Abstract
We investigated the changes and the molecular mechanisms of cerebral vascular damage and tested the therapeutic effects of Niaspan in type-1 streptozotocin induced diabetic (T1DM) rats after stroke. T1DM-rats were subjected to transient middle cerebral artery occlusion (MCAo) and treated without or with Niaspan. Non-streptozotocin rats (WT) were also subjected to MCAo. Functional outcome, blood–brain-barrier (BBB) leakage, brain hemorrhage, immunostaining, and rat brain microvascular endothelial cell (RBEC) culture were performed. Compared to WT-MCAo-rats, T1DM-MCAo-rats did not show an increase lesion volume, but exhibited significantly increased brain hemorrhage, BBB leakage and vascular damage as well as decreased functional outcome after stroke. Niaspan treatment of stroke in T1DM-MCAo-rats significantly attenuated BBB damage, promoted vascular remodeling and improved functional outcome after stroke. T1DM-MCAo-rats exhibited significantly increased Angiopoietin 2 (Ang2) expression, but decreased Ang1 expression in the ischemic brain compared to WT-MCAo-rats. Niaspan treatment attenuated Ang2, but increased Ang1 expression in the ischemic brain in T1DM-MCAo-rats. In vitro data show that the capillary-like tube formation in the WT-RBECs marginally increased compared to T1DM-RBEC. Niaspan and Ang1 treatment significantly increased tube formation compared to non-treatment control. Inhibition of Ang1 attenuated Niacin-induced tube formation in T1DM-RBECs. Niaspan treatment of stroke in T1DM-rats promotes vascular remodeling and improves functional outcome. The Ang1/Ang2 pathway may contribute to Niaspan induced brain plasticity. Niaspan warrants further investigation as a therapeutic agent for the treatment of stroke in diabetics.
Keywords: Type-one diabetes rats, Stroke, Angiopoietin, Vascular remodeling, Niaspan
Introduction
Diabetes mellitus (DM) is a major health problem associated with both microvascular and macrovascular diseases and leads to 3–4 fold higher risk of experiencing ischemic stroke (Mast et al., 1995). Hyperglycemia and diabetes instigate a cascade of events leading to vascular endothelial cell dysfunction, increased vascular permeability (Li et al., 2010) and poor recovery after ischemic stroke (Capes et al., 2001). Therefore, the mechanisms by which diabetes increase vascular damage are primary targets of diabetes-stroke research.
Disequilibrium of angiogenesis promoters and inhibitors in diabetes may lead to exuberant but dysfunctional neovascularization. Angiopoietin-1 (Ang-1), a family of endothelial growth factors, mediates vascular remodeling (Suri et al., 1996) and plays a role in the recruitment of vascular smooth muscle cells (VSMCs) and pericytes (Sato et al., 1995; Suri et al., 1996). Angiopoietin-2 (Ang2), as an antagonist for Ang1, inhibits Ang1-promoted Tie2 signaling and decreases blood vessel maturation and stabilization. Retinal overexpression of Ang-2 mimics diabetic retinopathy and enhances vascular damage in hyperglycemia (Pfister et al., 2010). However, the changes of Ang1 and Ang2 expression after brain ischemia in DM have not been investigated.
Treatment of stroke has historically focused on neuroprotection, which has yielded failed clinical trials, except for the NINDS recombinant tissue plasminogen activator (rtPA) trial (Adams et al., 1996). However, tPA treatment in stroke patients with DM induced an incremental risk of death and spontaneous intracerebral hemorrhage and unfavorable 90-day outcomes in patients with hyperglycemia (Alvarez-Sabin et al., 2003; Poppe et al., 2009). Therefore, effective therapy of stroke in the normal blood glucose population may not necessarily transfer to the diabetic population, prompting the need to specifically test therapeutic agents for stroke in the diabetic population. Niacin (nicotinic acid) is the most effective medication in clinical use for increasing high density lipoprotein (HDL) cholesterol (Elam et al., 2000). Niacin improves endothelium-dependent vasodilation in coronary heart disease patients (Chapman et al., 2004). Niaspan is a prolonged release formulation of Niacin. Niaspan treatment of stroke increases Ang1/Tie2 axis activity and promotes vascular maturation after stroke in normal blood glucose animals (Chen et al., 2007). However, whether Niaspan treatment decreases brain hemorrhage, regulates Ang1 and Ang2 expression or impacts recovery after stroke in diabetic rats have not been investigated.
In this study, we investigated the differences of vascular and Ang1 and Ang2 angiogenic factor changes between streptozotocin induced type-1 diabetic (T1DM) and Non-streptozotocin (WT) rats subjected to stroke. We also tested whether Niaspan treatment of stroke in T1DM rats improves neurological functional outcome and vascular remodeling in a rat model of middle cerebral artery occlusion (MCAo). Molecular mechanisms underlying vascular remodeling induced by Niaspan are described.
Materials and methods
All experiments were conducted in accordance with the standards and procedures of the American Council on Animal Care and Institutional Animal Care and Use Committee of Henry Ford Health System.
Diabetes induction
Adult Male Wistar rats (225–250 g) purchased from Charles River (Wilmington, MA) were used. Diabetes was induced by a single intraperitoneal injection of streptozotocin (STZ, 60 mg/kg, Sigma Chemical Co., St. Louis, MO) to rat. The fasting blood glucose level was tested by using a glucose analyzer (Accu-Chek Compact System; Roche Diagnostics, Indianapolis, IN). Animals were subjected MCAo 2 weeks after diabetes induction (fasting blood glucose > 300 mg/dl) (Ye et al., 2011).
MCAo model and experiment groups
WT and T1DM rats were anesthetized and transient (2 h) MCAo was induced by using an intraluminal vascular occlusion (Chen et al., 2001a, 2001b). Briefly, rats were anesthetized with 2% isoflurane in a jar for pre anesthetic, and spontaneously respired with 1.5% isoflurane in 2:1 N2O:O2 mixture using a facemask connected and regulated with a modified FLUOTEC 3 Vaporizer (Fraser Harlake, Orchard Park, NY 14127). Rectal temperature was maintained at 37 °C throughout the surgical procedure using a feedback regulated water heating system (a recirculating pad and K module and monitored via an intrarectal type T thermocouple). A 4-0 nylon suture with its tip rounded by heating near a flame, was inserted into the external carotid artery (ECA) through a small puncture. The microsurgical clips were removed. The length of nylon suture, determined according to the animal’s weight, was gently advanced from the ECA into the lumen of the internal carotid artery (ICA) until the suture blocked the origin of the middle cerebral artery (MCA). The nylon filament was retained inside the ICA for 2 h and the neck incision was closed. The animals were moved to their cage to awaken. After 2 h of MCAo, animals were reanesthetized with isoflurane, and restoration of blood flow was performed by withdrawal of the filament until the tip cleared the lumen of the ECA. The incision was then closed. Rats were randomized and assigned to different groups and were gavaged starting 24 h after MCAo with: 1) saline for T1DM-MCAo-control (n=25); 2) saline for WT-MCAo-control (n=16); 3) T1DM-Niaspan treatment: 40 mg/kg Niaspan (Kos pharmaceuticals, Inc. Cranbury. NJ; dissolved in saline) daily for 14 days in T1DM-rats (n=17). Our previous study has shown that treatment of stroke with 40 mg/kg of Niaspan starting at 24 h after MCAo does not alter lesion volume (Chen et al., 2007). Therefore, any beneficial effect derives from induction of neurorestorative mechanisms. In this study, we investigate the neurorestorative effect of Niaspan; therefore, Niaspan treatment was initiated at 24 h after stroke. Rats received repeated intraperitoneal injections of the cell proliferation-specific marker 5-bromodeoxyuridine (BrdU, 100 mg/kg) daily after 24 h of stroke induction until they were euthanized 14 days after MCAo. A battery of functional tests was performed before MCAo and at 1, 7 and 14 days after MCAo. Rats were sacrificed at 14 days after MCAo, and lesion volume and brain hemorrhage were measured.
