Abstract
The boundary between ice and basalt on Earth is an analogue for some near-surface environments of Mars. We investigated neutrophilic iron-oxidizing microorganisms from the basalt-ice interface in a lava tube from the Oregon Cascades with perennial ice. One of the isolates (Pseudomonas sp. HerB) can use ferrous iron Fe(II) from the igneous mineral olivine as an electron donor and O2 as an electron acceptor. The optimum growth temperature is ∼12–14°C, but growth also occurs at 5°C. Bicarbonate is a facultative source of carbon. Growth of Pseudomonas sp. HerB as a chemolithotrophic iron oxidizer with olivine as the source of energy is favored in low O2 conditions (e.g., 1.6% O2). Most likely, microbial oxidation of olivine near pH 7 requires low O2 to offset the abiotic oxidation of iron. The metabolic capabilities of this bacterium would allow it to live in near-surface, icy, volcanic environments of Mars in the present or recent geological past and make this type of physiology a prime candidate in the search for life on Mars. Key Words: Extremophiles—Mars—Olivine—Iron-oxidizing bacteria—Redox. Astrobiology 12, 9–18.
1. Introduction
The present-day temperature of Mars' surface is mostly below the freezing point of water, the thin atmosphere leaves the surface exposed to UV radiation, and the absence of a magnetic field exposes the surface to ionizing radiation. Because of inhospitable conditions, primary production through photosynthesis is assumed not to occur. Yet the shallow subsurface of the Red Planet, where temperatures are above freezing, could harbor chemolithoautotrophic microorganisms. In the recent geological past, Mars' surface could have been above freezing because of residual geothermal heat, orbital forcing, or greenhouse gas effects (Carr, 1995; Fogg, 1996; Abramov and Kring, 2005). Liquid water could have existed on Mars over much of the planet's history, and it may still exist at the rock-ice interface, in rocks and soil as a result of impact events, and in brines (Travis et al., 2003; Clifford et al., 2010; Fairén, 2010; Samarkin et al., 2010). Much of Mars' surface is composed of igneous rocks similar to basalt on Earth (Bandfield et al., 2000; Edwards et al., 2008). As in terrestrial basalts, a prominent component is Fe(II), which is present in the minerals olivine and pyroxene and in glass (Hoefen et al., 2003; Edwards et al., 2008).
The Mars-like terrestrial habitat that we have focused on in this study is the rock-ice interface from lava tube caves, which occur frequently in basalt flows. In this type of habitat on Mars, a film of liquid water can exist at the rock's surface, where life would be protected from intense solar irradiation. Yet because it is exposed to the atmosphere, this habitat also has the benefit of an abundant source of energy in the form of redox disequilibrium between the oxidized surface of Mars and Fe(II)-bearing minerals such as olivine and pyroxene. Although iron oxidation can also occur by phototrophy, the most common process to extract energy from Fe(II) minerals on Earth is with oxidants such as dioxygen (O2) and nitrate (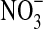 ) (Widdel et al., 1993; Kappler and Newman, 2004; Schippers et al., 2005; Miot et al., 2009; Newman, 2010). On Mars, electron acceptors for Fe(II) may include putative superoxides and
) (Widdel et al., 1993; Kappler and Newman, 2004; Schippers et al., 2005; Miot et al., 2009; Newman, 2010). On Mars, electron acceptors for Fe(II) may include putative superoxides and 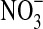 from rock surfaces and atmospheric O2 (∼8–13 μbar).
from rock surfaces and atmospheric O2 (∼8–13 μbar).
Microbes can influence (trigger or limit) the dissolution of olivine, pyroxene, or basalt (Santelli et al., 2001; Welch and Banfield, 2002; Benzerara et al., 2004; Josef et al., 2007; Wu et al., 2007). Weathering features and chemical signatures that are indicative of life were reported in olivine from Earth, and similar features were also observed in martian meteorites (Fisk et al., 2006). We proposed that some of these features are produced by neutrophilic iron-oxidizing (nFeO) microorganisms that use Fe(II) from olivine (Fisk et al., 2006). Neutrophilic iron-oxidizing bacteria (nFeOB) are common in freshwater ecosystems (Straub et al., 1996, 2004) and marine basalts (Stevens, 1997; Emerson and Moyer, 2002; Edwards et al., 2003a, 2003b; Lehman et al., 2004; Bailey et al., 2009). The most recognized phylotypes belong to the genera Gallionella, Lepthotrix, Sideroxydans, Marinobacter, Mariprofundus, and Sphaerotilus. Although best studied in bacteria, this physiotype is also present in some archaea such as Ferroglobus placidus (Hafenbradl et al., 1996). Recently, a diverse collection of α-, γ-, and ζ-proteobacteria were found that are capable of such activity, although they are not closely related to any previously known nFeO microorganisms (Edwards et al., 2004; Emerson and Floyd, 2005; Duckworth et al., 2009; Wang et al., 2009). Even phylogroups that are dominated by heterotrophic species, such as Pseudomonas or Acidovorax, contain strains that are facultatively or even obligate nFeO microorganisms (Kappler et al., 2005; Bailey et al., 2009). In a recent paper, we reported that bacteria from a basalt subseafloor habitat (Juan de Fuca Ridge) preferentially colonize olivine above all other igneous minerals and that many heterotrophic oligotrophic isolates colonizing basalt minerals and glass are facultative nFeO microorganisms (Smith et al., 2011). The presence of olivine in basalts led us to suspect that nFeO microorganisms play an important role in the ecology and biogeochemical cycles of basalt-hosted subsurface ecosystems.
Olivine ((Mg,Fe)2SiO4) is a class of minerals that has a variable iron-to-magnesium ratio. The abundance of iron relative to magnesium ([Fe/(Fe+Mg)]·100) ranges from 0% Fe(II) in forsterite to 100% Fe(II) in fayalite. Most commonly, olivine contains about 10% Fe(II). Although olivine only contains iron in reduced form (Fe(II)), no strain of nFeO microorganism has ever been reported to have the capacity to use this mineral as a source of energy. Such a finding would be invaluable for the study of olivine bioweathering; the identification of biosignatures and microfossils; determination of whether life and associated microhabitats were, or are, in existence on Mars; and the search for extraterrestrial life. Here, we report that olivine-oxidizing nFeO bacteria (nFeOB) are present in basalt in cold, near-surface, aphotic environments such as caves (especially lava tubes) with permanent ice, and we show the olivine-dependent growth characteristics of one such isolate. The similarity of this environment to environments on Mars suggests that nFeO microorganisms that live at the basalt-ice interface could survive on Mars, or may have thrived on Mars in the past when the temperature, atmospheric pressure, and (possibly) the O2 partial pressure (PO2) were higher than they are today and appropriate for olivine-dependent growth of nFeO microorganisms.
2. Materials and Methods
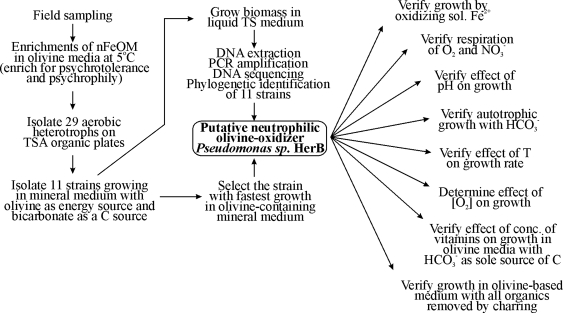
We collected ice and rock fragments from the rock-ice interface in South Ice Cave in the Oregon Cascades (Lat. 43°34′59″N, Long. 121°04′38″W). South Ice Cave, a basalt lava tube at an elevation of 1530 m, is the result of an eruption on the southern flank of Newberry Caldera and contains permanent ice. This basalt flow contains ∼9.0% iron as FeO, and its mineralogy is primarily plagioclase feldspar, pyroxene, and olivine (personal communication, Julie Donnelly-Nolan). The rock/ice samples were stored in sterile bags and packed on ice for transportation to the lab. Culture media were inoculated with melted ice and rock fragments within 2 days of collection. Our overall strategy for isolation of a microorganism capable of olivine-dependent growth is summarized in the flow chart from Fig. 1.
