Abstract
Numerous inflammatory cytokines have been implicated in the pathogenesis of cardiovascular diseases. Monocyte chemoattractant protein (MCP)-1/CCL2 that is expressed by mainly inflammatory cells and stromal cells such as endothelial cells, and its expression is upregulated following pro-inflammatory stimuli and tissue injury. MCP-1 can function as a traditional chemotactic cytokine and also regulates gene transcription. The recently discovered novel zinc-finger protein, called MCPIP (MCP-1-induced protein), which initiates a series of signaling events that causes oxidative and endoplasmic reticulum (ER) stress, leading to autophagy that can result in cell death or differentiation depending on the cellular context. After briefly reviewing the basic processes involved in inflammation, ER stress and autophagy, the recently elucidated role of MCP-1 and MCPIP in inflammatory diseases is reviewed. MCPIP was found to be able to control inflammatory response by inhibition of NFκB activation via its deubiquitinase activity or by degradation of mRNA encoding a set of inflammatory cytokines via its RNase activity. The potential inclusion of such a novel deubiquitinase in the emerging anti-inflammatory strategies for the treatment of inflammation related diseases such as cardiovascular diseases and type 2 diabetes is briefly discussed.
Keywords: cardiovascular disease, type 2 diabetes, angiogenesis, adipogenesis, osteoclastogenesis
1. Introduction
Inflammation is widely recognized to be at the root of a host of serious human diseases from heart disease to diabetes and cancer. Inflammation involves permeability changes in vascular walls leading to enhanced movement of immune cells from the vascular lumen into the tissue. Acute inflammation can occur in response to pathogen attack (infection) or tissue injury. This process involves activation of leukocytes and production of reactive oxygen that kills invading pathogens. The pathogen and cell debris resulting from tissue injury are phagocytosed into the leukocytes in which they undergo degradation. This acute inflammatory process is short lived as it clears up when the injury or infection is resolved. Persistent inflammatory stimuli or dysfunction of resolution phase results in chronic inflammation, which has been recognized as the underlying factor in the development of various diseases including cardiovascular diseases, obesity-induced type 2 diabetes, rheumatoid arthritis (RA), and cancer.1–3 Consequently, anti-inflammatory therapies are being explored for prevention and treatment of these diseases.4–6 As heart disease progresses, the inflammatory response is activated, contributing to the deleterious effects on the heart and vasculature, progression of ventricular dysfunction and heart failure.7,8 Key players that contribute to the inflammatory response include agents that mediate blood leukocyte recruitment into tissues and cause local activation and interaction with resident tissue cells. Monocyte chemoattractant protein, MCP-1/CCL2, is one of the best-studied chemokines that is expressed by mainly inflammatory cells and stromal cells such as endothelial cells, and its expression is upregulated following pro-inflammatory stimuli and tissue injury.9,10 MCP-1 can function as a traditional chemotactic cytokine and also regulates gene transcription.9,10 Many reports indicate that endoplasmic reticulum (ER) stress and autophagy are involved in the induction of inflammatory response and contributes to the pathogenesis of chronic inflammatory diseases.11,12. Understanding the mechanisms involved in the regulation of inflammation may help to identify novel therapeutic targets with important clinical applications. This review will discuss the pathogenesis of cardiovascular and associated diseases involving MCP-1-mediated processes. We focus on the key inflammatory signaling pathways related to the development of cardiovascular diseases. We present experimental evidence which suggest that MCP-1 triggers the expression of a recently identified novel zinc-finger protein which induces ER stress that leads to autophagy and controls inflammatory response by negatively regulating nuclear factor-κB (NFκB), a master controller of inflammation, by catalyzing deubiquitination. Potential novel strategies to treat cardiovascular disease using agents that target MCP-1/CCR2 signaling are also briefly reviewed.
2. Inflammatory signaling pathways in cardiovascular disease
There is abundant evidence that inflammatory processes are involved in cardiovascular injury resulting from ischemia and/or reperfusion, thrombosis and infection.7,8,13. For example, myocardial inflammation has been implicated as a secondary injury mechanism following ischemia and reperfusion.13 Inflammation is also a major component of the damage caused by infectious diseases such as myocarditis and rheumatic heart disease, and is also a fundamental contributor to atherosclerosis, ischemic heart disease and heart failure as well as transplant vasculopathy and stroke.7,8 The inflammatory response is a complex process consisting of many components and their interactions, and these inflammatory molecules and pathways are tightly interrelated and alter cellular physiology leading to various pathologies in the cardiovascular system.7,8
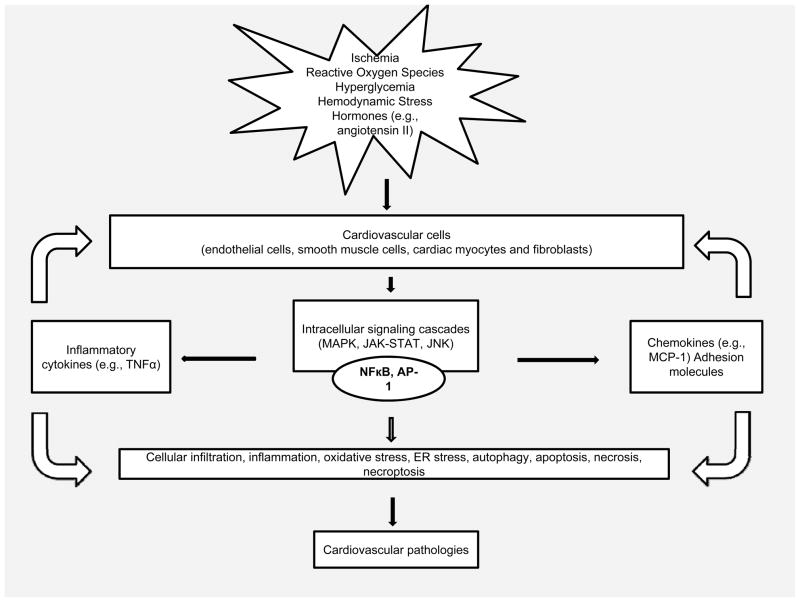
The major cellular players involved in the inflammatory reaction are monocytes/macrophages, neutrophils, mast cells, blood platelets and T cells. They are activated rapidly in response to tissue injury or infection, and exert diverse actions. In the cardiovascular system, endothelial and smooth muscle cells, cardiomyocytes as well as fibroblasts participate in the inflammatory reaction.8 Tissue macrophages are the primary cells implicated in cardiovascular inflammation. The presence of damaged cells and cell debris can cause the resident macrophages to be activated, leading to release of pro-inflammatory cytokines and other molecules, like reactive oxygen species (ROS) and proteases, which can cause damaging or protective effects on neighboring cells (e.g., cardiomyocytes). In response to injury, both endothelial cells and cardiomyocytes can change into a pro-inflammatory phenotype and produce some inflammatory mediators, such as MCP-113,14,15 These molecules are key contributors to tissue injury by their ability to attract circulating blood cells (e.g., monocytes, neutrophils and lymphocytes) and facilitate the transmigration of these cells into the site of damage. These infiltrating cells can also produce inflammatory mediators that can locally activate and interact with resident tissue cells, resulting in the initiation and progression of cardiovascular disease (Figure 1).
Figure 1.
Schematic representation of inflammatory response leading to cardiovascular pathologies.
The inflammatory reaction is generally triggered by the activation of pattern recognition receptors (PRRs), e.g. toll-like receptors (TLRs) and nucleotide oligomerization domain-like receptors (NLRs), which recognize pathogens or pathogen-associated molecular patterns (PAMPs).16,17 Endogenous products derived from damaged cells and tissues, termed damage-associated molecular pattern (DAMP), can initiate an intense inflammatory response in the same way as pathogens.18,19 We demonstrated that Fas ligands released by dying infiltrating inflammatory cells can initiate an intense inflammatory response and contribute to the development of ischemic cardiomyopathy and heart failure.20–22 PRRs are present not only in immune cells such as macrophages and dendritic cells, but also in the “non-professional” immune cells such as endothelial cells, vascular smooth muscle cells and cardiomyocytes.16 Currently, very little is known about the effects of NLRs in the cardiovascular system. TLRs are implicated in a range of cardiovascular diseases and syndromes including atherosclerosis, sepsis, ischemia, cardiac remodeling and heart failure.23 TLR signaling network has already been reviewed.14,24 Briefly, the binding of PAMP/DAMP to a PRR leads to a sequential cascade of different transcriptional regulatory events, resulting in inflammatory responses. NFκB, which is activated by many stimuli, is a central player involved in the inflammatory processes associated with the development of cardiovascular diseases.25,26 NFκB activation triggers the production of molecules such as adhesion molecules, chemokines, and proinflammatory cytokines, all of which are involved in inflammatory responses. In turn, these responses lead to infiltration of neutrophils, monocytes, and lymphocytes into the sites of tissue damage, where activation and interaction with resident tissue cells, result in sustained inflammation. Other distinctive signaling pathways, such as the mitogen-activated protein kinase (MAPK) pathway, phosphoinositide 3-kinase (PI3K)-related signaling pathway, and Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathway, interacting with the TLR4 signaling pathway, were also implicated in the pathogenesis of cardiovascular diseases.27,28 These different pathways activate a series of downstream transcriptional factors, produce a great quantity of inflammatory cytokines, such as interleukin (IL)-1, tumor necrosis factor (TNF)-α, and initiate inflammatory responses.