Neurological functional tests
Footfault, adhesive removal test and a modified neurological severity score (mNSS) evaluation were performed before MCAo, and at 1 day after MCAo before treatment and 7, 14 days after MCAo by an investigator who was blinded to the experimental groups (Chen et al., 2001a, 2001b).
mNSS is a composite of motor, sensory, balance and reflex tests. Neurological function was graded on a scale of 0 to 18 (normal score 0; maximal deficit score 18) with one point awarded for the exhibition of specific abnormal behavior or for lack of a tested reflex. A greater impairment of normal function results in a higher score. Rats were excluded if mNSS score less than 6 or over 13 24 h after MCAo.
HDL-cholesterol measurements
Serum HDL was measured (n=6/group) before MCAo and 14 days after Niaspan treatment using the EZ HDL-Cholesterol kit (Trinity Biotech USA, Jamestown, NY 14701 USA). The data are presented as μg/ml values. The animals were fasted 6 h before blood sample collection.
Quantitative evaluation of Evans blue dye extravasation
Rats (n=4/group) were sacrificed at 5 days after MCAo. 2% Evans blue dye in saline was injected intravenously as a BBB permeability tracer at 2 h before sacrifice. Evans blue dye fluorescence intensity was determined by a microplate fluorescence reader (excitation 620 nm and emission 680 nm). Calculations were based on the external standards dissolved in the same solvent (Zhang et al., 2002a, 2002b).
Lesion volume, histological and immunohistochemical assessment
The brains were fixed by transcardial perfusion with saline, followed by perfusion and immersion in 4% paraformaldehyde before being embedded in paraffin. Seven coronal sections (a–g, see Fig. 1D) of tissue were processed and stained with hematoxylin and eosin for calculation of volume of cerebral infarction and presented as a percentage of the lesion compared with the contralateral hemisphere (Swanson et al., 1990).
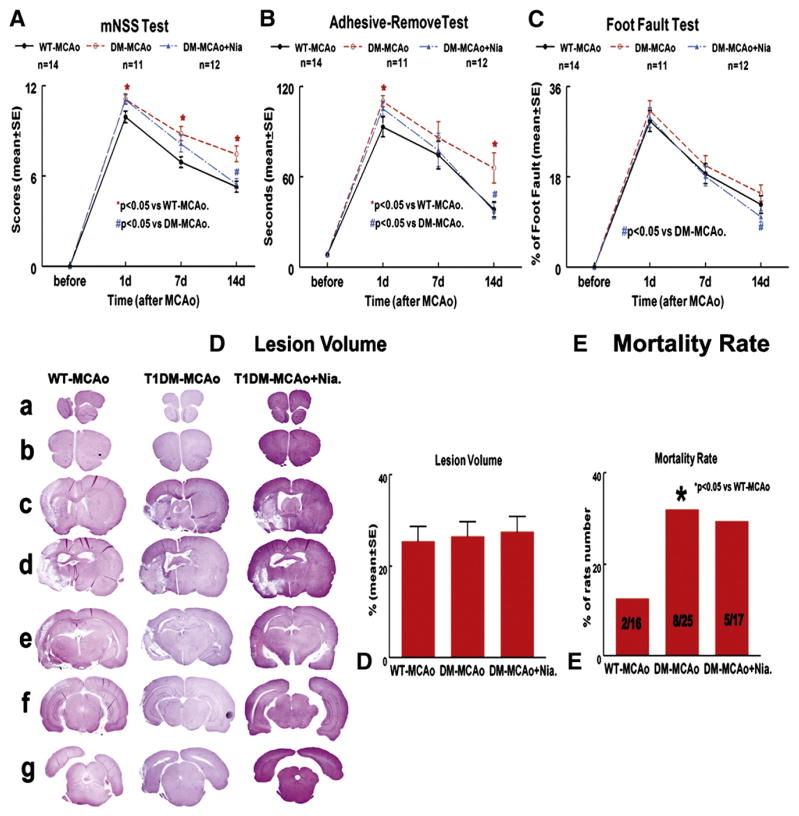
Fig. 1.
Lesion volume is not increased in T1DM rats after stroke, but functional outcome is significantly attenuated after stroke compared to WT-MCAo rats. Niaspan treatment promotes functional outcome after stroke in T1DM rats. A–C: mNSS test (A), adhesive removal-test (B) and foot-fault test (C) were measured before MCAo and at 1, 7 and 14 days after MCAo. D: Seven sections of HE staining for lesion volume measurement and quantitative data 14 days after MCAo. E: Mortality rate.
A series of 6 μm thick sections was cut from the standard paraffin block (bregma −1 mm to +1 mm). Every 10th coronal section for a total 5 sections was used for immunohistochemical staining. Antibody against Von Willebrand Factor (vWF, a endothelial cell marker, 1:400; Dako, Carpenteria, CA) (Chen et al., 2003a, 2003b), α-smooth muscle actin (α-SMA, mouse monoclonal IgG 1:800, Dako), a SMC marker (Ho et al., 2006), BrdU (a marker of proliferating cells, 1:100; Boehringer Mannheim), membrane-associated zona occluden-1 (ZO-1, rabbit polyclonal IgG, 1:50, Zyemd, Camarillo, CA), a marker of tight junction protein (Mark and Davis, 2002); endothelial barrier antigen (EBA, specifically expressed by rat endothelial cells, 1:1000; Sternberg Monoclonals, Lutherville, MD) (Morris et al., 2010), Desmin (rabbit monoclonal IgG, 1:700, Chemicon, Billerica, MA), a pericyte marker (Hughes et al., 2006), Ang1 (rabbit polyclonal IgG, 1:2000, Abcam, Cambridge, MA) and Ang2 (rabbit polyclonal lgG, 1:500, Abcam, Cambridge, MA) (Nourhaghighi et al., 2003; Sandhu et al., 2004) were performed. Control experiments consisted of staining brain coronal tissue sections as outlined above, but non-immune serum was substituted for the primary antibody (Li et al., 1998). The immunostaining analysis was performed by an investigator blinded to the experimental groups.