FIG. 1.
Flow chart of activities used to isolate, identify, select, and characterize a neutrophilic iron-oxidizing microorganism (nFeOM) that is a facultative organotroph, psychrotolerant or psychrophilic, using O2 as an electron acceptor, and capable of growing with bicarbonate as a sole source of carbon and olivine-iron(II) as a sole source of energy. The strain selected for this study is Pseudomonas sp. HerB.
For enrichments, we used test tubes with 5 mL sterile 0.2 micron-filtered cave water and olivine sand with 9% Fe/(Fe+Mg). The enrichments were incubated at 5°C for about 4 weeks to favor the growth of nFeO microorganisms that are also psychrophilic or psychrotolerant. The enrichments were inoculated by streaking on tryptone soy agar (TSA) organotrophic oligotrophic plates. Colonies that exhibited differing morphologies were selected, saved in a library, and preserved at −80°C in 50% glycerol.
The mineral medium used for culturing isolates contained per liter 1 mL trace mineral solution, 1 mL vitamin mix, 30 mmol phosphate buffer (pH 7), 20 mmol bicarbonate, 30 mmol nitrate, and 100 g olivine. The trace mineral solution contained 6.72 mM Na2EDTA, 5.6 mM H3BO3, 1 mM NaCl, 0.54 mM FeSO4, 0.5 mM CoCl2, 0.5 mM NiSO4, 0.39 mM Na2MoO4, 0.15 mM NaSeO4, 0.13 mM MnCl2, 0.13 mM ZnCl2, and 0.02 mM CuCl2. The vitamin mix contained per milliliter 5 μg p-aminobenzoic acid, 5 μg biotin, 5 μg cyanocobalamin, 5 μg folic acid, 100 μg i-inositol, 100 μg nicotinic acid, 100 μg pyridoxine, 100 μg panthotenic acid, 100 μg riboflavin, and 1 μg thiamine. The vitamin mix was added filter-sterilized after autoclavation. All chemicals were reagent grade. Sand (0.2–0.8 mm grain size) composed of 100% olivine (Fo91) that contained 8 wt % FeO was provided by Unimin Corporation. Tumbled olivine (∼Fo90 beads 1–3 mm in size, ∼24 grains/g) were obtained from a local supplier of minerals and gems. For most experiments (including enrichments), the olivine was washed with dH2O and autoclaved in the culture medium. Throughout this work, we used un-inoculated controls and controls that represent media with and without various chemical modifications. The controls for each experiment (whenever applicable) are explained in the Results section.
In some experiments, organic-free olivine was used; this was obtained by heating olivine in a furnace at 500°C for 90 min in air. After cooling, the olivine showed evidence of surface oxidation (uneven small patches with a yellow-rusty appearance). Part of the iron oxides were removed by acid dissolution in three 24 h long washes with occasional stirring at room temperature. The acid washing solution contained 0.25 ml/L H2SO4 and 20 mM Na2SO4, pH ∼2.5, and was used in a proportion of 100 mL solution to about 10 g olivine. We compared the UV spectra of the various washes with the spectra of control unheated olivine and calculated the concentration of Fe(III) relative to a standard. Under these conditions, ferric iron absorbs strongly in the 295–304 nm range, while ferrous iron absorbs mostly in the 220–250 nm range (Steiner and Lazaroff, 1974). In this method, 304 nm peaks in solutions containing Fe(II) are used as evidence of Fe(III). This method allows detection of concentrations of Fe(III) as low as 20 μM even in the presence of high concentrations of Fe(II), because the absorbance of Fe(III) at 304 nm is ∼300 times larger than that of Fe(II). After the acid treatment, the olivine was washed with dH2O and dried in a 55°C oven. Macroscopically, the heated olivine retained a yellow-green appearance with pink-rusty patches. Under a dissecting scope, most oven-heated and acid-washed olivine sand particles appeared transparent and colorless, while the similarly treated olivine beads appeared pale green and translucent.
For phylogenetic identification, we obtained biomass by growing cells in liquid tryptone soy broth (TSB) medium in aerobic conditions. Cells were separated by centrifugation (14,000 rpm, 2°C, 5 min), and genomic DNA was extracted with a Qiagen genomic tip kit and quantified with a NanoDrop1000 instrument. A fragment of the SSU rRNA gene was amplified by PCR with the primers 8F (5′-AGAGTTTGATCCTGGCTCAG) and 1492R (5′-GGTTACCTTGTTACGACTT) (Baker et al., 2003). We used 20 μL PCR volumes containing 10 μL Fermentas mix, 0.8 μL of each μM primer, 6.5 μL of dH2O, and 2 μL of 100 ng/μL genomic DNA. The PCR conditions were as follows: denaturing at 95°C for 5 min, 40 cycles of 94°C for 30 s, 50°C for 30 s, and 72°C for 2 min, and final extension at 72°C for 7 min. The size of the PCR products was verified by 0.7% agarose electrophoresis, and the remaining 15 μL PCR product was cleaned with an UltraClean PCR DNA purification kit (MoBio). The amplicons were sequenced at the DNA Sequencing Core facility of Oregon Health and Sciences University with three primers: 8F, 515F (5′-GTGCCAGCMGCCGCGGTAA), and 1492R (Baker et al., 2003) by capillary electrophoresis on an ABI 3130xl instrument. Duplicate sequences were manually aligned, and when differences between duplicates were found, we repeated the PCR and sequencing to compare triplicates for each sequence. The sequences of each isolate were assembled into contiguous DNA fragments and blasted in the Ribosomal Database for phylogenetic identification. Sequences were imported in MEGA 4 (Tamura et al., 2007) and aligned versus phylogenetic relatives. The evolutionary history was inferred by using the neighbor-joining method (Saitou and Nei, 1987). The evolutionary distances (base substitutions per site) were computed by using the maximum composite likelihood method (Tamura et al., 2004).
To characterize the O2 preference of the isolates, we inoculated tryptone soy semisolid agar gradient tubes containing 0.15% agar and 2 mg/L resazurin. The capacity of the isolates to grow as microaerophilic iron oxidizers was verified in gradient tubes with semisolid medium (0.15% agar) and 2% agar plug containing 36 mM FeCO3 or olivine sand as Fe(II) sources (modified after Emerson and Moyer, 1997; Emerson and Floyd, 2005). When growth was seen in a gradient tube, we repeated the inoculation a couple of times from tubes with growth into fresh tubes by using a stabbing needle. Because some cells may grow in FeCO3 gradient tubes by using the agar or agar contaminants as energy sources, when necessary, growth by neutrophilic iron oxidation was also verified in liquid mineral medium with 5 mM soluble ferrous sulfate, at pH 7 and under 1.6% O2. When testing for nitrate reduction capabilities, we used a medium with 10 mM 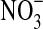 at pH 7 (DIFCO Catalog #226810) in culture tubes containing an inverted Durham tube to capture N2 gas that may have been produced by denitrification. After 5 days of incubation, the cultures were examined for evidence of denitrification and tested for nitrate and nitrite reduction (Leboffe and Pierce, 2010). To verify growth by olivine oxidation, we incubated cells in test tubes with mineral medium with or without olivine.
at pH 7 (DIFCO Catalog #226810) in culture tubes containing an inverted Durham tube to capture N2 gas that may have been produced by denitrification. After 5 days of incubation, the cultures were examined for evidence of denitrification and tested for nitrate and nitrite reduction (Leboffe and Pierce, 2010). To verify growth by olivine oxidation, we incubated cells in test tubes with mineral medium with or without olivine.