3. ER stress in inflammation
In recent years, considerable evidence has demonstrated that inflammation within the cardiovascular system is linked to the endoplasmic reticulum (ER) stress and unfolded protein response (UPR), which alters gene expression and translational programs to overcome stressful conditions and to restore ER homeostasis.11,12,29,30 Recent evidence suggests that ER stress is a key factor in the inflammatory response, and a potential mediator of inflammation in cardiovascular disease.31–33
3.1. ER stress and UPR signaling
The intracellular pathways that mediate UPR have been reviewed.34,35 These UPR signaling cascades are initiated via three ER-localized transmembrane proteins, namely, inositol-requiring enzyme 1 (IRE1), pancreatic ER kinase (PERK), and activating transcription factor 6 (ATF6). In the absence of stress, these three proteins associate with the 78 kDa glucose-regulated protein (GRP78) and remain in an inactive state. Under ER stress, GRP78 preferentially binds to unfolded or misfolded proteins, resulting in the release and activation of these transmembrane proteins. In response to ER stress, IRE1α autophosphorylation can elicit endoribonuclease activity that cleaves off a 26-base intron from the mRNA encoding X-box binding protein-1 (XBP1), resulting in expression of active XBP1 with potent transcriptional activity that activates expression of UPR target genes.36
PERK is one of the stress kinases responsible for phosphorylation of eIF2 protein and consequent inhibition of protein synthesis.37 Phosphorylated eIF2α can also increase the expression of ATF4 by alternate translation.38 This translational induction of ATF4 can induce expression of UPR target genes involved in oxidative stress. PERK phosphorylation can also activate nuclear erythroid 2-related factor 2-driven expression of genes that encode antioxidant enzymes that are thought to counteract oxidative stress initiated by ER stress.39
Upon release of ATF6 from GRP78, it translocates from ER to the Golgi where site-1 protease (S1P) and S2P cleaves off the cytosolic domain of ATF6 from the membrane, resulting in the translocation of its functional fragment containing a basic leucine zipper transcription factor to the nucleus, leading to the activation of transcription of a set of UPR genes encoding chaperones and protein modifying enzymes related to protein folding and ER-associated degradation (ERAD).40,41 If these signaling mechanisms fail to resolve the mismatch between protein load and handling capacity over a sustained period of time, the UPR induces autophagy as an alternative coping mechanism to remove unfolded or misfolded proteins in the ER lumen that cannot be degraded by ERAD. Although the proteins targeted by autophagy and the ubiquitin proteasome system are different, the two systems serve a similar purpose contributing to maintenance of cellular homeostasis.
3.2. Integration of ER stress and inflammation
Upon tissue injury, inflammatory cells (e.g., neutrophils and macrophages) are recruited to the site of damage, leading to the production of inflammatory cytokines and generation of ROS. Such factors could trigger ER stress. It has been shown that activation of TLR signal can activate IRE1 and its downstream target XBP1 that is required for the production of proinflammatory cytokines such as TNF-α, MCP-1, IL-6, IL-8 and CXCL3 in macrophages and endothelial cells, resulting in enhanced TLR responses contributing to inflammation.42–44 XBP1 is also required for the differentiation of B lymphocytes and dendritic cells, both of which are critical in mediating inflammatory response and production of cytokines.45
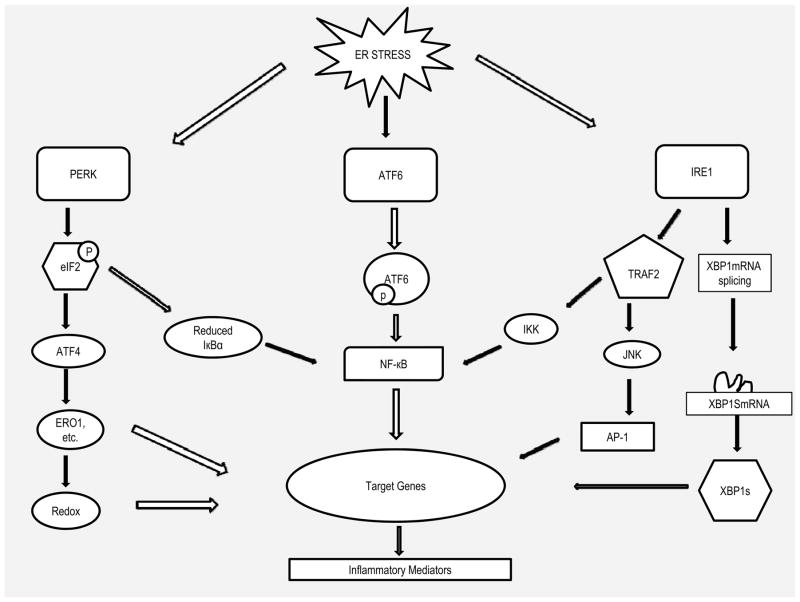
The three UPR signal pathways are tightly interrelated with inflammatory pathways (Figure 2). An increase in the protein load in the ER has been shown to induce NFκB activation.29,32 In response to ER stress, PERK-eIF2α-mediated translation suppression of IκB can directly induce NFκB activation by increasing the ratio of NF-κB to IκB, thereby allowing the excess NFκB to enter the nucleus to trigger expression of inflammatory cytokines.46 IRE1α has been increasingly recognized as a key molecule integrating ER stress signaling to inflammatory signaling pathway by interaction with TRAF2 (TNF receptor-associated factor 2), which activates protein kinases, resulting in the activation of several inflammatory signals.47–49 Upon ER stress, the autophosphorylation of IRE1α leads to its binding to TRAF2 leading to recruitment of IκB kinase (IKK) and promote NFκB-mediated inflammation. The IRE1α-TRAF2 complex can also interact with JNK (Jun N-terminal Kinase), which activates transcription factor activator protein 1 (AP-1), resulting in expression of inflammatory genes.50 ATF6 has been linked to acute-phase-response, a broad immunological process associated with infiltration and activation of inflammatory cells and production of inflammatory mediators.51 Cleavage of ATF6 leads to transcriptional activation of a set of acute-phase proteins such as C-reactive protein (CRP), which is a well-known marker of systemic inflammatory response. CRP can cause elevated expression of MCP-1 receptor and thus contribute to inflammation.52,53 In addition, ATF6 itself can trigger NFκB-mediated inflammation via Akt kinase phosphorylation.54
Figure 2.
Signal transduction events that link ER stress to inflammation. Unfolded proteins in the ER cause release of IRE1, PERK, and ATF6. Once released, IRE1α binds TRAF2, activating signaling downstream kinases that activate NFκB and AP-1, causing expression of genes associated with inflammatory response. The intrinsic ribonuclease activity of IRE1α also results in production of XBP1, inducing production of inflammatory cytokines by enhancing TLR signaling and differentiation of B lymphocytes and dendritic cells. PERK activates its intrinsic kinase activity, causing phosphorylation of eIF2 and attenuation of translation of IκB, resulting in excess of NFκB moving to the nucleus and induces expression of genes involved in inflammatory pathways. Selective activation ATF4 by PERK induces production of inflammatory cytokines and regulates redox homeostasis. Activation of ATF6 also induces activation of NFκB. ATF6 can also induce production of XBP1 (not shown).
Inflammatory pathways and ER stress are integrated also via the intracellular calcium and the generation of ROS.12,32 NO synthesized by inducible NO synthase (iNOS) in inflammatory cells can activate ER stress signaling pathways through disturbance of ER Ca2+ homeostasis, or enhancing ROS generation, or inhibiting protein disulfide isomerase (PDI) by NO-induced S-nitrosylation.33 Under ER stress condition, the increased protein folding triggered by UPR can result in the generation of ROS due to Ca2+ release from ER via inositol-trisphosphate receptors.32 ROS generated by inflammation can also induce ER stress by inhibition of protein disulfide isomerase (PDI) and subsequent accumulation of polyubiquitinated proteins, which can trigger NFκB signaling activation and inflammatory response.55 Thus, ER stress is an important component of chronic inflammation, which plays a critical role in the development of cardiovascular diseases.
3.3. ER stress-mediated autophagy in cardiovascular disease
Multiple components of the UPR signaling link ER stress to cell death, including activation of transcription factors, proteases, kinases, and Bcl-2 family proteins.35 ER stress-mediated signaling can often induce apoptotic and non-apoptotic cell death.35,56 Several UPR signaling pathways have been linked to the induction of autophagy.57 For example, activation of IRE1 can induce autophagy probably through interaction with TRAF2 and activation of JNK, which are known to control the key autophagy regulator Beclin-1 expression.58 PERK-dependent eIF2α phosphorylation also plays an important role in the induction of autophagy (Atg) via upregulation of Atg12.57 It was recently reported that XBP1 ablation induces autophagy and protects against amyotrophic lateral sclerosis.59 A basal level autophagy plays an important role in the normal healthy functioning of cardiomyocytes. Thus, cardiac specific deletion of the autophagy gene, atg5, in adult mice induced rapid development of cardiac hypertrophy and contractile dysfunction.60 The human disease resulting from the impairment of autophagy manifests severe cardiomyopathy with left ventricular dysfunction.61,62 Autophagy is also thought to play a key role in hypoxic adaptation that can be critical for survival of the heart through mild ischemia conditions.61,63 Autophagy helps generate ATP from the digestion products and this helps cells to survive through the hypoxic period, thus allowing full recovery when hypoxia is eased. Autophagy can protect against cell death by removing damaged mitochondria and consequently reducing pro-apoptotic factors such as ROS and cytochrome C, as well as, by removing toxic protein aggregates.63,64 Thus, autophagy contributes to the survival of cardiac myocytes during transient mild hypoxia or chronic mild hypoxia.
On the other hand, under the nearly complete ischemia that occurs in the risk area in myocardial infarction, autophagy is unable to prevent cardiomyocyte death except in the border zone that may get some oxygen from adjacent areas through diffusion.61 At the reperfusion phase, autophagy occurs but the cells die as autophagy is no longer capable of protecting against death caused by the extreme stress. Thus, ischemia/reperfusion induced infarct size and the number of autophagosomes were smaller in beclin-1 deficient mice.65 In vitro, inhibition of autophagy in cardiomyocytes with chemical inhibitors or gene knock down protected against ischemia reperfusion.66 There is good evidence for the role of autophagy in cell death caused by ischemia/reperfusion in the brain. Focal cerebral ischemia induces delayed cell death of neurons in peri-infarct zone.67 In such cells, elevated expression of beclin-1 and accumulation of autophagosomes/autolysosomes were found. Furthermore, brain specific knockout of atg7 in mice caused inhibition of ischemia-induced death of neurons and autophagy. Thus, autophagy is involved in cell death in both the heart and the brain subjected to the extreme stress of ischemia/reperfusion. It appears that autophagy can be protective under mild stress whereas it contributes to damage by causing cell death under very severe stress.