Mortality/hemorrhage rate and brain hemorrhage volume measurement
The number of dead animals in each group was counted (2 WT, 8 T1DM, and 5 Niaspan-treatment), and the mortality rate is presented as a percentage of rats that died between 24 h (initiation of Niaspan treatment) and 14 days (sacrifice time) after MCAo to the total animals in each group. Brain hemorrhage rate and hemorrhage volume were measured by using H&E staining under light microscopy in all animals including all the animals that died in the early stage of (within 72 h) after stroke.
EBA, ZO1, Desmin, Ang1 and Ang2 expression quantification
Five slides from each brain, with each slide containing 8 fields from the ischemic boundary zone (IBZ, Fig. 1F) were digitized under a 20× objective using a 3-CCD color video camera interfaced with an MCID image analysis system (Calza et al., 2001; Chen et al., 2003a, 2003b). For quantitation, EBA-, ZO1- and Desmin-immunoreactive positive cells or positive area were measured in total 20 enlarged positive blood vessels. Ang1 and Ang2 expression in the IBZ was digitized under a 20× objective (Olympus BX40). The data are presented as percentage of positive cell number or positive area of vessels or area in the IBZ, respectively (Cui et al., 2011).
Vascular perimeter and density measurement
Eight fields of view of vWF immunostaining from the IBZ were digitized using a 20× objective via the MCID computer imaging analysis system. The total perimeter of ten enlarged thin walled vessels, and vascular density in the IBZ was measured (Chen et al., 2003a, 2003b).
α-SMA positive coated vessel density and arterial diameter measurement
α-SMA immunoreactivity was employed as a marker to identify arteries (Ho et al., 2006). The density of α-SMA-stained vessels was analyzed with regard to small and large vessels (≥ 10 μm diameter) in the IBZ. Twenty largest within-lumen arterial diameters (minimum diameter) were measured. The numbers of α-SMA immunoreactive vessels and artery diameters were counted.
Vascular endothelial cell (VEC) proliferation
For quantitation of VEC proliferation, 8 fields from the IBZ were digitized under a 40× objective, and the percentage of BrdU-positive VECs to a total of VECs in 10 enlarged vessels located in the IBZ was measured in each section using the MCID imaging analysis system.
Western blot
An additional set of rats was sacrificed at 14 days after MCAo (n=4/group). Ischemic brain tissues were extracted from the ischemic core (IC) and IBZ (Chen et al., 2007) (please see Fig. 4C). Equal amounts of cell lysate were subjected to Western blot analysis, as previously described. The following primary antibodies were used: anti-β-actin (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA), anti-Ang1 (1:1000; Abcam, Cambridge, MA), and anti-Ang2 (1:1000; Abcam, Cambridge, MA).
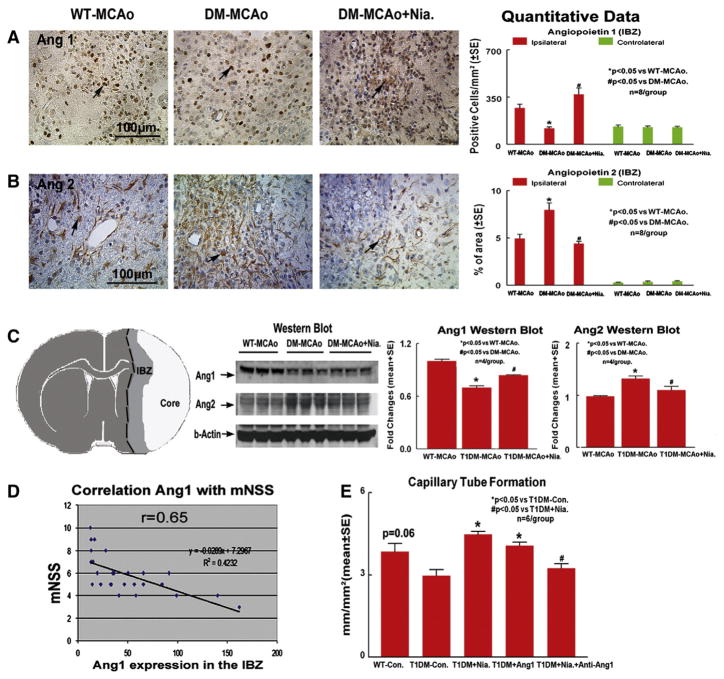
Fig. 4.
T1DM-MCAo rats show decreased Ang1 and increased Ang2 expression in the ischemic brain compared to WT-MCAo rats. Niaspan treatment increases Ang1 and decreases Ang2 expression in the ischemic brain compared to non-Niaspan treatment T1DM-MCAo rats. A: Ang1 expression in the IBZ (n=8/group). B: Ang2 expression in the IBZ (n=8/group). C: Schematic map shows ischemic brain tissue extraction from IC and IBZ for Western blot assay and Western blot quantitative data (n=4/group). D: Correlation Ang1 expression in the IBZ and mNSS. E: Capillary-like tube formation measurements in the rat brain endothelial cells (RBECs) in vitro.
Regional cerebral blood flow (rCBF) measurements (Zhang et al., 1997)
Relative rCBF was measured using laser Doppler flowmetry (LDF), which was performed with a PeriFlux PF4 flowmeter (Perimed AB, Järfälla, Sweden) with relative flow values expressed as perfusion units. Under anesthesia with an intraperitoneal injection of Ketamine (80 mg/kg body weight) and Xylazine (13 mg/kg body weight), animals underwent a right unilateral craniotomy. Core body temperature was maintained at 37 °C to 38 °C by a feedback controlled heating pad. Using a micromanipulator, one probe was positioned 2 mm posterior to the bregma, 6 mm to right side of midline, being careful to avoid pial vessels after reflection of the skin overlying the calvarium. Regional CBF within the right hemisphere was simultaneously measured before MCAo (to calculate the baseline flow) and at approximately 24 h after MCAo prior to and 2 h after Niaspan treatment (n=4/group). The data were analyzed using Perimed data acquisition and analysis system (Perimed AB, Järfälla, Sweden). Regional CBF is expressed as a percentage of preischemic baseline values of WT rats.
Rat brain microvascular endothelial cell (RBEC) culture
RBEC culture was performed, as previously described (Chen et al., 2009). Briefiy, the cortical brain tissue was isolated from WT and T1DM rats and digested in collagenase/dispase, and the microvessels separated by centrifugation in a Percoll (Sigma) gradient. Microvessels were seeded in flasks coated with rat-tail collagen and the medium was changed every 2–3 days. RBECs were used for tube formation assay.
