 1.36. CAT activity exhibited a higher increase than SOD and TPX activities, which is very advantageous as in general it is recognized that a rapid increase in the activity of CAT and SOD is critical to quickly remove reactive oxygen species and prevent oxidative cell damage.
Table 6.
Antioxidant Effect of the 80:20 Methanol:Water Extract Acidified with 1% HCl (1 N) of Purple Corn (Z. mays L.) on Cellular Antioxidant Response and Hydrogen Peroxide–Induced Lipid Peroxidation in Isolated Mouse Organs
| Organ, treatment | CAT | TPX | SOD | TBARS |
|---|---|---|---|---|
| Kidney | ||||
| PCEx+H2O2 | 11.3±2.2a | 66.1±1.3a | 32.1±0.7a | 1.11±0.02b |
| PCEx | 5.86±2.3b | 61.7±1.5b | 26.0±0.5b | 0.17±0.03a |
| H2O2 | 1.52±0.3c | 48.5±1.1c | 10.3±0.2c | 7.59±0.08c |
| H2O | 1.01±2.1 | 58.0±1.1 | 17.9±0.3 | 3.26±0.04 |
| F ratio | 21.2 | 146 | 1,460 | 19,100 |
| P | .001** | .001** | .001** | .001** |
| Liver | ||||
| PCEx+H2O2 | 21.1±4.9b | 51.8±1.6b | 35.0±1.5a | 4.34±0.07a |
| PCEx | 34.4±3.9a | 57.7±1.8a | 36.2±0.6a | 4.22±0.06a |
| H2O2 | 1.34±2.8c | 43.8±1.3c | 10.6±0.6b | 25.1±0.3b |
| H2O | 28.2±2.4 | 42.4±1.5 | 28.6±1.5 | 14.0±0.14 |
| F ratio | 55.8 | 58.8 | 633 | 13,200 |
| P | .001** | .001** | .001** | .001** |
| Brain | ||||
| PCEx+H2O2 | 18.0±1.2a | 33.9±0.6b | 11.6±0.3b | 2.34±0.04a |
| PCEx | 15.8±0.9b | 37.8±0.7a | 21.7±0.5a | 2.65±0.04a |
| H2O2 | 9.58±0.8c | 31.6±0.6c | 6.40±0.1c | 33.6±0.4b |
| H2O | 10.0±0.6 | 34.7±0.6 | 8.29±0.2 | 2.19±0.04 |
| F-ratio | 59.3 | 74.7 | 1,560 | 17,700 |
| P | .001** | .001** | .001** | .001** |
Data are mean±SD values. Catalase (CAT), total peroxidase (TPX), and superoxide dismutase (SOD) activities were measured as the change in absorbance (minute–1×1,000). The thiobarbituric acid–reactive substances (TBARS) level was measured as the malondialdehyde concentration (in μM).
Significant difference t P<.001 compared to H2O2 (water) treatment.
Significant at the P<.001 level.
Conclusions
The efficiency of the extraction system for the extraction of bioactive compounds from purple corn (Z. mays L.) is influenced by the hydrophilic or lipophilic behavior of these compounds and their affinity to the extracting solvent. On our study it was observed that the most effective extracting solvent was 80:20 methanol:water, acidified with 1% 1 N HCl. Purple corn is considered a natural pigment source due to its high anthocyanin content, which we reported in the range from 0.86 to 2.87 g/kg of sample across all the different extraction systems. The antioxidant activity, measured as the IC50, ranged between 66.3 and 79.8 μg/mL for the DPPH assay, 219 and 799 μg/mL for the ABTS assay, and 13.1 and 26.1 μM TE/g for the FRAP assay, whereas its protective effect of deoxyribose ranged between 67.7% and 93.8%. The highest antioxidant activity and total content of polyphenols, anthocyanins, flavonoids, flavonols, and flavanols were obtained with the 80:20 methanol:water extract acidified with 1% HCl (1 N). Thus, the 80:20 methanol:water extract acidified with 1% HCl (1 N) was used for the remaining studies. Eight phenolic compounds were identified by HPLC—chlorogenic acid, caffeic acid, rutin, ferulic acid, morin, quercetin, naringenin, and kaempferol—but gallic acid, catechin, and hesperidin were not detected (limit of quantification=0.25 μg/mL). In our in vitro study using isolated mouse kidney, liver, and brain homogenates, it was observed that purple corn extract increased the levels of endogenous enzymes such as SOD, CAT, and TPX and decreased MDA formation. This might be in great part due to the presence of bioactive compounds that can penetrate the membrane and participate in the stability and protection of lipid membranes, reducing the damage caused by free radicals. On the basis of the results, further in vivo studies with purple corn extracts are warranted to assess its antioxidant activity and other bioactivities for possible applications in various fields of study.