There is increasing evidence that autophagy plays a role in differentiation, development and cell death.68 Studies have shown that autophagy is important for cell differentiation during erythropoiesis,69,70 lymphopoeisis71,72 and adipogenesis.73,74 This aspect is discussed in a later section. When autophagy cannot control protein and organelle quality the result is a form of non-apoptotic cell death known as “autophagy-dependent necroptosis” or “necroptosis”, which involves Fas/TNF-α death domain receptor activation and inhibition of receptor-interacting protein (RIP) 1 kinase.75,76 Such a death process contributes to the pathogenesis of cardiovascular diseases, including ischemic injury and cardiomyopathy.77,78 It has been also shown that necroptosis is a driver of injury-induced inflammation by death receptors.75,79 Thus, prolonged ER stress can lead to autophagy and apoptotic or necroptotic cell death or differentiation depending on cellular context.
3.4. ER stress in cardiovascular disease
There is compelling evidence that ER stress plays fundamental roles in the development and progression of cardiovascular diseases such as ischemic heart diseases and atherosclerosis. In the heart, hypoxia, ischemia/reperfusion (I/R), pressure overload, and inflammation can result in activation of ER stress.80 Several studies have demonstrated that ER stress is activated in rodent models of myocardial ischemia/reperfusion,81 pressure overload,82 and myocardial infarction.83 In humans with heart failure, cardiac ER stress has been evidenced by the existence of spliced XBP1 and induction of GRP78, ATF4, and CHOP gene expression.82,84 We have reported that ER stress-associated genes, including CHOP, are induced in the heart of a mouse model with ischemic heart disease.85 It was demonstrated that myocardial I/R induce ER stress response and CHOP-mediated signaling that subsequently causes cardiomyocyte apoptosis and enhances myocardial inflammation, possibly by the transcriptional induction of proinflammatory cytokine genes such as IL-1β and IL6.86 However, CHOP deficiency almost completely inhibited cardiomyocyte apoptosis in the reperfused myocardium86 and neuronal cell death in the reperfused mice brain.87 CHOP deficiency also suppressed cardiac hypertrophy and heart failure induced by pressure overload.88 Overexpression of tamoxifen-activated form of ATF6, one of the ER stress sensors on the ER membrane, induced expression of ER chaperones such as GRP78 and GRP94, resulting in protection against myocardial reperfusion injury.86 Treatment of mice with ATF6-specific inhibitor, 4-(2-aminoethyl) benzenesulfonyl fluoride, led to myocardial dysfunction and an increased mortality rate after myocardial infarction.89 In addition, general inhibition of ER stress response by a protein kinase C inhibitor led to suppressed cardiomyocyte apoptosis and limited infarct size.90 Experimental autoimmune cardiomyopathy induced by injection of the β1 adrenergic receptor peptide has been also shown to be associated with cardiac ER stress as indicated by induction of Grp78 and CHOP gene expression.91 Furthermore, transgenic mice expressing a mutant of KDEL receptor (a retrieval receptor for ER chaperone in the early secretory pathway) or expressing dominant negative mutant of ATF6 showed dilated cardiomyopathy, enhanced expression of CHOP and apoptotic cardiomyocyte death, and compromised cardiac function.89,92
ER stress-mediated inflammatory pathway has been suggested to play a role in the development of atherosclerosis. Activation of ER stress is implicated in atherosclerotic lesion at all stages of atherosclerosis.93 Increased expression of ER stress-associated genes, including CHOP, was detected in endothelial cells subjected to atherosclerosis-prone shear stress,94 and macrophages and smooth muscle cells within ruptured plaques.95 The levels of expression of ER stress-associated genes in atherectomy specimens from patients with unstable angina pectoris are higher than those from patients with stable angina pectoris.95 A causal link between ER stress and progression of atherosclerosis was indicated by the finding that the development of atherosclerosis was suppressed in the CHOP-deficient mice mated with two distinct models of atherosclerotic mice.96 Accumulation of free cholesterol in the ER is considered as one of the main causes of ER stress leading to activation of UPR and CHOP-induced apoptosis in atherosclerotic lesion,97 and ER stress induced apoptosis in macrophages is thought to be a major contributor to the instability of atherosclerotic plaques.98,99
4. MCP-1 links ER stress and cardiovascular inflammation
Several studies have linked ER stress with production of various pro-inflammatory molecules such as IL-8, IL-6, MCP-1 and TNF-α.100,101 Of these, MCP-1 is widely recognized to be a major component of chronic inflammation associated with a variety of major diseases including cancer, obesity-associated type 2 diabetes and cardiovascular diseases.9 Here we review the key role of MCP-1 in inflammation, ER stress and the development of cardiovascular disease, with brief reference to a major risk factor, obesity and diabetes.
4.1. Role of MCP-1 in ER stress in atherosclerosis
The pathogenic role of MCP-1 in atherosclerosis is well-established.9,102 MCP-1 is produced by many cell types, including fibroblasts, cardiomyocytes, endothelial and smooth muscle cells, and monocytic cells.9,102 Many stimuli such as oxidative stress, cytokines, metabolic factors and shear stress can cause the production of MCP-1. Hyperlipidaemia and oxidized lipids can induce MCP-1 production in vascular cells and stimulate the local accumulation of monocytic cells in the vascular wall, resulting in increased foam cell formation, inflammation and progression of atherosclerosis.9 Inflammatory cell infiltration of the vascular wall plays a critical role in both hemorrhagic and ischemic stroke103,104 and MCP-1 knockout mice were shown to have reduced incidence of intracranial aneurysm.104 Inflammatory factors cause ER stress and activation of the UPR.31–33 Acrolein, a ubiquitous environmental pollutant and an endogenous product of lipid peroxidation, can induce ER stress and trigger the UPR and stimulate MCP-1 production in endothelial cells through a pathway which involves activation of NFκB signaling.101 ER stress-induced transcription factors, ATF4 and XBP1, are involved in the production of MCP-1, IL-6, IL-8 and CXCL3 by human aortic endothelial cells in the normal state and in the presence of oxidized lipids.42 In cholesterol-fed macrophage, UPR is activated resulting in increased CHOP expression,105 which could lead to the death of macrophages and smooth muscle cells, contributing to development of atherosclerotic lesions and plaque rupture.96 A strong association between up-regulation of ER stress markers and atherosclerotic plaque rupture was also found in human coronary artery autopsy.95 MCP-1 was reported to induce plaque destabilization by increasing activity of the ubiquitin-proteosome system in the inflammatory macrophages.106 These findings suggest that MCP-1 may link ER stress to inflammation that leads to atherosclerosis.
4.2. Role of MCP-1 in ER stress involved in heart disease
MCP-1 induction is a prominent feature of ischemic myocardium, and increased expression of MCP-1 contributes to the pathogenesis of cardiac hypertrophy, inflammation and heart failure induced by chronic mechanical overload, myocardial infarction and diabetes.9,107 Disruption of MCP-1/CCR2 axis decreases and delays macrophage infiltration, reduces production of cytokines, TNF-α, IL-1β, and transforming growth factor (TGF)-β, as well as attenuates cardiac remodeling. MCP-1 gene knockout107 or inhibition of MCP-1 function, by overexpression of N-terminal deleted MCP-1 gene,108 and genetic disruption of CCR2109 also demonstrate the central role of MCP-1/CCR2 in cardiovascular diseases. To investigate the role of chronic inflammation in the development of cardiovascular disease we generated mice with cardiac-specific expression of MCP-1.110 These mice manifested many pathological and molecular alterations of human ischemic cardiomyopathy.9 This transgenic mice model manifested features found in human diseases such as intraarterial thrombosis9 that are said to be not usually manifested in animal models.5 These transgenic mice manifested activation of ER stress, inflammation and apoptotic cell death in the myocardium, resulting in the development of ischemic cardiomyopathy and heart failure at 6 to 7 months of age,9,85 suggesting that ER stress is likely involved in the development of myocardial inflammation and dysfunction. In support of this conclusion was the finding that cerium oxide nanoparticles, a free radical scavenger, attenuated oxidative and ER stress and ischemic cardiomyopathy.111 As indicated below hyperglycemia-induced cardiomyocyte death is also mediated via ER stress.112
The young transgenic animals expressing MCP-1 in the heart manifested a preconditioning-like cardioprotection against I/R injury and post-infarction cardiac dysfunction.113,114 It appears that MCP-1-induced ER chaperones may contribute to “preconditioning” of the heart against subsequent myocardial oxidative- and ER-stress as well as inflammation caused by ischemia. This conclusion is supported by the recent findings that ischemic preconditioning or postconditioning reduced cardiac damage associated with activation of UPR.115–117 Overexpression of GRP94 attenuates myocyte necrosis induced by ischemia in cultured H9c2 cardiac myoblasts.118 Cardioprotection by ischemic preconditioning is found to be closely related to induction of GRP78.115,119 Overexpression of the ER-stress induced protein, PDI, in the mouse heart showed protection against cardiomyocyte apoptosis and adverse cardiac remodeling in a mouse model of myocardial infarction.120,121 Preconditioning with ER stress mitigates retinal endothelial inflammation, which is likely through activation of XBP1-mediated UPR and inhibition of NFκB activation.122 Priming of the non-immune cells with ER stress inducers, like tunicamycin and thapsigargin, caused blunted induction of MCP-1 in response to TNF-α or IL-1β, and this suppression of MCP-1 production under ER stress conditions was correlated with the level of GRP78.123
5. Discovery of a novel CCCH zinc-finger protein, MCPIP, and its function
How MCP-1 causes migration of cells via binding to its receptor CCR2, present on the target cells has been studied extensively.9,10,102 However, the evidence that has been accumulating on the role of MCP-1 in a variety of inflammatory diseases indicates that MCP-1 probably plays additional roles other than its well-established chemotactic function. The signal transduction events initiated by MCP-1 binding to CCR2 could result in induction of genes that could play a significant role in the development of inflammatory processes. However, the nature of the genes induced by MCP-1 and their potential role in inflammatory processes is not well understood. Efforts to fill this void resulted in our discovery of MCP-1 induction of a novel class of zinc-finger proteins in human peripheral blood monocytes as a consequence of the chain of events triggered by MCP-1 binding to CCR2.124 This MCP-1 induced protein, called MCPIP turned out to be the first member of a novel family of CCCH zinc finger proteins containing four members that we designate MCPIP 1, 2, 3 and 4 encoded by zc3h12a, zc3h12b, zc3h12c and z2ch12d, respectively.125 The best-studied member MCPIP1 is often simply called MCPIP. A genome-wide analysis of the CCCH zinc finger gene family revealed 58 such genes in mice and 55 in humans. At least seven of them were found to be expressed in macrophage related organs such as thymus, spleen, lung, intestine and adipose tissues.125
5.1. MCPIP mediates inflammation-associated differentiation via oxidative and ER stress and autophagy
Differentiation associated with inflammation is involved in at least three pathophysiological processes a) angiogenesis associated with cardiovascular diseases, obesity/type 2 diabetes and tumor growth b) adipogenesis associated with obesity c) osteoclastogenesis associated with inflammatory bone erosion. Recent evidence shows that MCP-1/MCPIP system plays a critical role in all of these differentiation processes.