Capillary-like tube formation assay
Capillary-like tube formation assay was performed as previously described (Rikitake et al., 2002). Briefiy, 0.1 ml growth factor reduced Matrigel (Becton Dickinson) was added per well. RBECs (2×104 cells) were incubated in (n=6/group): 1) WT-RBECs; 2) T1DM-RBECs; 3) T1DM-RBECs+Niacin (1 mM); 4) T1DM-RBECs+ Ang1 (200 ng, mouse Ang1 peptide, Millipore, Temcula, CA); 5) T1DM-RBECs+Niacin+anti-Ang-1 antibody (1.125 μg/ml, Rabbit anti-angiopoietin-1 affinity purified polyclonal antibody, Millipore, Temcula, CA) for 5 h. Matrigel wells were digitized under a 4× objective (Olympus BX40) for measurement of total tube length of capillary tube formation.
Statistical analysis
The global test using Generalize Estimating Equation (GEE) was implemented to analyze three correlated functional tests per animal. All measurements and analyses were performed by normality of distribution, and the homogeneity of variances was tested including the functional outcome, biochemistry, immunostaining, Western blot, and cell culture. One-way ANOVA was used for the evaluation of lesion volume, mortality, hemorrhage rate and volume, BBB leakage, immunostaining, Western blot and tube-formation analysis, respectively. The analysis started with overall group effect, followed by “Contract/estimate” statement to test the group difference if the overall group effect was detected at 0.05 level. Spearman partial correlation coefficient analysis was employed for the correlation between functional tests and histology evaluations at day 14 after MCAo adjusting for the study groups. Chi-square or Fisher exact test was used to test the mortality and the incidence of hemorrhage among the groups. All data are presented as mean±standard error (SE).
Results
Blood glucose level
T1DM rats had significantly increased blood glucose before and after MCAo (before-MCAo: 374.1 ±30.1 mg/dl; 1 day after-MCAo: 376.2 ±28.3 mg/dl) and compared to WT-rats (before-MCAo: 90.6±1.8 mg/dl, 1 day after-MCAo: 93.5 ±7.6 mg/dl; p<0.05). Niaspan treatment of stroke did not alter blood glucose 14 days after stroke in T1DM-MCAo rats (427.4 ±52.9 mg/dl) compared to non-treated T1DM-MCAo rats (383.6 ±25.3 mg/dl, p > 0.05).
HDL level
T1DM rats had significantly decreased blood HDL level (before-MCAo: 32.0±0.9 μg/ml; after-MCAo: 32.3±1.2 μg/ml) compared to WT rats (before-MCAo: 38.0±2.5 μg/ml; after-MCAo: 37.5±4.1 μg/ml; p<0.05). Niaspan treatment significantly increased HDL level (38.3± 2.4 μg/ml) in T1DM-MCAo rats compared to non-treated T1DM-MCAo rats (30.7±4.2 μg/ml, p<0.05).
Neurological outcome, lesion volume and mortality rate
To test whether Niaspan treatment regulates functional outcome after stroke in T1DM rats, a battery of functional tests was performed. Fig. 1 shows that T1DM-MCAo rats exhibited significantly attenuated functional outcome (A–C, p<0.05), but not an increase in lesion volume (D) after stroke compared with WT-MCAo rats. However, Niaspan treatment starting at 24 h after MCAo significantly improved functional outcome after stroke in T1DM-MCAo rats compared to non-treated T1DM-MCAo control rats (A–C, p<0.05). Fig. 1E shows that the mortality rate in the T1DM rats was significantly higher than that in the WT rats (F=7.644, p=0.014), but no difference was observed between T1DM-MCAo groups with or without Niaspan treatment.
Cerebral hemorrhage
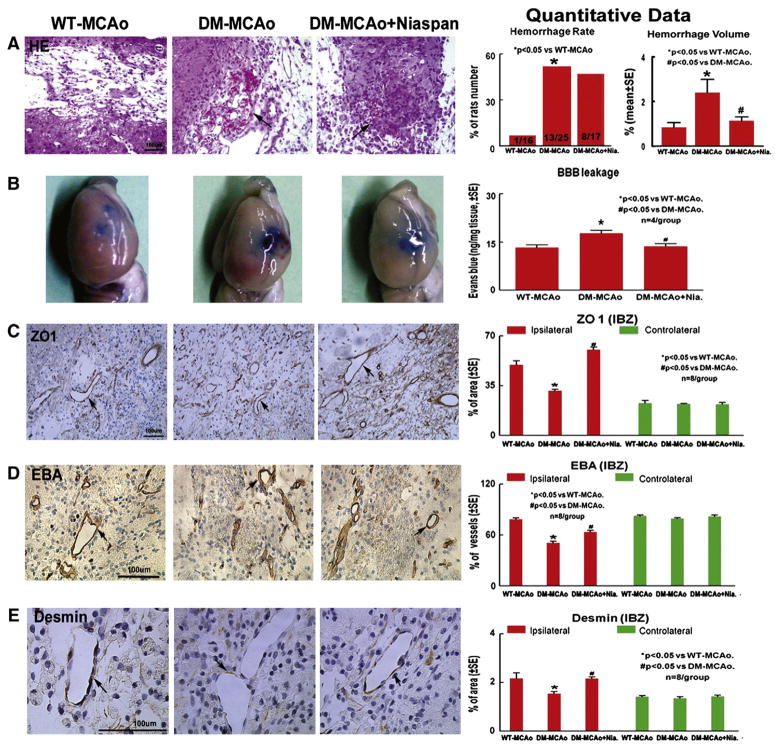
To test whether Niaspan regulates brain hemorrhage in T1DM rats, brain hemorrhage rate and volume using H&E staining were measured under light microscopy. Fig. 2A shows that both the cerebral hemorrhagic rate and hemorrhage volume in T1DM rats were significantly higher than in WT rats (p<0.05). The brain hemorrhage, including animals that died between 24 h and 14 days post stroke was evaluated. Niaspan treatment in T1DM rats did not significantly decrease brain hemorrhage rates, but significantly decreased the hemorrhage volume compared with non-treatment rats (F=5.58, P=0.012). We found that most rats died during the early stage after stroke (24–72 h), and all animals that died exhibited cerebral hemorrhage. Moreover, the cerebral hemorrhage volume was significantly correlated with animal death (r=0.85, p<0.05).
Fig. 2.
Brain hemorrhage, BBB leakage and vascular damage are increased in T1DM-MCAo rats compared to WT-MCAo rats. Niaspan treatment of T1DM-MCAo rats decreases BBB leakage, brain hemorrhage and vascular damage. A: HE staining for brain hemorrhage rate and hemorrhage volume measurement. B: Evans blue assay for BBB leakage (n=4/group). C–E: ZO1, EBA and Desmin expression in the IBZ (n=8/group).