Growth on TSB medium was monitored by spectrophotometry (Abs600) and microscopy. Media with olivine sand contain suspended mineral particles, which make spectrophotometry readings difficult to interpret. Therefore, growth on olivine-containing media was determined only by microscopy. To study the effect of temperature on growth, we analyzed growth at 2°C, 5°C, 10°C, 15°C, 25°C, 30°C, 37°C, and 40°C. To test for autotrophic growth in olivine-containing mineral media, we incubated cells with various concentrations of 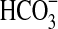 as the sole source of carbon. Incubations in liquids under microaerophilic conditions were done in serum bottles sealed with a 1 cm thick butyl stopper and purged prior to autoclaving with dinitrogen gas containing 1.6% O2. The O2 concentration in the headspace was measured by gas chromatography (SRI 310C instrument, molecular sieve column and thermal conductivity detector). The gas pressure was measured with an Omega pressure meter (Omega Engineering, Inc., CT). The concentration of O2 in the liquid phase was calculated knowing that in equilibrium with air at 760 mmHg and at 30°C freshwater contains 236 μM O2.
as the sole source of carbon. Incubations in liquids under microaerophilic conditions were done in serum bottles sealed with a 1 cm thick butyl stopper and purged prior to autoclaving with dinitrogen gas containing 1.6% O2. The O2 concentration in the headspace was measured by gas chromatography (SRI 310C instrument, molecular sieve column and thermal conductivity detector). The gas pressure was measured with an Omega pressure meter (Omega Engineering, Inc., CT). The concentration of O2 in the liquid phase was calculated knowing that in equilibrium with air at 760 mmHg and at 30°C freshwater contains 236 μM O2.
We also verified whether the growth of one of the olivine-using isolates (Pseudomonas sp. HerB) may be explained by organic contaminants present on olivine surfaces. In this experiment, we used 5 mL liquid mineral medium (composition shown above) in Hungate tubes, with 20 mM 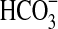 , 0 mM nitrate (as Pseudomonas sp. HerB does not reduce nitrate), pH 7, ∼1 g olivine per tube, sealed and crimped, purged with 1.6% O2, and autoclaved. A volume of diluted vitamin mixture solution was injected filter-sterilized after autoclavation to a proportion of 1 mL/L. We inoculated washed cell pellets, from serial dilutions into tubes containing heat-treated versus non-heat-treated olivine and sand versus beads, as well as medium without olivine.
, 0 mM nitrate (as Pseudomonas sp. HerB does not reduce nitrate), pH 7, ∼1 g olivine per tube, sealed and crimped, purged with 1.6% O2, and autoclaved. A volume of diluted vitamin mixture solution was injected filter-sterilized after autoclavation to a proportion of 1 mL/L. We inoculated washed cell pellets, from serial dilutions into tubes containing heat-treated versus non-heat-treated olivine and sand versus beads, as well as medium without olivine.
3. Results
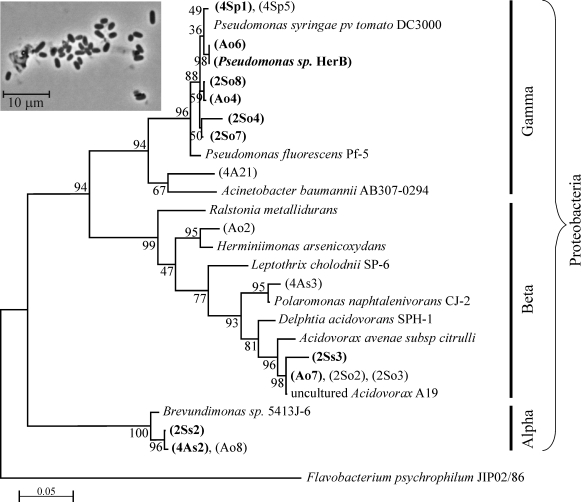
Of 29 aerobic heterotrophs that we isolated from South Ice Cave, 11 strains showed growth in a mineral medium with olivine as the source of energy (Fig. 2). In these incubations, vitamins were also present, and the olivine had not been heated to remove traces of organics. Hence, alternative physiologies could not be excluded (e.g., oligotrophic organotrophs using vitamins or traces of organics from olivine surfaces). Seven of these strains were mesophilic γ-proteobacteria from the genus Pseudomonas, two strains were cryophilic Brevundimonas (α-proteobacteria), and two strains (also cryophilic) belong with Acidovorax (β-proteobacteria) (Fig. 2). One of these strains, Pseudomonas sp. HerB, was selected for further work because among all isolates it reached the highest density while growing in a mineral medium with 10% w:v olivine sand, 20 mM 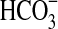 , 1 mL vitamin mix per liter, pH 7, and 30°C. Starting from ∼103 cells/mL, this strain reached ∼5·107 cells/mL in one week.
, 1 mL vitamin mix per liter, pH 7, and 30°C. Starting from ∼103 cells/mL, this strain reached ∼5·107 cells/mL in one week.
FIG. 2.
Tree indicating the phylogenetic position of 29 aerobic heterotrophic strains, in parentheses, isolated from South Ice Cave. Eleven of these strains, in bold font (seven Pseudomonas, two Acidovorax, and two Brevundimonas) also showed growth when incubated in a mineral medium with olivine as the sole source of reducing power. The sequences of these 11 isolates were submitted to GenBank under the accession numbers JN399075 through JN399085. The evolutionary history was inferred based on partial 16S rRNA gene sequences using neighbor-joining analysis. Bootstrap percentages above 50%, based on 500 replicates (Felsenstein, 1985) are shown next to the branches. Bar, 5 substitutions per 100 nucleotides. The insert image shows cells of Pseudomonas sp. HerB grown in R2A medium seen by phase contrast optical microscopy at 1000×. The same cell shape and size was seen when HerB cells grew in FeCO3 gradient tubes and in olivine-containing liquid mineral medium.
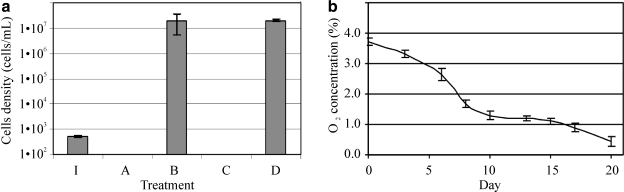
Cells of Pseudomonas sp. HerB are aerobic under heterotrophic conditions. While on TSA plates and TSB tubes, growth was faster in air than in a 1.6% O2 atmosphere. However, in mineral media with olivine sand as the source of energy and at pH 7, growth was very slow at 21% O2, better at ∼5% O2, and best at ∼1.6% O2, where cultures reached densities of ∼3·107 cells/mL after 7 days of incubation. Figure 3a shows the effect of olivine on the growth of Pseudomonas sp. HerB in mineral medium (with/without olivine, and with/without 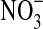 ). No growth occurred when olivine was absent, but in the presence of olivine the culture reached 2.5·107 cells/mL without
). No growth occurred when olivine was absent, but in the presence of olivine the culture reached 2.5·107 cells/mL without 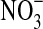 and 3.7·107 cells/mL with
and 3.7·107 cells/mL with 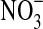 . The difference between with/without
. The difference between with/without 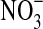 was within one standard deviation (SD) (based on triplicates); thus significant statistical difference between these treatments could not be confirmed. Verifying nitrate-reducing capabilities by using the protocol shown in Smith et al. (2011), we found that Pseudomonas sp. HerB was not capable of such activity (results not shown). Regarding O2 consumption, 10 mL culture of Pseudomonas sp. HerB used ∼180 μmol O2 in 20 days (Fig. 3b).
was within one standard deviation (SD) (based on triplicates); thus significant statistical difference between these treatments could not be confirmed. Verifying nitrate-reducing capabilities by using the protocol shown in Smith et al. (2011), we found that Pseudomonas sp. HerB was not capable of such activity (results not shown). Regarding O2 consumption, 10 mL culture of Pseudomonas sp. HerB used ∼180 μmol O2 in 20 days (Fig. 3b).
FIG. 3.