Angiogenesis plays a major role in the reparative and remodeling processes following myocardial injury as well as in the pathogenesis of atherosclerosis and in providing blood to the growing adipose tissue and tumors. MCP-1 is an angiogenic factor associated with the recruitment of monocytic cells.126–128 MCP-1 mobilizes and transdifferentiates bone marrow monocyte lineage cells into endothelial-like cells.126,127 Recent reports strongly suggest that increased expression of MCP-1 in adipose tissue causes macrophage infiltration into adipose tissue, insulin resistance and hepatic steatosis associated with obesity in mice.129,130 It was shown that an acute increase in circulating concentration of MCP-1 elicited systemic insulin resistance irrespective of adipose tissue inflammation in mice.131 That the role of MCP-1 in type 2 diabetes is mediated via its binding to the receptor, CCR2, was demonstrated by results obtained with CCR2−/− mice.132,133 CCR2 deficiency attenuated the development of obesity in high fat-fed mice. CCR2 deficiency resulted in reduced macrophage content, increased adiponectin expression, amelioration of hepatic steatosis and insulin resistance. Treatment with a pharmacological antagonist of CCR2 lowered macrophage content of adipose tissue and improved insulin sensitivity in obese mice.134 Thus, MCP-1/CCR2 system plays a critical role in obesity-induced diabetes in mice. Many recent observations suggest that in humans, MCP-1 plays a similar role in obesity-induced diabetes. The increased level of serum MCP-1 levels found in humans correlated with markers of the metabolic syndrome, including obesity, insulin resistance, type 2 diabetes, hypertension, and increased serum concentration of triacylglycerol.135 In diabetic patients CCR2 expression in monocytes is elevated.136
MCP-1/MCPIP plays a major role in angiogenesis, as indicated by the finding that tube formation in human umbilical vein endothelial cells (HUVEC) was induced by forced expression of MCPIP.137 MCP-1-induced tube formation was inhibited by knockdown of MCPIP indicating that MCP-1-induced angiogenesis is mediated via MCPIP. In this case the transcription factor activity of MCPIP probably plays an important role. Chromatin immunoprecipitation analysis indicated that cadherins, (cdh)12 and cdh19, are targets of MCPIP. Knockdown of cdh12 and cdh19 caused marked inhibition of MCPIP-induced tube formation by HUVEC. Since cadherins are likely to be involved in cell-cell adhesion of endothelial cells required for tube formation, it is reasonable to conclude that MCPIP induced expression of these cadherins is important for angiogenesis. Angiogenesis also involves MCPIP-mediated induction of hypoxia inducible factor, (HIF)-1α, that induces vascular endothelial growth factor (VEGF) that causes induction of oxidative stress leading to ER stress and autophagy required for the differentiation. Selective inhibition of each of these postulated steps with chemicals or gene knockdown inhibited subsequent steps in the sequence and tube formation (A. Roy and P.E. Kolattukudy, unpublished). Additional interplay between the intermediary processes is probably involved in this differentiation process. For example, the ER stress induced UPR can contribute to VEGF expression. Three UPR signaling pathways can independently regulate VEGF expression under ER stress via different regulatory regions of the VEGF gene.138
MCP-1/MCPIP is involved in adipogenesis.139 MCP-1 treatment or forced expression of MCPIP caused induction of CCAAT/enhancer-binding protein (C/EBP) family of transcription factors and peroxisome proliferator-activated receptor (PPAR)-γ ays well as adipocyte markers and a robust accumulation of lipid droplets in 3T3-L1 cells in the absence of the classical adipogenesis-inducing ingredients. Surprisingly, MCPIP expression was found to induce robust adipogenesis in the absence of PPARγ that had been thought to be indispensable for adipogenesis.139 MCPIP expression in 3T3-L1 cells caused inducible NO synthase (iNOS) induction and elevated reactive oxygen/nitrogen species production that caused ER stress that led to autophagy required for differentiation.140 Inhibition of any of these steps with selective chemical inhibitors or gene knockdown, inhibited the postulated subsequent steps and adipogenesis (C Younce and P.E. Kolattukudy, unpublished). Involvement of autophagy in adipogenesis was demonstrated by the inhibition of adipogenesis by knockdown of Atg7 or Atg5 in 3T3-L1 cells.140 The role of autophagy in adipogenesis in vivo was demonstrated by the finding that Atg deficient mice had fewer adipocytes141 and adipocyte specific knockout of Atg7 resulted in a decrease in white adipose tissue.142
MCP-1 induced osteoclast differentiation is involved in inflammatory bone erosion involved in diseases such as rheumatoid arthritis, multiple myeloma and bone metastasis.143,144 MCP-1 was reported to cause differentiation of monocytic cells into osteoclast precursors145 and MCP-1 deficiency caused a reduction in osteoclasts and such mice showed osteopetrosis.146 The MCP-1-mediated differentiation of bone marrow-derived monocytic cells to osteoclast precursors was recently found to be mediated via MCPIP induced oxidative and ER stress that resulted in autophagy required for the differentiation.147 Involvement of autophagy in hypoxia-induced osteoclastogenesis was recently reported.148
5.2. MCPIP-induced death mediated via oxidative and ER stress and autophagy
MCPIP was found to induce death in HEK293 cells.124 Microarray analysis showed that MCPIP expression caused elevation of expression of death-associated genes such as components of caspase activation, cytochrome C release, unique subsets of the Bcl-2 family of genes and the TNF receptor family members.149 In transgenic animals with cardiomyocyte targeted MCP-1 expression, MCPIP was found in dying cardiomyocytes suggesting that cardiomyocyte death might have been mediated via MCPIP.124 Forced expression of MCPIP in the H9c2 cardiomyoblasts caused cell death as indicated by caspase 3 activation and TUNEL assay.149 Hyperglycemia-induced cardiomyocyte death, that probably plays a critical role in diabetic cardiomyopathy, was found to be mediated via production of MCP-1 and induction of MCPIP.112
In terminally differentiated cardiomyocytes, the MCP-1-initiated processes, mediated via MCPIP, leads to death. The intermediate processes initiated by MCPIP that leads to cardiomyocyte death were recently elucidated.112,149 MCPIP expression causes induction of reactive oxygen/nitrogen species, that caused ER stress, that lead to autophagy. In cardiomyocytes, autophagy is not able to cause cell survival but causes cell death. This postulate regarding the sequence of events is supported by the finding that inhibition of oxidative stress, ER stress, or autophagy by chemical inhibitors or by gene knockdown prevented each subsequent step and cell death. Activation of JNK and p38 and induction of p53 and PUMA (p53 upregulated modulator of apoptosis) by MCPIP appears to be involved in the MCPIP induction of apoptosis. A similar chain of events is involved in hyperglycemia-induced MCP-1/MCPIP-mediated cell death in H9c2 cells and in neonatal rat cardiomyocytes.112 Many forms of stress on the myocardium, such as pressure overload, chronic ischemia and I/R, induce autophagy and cell death and thus contribute to the cardiovascular pathology.65,66,150 Histone deacetylase inhibitors were shown to attenuate cardiac hypertrophy by suppressing autophagy.151
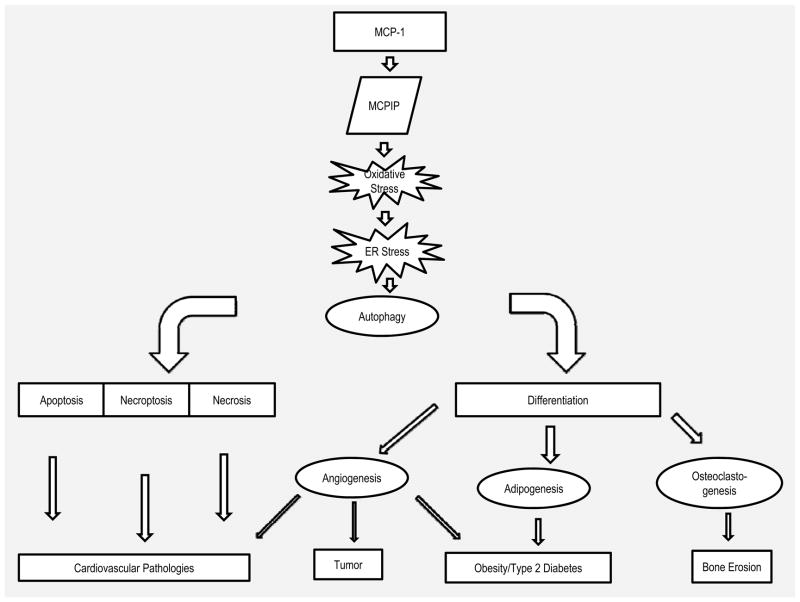
It has been recognized that death and differentiation share some common components such as caspases.152 However, the biological processes shared between death and differentiation pathways remain obscure. Although a role for individual processes such as oxidative stress, ER stress and autophagy in cell death and differentiation has been recognized in scattered reports, how these processes are interrelated in bringing about death or differentiation has not been clear. Inflammatory processes lead to death of cells in cardiomyopathy whereas inflammation can also lead to differentiation processes such as, angiogenesis, adipogenesis and osteoclastogenesis. We have found that in all of these processes oxidative stress resulting from inflammation causes ER stress that leads to autophagy that can lead to cell death in the case of terminally differentiated cells such as cardiomyocytes or differentiation in preadipocytes and monocytic cells (Figure 3).