BBB leakage and BBB function
Fig. 2B shows that BBB leakage significantly increased in T1DM-MCAo rats compared to WT-MCAo rats (p<0.05). Niaspan treatment significantly attenuated the increased BBB leakage in T1DM-MCAo rats (p<0.05). To test BBB integrity, ZO1, EBA and Desmin were measured in the ischemic brain and contralateral hemisphere. Figs. 2C–E show that T1DM-MCAo rats had significantly decreased BBB functional integrity identified by ZO1 (C), EAB (D), expression, and Desmin (E) positive cells around vessels compared to WT-MCAo rats (p<0.05). Niaspan treatment significantly increased EAB, ZO1 and Desmin expression in the ipsilateral vessels in T1DM-MCAo rats compared to non-treated T1DM-MCAo control rats (p<0.05).
Vascular changes in the ischemic brain
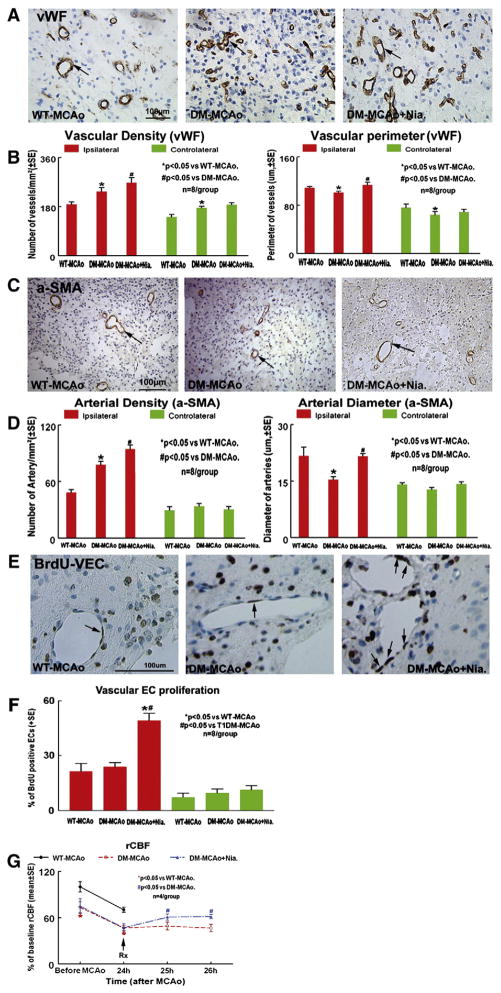
To test whether diabetes affects vascular change, vWF and α-SMA immunostaining were performed. Figs. 3A–D show that the vascular density was significantly increased in the ipsilateral hemisphere of T1DM-MCAo rats compared with the WT-MCAo rats after stroke (Fig. 3B. vWF-density: F=9.26, p=0.01; Fig. 3D. α-SMA-density: F=48.874, p<0.001). However, the vascular perimeter and arterial diameter were significantly decreased in the ipsilateral hemisphere in T1DM-MCAo rats compared to WT-MCAo rats (Fig. 3B. Vascular perimeter: F=6.125, p=0.008; Fig. 3D. arterial diameter: F=9.961, p=0.001). Niaspan treatment in T1DM-MCAo rats exhibited significantly increased vascular and arterial density as well as increased vessel perimeter and arterial diameter in the ischemic brain (Figs. 3A–D). Figs. 3E and F show that there is no significant difference in vascular en-dothelial cell (VEC) proliferation between WT-MCAo and DM-MCAo groups (p > 0.05). However, Niaspan-treatment significantly increased VEC proliferation compared with the non-treatment group in T1DM-MCAo rats (F=23.435, p<0.001).
Fig. 3.
T1DM-MCAo rats have increased vascular density, but decreased vascular perimeter, arterial diameter compared to WT-MCAo rats. Niaspan treatment of stroke increases vascular remodeling in the ischemic brain in T1DM-MCAo rats. A–B: Vascular density and vascular perimeter were measured by vWF immunostaining in the IBZ (n=8/group). C–D: Artery density and arterial diameter was measured by α-SMA immunostaining in the IBZ (n=8/group). The total number of α-SMA positive coated vessels per mm2 area is presented. E–F: BrdU-positive VECs and quantatitive data. G: rCBF was measured before MCAo, after MCAo and Niaspan treatment in WT-MCAo, T1DM-MCAo and T1DM-MCAo+Niaspan treatment rats. The data are presented as percentage of baseline (before MCAo in WT rats).
rCBF measurement
To test whether Niaspan treatment in T1DM alters rCBF, rCBF was measured before and after MCAo. Fig. 3G shows that T1DM rats exhibited significantly decreased rCBF before and after MCAo compared to WT rats (p<0.05). Niaspan treatment in T1DM rats significantly increased rCBF in the ipsilateral hemisphere (p<0.05) compared to non-treatment T1DM control.
Ang1 and Ang2 expression
To test the mechanism of Niaspan decrease of BBB leakage in T1DM rats, Ang1 and Ang2 expression was measured in the ischemic brain. Figs. 4A–B show that T1DM-MCAo rats exhibit significantly decreased Ang1, and increased Ang2 expression in the ischemic brain compared to WT-MCAo rats (p<0.05, n=8/group). Niaspan treatment significantly increased Ang1 (Fig. 4A, F=18.326, p<0.01) and decreased Ang2 (Fig. 4B, F=19.829, p<0.01) expression in the ischemic brain in T1DM-MCAo rats compared to non-treated T1DM-MCAo rats. In addition, the functional outcome (mNSS) at 14 days after MCAo significantly correlated with Ang1 level in the ischemic brain (Fig. 4D. p<0.05, r=0.65).
Western blot assay
Fig. 4C shows that the Western blot data were consistent with the immunostaining; T1DM-MCAo rats exhibited decreased Ang1, but increased Ang2 protein expression in the ischemic brain. Niaspan treatment in T1DM-MCAo rats significantly increased Ang1 (F=33.794, p=0.001) and decreased Ang2 (F=9.961, p=0.012) expression in the ischemic brain.
Capillary tube formation assays
Fig. 4E shows that WT-RBECs (rats brain endothelial cells) marginally (p=0.06) increased capillary tube formation, compared to T1DM-RBEC. Niaspan and Ang1 treatment significantly increased tube formation compared to non-treatment control (p<0.05). Inhibition of Ang1 attenuated Niacin-induced tube formation in T1DM-RBECs (p<0.05).