(a) The growth of Pseudomonas sp. HerB with olivine as a source of energy. Incubation in Hungate tubes with 10 mL medium, 20 mM 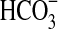 , 1 mL/L vitamin mix, pH 7, 1.6% O2 at 30°C. In this experiment we compared growth with/without 10% w:v olivine sand and with/without 10 mM
, 1 mL/L vitamin mix, pH 7, 1.6% O2 at 30°C. In this experiment we compared growth with/without 10% w:v olivine sand and with/without 10 mM 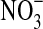 . I=Initial cell density (∼7.7·102 cells/mL). A=No olivine, no
. I=Initial cell density (∼7.7·102 cells/mL). A=No olivine, no 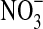 . B=with olivine, no
. B=with olivine, no 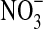 . C=No olivine, with
. C=No olivine, with 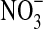 . D=with olivine, with
. D=with olivine, with 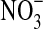 . Cell counts are based on triplicates and were determined by microscopy after 7 days of incubation. Error bars are 1 SD from triplicates. (b) Evolution of the O2 concentration in gas phase during the growth of Pseudomonas sp. HerB in a mineral medium with olivine (subtracted from an un-inoculated control). Incubations were in 140 mL serum bottles with 10 mL mineral medium, 10% w:v olivine sand, 20 mM
. Cell counts are based on triplicates and were determined by microscopy after 7 days of incubation. Error bars are 1 SD from triplicates. (b) Evolution of the O2 concentration in gas phase during the growth of Pseudomonas sp. HerB in a mineral medium with olivine (subtracted from an un-inoculated control). Incubations were in 140 mL serum bottles with 10 mL mineral medium, 10% w:v olivine sand, 20 mM 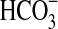 , 1 mL/L vitamin mix, pH 7, and ∼1.1 bar initial pressure at 30°C. The error bars are 1 SD based on triplicate readings of one bottle.
, 1 mL/L vitamin mix, pH 7, and ∼1.1 bar initial pressure at 30°C. The error bars are 1 SD based on triplicate readings of one bottle.
The ability of Pseudomonas sp. HerB to grow as a neutrophilic iron oxidizer was also seen in gradient tubes with iron carbonate and in liquid mineral medium with 5 mM Fe(II) at pH 7 and under 1.6% O2. Growth was also observed during serial inoculation of gradient tubes that contained only inorganic media. We compared growth on a mineral medium with 10% w:v olivine sand relative to growth in the same mineral medium with 5 mM soluble Fe(II) initial concentration, 20 mM 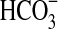 , pH 7, 1.6% O2, incubated at 30°C. The extent of growth was similar for these two media. The cultures containing olivine reached ∼8·106 cells/mL in 10 days.
, pH 7, 1.6% O2, incubated at 30°C. The extent of growth was similar for these two media. The cultures containing olivine reached ∼8·106 cells/mL in 10 days.
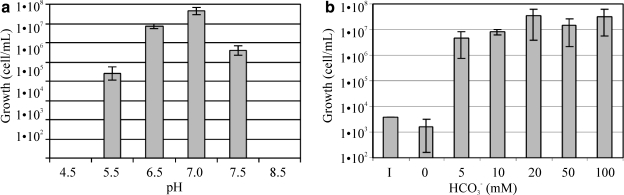
We verified the growth of Pseudomonas sp. HerB in a mineral medium containing olivine at six pH values (Fig. 4a). No growth was observed at pH 4.5 and pH 8.5, and the largest cell density (after 1 week of incubation) was seen at pH 7. Fig. 4b shows the effect of 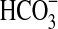 on the growth of Pseudomonas sp. HerB in mineral media containing olivine. No growth was observed without
on the growth of Pseudomonas sp. HerB in mineral media containing olivine. No growth was observed without 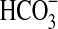 ,∼1·107 cells/mL at 10 mM
,∼1·107 cells/mL at 10 mM 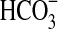 , and little variation in cell density above 10 mM
, and little variation in cell density above 10 mM 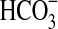 . In this experiment, we also incubated cells in controls without olivine, and (similar to above) no measurable growth was seen.
. In this experiment, we also incubated cells in controls without olivine, and (similar to above) no measurable growth was seen.
FIG. 4.
(a) The growth of Pseudomonas sp. HerB in olivine-containing mineral media at different pH. The media contained 20 mM 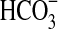 , 10% w:v olivine sand, and 1 mL/L vitamin mix, and incubation occurred under 1.6% O2 at 30°C for 7 days. (b) Growth in the same mineral medium at pH 7 and with various concentrations of
, 10% w:v olivine sand, and 1 mL/L vitamin mix, and incubation occurred under 1.6% O2 at 30°C for 7 days. (b) Growth in the same mineral medium at pH 7 and with various concentrations of 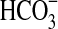 , incubated for 14 days at 20°C. I=Initial cell density (∼3.8·103 cells/mL). The error bars are 1 SD from triplicates.
, incubated for 14 days at 20°C. I=Initial cell density (∼3.8·103 cells/mL). The error bars are 1 SD from triplicates.
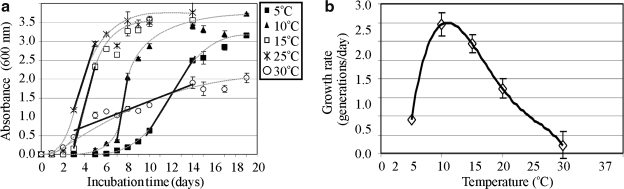
We compared growth rates at different temperatures, using the slopes of the exponential growth phases (Fig. 5a, 5b). We found the following temperatures for olivine growth: ∼4–5°C minimum, ∼12–14°C optimum, and ∼30–31°C maximum (Fig. 5b).
FIG. 5.
(a) Growth profiles of Pseudomonas sp. HerB in TSB medium at five temperatures (5°C; 10°C; 15°C; 25°C, and 30°C). No growth was seen at 2°C, 37°C, and 40°C. The values shown are averages of triplicates, and the errors bars equal 1 SD. The interrupted lines are hand drawn and help observe the general trend of each set of data. The straight lines are linear regression slopes for the data points situated near and opposite sides of the inflexion point of the polynomial fit, in the part of the curve that represents the exponential growth phase. All cultures started from ∼103 cells mL−1 and were incubated in 18 mm diameter test tubes with 10 mL medium. (b) The effect of temperature on the growth rate, calculated based on the slope of the exponential phase shown in (a). The error bars from (b) are 1 SD of the expected variation in the slope of exponential growth in (a) based on±1 SD of cell density.
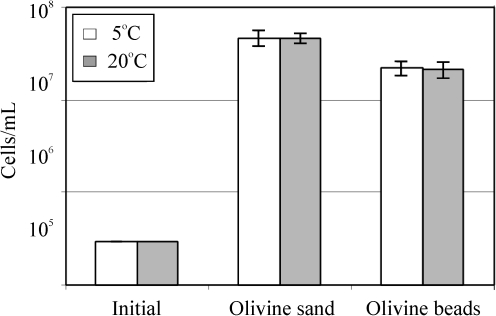
To determine whether the olivine surface is a limiting factor in the growth of Pseudomonas sp. HerB, we compared growth in the presence of olivine sand versus olivine beads. In these experiments, we also compared growth at 5°C versus 20°C (Fig. 6). We expected to find higher cell density with olivine sand than with beads and higher cell density at 20°C relative to 5°C. Olivine beads with 3 mm diameter have about 120 mm2/g, while olivine sand with 0.4 mm particle diameter have about ∼7000 mm2/g, (i.e., about a 55-fold increase in surface:mass ratio). After 7 days of incubation, we found only about a 2-fold increase in cell density on sand (4.6·107 cells/mL) versus beads (2.1·107 cells/mL), and no significant differences in cell density between the 5°C and 20°C treatments.
FIG. 6.
The growth of Pseudomonas sp. HerB in mineral medium with olivine of two particle sizes (olivine sand and olivine beads) and at two temperatures (5°C and 20°C). These two olivine samples are different with regard to size (and thus surface area) but are similar in composition. Incubation occurred for 7 days in Hungate tubes, 5 mL mineral medium, 10% w:v olivine, 20 mM 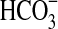 , pH 7, 1.6% O2, and 1 mL/L vitamin mix. The error bars are 1 SD from triplicates.
, pH 7, 1.6% O2, and 1 mL/L vitamin mix. The error bars are 1 SD from triplicates.