Figure 3.
Schematic representation of the MCP-1/MCPIP-induced processes in inflammatory diseases. MCPIP promotes oxidative stress that leads to autophagy that can cause different forms of cell death or differentiation that leads to angiogenesis, adipogenesis and osteoclastogenesis.
6. MCPIP controls inflammation by inhibition of NFκB activation
Assessment of changes in the expression of all of the CCCH zinc-finger gene family during LPS activation of mouse macrophages showed that most of this family of genes is associated with macrophage activation.153 Induction of MCPIP by inflammatory agents such as LPS, TNF-α and IL-1 appears to be a mechanism by which inflammation can be controlled, and thus can be viewed as an autoregulatory feedback mechanism. For example, macrophage activation induced by LPS and cytokines was inhibited by MCPIP expression.153 Thus; forced expression of MCPIP inhibited LPS-induced production of inflammatory cytokines and oxidative stress. Knockdown of MCPIP expression with siRNA enhanced LPS-induced inflammatory gene expression. Reporter gene expression controlled by TNF-α and iNOS promoters were severely inhibited by MCPIP. NFκB is the well-known integrator of inflammation caused by a variety of inflammatory agents including LPS, IL-1, and TNF-α25,26 and thus could be a target of MCPIP action. In fact, forced expression of MCPIP inhibited LPS-induced NFκB activation.153 Expression of MCPIP inhibited p65-induced activation of TNF-α, iNOS and NFκB promoters both in vitro and in vivo.
How MCPIP regulates NFκB activation is only beginning to be explored. Mice lacking MCPIP (MCPIP−/−) showed systemic inflammation with elevated serum levels of TNF-α and MCP-1.154,155 MCPIP−/− bone marrow-derived macrophages secreted greater amounts of proinflammatory cytokines. Elevated levels of TNF-α, IL-β, IL-6 and MCP-1 were found in MCPIP−/− macrophages and the LPS-induced levels of such inflammatory cytokines were much higher in MCPIP−/− macrophages.154 Expression of such cytokines and iNOS in intestine, lung and thymus was much higher in MCPIP−/− mice that showed stunted growth and shortened life span of less than four months. Lungs of the MCPIP−/− mice showed elevated phosphorylation of JNK and IKKβ suggesting that the absence of MCPIP caused constitutive activation of JNK and NFκB signaling. LPS-induced phosphorylation of JNK, ERK 1/2 and IKKβ was higher in MCPIP−/−indicating that MCPIP is essential for down regulation of LPS-induced JNK and NFκB activation. Forced expression of MCPIP in Raw 264.7 macrophage cell line blocked LPS-induced phosphorylation of JNK and IKKβ and nuclear translocation of p65. Gene array analysis of LPS-induced gene expression changes in Raw 264.7 cells showed that expression of genes encoding inflammatory agents, TNF, IL-1β, IL-6 and MCP-1, whose transcription is known to be controlled by NFκB and JNK signaling, were repressed by MCPIP.153 Thus, NFκB, the master controller of inflammation is probably the target of MCPIP.
The in vivo role of MCPIP as an inhibitor of NFκB has been recently demonstrated.155 Cardiac specific expression of MCPIP in mice resulted in marked attenuation of LPS-induced deterioration of myocardial contractile function.156 MCPIP expression caused marked reduction in the LPS-induced production of inflammatory cytokines, iNOS expression and peroxynitrite formation as well as reduced caspase activation and apoptosis in the heart. That these protective effects were mediated via inhibition of IKK activation was suggested by the decreased phosphorylation of IKKα/β, reduced degradation of IκBα and decreased nuclear translocation of p65 subunit of NFκB.156
7. MCPIP inhibits NFκB activation via its deubiquitinase activity
Ubiquitination plays critical roles in JNK and NFκB signaling.157 Expression of MCPIP in HEK293 cells caused a decrease in ubiquitinated protein levels suggesting that MCPIP might either inhibit ubiquitination or deubiquitinate proteins.154 Direct test for deubiquitinase activity showed that purified MCPIP cleaved both K48 and K63 linked polyubiquitin with a preference for higher molecular weight polyubiquitin chains such as Ub6 and Ub8. N-ethyl maleimide inhibited polyubiquitin hydrolase activity of MCPIP suggesting that it is a Cys proteinase type enzyme. In vivo substrates of MCPIP have not been clearly identified. TRAFs are known to be ubiquitinated.157 Stimulus-dependent autoubiquitination of TRAF2 and TRAF6 activates TAK 1 that plays a key role in NFκB activation (Figure 4). Ubiquitinated TRAF2 and TRAF6 levels were lowered by expression of MCPIP in HEK293 cells.154 MCPIP also affected RIP that modulates NFκB activity. MCPIP expression caused a major decrease in IkBα ubiquitination. MCPIP−/− caused a drastic increase in basal levels of ubiquitinated TRAF2, TRAF3 and TRAF6 in splenocytes and LPS-treatment caused a further increase in these polyubiquitinated proteins. Isolated polyubiquitinated TRAF2 and TRAF3 were deubiquitinated by incubation with MCPIP.154 Thus, TRAF family members involved in NFκB signaling are deubiquitinated by MCPIP.
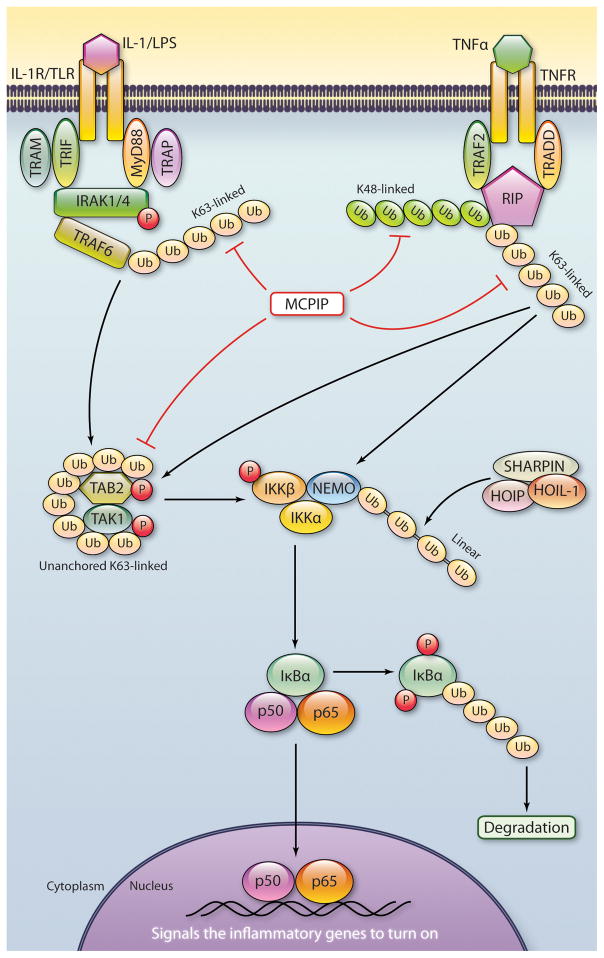
Figure 4.
Schematic representation of the ways in which the deubiquitinating activity of MCPIP plays a role in termination of NFκB activation. The K63-linked polyubiquitin attached to TRAF6 and K48- and K63-linked polyubiquitin attached to RIP and unanchored K63-linked polyubiquitins are involved in activation of IKK complex that leads to phosphorylation and subsequent ubiquitination IKBα that releases P50/p65 dimer for entry into the nucleus to cause transcriptional activation of genes encoding inflammatory agents. MCPIP hydrolyzes all of these K48- and K63-linked polyubiquitins to block NFκB activation.(Illustration Credit: Cosmocyte/Ben Smith).
Ligand activated NFκB activation is a very complex process with a major role for phosphorylation and different types of polyubiquitination of the different components involved in NFκB activation (Figure 4).157 TRAF6 is a ubiquitin ligase involved in NFκB activation triggered by binding of IL-1 receptor or TLRs. TRAF6 works with a ubiquitin conjugating enzyme complex (Ubc13) to catalyze K63 polyubiquitination that activates the TAK1 kinase complex. This activated kinase phosphorylates IKK that phosphorylates IκB leading to its K48 ubiquitination and degradation that leads to translocation of the p65/p50 to the nucleus for triggering the expression of a set of NFκB target genes (Figure 4). The nature of polyubiquitin chains required for activation of TAK1 or IKK activation is not fully understood. Using a reconstituted system, consisting of purified proteins, it was recently demonstrated that unanchored large K63 polyubiquitin chains can directly activate TAK1 by binding to the ubiquitin receptor TAB2 causing autophosphorylation and consequent activation of TAK1.158 Treatment of the K63 polyubiquitin with IsoT, an enzyme that cleaves the polyubiquitin, prevented the activation suggesting that the intact polyubiquitin was necessary for TAK1 activation. Treatment of high molecular weight K63-linked polyubiquitin with purified MCPIP caused hydrolysis of the polyubiquitin and inhibition of TAK1 phosphorylation (M. Zhang, C. Menaa and P.E. Kolattukudy unpublished). Since such unanchored polyubiquitin chains were also shown to activate IKK complex, 158 hydrolytic removal of such polyubiquitin is probably one mechanism by which MCPIP inhibits NFκB activation.