Discussion
We demonstrate that T1DM-MCAo rats exhibit increased mortality rate, BBB leakage, brain hemorrhage and worse functional outcome after stroke compared to WT-MCAo rats. T1DM-MCAo rats also show decreased BBB integrity as well as reduced relative levels of Ang1 and increased Ang2 compared to WT-MCAo rats. Our data are the first to demonstrate that Niaspan treatment of stroke starting 24 h after MCAo in T1DM rats significantly reduces BBB leakage and promotes functional outcome but does not decrease lesion volume compared to non-treated T1DM-MCAo control rats. Treatment of T1DM-MCAo rats with Niaspan significantly increases the levels of Ang1 and decreases Ang2 in the ischemic brain. Ang1 level is significantly correlated with functional outcome after stroke. Complementary in vitro data support the hypothesis that Niaspan-induced increase of Ang1 and decrease of Ang2 in T1DM-MCAo rats contribute to vascular remodeling in the is-chemic brain, which plays an important role in functional outcome after stroke in T1DM-MCAo rats.
Intracerebral hemorrhagic transformation is a multifactorial process in which ischemic brain tissue converts into a hemorrhagic lesion with blood-vessel leakage, extravasation, and further brain injury. We found that T1DM-MCAo increases brain hemorrhage and evokes worse functional outcome after stroke, which is consistent with data from Goto-Kakizaki type-2 diabetic rats (Ergul et al., 2007). BBB rupture and brain hemorrhage occur in the acute stage after stroke (Cui et al., 2011). Our previous study has found that BBB leakage can be measured at 5 days after stroke (Chen et al., 2009). In addition, using MRI, Lee et al. examined vascular permeability and spontaneous intracerebral hemorrhage in stroke-prone spontaneous hypertensive rats (SHRsp) and found that all 12 rats that developed spontaneous hemorrhages demonstrated concurrent or prior vascular permeability at the site of the hemorrhage. In 4 of the 7 hemorrhages, evidence of vascular permeability was found prior to the detection of hemorrhage, preceding it by up to 2 weeks (Lee et al., 2007). Therefore, in the present study, BBB leakage was measured at 5 days and brain hemorrhage was measured 1 to 14 days after MCAo. Early BBB disruption promotes intracerebral hemorrhage. In this study, we found that most animals that died did so within 3 days after MCAo. In addition, brain hemorrhage is significantly correlated with animal death (p<0.05, r=0.85). The BBB function consists of a combination of endothelial cells with tight junctions and pericytes. Tight junctions provide barriers between adjacent brain capillary endothelial cells at the BBB, and stabilize angiogenic vessels (Hori et al., 2004). We found that T1DM-MCAo rats exhibit significantly decreased EBA, tight junction protein and pericyte expression in the ischemic brain vessels and increase brain hemorrhage and BBB leakage in the ischemic brain. Therefore, diabetes decreases BBB function and promotes BBB leakage and brain hemorrhage after stroke which may contribute to the decrement of functional outcome after stroke in T1DM-MCAo rats.
CBF alterations have a significant impact on brain homeostasis in pathological states. Autoregulation of cerebral blood flow and vasodilation of cerebral arteries are impaired in T1DM patients (Hoffman et al., 2004). Lower extremity and retinal capillary perfusion are also impaired in DM rats and lead to diabetic retinopathy and neuropathy (Arora et al., 2002; Ben-nun et al., 2004). We found that T1DM-MCAo rats exhibit increased vascular density but significantly decreased vascular perimeter and artery diameter compared to WT-MCAo rats. Niaspan treatment increased vascular remodeling. Consistent with in vivo findings, tube formation in RBECs from T1DM-MCAo rats was significantly increased compared to WT-MCAo rats. These data suggest that the increased vessels in diabetic rats are not necessarily functional vessels. T1DM-MCAo rats show increased brain hemorrhage and decreased endothelial cell and mural cell interaction which in concert may contribute to pathological cerebral neovascularization and arteriogenesis and also disrupt the integrity of the BBB and thereby contribute to the reduced functional outcome after stroke.
Niacin is an effective medication in clinical use for increasing HDL-C (Elam et al., 2000). In this study, we found that Niaspan significantly increased HDL level, increases VEC proliferation and decreased BBB leakage after stroke in T1DM rats. Growth of functional arteries is essential for the restoration of blood flow to ischemic organs. Arteriogenesis serves as the most efficient mechanism to restore flow after arterial occlusion (Hershey et al., 2001). Niaspan treatment promotes arteriogenesis, as demonstrated by increased functional arterial density and diameter in the ischemic brain in T1DM-MCAo rats. These data indicate that Niaspan treatment increases vascular remodeling, decreases BBB damage and improves functional outcome after stroke in T1DM rats.
Many factors regulate BBB leakage after stroke. The Ang–Tie system modulates pericyte loss in diabetic retinopathy (Pfister et al., 2008). Ang-1 promotes pericyte recruitment, remodeling, maturation, and stabilization of blood vessels (Iurlaro et al., 2003; Suri et al., 1998), and prevents plasma leakage in the ischemic brain (Metheny-Barlow et al., 2004; Zhang et al., 2002a, 2002b). Ang-1 gene therapy normalizes immature vasculature in type II db/db mice (Chen and Stinnett, 2008). Ang-2 is linked to pericyte loss and subsequent induces diabetic retinopathy. We found that T1DM-MCAo rats exhibit significantly decreased Ang1 expression and increased Ang2 expression in the ischemic brain as well as increased BBB leakage and brain hemorrhage compared to WT-MCAo rats. Niaspan treatment of T1DM-MCAo rats attenuated the disequilibrium angiogenic factor expression, by increasing Ang1 and decreasing Ang2 expression in the ischemic brain. Therefore, Ang1/Ang2 disequilibrium may contribute to T1DM-induced BBB damage, brain hemorrhage and thereby worsen functional outcome after stroke. Increasing Ang1 expression may contribute to Niaspan-induced functional outcome after stroke in T1DM-MCAo rats. However, the molecular mechanism of how Niaspan regulates Ang1 and Ang2 is not known and requires further investigation. In addition, many factors may regulate the increased BBB leakage and brain hemorrhage in diabetic animals, such as VEGF, MMP9 and tPA/PAI signaling pathways (Rosell et al., 2008; Yepes et al., 2003; Zhang et al., 2000, 2002a, 2002b). Whether Niaspan regulates these factors requires investigation.
There are a number of limitations and caveats to the present study. In this study, we used a transient 2 h MCAo model in 2 week Type 1 diabetes induced rats which is an early stage of diabetes. Additional studies are warranted under conditions of permanent focal stroke and a late stage of diabetes animals. In addition, using perfusion-fixed brains, but not the irreversible vasodilator may, at least in part, influence artery diameter measurement. However, we found a difference between control and treated populations in the tissue prepared in identical ways and the use of irreversible vasodilators such as papaverine may induce endothelial denudation (Mayranpaa et al., 2004).