Acknowledgments
We thank the National Council for Science, Technology and Technological Innovation for financial support of the project (number PROCYT 331-2007) and the Program on Science and Technology for the grant of doctoral studies (number 124-2009-FINCyT-BDE) for F.R.-E.
Author Disclosure Statement
J.A.Y. is an employee of Alcon Research, Ltd. F.R-E., A.M.M., and C.A.-O. have no competing financial interests.
References
- 1.Kyle JAM. Duthie GG. Flavonoids in foods. In: Andersen OM, editor; Markham KR, editor. Flavonoids: Chemistry, Biochemistry and Applications. CRC Press, Taylor and Francis; Boca Raton, FL: 2006. pp. 219–262. [Google Scholar]
- 2.Naczk M. Shahidi F. Phenolics in cereals, fruits and vegetables: occurrence, extraction and analysis. J Pharm Biomed Anal. 2006;41:1523–1542. doi: 10.1016/j.jpba.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Yanez JA. Andrews PK. Davies NM. Methods of analysis and separation of chiral flavonoids. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;848:159–181. doi: 10.1016/j.jchromb.2006.10.052. [DOI] [PubMed] [Google Scholar]
- 4.Duthie GG. Duthie SJ. Kyle JA. Plant polyphenols in cancer and heart disease: implications as nutritional antioxidants. Nutr Res Rev. 2000;13:79–106. doi: 10.1079/095442200108729016. [DOI] [PubMed] [Google Scholar]
- 5.Mejia-Meza EI. Yanez JA. Davies NM, et al. Improving nutritional value of dried blueberries (Vaccinium corymbosum L.) combining microwave-vacuum, hot-air drying and freeze-drying technologies. Int J Food Eng. 2008;4:1–6. [Google Scholar]
- 6.Mejia-Meza EI. Yanez JA. Remsberg CM, et al. Effect of dehydration on raspberries: polyphenol and anthocyanin retention, antioxidant capacity, and antiadipogenic activity. J Food Sci. 2010;75:H5–H12. doi: 10.1111/j.1750-3841.2009.01383.x. [DOI] [PubMed] [Google Scholar]
- 7.Ramos-Escudero DF. Condezo-Hoyos LA. Ramos-Escudero M. Yanez JA. Design, assessment of the in vitro anti-oxidant capacity of a beverage composed of green tea (Camellia sinensis L), lemongrass (Cymbopogon citratus Stap) In: McKinley H, editor; Jamieson M, editor. Handbook of Green Tea and Health Research. Nova Science Publishers; New York: 2009. pp. 81–101. [Google Scholar]
- 8.Ramos-Escudero M. Ramos-Escudero DF. Remsberg CM. Takemoto JK. Davies NM. Yanez JA. Identification of polyphenols and anti-oxidant capacity of Piper aduncum L. Open Bioact Compd J. 2008;1:18–21. [Google Scholar]
- 9.Pedreschi R. Cisneros-Zevallos L. Phenolic profiles of Andean purple corn (Zea mays L) Food Chem. 2007;100:956–963. doi: 10.1021/jf0531050. [DOI] [PubMed] [Google Scholar]
- 10.Pascual-Teresa S. Santos-Buelga C. Rivas-Gonzalo JC. LC-MS analysis of anthocyanins from purple corn cob. J Sci Food Agric. 2002;82:1003–1006. [Google Scholar]
- 11.Yang Z. Chen Z. Yuan S. Zhai W. Piao X. Piao X. Extraction and identification of anthocyanin from purple corn (Zea mays L.) Int J Food Sci Technol. 2009;44:2485–2492. [Google Scholar]
- 12.Gonzalez-Manzano S. Perez-Alonso JJ. Salinas-Moreno Y, et al. Flavanol-anthocyanin pigments in corn: NMR characterisation and presence in different purple corn varieties. J Food Compos Anal. 2008;21:521–526. [Google Scholar]
- 13.Sosulski F. Krygier K. Hogge L. Free, esterified, and insoluble-bound phenolic acids. 3. Composition of phenolic acids in cereal and potato flours. J Agric Food Chem. 1982;30:337–340. [Google Scholar]
- 14.Shahidi F. Naczk M. Cereals, legumes, nuts. In: Shahidi F, editor; Naczk M, editor. Phenolic in Food and Nutraceuticals. CRC Press; Boca Raton, FL: 2003. pp. 17–83. [Google Scholar]
- 15.Ranilla LG. Apostolidis E. Genovese MI. Lajolo FM. Shetty K. Evaluation of indigenous grains from the Peruvian Andean region for antidiabetes and antihypertension potential using in vitro methods. J Med Food. 2009;12:704–713. doi: 10.1089/jmf.2008.0122. [DOI] [PubMed] [Google Scholar]