In spite of the evidence for the participation of k63-linked polyubiquitin in NFκB activation, knockout of Ubc13, encoding the E2 ligase needed to generate K63-linked chains showed little effect on NFκB activation indicating that NFκB can be activated independent of K63-linked polyubiquitin chains.159 LUBAC, composed of the two RING finger protein HOIL-IL and HOIP, activates NFκB pathway by binding to NEMO and conjugating linear polyubiquitination on to NEMO (Figure 4). In this case ubiquitin moieties are linked by peptide bonds between the carboxyl terminal Gly of one ubiquitin with the amino group of the N-terminal Met of another. This linear ubiquitination of NEMO is independent of Ubc13. Absence of HOIL-1 that encodes HOIL-1L and HOIL-1 caused severe impairment of TNFα and IL-1β induced NFκB activation in cells and in intact mice demonstrating the involvement of linear polyubiquitination in NFκB activation.159 MCPIP deubiquitinates, both K63- and K48-linked polyubiquitin, attached to the proteins and unanchored large K63-linked polyubiquitin chains involved in NFκB activation as indicated above.154 Expression of MCPIP severely inhibited phosphorylation of TAK1 in cells, in which IL-1β activates NFκB (C. Menaa and P.E. Kolattukudy, unpublished). Thus, it appears likely that MCPIP can inhibit NFκB activation by hydrolyzing protein-bound and unanchored polyubiquitin chains involved in NFκB activation (Figure 4). Our results showed that MCPIP expression caused drastic decrease in RIP ubiquitination but showed much less effect on NEMO ubiquitination.154 Consistent with this finding we found that purified MCPIP does not hydrolyze linear polyubiquitin (M. Zhang and P.E. Kolattukudy, unpublished). Whether deubiquitinase activity of MCPIP is critical for its inhibitory activity on NFκB activation was examined with deletion mutants of MCPIP.154 Mutants that lost deubiquitinase activity also lost the ability to inhibit TNF-α induced NFκB activation suggesting that inhibition of NFκB activation by MCPIP is via its deubiquitinase activity.154
NFκB activation is also regulated by other deubiquitinases.160 For example, overexpression of A20 inhibits LPS-induced activation of NFκB. A20 expression also inhibited NFκB reporter activation caused by forced expression of Myo 88, IRAK1, IRAK2, TRAF6 and TAK1/TAB2.161 Cylindromatosis (CYLD) has been shown to inhibit NFκB activation160 and more recent results indicate that this inhibition might be mediated via the ability of CYLD to hydrolyze unanchored polyubiquitin chains and thus inhibit TAK1 and IKK activation.162 Both A20 and CYLD are known to play an anti-inflammatory protective role in cardiovascular diseases by inhibiting NFκB activation.163–165 The ligand-induced NFκB activation involves polyubiquitins held together by different linkages such as K48, K63 and linear. Deubiquitinases show different specificities for cleavage of these polyubiquitins.166 For example, CLYD cleaves linear and K63 linkages, whereas A20 cleaves K48160 and MCPIP cleaves K63 and K48. The roles of the various deubiquitinases in the regulation of NFκB activation triggered by various inflammatory agents in different cell types involved in the development of human diseases are yet to be fully elucidated.
MCPIP may inhibit inflammation also by mechanisms other than the inhibition of NFκB activation. Another demonstrated mechanism involves degradation of mRNA encoding inflammatory cytokines.155,167. Thus, in MCPIP deficient mice, IL-6 mRNA decay was severely impaired in the macrophages. Overexpression of MCPIP accelerated IL6 mRNA degradation at the 3′-untranslated region (UTR). The N-terminus of MCPIP contains a nuclease domain. Expressed MCPIP protein showed direct binding of the UTR of IL-6 mRNA and manifested RNase activity against IL-6 mRNA. Selectivity of MCPIP for degradation of mRNA was demonstrated by the finding that the expression of TNF-α mRNA was not affected by the absence of MCPIP in the macrophages155. Another zinc-finger protein, tristetrapolin, controls TNFα mRNA stability.168 When IL-6 mRNA degradation by MCPIP was discovered, this activity was considered inadequate to account for the profound pathological changes found in MCPIP deficient mice.166 Probably inhibition of activation of the master controller of inflammation, that was not discovered until later, can account for the other pathological changes, not explained by the IL-6 RNase activity of MCPIP. Thus, MCPIP controls inflammation not only by preventing the production of inflammatory cytokines by inhibiting NFκB activation but also by degrading mRNA for some of the inflammatory cytokines.
How MCPIP carries out RNase and polyubiquitinase activities remains to be elucidated. Direct binding of the UTR of IL-6 mRNA was demonstrated.155 MCPIP binding to ubiquitin has been demonstrated by pull down experiments.154 Incubation of ubiquitin vinyl sulfone with MCPIP caused covalent cross-linking of MCPIP with ubiquitin probably via the catalytic Cys residue (M. Zhang and P.E. Kolattukudy unpublished). The N-terminal region of MCPIP contains ubiquitin association domain and deletion of this domain abolished ubiquitin binding.154 Several putative Cys boxes and Asp boxes, usually found in Cys proteinases, are present in MCPIP. The Cys box of MCPIP is conserved in organisms from fly to human. Mutagenesis analysis showed that D141N, C157A, D278A, D279A and C306R had lost the inhibitory activity on NFκB activation and the corresponding proteins demonstrated loss of deubiquitinase activity.154 Our study confirmed the previously reported the loss of RNase activity by D141N mutation.155 However, the H88A, C157A and C306R mutants retained RNase activity but lost deubiquitinase activity and the ability to inhibit NFκB activation.154 Forced expression of MCPIP in MCPIP−/− fibroblasts suppressed LPS-induced TNF-α and MCP-1 release, but the C157A mutant lost this activity. All of these results suggest that deubiquitinase activity probably plays a more major role in the anti-inflammatory function of MCPIP than the RNase activity. Since NFκB is the integrator of many inflammatory signals, inhibition of NFκB activation would be an effective means to control inflammation caused by multiple inflammatory agents.
8. MCP-1/CCR2 pathway: a novel therapeutic target in cardiovascular disease
With the realization that inflammation is a key factor in the initiation and progression of the cardiovascular diseases, anti-inflammatory approaches to prevent and treat such diseases have become an avenue under active investigation.169 The recently designed Cardiovascular Inflammation Reduction Trial with low doses of methotrexate or placebo is an example.169 Another trial “The canakinumab anti-inflammatory Thrombosis Outcome study” tests whether IL-1β inhibition with a human monoclonal antibody is effective against recurrent myocardial infarction, stroke and death from cardiovascular diseases.4,5 Statins constitute the best-characterized anti-inflammatory class of drugs for the prevention of coronary heart disease. In addition to their lipid-lowering activity, statins also exert anti-inflammatory effects.171 Clinical evidence has indicated that that statin-induced decrease in CRP, a marker of inflammation, was significantly correlated with reduced atherosclerosis progression, independent of LDL cholesterol lowering.172 Further studies by the JUPITER (The Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) trial demonstrated that the decrease in the levels of CRP was in parallel with the clinical benefit,170 suggesting a protective effect of targeting inflammation in the prevention of cardiovascular events.
MCP-1/CCR2 pathway was recently recognized as a new therapeutic target for treatment of cardiovascular diseases. Interference with MCP-1 interaction with CCR2 has shown beneficial effects against the development of cardiovascular disease.9,102,107 In recent years, small molecules that interfere with MCP-1/CCR2 interaction have been developed and shown to suppress inflammation in the mouse model of multiple sclerosis, renal ischemia-reperfusion injury, and diabetic nephropathy.173–175 A recent clinical study also demonstrated that MLN1202, a humanized monoclonal antibody that blocks MCP-1/CCR2 interaction, can reduce the levels of inflammation in patients with high risk for major adverse cardiovascular events.176 The synthetic peptide fragment (65–76) from the C-terminal domain of MCP-1 was recently reported to inhibit monocyte migration and has anti-inflammatory activity in patients with stable angina after coronary stenting.177 Recent findings that a novel CCR2 antagonist ameliorated insulin resistance in high fat fed mice suggests that MCP-1/CCR2 pathway could be a target for drugs against type 2 diabetes.131,134 Future clinical trials are needed to test MCP-1/CCR2 pathway as a potential therapeutic target to reduce cardiovascular events and risk factors such as obesity/diabetes.
9. Conclusion and future direction
The realization that chronic inflammation is a critical factor in the development of many of the major human diseases such as cardiovascular diseases, type 2 diabetes, rheumatoid arthritis and cancer, opens up new avenues for therapeutic intervention. Obesity, that affects a rapidly increasing segment of human population, is closely associated with some of the leading causes of death including heart disease, stroke, type 2 diabetes and cancer. The maladies caused by obesity probably result from the fact that obesity generates a chronic inflammatory condition. It is probable that the chronic inflammation generates oxidative stress that can cause DNA damage leading to cancer. Such a mechanism might help to explain how obesity causes up to 20% of all human cancers, a major issue that attracts great attention at the National Cancer Institute. Inflammation involves oxidative stress that causes ER stress that leads to autophagy involved in the development of many of the pathologies associated with the obesity-related major diseases. The complex sets of molecular players involved in these processes are incompletely understood and novel players critical to inflammation-associated processes are being discovered. The involvement of deubiquitination in inflammatory processes present many unanswered questions. Inflammation-induced oxidative stress can lead to cell death or differentiation depending on the cellular context. What determines the cellular fate remains, unknown. Whether deubiquitination or NFκB is involved in both cellular fates is not known. The biological functions of the many deubiquitinases present in different cell types in the development of human diseases remain to be elucidated. Elucidation of the critical steps involved in this complex series of events will reveal novel targets for therapeutic intervention. Thus, the anti-inflammatory approaches that are currently in clinical trials constitute only a beginning, and future elucidation of the critical steps will lead to an increasing number of opportunities to develop effective anti-inflammatory drugs to treat major human disease.
Acknowledgments
Source of Funding
Work from the authors’ laboratory was supported in part by grant HL-69458 from National Institutes of Health.