Recently, clinical studies have found that high dose (2000 mg/daily) of extended-release niacin offered no benefits beyond statin therapy alone in reducing cardiovascular-related complications and induced small and unexplained increase in ischemic stroke rates (NIH News, May 26, 2011). These data actually are consistent with our previous pre-clinical findings using statins and Niaspan, that high doses of statins (atorvastatin 8 mg/kg) and Niaspan (80 mg/kg) do not improve functional outcome after stroke and may have an adverse effect on neurological recovery (Chen et al., 2003a, 2003b; Shehadah et al., 2010). Our present study employs a low dose of Niaspan (40 mg/kg) in rats which is equivalent to a human dose of (400–500 mg/day) as a neurorestorative treatment of stroke. Recent studies have found that the rat MCAO model has failed to predict the outcome of clinical trials for neuroprotective agents. This problem persists and has not yet been adequately addressed.
In conclusion, Niaspan treatment increases Ang1 and decreases Ang2 expression and promotes vascular remodeling as well as improves functional outcome after stroke in T1DM rats. Regulation of Ang1/Ang2 expression by Niaspan treatment may contribute to the beneficial effect after stroke in diabetic rats. Further investigations into the use of Niaspan as a therapeutic agent for the treatment of stroke in diabetics are warranted.
Acknowledgments
The authors wish to thank Qinge Lu and Sutapa Santra for technical assistance.
Footnotes
Sources of Funding: This work was supported by National Institute on Aging RO1 AG031811 (JC), National Institute of Neurological Disorders and Stroke PO1 NS23393 (MC) and 1R41NS064708 (JC), and American Heart Association grant 09GRNT2300151 (JC).
Disclosures: None.
References
- Adams HP, Jr, Brott TG, Furlan AJ, Gomez CR, Grotta J, Helgason CM, Kwiatkowski T, Lyden PD, Marler JR, Torner J, Feinberg W, Mayberg M, Thies W. Guidelines for thrombolytic therapy for acute stroke: a supplement to the guidelines for the management of patients with acute ischemic stroke. A statement for health-care professionals from a Special Writing Group of the Stroke Council, American Heart Association. Circulation. 1996;94:1167–1174. doi: 10.1161/01.cir.94.5.1167. [DOI] [PubMed] [Google Scholar]
- Alvarez-Sabin J, Molina CA, Montaner J, Arenillas JF, Huertas R, Ribo M, Codina A, Quintana M. Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator-treated patients. Stroke. 2003;34:1235–1241. doi: 10.1161/01.STR.0000068406.30514.31. [DOI] [PubMed] [Google Scholar]
- Arora S, Pomposelli F, LoGerfo FW, Veves A. Cutaneous microcirculation in the neuropathic diabetic foot improves significantly but not completely after successful lower extremity revascularization. J Vasc Surg. 2002;35:501–505. doi: 10.1067/mva.2002.121126. [DOI] [PubMed] [Google Scholar]
- Ben-nun J, Alder VA, Constable IJ. Retinal microvascular patency in the diabetic rat. Int Ophthalmol. 2004;25:187–192. doi: 10.1007/s10792-004-5815-x. [DOI] [PubMed] [Google Scholar]
- Calza L, Giardino L, Giuliani A, Aloe L, Levi-Montalcini R. Nerve growth factor control of neuronal expression of angiogenetic and vasoactive factors. Proc Natl Acad Sci U S A. 2001;98:4160–4165. doi: 10.1073/pnas.051626998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–2432. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- Chapman MJ, Assmann G, Fruchart JC, Shepherd J, Sirtori C. Raising high-density lipoprotein cholesterol with reduction of cardiovascular risk: the role of nicotinic acid—a position paper developed by the European Consensus Panel on HDL-C. Curr. Med Res Opin. 2004;20:1253–1268. doi: 10.1185/030079904125004402. [DOI] [PubMed] [Google Scholar]
- Chen JX, Stinnett A. Ang-1 gene therapy inhibits hypoxia-inducible factor-1alpha (HIF-1alpha)-prolyl-4-hydroxylase-2, stabilizes HIF-1alpha expression, and normalizes immature vasculature in db/db mice. Diabetes. 2008;57:3335–3343. doi: 10.2337/db08-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001a;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001b;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003a;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003b;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- Chen J, Cui X, Zacharek A, Jiang H, Roberts C, Zhang C, Lu M, Kapke A, Feldkamp CS, Chopp M. Niaspan increases angiogenesis and improves functional recovery after stroke. Ann Neurol. 2007;62:49–58. doi: 10.1002/ana.21160. [DOI] [PubMed] [Google Scholar]
- Chen J, Cui X, Zacharek A, Chopp M. Increasing Ang1/Tie2 expression by simvastat-in treatment induces vascular stabilization and neuroblast migration after stroke. J Cell Mol Med. 2009;13:1348–1357. doi: 10.1111/j.1582-4934.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Chopp M, Zacharek A, Ye X, Roberts C, Chen J. Angiopoietin/Tie2 pathway mediates type 2 diabetes induced vascular damage after cerebral stroke. Neurobiol Dis. 2011;43:285–292. doi: 10.1016/j.nbd.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam MB, Hunninghake DB, Davis KB, Garg R, Johnson C, Egan D, Kostis JB, Sheps DS, Brinton EA. Effect of niacin on lipid and lipoprotein levels and glycemic control in patients with diabetes and peripheral arterial disease: the ADMIT study: a randomized trial. Arterial Disease Multiple Intervention Trial. JAMA. 2000;284:1263–1270. doi: 10.1001/jama.284.10.1263. [DOI] [PubMed] [Google Scholar]
- Ergul A, Elgebaly MM, Middlemore ML, Li W, Elewa H, Switzer JA, Hall C, Kozak A, Fagan SC. Increased hemorrhagic transformation and altered infarct size and localization after experimental stroke in a rat model type 2 diabetes. BMC Neurol. 2007;7:33. doi: 10.1186/1471-2377-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey JC, Baskin EP, Glass JD, Hartman HA, Gilberto DB, Rogers IT, Cook JJ. Revascularization in the rabbit hindlimb: dissociation between capillary sprouting and arteriogenesis. Cardiovasc Res. 2001;49:618–625. doi: 10.1016/s0008-6363(00)00232-7. [DOI] [PubMed] [Google Scholar]
- Ho TK, Rajkumar V, Black CM, Abraham DJ, Baker DM. Increased angiogenic response but deficient arteriolization and abnormal microvessel ultrastructure in critical leg ischaemia. Br J Surg. 2006;93:1368–1376. doi: 10.1002/bjs.5496. [DOI] [PubMed] [Google Scholar]
- Hoffman WH, Litaker MS, Pluta RM, Camens ML. Cerebral vasoreactivity in children and adolescents with type 1 diabetes mellitus. Endocr Res. 2004;30:315–325. doi: 10.1081/erc-200033190. [DOI] [PubMed] [Google Scholar]