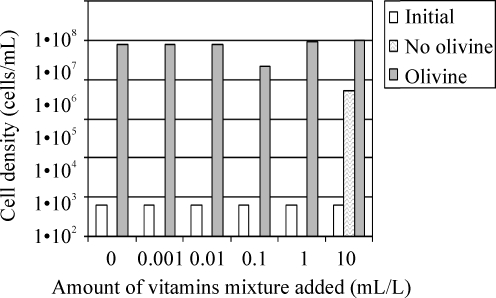
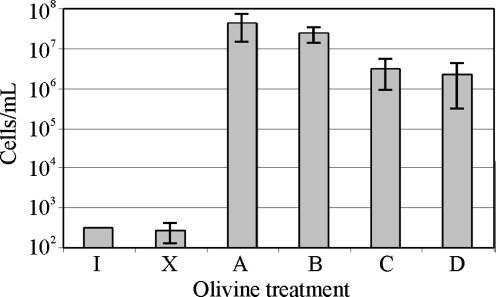
Because pseudomonads can metabolize a wide variety of organic molecules, excess of vitamins in the culture media can represent an additional source of carbon and energy. Fig. 7 shows the growth of Pseudomonas sp. HerB in mineral media with olivine and various concentrations of vitamins. In the olivine-containing media, good growth (>107 cells/mL) was seen in all treatments. In the olivine-absent media, growth was not observed when the vitamin mix was≤1 mL/L. We also found that Pseudomonas sp. HerB grew in olivine freed of organics and that no significant growth differences existed (within±1 SD) between heat-treated and non-heat-treated olivine (Fig. 8).
FIG. 7.
Growth of Pseudomonas sp. HerB in mineral medium (with/without olivine) and various abundances of vitamin mix. Incubation occurred in test tubes with 5 mL mineral medium, 10 mM 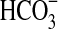 , with/without 20 olivine beads (∼840 mg olivine per tube), and at pH 7. All cultures started from ∼5.3·103 cells/mL. The graph shows cell densities after 7 days at 1.6% O2 and 20°C.
, with/without 20 olivine beads (∼840 mg olivine per tube), and at pH 7. All cultures started from ∼5.3·103 cells/mL. The graph shows cell densities after 7 days at 1.6% O2 and 20°C.
FIG. 8.
The growth of Pseudomonas sp. HerB in mineral medium with olivine sand and olivine beads after removing traces of organics by heating the crystals at 500°C. Incubation occurred for 14 days in Hungate tubes, with 5 mL mineral medium, 10% w:v olivine, 20 mM 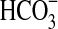 , pH 7, 1 ml/L vitamin mix, and 1.6% O2. The treatments shown in the graph are I=initial cell density; X=no olivine present; A=non-heat-treated olivine sand; B=heat-treated olivine sand; C=non-heat-treated olivine beads; and D=heat-treated olivine beads. The values shown are averages of triplicates, and error bars equal 1 SD.
, pH 7, 1 ml/L vitamin mix, and 1.6% O2. The treatments shown in the graph are I=initial cell density; X=no olivine present; A=non-heat-treated olivine sand; B=heat-treated olivine sand; C=non-heat-treated olivine beads; and D=heat-treated olivine beads. The values shown are averages of triplicates, and error bars equal 1 SD.
4. Discussion
Neutrophilic iron-oxidizing bacteria have been reported to inhabit seawater, freshwater, groundwater, terrestrial basalts, subseafloor basalt, hydrothermal systems, iron oxyhydroxide mats, and the surface of glass and Fe(II)-containing minerals from a wide variety of sources (Emerson and Moyer, 2002; Edwards et al., 2003a, 2003b; Kappler et al., 2005; Gronstal et al., 2009; Miot et al., 2009). Our finding extends the palette of environments where nFeOB exist to the basalt/ice boundary habitat in a lava-tube ice cave and to olivine minerals as a source of energy. The properties of this habitat (near 0°C, dark, oligotrophic, circumneutral pH, and at the interface between basalt and ice near an oxidized atmosphere) makes it a terrestrial analogue for a near-surface aphotic environment on Mars, where life may exist today and could have thrived in the past when the atmospheric pressure and surface temperature of Mars were higher than today.
Of 11 strains of putative nFeOB we isolated, we report the olivine-dependent growth characteristics of one: Pseudomonas sp. HerB. Regarding the source of carbon, this microorganism is a heterotroph and facultative autotroph with the capacity to use CO2 as a sole source of carbon. Regarding the source of energy, it is an organotroph and facultative chemolithotroph, capable of neutrophilic iron oxidation with Fe(II) from olivine as the sole electron donor and O2 as the electron acceptor. The optimum pH for growth by olivine oxidation was ∼7, but we did not verify to what extent this optimum was due to competition with iron autooxidation or to metabolic preference for circumneutral pH. Regarding temperature preference, Pseudomonas sp. HerB is submesophilic. The optimum growth in TSB medium was about 14–15°C, but slow growth was seen at temperatures as low as ∼5°C. Based on the growth profile, we predict that the minimum temperature for growth is ∼4°C. The growth rate versus temperature profile is skewed toward higher temperatures (Fig. 5b), a profile that is difficult to explain with the data at hand. Regarding O2 tolerance, this strain is microaerophilic and a facultative aerobe, and the growth in olivine-containing mineral medium is faster at low O2 concentration (1.6%) than at higher O2 concentrations (5% and 21%). The fact that most of our isolates were from the genus Pseudomonas is not unexpected. Pseudomonads are versatile and show all metabolic capabilities presented above. Some strains of Pseudomonas were shown to be nFeO (Bailey et al., 2009). Pseudomonads are important denitrifiers in soil (Chan et al., 1994; Smil, 2000), albeit Pseudomonas sp. HerB strain is not a denitrifier and cannot reduce nitrate. We did not study the source of nitrogen used by Pseudomonas sp. HerB for assimilation. The pathway for carbon fixation in Pseudomonas sp. HerB is unknown, though some facultative autotrophic pseudomonads have already been shown to fix CO2 by using RuBisCo (Morikawa and Imanaka, 1993; Yuliar, 1997; Mahmood et al., 2009).
The mechanism of dissolution of olivine during the activity of Pseudomonas sp. HerB is unclear. Iron-oxidizing bacteria are known to dissolve a variety of Fe(II)-containing minerals, including pyrite, iron monosulfides, magnetite, siderite, and vivianite at rates usually controlled by the solubility of the phase (Schippers and Jorgensen, 2002; Kappler and Newman, 2004; Miot et al., 2009). Organic ligands such as citrate, oxalate, malonate, gallate, salicylate, and phthalate (some of which are known metabolic by-products of pseudomonads) were shown to dissolve basalt (Neaman et al., 2005). To obtain iron, many bacteria produce siderophores, which also promote mineral dissolution (Buss et al., 2007; Luo and Gu, 2011). Pseudomonads are known to produce a wide diversity of siderophores (Cornelis and Matthijs, 2002), but siderophores transport iron mostly in Fe(III) form (Martínez et al., 2000), while the iron from olivine is valuable as an energy source to Pseudomonas sp. HerB in the Fe(II) form. If a specialized mechanism to extract Fe(II) from olivine crystals does not exist, then the growth of the olivine-using Pseudomonas sp. HerB should be controlled predominantly by the rate of olivine dissolution and by the kinetics of chemical Fe(II) oxidation (which are probably low). The growth of olivine-oxidizing nFeOB is probably favored by low temperature, low O2, and the presence of 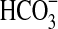 or other iron-binding agents. Low temperature and low O2 decrease the rate of iron oxidation while increasing the availability of soluble Fe(II). The role of low temperatures in controlling the growth of nFeOB was little studied. The fact that nFeOB prefer low O2 conditions is well known; it is due to competition between microbial iron oxidation and iron autooxidation (Edwards et al., 2003b). The pH may also play an important role in the olivine-dependent growth of Pseudomonas sp. HerB. Because final mineral products vary significantly with the pH, the redox potential (Eo) of the Fe3+/Fe2+ couple is pH-dependent, taking more positive values in acidic conditions (Thauer et al., 1977). Iron oxidation is more exergonic at neutral pH than at acidic pH.
or other iron-binding agents. Low temperature and low O2 decrease the rate of iron oxidation while increasing the availability of soluble Fe(II). The role of low temperatures in controlling the growth of nFeOB was little studied. The fact that nFeOB prefer low O2 conditions is well known; it is due to competition between microbial iron oxidation and iron autooxidation (Edwards et al., 2003b). The pH may also play an important role in the olivine-dependent growth of Pseudomonas sp. HerB. Because final mineral products vary significantly with the pH, the redox potential (Eo) of the Fe3+/Fe2+ couple is pH-dependent, taking more positive values in acidic conditions (Thauer et al., 1977). Iron oxidation is more exergonic at neutral pH than at acidic pH.