Non-standard Abbreviations and Acronyms
- RA
rheumatoid arthritis
- ER
endoplasmic reticulum
- ROS
reactive oxygen species
- NO
nitric oxide
- PRR
pattern recognition receptor
- TLR
toll-like receptor
- NLR
nucleotide oligomerization domain-like receptor
- PAMP
pathogen-associated molecular pattern
- DAMP
damage-associated molecular pattern
- MAPK
mitogen-activated protein kinase
- PI3K
phosphoinositide 3-kinase
- JAK/STAT
Janus kinase/signal transducers and activators of transcription
- UPR
unfolded protein response
- IRE1
inositol-requiring enzyme 1
- PERK
pancreatic ER kinase
- ATF6
activating transcription factor 6
- GRP78
glucose-regulated protein
- XBP1
X-box binding protein-1
- S1P
site-1 protease
- ERAD
ER-associated degradation
- TRAF2
TNF receptor-associated factor 2
- IKK
IκB kinase
- JNK
Jun N-terminal kinase
- AP-1
activator protein 1
- CRP
C-reactive protein
- iNOS
inducible NO synthase
- PDI
protein disulfide isomerase
- Atg
autophagy
- RIP
receptor-interacting protein
- I/R
ischemia/reperfusion
- CHOP
C/EBP homologous protein
- LPS
lipopolysaccharide
- ERK
extracellular signal-regulated kinases
- TAK1
transforming growth factor β–activated kinase 1
- TGF
transforming growth factor
- HUVEC
human umbilical vein endothelial cells
- cdh
cadherins
- VEGF
vascular endothelial growth factor
- C/EBP
CCAAT/enhancer-binding protein
- PPAR
peroxisome proliferator-activated receptor PUMA- p53 upregulated modulator of apoptosis
- CYLD
cylindromatosis
- UTR
untranslated region
Footnotes
Conflict of Interest: None.
References
- 1.Maskrey BH, Megson IL, Whitfield PD, Rossi AG. Mechanisms of resolution of inflammation: a focus on cardiovascular disease. Arterioscler Thromb Vasc Biol. 2011;31:1001–1006. doi: 10.1161/ATVBAHA.110.213850. [DOI] [PubMed] [Google Scholar]
- 2.Harvey AE, Lashinger LM, Hursting SD. The growing challenge of obesity and cancer: an inflammatory issue. Ann N Y Acad Sci. 2011;1229:45–52. doi: 10.1111/j.1749-6632.2011.06096.x. [DOI] [PubMed] [Google Scholar]
- 3.González-Chávez A, Elizondo-Argueta S, Gutiérrez-Reyes G, León-Pedroza JI. Pathophysiological implications between chronic inflammation and the development of diabetes and obesity. Cir Cir. 2011;79:209–216. [PubMed] [Google Scholar]
- 4.Charo IF, Taub R. Anti-inflammatory therapeutics for the treatment of atherosclerosis. Nat Rev Drug Discov. 2011;10:365–76. doi: 10.1038/nrd3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Li L, Kim SH, Hagerman AE, Lü J. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm Res. 2009;26:2066–2080. doi: 10.1007/s11095-009-9932-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hohensinner PJ, Niessner A, Huber K, Weyand CM, Wojta J. Inflammation and cardiac outcome. Curr Opin Infect Dis. 2011;24:259–264. doi: 10.1097/QCO.0b013e328344f50f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linde A, Mosier D, Blecha F, Melgarejo T. Innate immunity and inflammation--New frontiers in comparative cardiovascular pathology. Cardiovasc Res. 2007;73:26–36. doi: 10.1016/j.cardiores.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Niu J, Kolattukudy PE. Role of MCP-1 in cardiovascular disease: molecular mechanisms and clinical implications. Clin Sci (Lond) 2009;117:95–109. doi: 10.1042/CS20080581. [DOI] [PubMed] [Google Scholar]
- 10.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rath E, Haller D. Inflammation and cellular stress: a mechanistic link between immune-mediated and metabolically driven pathologies. Eur J Nutr. 2011;50:219–233. doi: 10.1007/s00394-011-0197-0. [DOI] [PubMed] [Google Scholar]
- 12.Zhang K. Integration of ER stress, oxidative stress and the inflammatory response in health and disease. Int J Clin Exp Med. 2010;3:33–40. [PMC free article] [PubMed] [Google Scholar]
- 13.Arslan F, de Kleijn DP, Pasterkamp G. Innate immune signaling in cardiac ischemia. Nat Rev Cardiol. 2011;8:292–300. doi: 10.1038/nrcardio.2011.38. [DOI] [PubMed] [Google Scholar]
- 14.Mann DL. The emerging role of innate immunity in the heart and vascular system: for whom the cell tolls. Circ Res. 2011;108:1133–1145. doi: 10.1161/CIRCRESAHA.110.226936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen G, Nunez G. Sterile inflammation: sensing and reacting to damage. Nature Reviews Immunol. 2010;10:832–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell JA, Ryffel B, Quesniaux VF, Cartwright N, Paul-Clark M. Role of pattern-recognition receptors in cardiovascular health and disease. Biochem Soc Trans. 2007;35:1449–1452. doi: 10.1042/BST0351449. [DOI] [PubMed] [Google Scholar]
- 17.Fukata M, Vamadevan AS, Abreu MT. Toll-like receptors (TLRs) and Nod-like receptors (NLRs) in inflammatory disorders. Semin Immunol. 2009;21:242–253. doi: 10.1016/j.smim.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 19.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 20.Niu J, Azfer A, Wang K, Wang X, Kolattukudy PE. Cardiac-targeted expression of soluble fas attenuates doxorubicin-induced cardiotoxicity in mice. J Pharmacol Exp Ther. 2009;328:740–748. doi: 10.1124/jpet.108.146423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niu J, Azfer A, Deucher MF, Goldschmidt-Clermont PJ, Kolattukudy PE. Targeted cardiac expression of soluble Fas prevents the development of heart failure in mice with cardiac-specific expression of MCP-1. J Mol Cell Cardiol. 2006;40:810–820. doi: 10.1016/j.yjmcc.2006.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niu J, Azfer A, Kolattukudy PE. Protection against lipopolysaccharide-induced myocardial dysfunction in mice by cardiac-specific expression of soluble Fas. J Mol Cell Cardiol. 2008;44:160–169. doi: 10.1016/j.yjmcc.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Vallejo JG. Role of toll-like receptors in cardiovascular diseases. Clin Sci (Lond) 2011;121:1–10. doi: 10.1042/CS20100539. [DOI] [PubMed] [Google Scholar]
- 24.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 25.Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of NF-κB in the heart: to be or not to NF-κB. Circ Res. 2011;108:1122–1132. doi: 10.1161/CIRCRESAHA.110.226928. [DOI] [PubMed] [Google Scholar]
- 26.Van der Heiden K, Cuhlmann S, Luong le A, Zakkar M, Evans PC. Role of nuclear factor kappaB in cardiovascular health and disease. Clin Sci (Lond) 2010;118:593–605. doi: 10.1042/CS20090557. [DOI] [PubMed] [Google Scholar]
- 27.Brown J, Wang H, Hajishengallis GN, Martin M. TLR-signaling networks: an integration of adaptor molecules, kinases, and cross-talk. J Dent Res. 2011;90:417–427. doi: 10.1177/0022034510381264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen P. Targeting protein kinases for the development of anti-inflammatory drugs. Curr Opin Cell Biol. 2009;21:317–324. doi: 10.1016/j.ceb.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 29.Kitamura M. Control of NF-κB and inflammation by the unfolded protein response. Int Rev Immunol. 2011;30:4–15. doi: 10.3109/08830185.2010.522281. [DOI] [PubMed] [Google Scholar]
- 30.Hotamisligil GS. Endoplasmic Reticulum Stress and the Inflammatory Basis of Metabolic Disease. Cell. 2010;140:900–927. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, Kaufman RJ. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124:587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 32.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gotoh T, Endo M, Oike Y. Endoplasmic reticulum stress-related inflammation and cardiovascular diseases. Int J Inflam. 2011;2011:259462. doi: 10.4061/2011/259462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. [DOI] [PubMed] [Google Scholar]
- 35.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 36.Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998;17:5708–5717. doi: 10.1093/emboj/17.19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 38.Lu PD, Jousse C, Marciniak SJ, Zhang Y, Novoa I, Scheuner D, Kaufman RJ, Ron D, Harding HP. Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. EMBO J. 2004;23:169–179. doi: 10.1038/sj.emboj.7600030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cullinan SB, Diehl JA. Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol. 2006;38:317–332. doi: 10.1016/j.biocel.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 41.Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gargalovic PS, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, Truong A, Baruch-Oren T, Berliner JA, Kirchgessner TG, Lusis AJ. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:2490–2496. doi: 10.1161/01.ATV.0000242903.41158.a1. [DOI] [PubMed] [Google Scholar]
- 43.Zeng L, Liu YP, Sha H, Chen H, Qi L, Smith JA. XBP-1 couples endoplasmic reticulum stress to augmented IFN-beta induction via a cis-acting enhancer in macrophages. J Immunol. 2010;185:2324–2330. doi: 10.4049/jimmunol.0903052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwakoshi NN, Lee AH, Vallabhajosyula P, Otipoby KL, Rajewsky K, Glimcher LH. Plasma cell differentiation and the unfolded protein response intersect at the transcription factor XBP-1. Nat Immunol. 2003;4:321–329. doi: 10.1038/ni907. [DOI] [PubMed] [Google Scholar]
- 46.Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 48.Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaneko M, Niinuma Y, Nomura Y. Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol Pharm Bull. 2003;26:931–935. doi: 10.1248/bpb.26.931. [DOI] [PubMed] [Google Scholar]
- 50.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 51.Watterson C, Lanevschi A, Horner J, Louden C. A comparative analysis of acute-phase proteins as inflammatory biomarkers in preclinical toxicology studies: implications for preclinical to clinical translation. Toxicol Pathol. 2009;37:28–33. doi: 10.1177/0192623308329286. [DOI] [PubMed] [Google Scholar]