- Hori S, Ohtsuki S, Hosoya K, Nakashima E, Terasaki T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem. 2004;89:503–513. doi: 10.1111/j.1471-4159.2004.02343.x. [DOI] [PubMed] [Google Scholar]
- Hughes S, Gardiner T, Hu P, Baxter L, Rosinova E, Chan-Ling T. Altered pericyte-endothelial relations in the rat retina during aging: implications for vessel stability. Neurobiol Aging. 2006;27:1838–1847. doi: 10.1016/j.neurobiolaging.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Iurlaro M, Scatena M, Zhu WH, Fogel E, Wieting SL, Nicosia RF. Rat aorta-derived mural precursor cells express the Tie2 receptor and respond directly to stimulation by angiopoietins. J Cell Sci. 2003;116:3635–3643. doi: 10.1242/jcs.00629. [DOI] [PubMed] [Google Scholar]
- Lee JM, Zhai G, Liu Q, Gonzales ER, Yin K, Yan P, Hsu CY, Vo KD, Lin W. Vascular permeability precedes spontaneous intracerebral hemorrhage in stroke-prone spontaneously hypertensive rats. Stroke. 2007;38:3289–3291. doi: 10.1161/STROKEAHA.107.491621. [DOI] [PubMed] [Google Scholar]
- Li Y, Jiang N, Powers C, Chopp M. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke. 1998;29:1972–1980. doi: 10.1161/01.str.29.9.1972. (discussion 1980–1971) [DOI] [PubMed] [Google Scholar]
- Li W, Prakash R, Kelly-Cobbs AI, Ogbi S, Kozak A, El-Remessy AB, Schreihofer DA, Fagan SC, Ergul A. Adaptive cerebral neovascularization in a model of type 2 diabetes: relevance to focal cerebral ischemia. Diabetes. 2010;59:228–235. doi: 10.2337/db09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia–reoxygenation. Am J Physiol Heart Circ Physiol. 2002;282:H1485–H1494. doi: 10.1152/ajpheart.00645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast H, Thompson JL, Lee SH, Mohr JP, Sacco RL. Hypertension and diabetes mellitus as determinants of multiple lacunar infarcts. Stroke. 1995;26:30–33. doi: 10.1161/01.str.26.1.30. [DOI] [PubMed] [Google Scholar]
- Mayranpaa M, Simpanen J, Hess MW, Werkkala K, Kovanen PT. Arterial endothelial denudation by intraluminal use of papaverine–NaCl solution in coronary bypass surgery. Eur J Cardiothorac Surg. 2004;25:560–566. doi: 10.1016/j.ejcts.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Metheny-Barlow LJ, Tian S, Hayes AJ, Li LY. Direct chemotactic action of angiopoietin-1 on mesenchymal cells in the presence of VEGF. Microvasc Res. 2004;68:221–230. doi: 10.1016/j.mvr.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Morris DC, Chopp M, Zhang L, Lu M, Zhang ZG. Thymosin beta4 improves functional neurological outcome in a rat model of embolic stroke. Neuroscience. 2010;169:674–682. doi: 10.1016/j.neuroscience.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourhaghighi N, Teichert-Kuliszewska K, Davis J, Stewart DJ, Nag S. Altered expression of angiopoietins during blood–brain barrier breakdown and angiogenesis. Lab Invest. 2003;83:1211–1222. doi: 10.1097/01.lab.0000082383.40635.fe. [DOI] [PubMed] [Google Scholar]
- Pfister F, Feng Y, vom Hagen F, Hoffmann S, Molema G, Hillebrands JL, Shani M, Deutsch U, Hammes HP. Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes. 2008;57:2495–2502. doi: 10.2337/db08-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister F, Wang Y, Schreiter K, vom Hagen F, Altvater K, Hoffmann S, Deutsch U, Hammes HP, Feng Y. Retinal overexpression of angiopoietin-2 mimics diabetic retinopathy and enhances vascular damages in hyperglycemia. Acta Diabetol. 2010;47:59–64. doi: 10.1007/s00592-009-0099-2. [DOI] [PubMed] [Google Scholar]
- Poppe AY, Majumdar SR, Jeerakathil T, Ghali W, Buchan AM, Hill MD. Admission hyperglycemia predicts a worse outcome in stroke patients treated with intravenous thrombolysis. Diabetes Care. 2009;32:617–622. doi: 10.2337/dc08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikitake Y, Hirata K, Kawashima S, Ozaki M, Takahashi T, Ogawa W, Inoue N, Yokoyama M. Involvement of endothelial nitric oxide in sphingosine-1-phosphate-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2002;22:108–114. doi: 10.1161/hq0102.101843. [DOI] [PubMed] [Google Scholar]
- Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J. MMP-9-positive neutrophil infiltration is associated to blood–brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39:1121–1126. doi: 10.1161/STROKEAHA.107.500868. [DOI] [PubMed] [Google Scholar]
- Sandhu R, Teichert-Kuliszewska K, Nag S, Proteau G, Robb MJ, Campbell AI, Kuliszewski MA, Kutryk MJ, Stewart DJ. Reciprocal regulation of angiopoietin-1 and angiopoietin-2 following myocardial infarction in the rat. Cardiovasc Res. 2004;64:115–124. doi: 10.1016/j.cardiores.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Shehadah A, Chen J, Cui X, Roberts C, Lu M, Chopp M. Combination treatment of experimental stroke with Niaspan and Simvastatin, reduces axonal damage and improves functional outcome. J Neurol Sci. 2010;294:107–111. doi: 10.1016/j.jns.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282:468–471. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–293. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Ye X, Chopp M, Liu X, Zacharek A, Cui X, Yan T, Roberts C, Chen J. Niaspan reduces high-mobility group box 1/receptor for advanced glycation end-products after stroke in type-1 diabetic rats. Neuroscience. 2011;190:339–345. doi: 10.1016/j.neuroscience.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood–brain barrier via the LDL receptor-related protein. J Clin Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Chopp M, Zhang RL, Goussev A. A mouse model of embolic focal cerebral ischemia. J Cereb Blood Flow Metab. 1997;17:1081–1088. doi: 10.1097/00004647-199710000-00010. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood–brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–838. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Yepes M, Jiang Q, Li Q, Arniego P, Coleman TA, Lawrence DA, Chopp M. Adjuvant treatment with neuroserpin increases the therapeutic window for tissue-type plasminogen activator administration in a rat model of embolic stroke. Circulation. 2002a;106:740–745. doi: 10.1161/01.cir.0000023942.10849.41. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Croll SD, Chopp M. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience. 2002b;113:683–687. doi: 10.1016/s0306-4522(02)00175-6. [DOI] [PubMed] [Google Scholar]