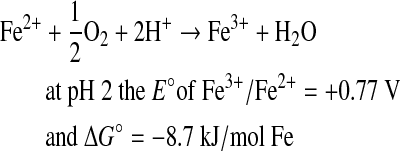 |
(1) |
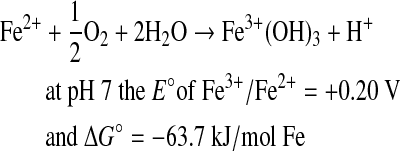 |
(2) |
According to Reaction 2 and the iron content of the olivine we have used, 180 μmol of O2 used by a 10 mL culture of Pseudomonas sp. HerB in 20 days is equivalent to oxidizing the iron from ∼290 mg olivine.
No direct evidence was ever found of olivine-related microbial activity on Mars, and we did not analyze secondary minerals produced by Pseudomonas sp. HerB while growing on olivine. Yet potential martian habitats that contain secondary minerals (such as iddingsite) produced by olivine weathering in the presence of water (Swindle et al., 2000) are important candidates to search for evidence of such microbial activity.
5. Conclusions
We report for the first time that a strain of nFeOB from the genus Pseudomonas is able to grow by using the mineral olivine as a source of energy. We propose that such microbes are common in nature and that microenvironments that support olivine-dependent growth have to satisfy a couple of specific requirements. Some of the most important are circumneutral pH, low  , low temperature, and low organic load. On Earth, such conditions can be encountered at basalt-ice interfaces where liquid water is also present. This finding is important for astrobiology because the environmental conditions in the recent geological past of Mars (higher pressure and temperature than today) would have allowed such microbes to thrive near the surface in lava tubes, under the ice sheet, and in the basalt subsurface where cells are protected from harmful ionizing radiation and UV radiation, yet still benefit from the oxidants of Mars's surface. Orbital and surface observations of Mars have confirmed that igneous rocks are exposed over significant areas (Bandfield et al., 2000; Edwards et al., 2008), and that some martian areas are dominated by olivine-bearing rocks (Hoefen et al., 2003; Edwards et al., 2008). In addition, skylights interpreted as entrances to lava tubes (a physical environment similar to South Ice Cave) have been observed on the flanks of martian volcanoes (Cushing et al., 2007). Subsurface environments and martian caves, which could contain permanent ice (Williams et al., 2010), have been proposed as astrobiological target sites (Boston et al., 1992; Boston, 2010; Northup et al., 2011).
, low temperature, and low organic load. On Earth, such conditions can be encountered at basalt-ice interfaces where liquid water is also present. This finding is important for astrobiology because the environmental conditions in the recent geological past of Mars (higher pressure and temperature than today) would have allowed such microbes to thrive near the surface in lava tubes, under the ice sheet, and in the basalt subsurface where cells are protected from harmful ionizing radiation and UV radiation, yet still benefit from the oxidants of Mars's surface. Orbital and surface observations of Mars have confirmed that igneous rocks are exposed over significant areas (Bandfield et al., 2000; Edwards et al., 2008), and that some martian areas are dominated by olivine-bearing rocks (Hoefen et al., 2003; Edwards et al., 2008). In addition, skylights interpreted as entrances to lava tubes (a physical environment similar to South Ice Cave) have been observed on the flanks of martian volcanoes (Cushing et al., 2007). Subsurface environments and martian caves, which could contain permanent ice (Williams et al., 2010), have been proposed as astrobiological target sites (Boston et al., 1992; Boston, 2010; Northup et al., 2011).
Calculations of autotrophic energy-producing reactions likely to occur on Mars suggest that the oxidation of Fe(II) by O2 or  could drive microbial ecosystems (Jepson et al., 2007). It has been proposed that the
could drive microbial ecosystems (Jepson et al., 2007). It has been proposed that the 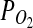 on Mars is sufficient to support microaerophiles (Fisk and Giovannoni, 1999). Applying ΔG=ΔGo′+TRlnQ to Reaction 2 (for T≈0°C), it can be shown that this reaction is exergonic (ΔG=− 4.2 kJ/mol) even at
on Mars is sufficient to support microaerophiles (Fisk and Giovannoni, 1999). Applying ΔG=ΔGo′+TRlnQ to Reaction 2 (for T≈0°C), it can be shown that this reaction is exergonic (ΔG=− 4.2 kJ/mol) even at 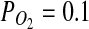 mbar. Notably, the
mbar. Notably, the 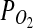 on Mars (derived from ∼7 mbar total pressure and ∼0.13% O2) is ∼10 μbar (Seiff and Kirk, 1977). Therefore, the oxidation of olivine and other iron-bearing silicates by nFeO microorganisms would be possible in the shallow subsurface of Mars where the pressure and temperature are higher than at the surface (e.g., P= 70 mbar).
on Mars (derived from ∼7 mbar total pressure and ∼0.13% O2) is ∼10 μbar (Seiff and Kirk, 1977). Therefore, the oxidation of olivine and other iron-bearing silicates by nFeO microorganisms would be possible in the shallow subsurface of Mars where the pressure and temperature are higher than at the surface (e.g., P= 70 mbar).
A key requirement for Earth-colonizing cellular life (including nFeOB) is the presence of liquid water. Even the present-day subsurface and the sub-ice conditions on Mars may harbor such microbes because thin films of water exist in soil, even below freezing (Anderson and Tice, 1973). Low-temperature brines (maintaining liquid water at temperatures as low as −20°C) could have existed over much of Mars's history (Fairén, 2010). Multiple lines of evidence indicate that Mars had liquid water at the surface in the past (Carr, 1995; Head et al., 2003; Carr and Head, 2010; Warner et al., 2010). Thus, some areas of the shallow subsurface of past Mars satisfy two requirements for nFeO-based cellular life: liquid water and redox energy in the form of olivine Fe(II) in disequilibrium with oxidized chemicals from the planet's surface. In the event of increases in temperature and pressure on the surface of Mars (such as during terraformation activities, orbital forcing, or release of greenhouse gas from buried hydrates), olivine-using nFeO microorganisms would be some of the first colonists and important primary producers of the newly formed martian ecosystems.
Acknowledgments
Funding for this research was provided by NASA Astrobiology grant NNX08AO22G NCE and The Cave Research Foundation. Unimin provided the olivine sand. We thank Gus Frederick for assistance with the fieldwork. Julie Donnelly-Nolan provided the chemical and petrographic information for the South Ice Cave lava flow. We also want to thank the four anonymous reviewers of this manuscript for helpful suggestions.
Abbreviations
nFeO, neutrophilic iron-oxidizing; nFeOB, neutrophilic iron-oxidizing bacteria; SD, standard deviation; TSA, tryptone soy agar; TSB, tryptone soy broth.