- 16.Lee CH. Garcia HS. Parkin KL. Bioactivities of kernel extracts of 18 strains of maize (Zea mays) J Food Sci. 2010;75:C667–C672. doi: 10.1111/j.1750-3841.2010.01784.x. [DOI] [PubMed] [Google Scholar]
- 17.Rent M. Antioxidant defences: endogenous, diet derived. In: Halliwell B, editor; Gutteridge J, editor. Free Radicals in Biology and Medicine. Oxford University Press; New York: 2007. pp. 79–185. [Google Scholar]
- 18.Shetty K. Role of proline-linked pentose phosphate pathway in biosynthesis of plant phenolics for functional food and environmental applications: a review. Process Biochem. 2004;39:789–803. [Google Scholar]
- 19.Singleton VL. Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- 20.Miliauskas G. Venskutonis PR. van Beek TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–237. [Google Scholar]
- 21.Djeridane A. Yousfi M. Nadjemi B. Maamri S. Djireb F. Stocker P. Phenolic extracts from various Algerian plants as strong inhibitors of porcine liver carboxylesterase. J Enzyme Inhib Med Chem. 2006;21:719–726. doi: 10.1080/14756360600810399. [DOI] [PubMed] [Google Scholar]
- 22.Arnous A. Makris DP. Kefalas P. Effect of principal polyphenolic components in relation to antioxidant characteristics of aged red wines. J Agric Food Chem. 2001;49:5736–5742. doi: 10.1021/jf010827s. [DOI] [PubMed] [Google Scholar]
- 23.Giusti MM. Wrolstad RE. Anthocyanins characterization, measurement with UV-visible spectroscopy. In: Wrolstad RE, editor. Current Protocols in Food Analytical Chemistry. John Wiley & Sons; New York: 2001. pp. 1–13. [Google Scholar]
- 24.de Campos LM. Leimann FV. Pedrosa RC. Ferreira SR. Free radical scavenging of grape pomace extracts from Cabernet Sauvingnon (Vitis vinifera) Bioresour Technol. 2008;99:8413–8420. doi: 10.1016/j.biortech.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 25.van Overveld FW. Haenen GR. Rhemrev J. Vermeiden JP. Bast A. Tyrosine as important contributor to the antioxidant capacity of seminal plasma. Chem Biol Interact. 2000;127:151–161. doi: 10.1016/s0009-2797(00)00179-4. [DOI] [PubMed] [Google Scholar]
- 26.Thaipong K. Boonprakob U. Crosby K. Cisneros-Zevallos L. Byrne DH. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compos Anal. 2006;19:669–675. [Google Scholar]
- 27.Benzie IF. Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 28.Sandoval M. Okuhama NN. Zhang XJ, et al. Anti-inflammatory and antioxidant activities of cat's claw (Uncaria tomentosa and Uncaria guianensis) are independent of their alkaloid content. Phytomedicine. 2002;9:325–337. doi: 10.1078/0944-7113-00117. [DOI] [PubMed] [Google Scholar]
- 29.Ciudad BC. Valenzuela BJ. Contenido de flavonoles en uvas para vino cultivadas en el valle de Casablanca, Chile. Agricultura Técnica. 2002;62:79–86. [Google Scholar]
- 30.Munoz AM. Ramos-Escudero DF. Alvarado-Ortiz C. Castaneda B. Evaluacion de la capacidad antioxidante y contenido de compuestos fenolicos en recursos vegetales promisorios. Rev Soc Quim Peru. 2007;73:142–149. [Google Scholar]
- 31.Zavaleta J. Muñoz AM. Blanco T. Alvarado C. Loja B. Capacidad antioxidante y principales ácidos fenólicos y flavonoides de algunos alimentos. Rev Horizonte Médico. 2005;2:29–38. [Google Scholar]
- 32.Laloue H. Weber-Lotfi F. Lucau-Danila A. Guillemaut P. Identification of ascorbate and guaiacol peroxidases in needle chloroplasts of spruce trees. Plant Physiol Biochem. 1997;35:341–346. [Google Scholar]
- 33.Marklund S. Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 34.Beers RF., Jr Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- 35.Tamagnone L. Merida A. Stacey N, et al. Inhibition of phenolic acid metabolism results in precocious cell death and altered cell morphology in leaves of transgenic tobacco plants. Plant Cell. 1998;10:1801–1816. doi: 10.1105/tpc.10.11.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuskoski EM. Vega JM. Rios JJ. Fett R. Troncoso AM. Asuero AG. Characterization of anthocyanins from the fruits of baguacu (Eugenia umbelliflora Berg) J Agric Food Chem. 2003;51:5450–5454. doi: 10.1021/jf030014z. [DOI] [PubMed] [Google Scholar]