References
- Abramov O. Kring D.A. Impact-induced hydrothermal activity on early Mars. J Geophys Res. 2005;110 doi: 10.1029/2005JE002453. [DOI] [Google Scholar]
- Anderson D.M. Tice A.R. The unfrozen interfacial phase in frozen soil water systems. In: Hadas A., editor; Swartzendruber D., editor; Rijtema P.E., editor; Fuchs M., editor; Yaron B., editor. Ecological Studies. Analysis and Synthesis. Vol. 4. Springer-Verlag; New York: 1973. pp. 107–124. [Google Scholar]
- Bandfield J.L. Hamilton V.E. Christensen P.R. A global view of martian surface compositions from MGS-TES. Science. 2000;287:1626–1630. [Google Scholar]
- Bailey B. Templeton A. Staudigel H. Tebo B. Utilization of substrate components during basaltic glass colonization by Pseudomonas and Shewanella isolates. Geomicrobiol J. 2009;26:648–656. [Google Scholar]
- Baker G.C. Smith J.J. Cowan D.A. Review and re-analysis of domain-specific 16S primers. J Microbiol Methods. 2003;55:541–555. doi: 10.1016/j.mimet.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Benzerara K. Barakat M. Menguy N. Guyot F. De Luca G. Audrain C. Heulin T. Experimental colonization and alteration of orthopyroxene by the pleomorphic bacteria Ramlibacter tataouinensis. Geomicrobiol J. 2004;21:341–349. [Google Scholar]
- Boston P.J. Location, location, location! Lava caves on Mars for habitat, resources, and the search for life. The Journal of Cosmology. 2010;12:3957–3979. [Google Scholar]
- Boston P.J. Ivanov M. McKay C.P. On the possibility of chemosynthetic ecosystems in subsurface habitats on Mars. Icarus. 1992;95:300–308. doi: 10.1016/0019-1035(92)90045-9. [DOI] [PubMed] [Google Scholar]
- Buss H.L. Luttge A. Brantley S.L. Etch pit formation on iron silicate surfaces during siderophore-promoted dissolution. Chem Geol. 2007;240:326–342. [Google Scholar]
- Carr M.H. Water on Mars. Oxford University Press; New York: 1995. [Google Scholar]
- Carr M.H. Head J.W. Geologic history of Mars. Earth Planet Sci Lett. 2010;294:185–203. [Google Scholar]
- Chan Y.K. Barraquio W.L. Knowles R. N2-fixing pseudomonads and related soil bacteria. FEMS Microbiol Rev. 1994;13:95–117. [Google Scholar]
- Clifford S.M. Lasue J. Heggy E. Boisson J. McGovern P. Max M.D. Depth of the martian cryosphere: revised estimates and implications for the existence and detection of subpermafrost groundwater. J Geophys Res. 2010;115 doi: 10.1029/2009JE003462. [DOI] [Google Scholar]
- Cornelis P. Matthijs S. Diversity of siderophore-mediated iron uptake systems in fluorescent pseudomonads: not only pyoverdines. Environ Microbiol. 2002;4:787–798. doi: 10.1046/j.1462-2920.2002.00369.x. [DOI] [PubMed] [Google Scholar]
- Cushing G.E. Titus T.N. Wynne J.J. Christensen P.R. THEMIS observes possible cave skylights on Mars. Geophys Res Lett. 2007;34 doi: 10.1029/2007GL030709. [DOI] [Google Scholar]
- Duckworth O.W. Holmstrom S.J.M. Pena J. Sposito G. Biogeochemistry of iron oxidation in a circumneutral freshwater habitat. Chem Geol. 2009;260:149–158. [Google Scholar]
- Edwards C.S. Christensen P.R. Hamilton V.E. Evidence for extensive olivine-rich basalt bedrock outcrops in Ganges and Eos chasmas, Mars. J Geophys Res. 2008;113 doi: 10.1029/2008JE003091. [DOI] [Google Scholar]
- Edwards K.J. Rogers D.R. Wirsen C.O. McCollom T.M. Isolation and characterization of novel psychrophilic, neutrophilic, Fe-oxidizing, chemolithoautotrophic alpha- and gamma-proteobacteria from the deep sea. Appl Environ Microbiol. 2003a;69:2906–2913. doi: 10.1128/AEM.69.5.2906-2913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K.J. Bach W. Rogers D.R. Geomicrobiology of the ocean crust: a role for chemoautotrophic Fe-bacteria. Biol Bull. 2003b;204:180–185. doi: 10.2307/1543555. [DOI] [PubMed] [Google Scholar]
- Edwards K.J. Bach W. McCollom T.M. Rogers D.R. Neutrophilic iron-oxidizing bacteria in the ocean: their habitats, diversity, and roles in mineral deposition, rock alteration, and biomass production in the deep-sea. Geomicrobiol J. 2004;21:393–404. [Google Scholar]
- Emerson D. Floyd M.M. Enrichment and isolation of iron-oxidizing bacteria at neutral pH. Methods Enzymol. 2005;397:112–123. doi: 10.1016/S0076-6879(05)97006-7. [DOI] [PubMed] [Google Scholar]
- Emerson D. Moyer C. Isolation and characterization of novel iron-oxidizing bacteria that grow at circumneutral pH. Appl Environ Microbiol. 1997;63:4784–4792. doi: 10.1128/aem.63.12.4784-4792.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson D. Moyer C.L. Neutrophilic Fe-oxidizing bacteria are abundant at the Loihi Seamount hydrothermal vents and play a major role in Fe oxide deposition. Appl Environ Microbiol. 2002;68:3085–3093. doi: 10.1128/AEM.68.6.3085-3093.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairén A.G. A cold and wet Mars. Icarus. 2010;208:165–175. [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Fisk M.R. Giovannoni S.J. Sources of nutrients and energy for a deep biosphere on Mars. J Geophys Res. 1999;104:11805–11815. [Google Scholar]
- Fisk M.R. Popa R. Mason O. Storrie-Lombardi M. Vicenzi E. Iron-magnesium silicate bioweathering on Earth (and Mars?) Astrobiology. 2006;6:48–68. doi: 10.1089/ast.2006.6.48. [DOI] [PubMed] [Google Scholar]
- Fogg M.J. The utility of geothermal energy on Mars. J Br Interplanet Soc. 1996;49:403–422. [Google Scholar]
- Gronstal A. Pearson V. Kappler A. Dooris C. Anand M. Poitrasson F. Kee T.P. Cockell C.S. Laboratory experiments on the weathering of iron meteorites and carbonaceous chondrites by iron-oxidizing bacteria. Meteorit Planet Sci. 2009;44:233–247. [Google Scholar]
- Hafenbradl D. Keller M. Dirmeier R. Rachel R. Roßnagel P. Burggraf S. Huber H. Stetter K.O. Ferroglobus placidus gen. nov., sp. nov., a novel hyperthermophilic archaeum that oxidizes Fe2+ at neutral pH under anoxic conditions. Arch Microbiol. 1996;166:308–314. doi: 10.1007/s002030050388. [DOI] [PubMed] [Google Scholar]
- Head J.W. Mustard J.F. Kreslavsky M.A. Milliken R.E. Marchant D.R. Recent ice ages on Mars. Nature. 2003;426:797–802. doi: 10.1038/nature02114. [DOI] [PubMed] [Google Scholar]
- Hoefen T.M. Clark R.N. Bandfield J.L. Smith M.D. Pearl J.C. Christensen P.R. Discovery of olivine in the Nili Fossae region of Mars. Science. 2003;302:627–630. doi: 10.1126/science.1089647. [DOI] [PubMed] [Google Scholar]
- Jepson S.M. Priscu J.C. Grimm R.E. Bullock M.A. The potential for lithoautotrophic life on Mars: application to shallow inerfacial water environments. Astrobiology. 2007;7:342–353. doi: 10.1089/ast.2007.0124. [DOI] [PubMed] [Google Scholar]
- Josef J.A. Fisk M.R. Giovanoni S. Perodotite dissolution rates in microbial enrichment cultures. Proc Ocean Drill Prog Sci Results. 2007;209 doi: 10.2973/odp.proc.sr.209.002.2007. [DOI] [Google Scholar]
- Kappler A. Newman D.K. Formation of Fe(III)-minerals by Fe(II)-oxidizing photoautotrophic bacteria. Geochim Cosmochim Acta. 2004;68:1217–1226. [Google Scholar]
- Kappler A. Schink B. Newman D.K. Fe(III) mineral formation and cell encrustation by the nitrate-dependent Fe(II)-oxidizer strain BoFeN1. Geobiology. 2005;3:235–245. [Google Scholar]
- Leboffe M.J. Pierce B.E. Microbiology Laboratory Theory and Application. 3rd. Morton Publishing Co.; Englewood, CO: 2010. [Google Scholar]
- Lehman R.M. O'Connell S.P. Banta A. Fredrickson J.K. Reysenbach A.L. Kieft T.L. Colwell F.S. Microbiological comparison of core and groundwater samples collected from a fractured basalt aquifer with that of dialysis chambers incubated in situ. Geomicrobiol J. 2004;21:169–182. [Google Scholar]
- Luo W.S. Gu B.H. Dissolution of uranium-bearing minerals and mobilization of uranium by organic ligands in a biologically reduced sediment. Environ Sci Technol. 2011;45:2994–2999. doi: 10.1021/es103073u. [DOI] [PubMed] [Google Scholar]
- Mahmood Q. Zheng P. Hu B.L. Jilani G. Azim M.R. Wu D.L. Liu D. Isolation and characterization of Pseudomonas stutzeri QZ1 from an anoxic sulfide-oxidizing bioreactor. Anaerobe. 2009;15:108–115. doi: 10.1016/j.anaerobe.2009.03.009. [DOI] [PubMed] [Google Scholar]
- Martínez J.S. Zhang G.P. Holt P.D. Jung H.T. Carrano C.J. Haygood M.G. Butler A. Self-assembling amphiphilic siderophores from marine bacteria. Science. 2000;287:1245–1247. doi: 10.1126/science.287.5456.1245. [DOI] [PubMed] [Google Scholar]
- Miot J. Benzerara K. Morin G. Kappler A. Bernard S. Obst M. Ferard C. Skouri-Panet F. Guigner J.M. Posth N. Galvez M. Brown G.E. Guyot F. Iron biomineralization by anaerobic neutrophilic iron-oxidizing bacteria. Geochim Cosmochim Acta. 2009;73:696–711. [Google Scholar]
- Morikawa M. Imanaka T. Isolation of a new mixotrophic bacterium which can fix CO2 and assimilate aliphatic and aromatic hydrocarbons anaerobically. J Ferment Bioeng. 1993;76:280–283. [Google Scholar]
- Neaman A. Chorover J. Brantley S.L. Implications of the evolution of organic acid moieties for basalt weathering over geological time. Am J Sci. 2005;305:147–185. [Google Scholar]
- Newman D.K. Feasting on minerals. Science. 2010;327:793–794. doi: 10.1126/science.1184229. [DOI] [PubMed] [Google Scholar]
- Northup D.E. Melim L.A. Spilde M.N. Hathaway J.J.M. Garcia M.G. Moya M. Stone F.D. Boston P.J. Dapkevicius M.L.N.E. Riquelme C. Lava cave microbial communities within mats and secondary mineral deposits: implications for life detection on other planets. Astrobiology. 2011;11:601–618. doi: 10.1089/ast.2010.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N. Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Samarkin V.A. Madigan M.T. Bowles M.W. Casciotti K.L. Priscu J.C. McKay C.P. Joye S.B. Abiotic nitrous oxide emission from the hypersaline Don Juan Pond in Antarctica. Nat Geosci. 2010;3:341–344. [Google Scholar]
- Santelli C.M. Welch S.A. Banfield J.F. The effect of Fe-oxidizing bacteria on Fe-silicate mineral dissolution rates. Chem Geol. 2001;180:99–115. [Google Scholar]
- Schippers A. Jorgensen B.B. Biogeochemistry of pyrite and iron sulfide oxidation in marine sediments. Geochim Cosmochim Acta. 2002;66:85–92. [Google Scholar]
- Schippers A. Neretin L.N. Kallmeyer J. Ferdelman T.G. Cragg B.A. Parkes R.J. Jørgensen B.B. Prokaryotic cells of the deep sub-seafloor biosphere identified as living bacteria. Nature. 2005;433:861–864. doi: 10.1038/nature03302. [DOI] [PubMed] [Google Scholar]
- Seiff A. Kirk D. Structure of the atmosphere of Mars in summer at mid-latitudes. J Geophys Res. 1977;82:4364–4378. [Google Scholar]
- Smil V. Cycles of Life: Civilization and the Biosphere. Scientific American Library; New York: 2000. [Google Scholar]
- Smith A.R. Popa R. Fisk M.R. Nielsen M.E. Wheat C.G. Jannasch H.W. Fisher A.T. Becker K. Sievert S.M. Flores G. In situ enrichment of ocean crust microbes on igneous minerals and glasses using an osmotic flow-through device. Geochemistry, Geophysics, Geosystems. 2011;12 doi: 10.1029/2010GC003424. [DOI] [Google Scholar]
- Steiner M. Lazaroff N. Direct method for continuous determination of iron oxidation by autotrophic bacteria. Appl Environ Microbiol. 1974;28:872–880. doi: 10.1128/am.28.5.872-880.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens T. Lithoautotrophy in the subsurface. FEMS Microbiol Rev. 1997;20:327–337. [Google Scholar]
- Straub K.L. Benz M. Schink B. Widdel F. Anaerobic, nitrate-dependent microbial oxidation of ferrous iron. Appl Environ Microbiol. 1996;62:1458–1460. doi: 10.1128/aem.62.4.1458-1460.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub K.L. Schonhuber W.A. Buchholz-Cleven B.E.E. Schink B. Diversity of ferrous iron-oxidizing, nitrate-reducing bacteria and their involvement in oxygen-independent iron cycling. Geomicrobiol J. 2004;21:371–378. [Google Scholar]
- Swindle T.D. Treiman A.H. Lindstrom D.J. Burkland M.K. Cohen B.A. Grier J.A. Li B. Olson E.K. Noble gases in iddingsite from the Lafayette meteorite: evidence for liquid water on Mars in the last few hundred million years. Meteorit Planet Sci. 2000;35:107–115. [Google Scholar]
- Tamura K. Nei M. Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA. 2004;101:11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K. Dudley J. Nei M. Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Thauer R.K. Jungermann K. Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis B.J. Rosenberg N.D. Cuzzi J.N. On the role of widespread subsurface convection in bringing liquid water close to Mars' surface. J Geophys Res. 2003;108 doi: 10.1029/2002JE001877. [DOI] [Google Scholar]
- Wang J.J. Muyzer G. Bodelier P.L.E. Laanbroek H.J. Diversity of iron oxidizers in wetland soils revealed by novel 16S rRNA primers targeting Gallionella-related bacteria. ISME J. 2009;3:715–725. doi: 10.1038/ismej.2009.7. [DOI] [PubMed] [Google Scholar]
- Warner N. Gupta S. Lin S.Y. Kim J.R. Muller J.P. Morley J. Late Noachian to Hesperian climate change on Mars: evidence of episodic warming from transient crater lakes near Ares Vallis. J Geophys Res. 2010;115 doi: 10.1029/2009JE003522. [DOI] [Google Scholar]
- Welch S.A. Banfield J.F. Modification of olivine surface morphology and reactivity by microbial activity during chemical weathering. Geochim Cosmochim Acta. 2002;66:213–221. [Google Scholar]
- Widdel F. Schnell S. Heising S. Ehrenreich A. Assmus B. Schink B. Ferrous iron oxidation by anoxygenic phototrophic bacteria. Nature. 1993;362:834–836. [Google Scholar]
- Williams K.E. McKay C.P. Toon O.B. Head J.W. Do ice caves exist on Mars? Icarus. 2010;209:358–368. [Google Scholar]
- Wu L.L. Jacobson A.D. Chen H.C. Hausner M. Characterization of elemental release during microbe-basalt interactions at T=28 degrees C. Geochim Cosmochim Acta. 2007;71:2224–2239. [Google Scholar]
- Yuliar. Autotrophic CO2 fixation in Pseudomonas sp. strain HD-1. Hayati Journal of Biosciences. 1997;4:59–61. [Google Scholar]