- 37.Sellappan S. Akoh CC. Krewer G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J Agric Food Chem. 2002;50:2432–2438. doi: 10.1021/jf011097r. [DOI] [PubMed] [Google Scholar]
- 38.Andersen OM. Chromatographic separation of anthocyanins in cowberry (lingonberry) Vaccinium vites-idea L. J Food Sci. 1985;50:1230–1232. [Google Scholar]
- 39.Benvenuti S. Pellati F. Melegari M. Bertelli D. Polyphenols, anthocyanins, ascorbic acid, and radical scavenging activity of Rubus, Ribes, and Aronia. J Food Sci. 2004;69:164–169. [Google Scholar]
- 40.Zheng W. Wang SY. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J Agric Food Chem. 2003;51:502–509. doi: 10.1021/jf020728u. [DOI] [PubMed] [Google Scholar]
- 41.Canadanovic-Brunet JM. Djilas SM. Cetkovic GS. Tumbas VT. Mandic AI. Canadanovic VM. Antioxidant activities of different Teucrium montanum L. extracts. Int J Food Sci Technol. 2006;41:667–673. [Google Scholar]
- 42.Lapidot T. Harel S. Akiri B. Granit R. Kanner J. pH-dependent forms of red wine anthocyanins as antioxidants. J Agric Food Chem. 1999;47:67–70. doi: 10.1021/jf980704g. [DOI] [PubMed] [Google Scholar]
- 43.Asuero AG. Sayago A. Gonzalez AG. The correlation coefficient: an overview. Crit Rev Anal Chem. 2006;36:41–59. [Google Scholar]
- 44.Gonzalez AG. Herrador MA. Asuero AG. Sayago A. The correlation coefficient attacks again. Accred Qual Assur. 2006;11:256–258. [Google Scholar]
- 45.Gil MI. Tomas-Barberan FA. Hess-Pierce B. Kader AA. Antioxidant capacities, phenolic compounds, carotenoids, and vitamin C contents of nectarine, peach, and plum cultivars from California. J Agric Food Chem. 2002;50:4976–4982. doi: 10.1021/jf020136b. [DOI] [PubMed] [Google Scholar]
- 46.Gardner PT. White TAC. McPhail DB. Duthie GG. The relative contributions of vitamin C, carotenoids and phenolics to the antioxidant potential of fruit juices. Food Chem. 2000;68:471–474. [Google Scholar]
- 47.Yang Z. Zhai W. Identification and antioxidant activity of anthocyanins extracted from the seed and cob of purple corn (Zea mays L.) Innov Food Sci Emerg Technol. 2010;11:169–176. [Google Scholar]
- 48.Aoki H. Kuze N. Kato Y. Anthocyanins isolated from purple corn (Zea mays L.) Foods Food Ingred J Jpn. 2002;199:41–45. [Google Scholar]
- 49.Lau FC. Shukitt-Hale B. Joseph JA. The beneficial effects of fruit polyphenols on brain aging. Neurobiol Aging. 2005;26(Suppl 1):128–132. doi: 10.1016/j.neurobiolaging.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 50.Rodrigo R. Bosco C. Oxidative stress and protective effects of polyphenols: comparative studies in human and rodent kidney. A review. Comp Biochem Physiol C Toxicol Pharmacol. 2006;142:317–327. doi: 10.1016/j.cbpc.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 51.Rossi L. Mazzitelli S. Arciello M. Capo CR. Rotilio G. Benefits from dietary polyphenols for brain aging and Alzheimer's disease. Neurochem Res. 2008;33:2390–2400. doi: 10.1007/s11064-008-9696-7. [DOI] [PubMed] [Google Scholar]
- 52.Vattem DA. Randhir A. Shetty K. Cranberry phenolics-mediated antioxidant enzyme response in oxidatively stressed porcine muscle. Process Biochem. 2005;40:2225–2238. [Google Scholar]
- 53.Moore K. Roberts LJ., 2nd Measurement of lipid peroxidation. Free Radic Res. 1998;28:659–671. doi: 10.3109/10715769809065821. [DOI] [PubMed] [Google Scholar]
- 54.Nielsen F. Mikkelsen BB. Nielsen JB. Andersen HR. Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem. 1997;43:1209–1214. [PubMed] [Google Scholar]
- 55.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]