- 52.Montecucco F, Steffens S, Burger F, Pelli G, Monaco C, Mach F. C-reactive protein (CRP) induces chemokine secretion via CD11b/ICAM-1 interaction in human adherent monocytes. J Leukoc Biol. 2008;84:1109–1119. doi: 10.1189/jlb.0208123. [DOI] [PubMed] [Google Scholar]
- 53.Devaraj S, Kumaresan PR, Jialal I. Effect of C-reactive protein on chemokine expression in human aortic endothelial cells. J Mol Cell Cardiol. 2004;36:405–410. doi: 10.1016/j.yjmcc.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 54.Yamazaki H, Hiramatsu N, Hayakawa K, Tagawa Y, Okamura M, Ogata R, Huang T, Nakajima S, Yao J, Paton AW, Paton JC, Kitamura M. Activation of the Akt-NF-kappaB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J Immunol. 2009;183:1480–1487. doi: 10.4049/jimmunol.0900017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature. 2006;441:513–517. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 56.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kouroku Y, Fujita E, Tanida I, Ueno T, Isoai A, Kumagai H, Ogawa S, Kaufman RJ, Kominami E, Momoi T. ER stress (PERK/eIF2alpha phosphorylation) mediates the polyglutamine-induced LC3 conversion, an essential step for autophagy formation. Cell Death Differ. 2007;14:230–239. doi: 10.1038/sj.cdd.4401984. [DOI] [PubMed] [Google Scholar]
- 58.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hetz C, Thielen P, Matus S, Nassif M, Court F, Kiffin R, Martinez G, Cuervo AM, Brown RH, Glimcher LH. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009;23:2294–2306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13:619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 61.Nishida K, Kyoi S, Yamaguchi O, Sadoshima J, Otsu K. The role of autophagy in the heart. Cell Death Differ. 2009;16:31–38. doi: 10.1038/cdd.2008.163. [DOI] [PubMed] [Google Scholar]
- 62.Saftig P, Tanaka Y, Lüllmann-Rauch R, von Figura K. Disease model: LAMP-2 enlightens Danon disease. Trends Mol Med. 2001;7:37–39. doi: 10.1016/s1471-4914(00)01868-2. [DOI] [PubMed] [Google Scholar]
- 63.Sciarretta S, Hariharan N, Monden Y, Zablocki D, Sadoshima J. Is autophagy in response to ischemia and reperfusion protective or detrimental for the heart? Pediatr Cardiol. 2011;32:275–281. doi: 10.1007/s00246-010-9855-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McLendon PM, Robbins J. Desmin-Related Cardiomyopathy: An Unfolding Story. Am J Physiol Heart Circ Physiol. 2011 Jul 22; doi: 10.1152/ajpheart.00601.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 66.Valentim L, Laurence KM, Townsend PA, Carroll CJ, Soond S, Scarabelli TM, Knight RA, Latchman DS, Stephanou A. Urocortin inhibits Beclin1-mediated autophagic cell death in cardiac myocytes exposed to ischaemia/reperfusion injury. J Mol Cell Cardiol. 2006;40:846–852. doi: 10.1016/j.yjmcc.2006.03.428. [DOI] [PubMed] [Google Scholar]
- 67.Rami A, Langhagen A, Steiger S. Focal cerebral ischemia induces upregulation of Beclin 1 and autophagy-like cell death. Neurobiol Dis. 2008;29:132–141. doi: 10.1016/j.nbd.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 68.Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol. 2010;12:823–830. doi: 10.1038/ncb0910-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang J, Ney PA. Autophagy-dependent and -independent mechanisms of mitochondrial clearance during reticulocyte maturation. Autophagy. 2009;5:1064–1065. doi: 10.4161/auto.5.7.9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mortensen M, Ferguson DJ, Edelmann M, Kessler B, Morten KJ, Komatsu M, Simon AK. Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc Natl Acad Sci U S A. 2010;107:832–837. doi: 10.1073/pnas.0913170107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pua HH, Dzhagalov I, Chuck M, Mizushima N, He YW. A critical role for the autophagy gene Atg5 in T cell survival and proliferation. J Exp Med. 2007;204:25–31. doi: 10.1084/jem.20061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miller BC, Zhao Z, Stephenson LM, Cadwell K, Pua HH, Lee HK, Mizushima NN, Iwasaki A, He YW, Swat W, Virgin HW., 4th The autophagy gene ATG5 plays an essential role in B lymphocyte development. Autophagy. 2008;4:309–314. doi: 10.4161/auto.5474. [DOI] [PubMed] [Google Scholar]
- 73.Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, Czaja MJ. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest. 2009;119:3329–3339. doi: 10.1172/JCI39228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Y, Goldman S, Baerga R, Zhao Y, Komatsu M, Jin S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc Natl Acad Sci U S A. 2009;106:19860–19865. doi: 10.1073/pnas.0906048106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Christofferson DE, Yuan J. Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol. 2010;22:263–268. doi: 10.1016/j.ceb.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 77.Martinet W, Knaapen MW, Kockx MM, De Meyer GR. Autophagy in cardiovascular disease. Trends Mol Med. 2007;13:482–491. doi: 10.1016/j.molmed.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 78.Kung G, Konstantinidis K, Kitsis RN. Programmed necrosis, not apoptosis, in the heart. Circ Res. 2011;108:1017–1036. doi: 10.1161/CIRCRESAHA.110.225730. [DOI] [PubMed] [Google Scholar]
- 79.Challa S, Chan FK. Going up in flames: necrotic cell injury and inflammatory diseases. Cell Mol Life Sci. 2010;67:3241–3253. doi: 10.1007/s00018-010-0413-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res. 2010;107:1071–1082. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- 81.Miyazaki Y, Kaikita K, Endo M, Horio E, Miura M, Tsujita K, Hokimoto S, Yamamuro M, Iwawaki T, Gotoh T, Ogawa H, Oike Y. C/EBP homologous protein deficiency attenuates myocardial reperfusion injury by inhibiting myocardial apoptosis and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1124–1132. doi: 10.1161/ATVBAHA.111.224519. [DOI] [PubMed] [Google Scholar]
- 82.Okada K, Minamino T, Tsukamoto Y, Liao Y, Tsukamoto O, Takashima S, Hirata A, Fujita M, Nagamachi Y, Nakatani T, Yutani C, Ozawa K, Ogawa S, Tomoike H, Hori M, Kitakaze M. Prolonged endoplasmic reticulum stress in hypertrophic and failing heart after aortic constriction: possible contribution of endoplasmic reticulum stress to cardiac myocyte apoptosis. Circulation. 2004;110:705–712. doi: 10.1161/01.CIR.0000137836.95625.D4. [DOI] [PubMed] [Google Scholar]
- 83.Thuerauf DJ, Marcinko M, Gude N, Rubio M, Sussman MA, Glembotski CC. Activation of the unfolded protein response in infarcted mouse heart and hypoxic cultured cardiac myocytes. Circ Res. 2006;99:275–282. doi: 10.1161/01.RES.0000233317.70421.03. [DOI] [PubMed] [Google Scholar]
- 84.Sawada T, Minamino T, Fu HY, Asai M, Okuda K, Isomura T, Yamazaki S, Asano Y, Okada K, Tsukamoto O, Sanada S, Asanuma H, Asakura M, Takashima S, Kitakaze M, Komuro I. X-box binding protein 1 regulates brain natriuretic peptide through a novel AP1/CRE-like element in cardiomyocytes. J Mol Cell Cardiol. 2010;48:1280–1289. doi: 10.1016/j.yjmcc.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 85.Azfer A, Niu J, Rogers LM, Adamski FM, Kolattukudy PE. Activation of endoplasmic reticulum stress response during the development of ischemic heart disease. Am J Physiol Heart Circ Physiol. 2006;291:H1411–1420. doi: 10.1152/ajpheart.01378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Martindale JJ, Fernandez R, Thuerauf D, Whittaker R, Gude N, Sussman MA, Glembotski CC. Endoplasmic reticulum stress gene induction and protection from ischemia/reperfusion injury in the hearts of transgenic mice with a tamoxifen-regulated form of ATF6. Circ Res. 2006;98:1186–93. doi: 10.1161/01.RES.0000220643.65941.8d. [DOI] [PubMed] [Google Scholar]
- 87.Tajiri S, Oyadomari S, Yano S, Morioka M, Gotoh T, Hamada JI, Ushio Y, Mori M. Ischemia-induced neuronal cell death is mediated by the endoplasmic reticulum stress pathway involving CHOP. Cell Death Differ. 2004;11:403–415. doi: 10.1038/sj.cdd.4401365. [DOI] [PubMed] [Google Scholar]
- 88.Fu HY, Okada K, Liao Y, Tsukamoto O, Isomura T, Asai M, Sawada T, Okuda K, Asano Y, Sanada S, Asanuma H, Asakura M, Takashima S, Komuro I, Kitakaze M, Minamino T. Ablation of C/EBP homologous protein attenuates endoplasmic reticulum-mediated apoptosis and cardiac dysfunction induced by pressure overload. Circulation. 2010;122:361–369. doi: 10.1161/CIRCULATIONAHA.109.917914. [DOI] [PubMed] [Google Scholar]
- 89.Toko H, Takahashi H, Kayama Y, Okada S, Minamino T, Terasaki F, Kitaura Y, Komuro I. ATF6 is important under both pathological and physiological states in the heart. J Mol Cell Cardiol. 2010;49:113–120. doi: 10.1016/j.yjmcc.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 90.Qi X, Vallentin A, Churchill E, Mochly-Rosen D. deltaPKC participates in the endoplasmic reticulum stress-induced response in cultured cardiac myocytes and ischemic heart. J Mol Cell Cardiol. 2007;43:420–428. doi: 10.1016/j.yjmcc.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mao W, Fukuoka S, Iwai C, Liu J, Sharma VK, Sheu SS, Fu M, Liang CS. Cardiomyocyte apoptosis in autoimmune cardiomyopathy: mediated via endoplasmic reticulum stress and exaggerated by norepinephrine. Am J Physiol Heart Circ Physiol. 2007;293:H1636–1645. doi: 10.1152/ajpheart.01377.2006. [DOI] [PubMed] [Google Scholar]
- 92.Hamada H, Suzuki M, Yuasa S, Mimura N, Shinozuka N, Takada Y, Suzuki M, Nishino T, Nakaya H, Koseki H, Aoe T. Dilated cardiomyopathy caused by aberrant endoplasmic reticulum quality control in mutant KDEL receptor transgenic mice. Mol Cell Biol. 2004;24:8007–8017. doi: 10.1128/MCB.24.18.8007-8017.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou J, Lhoták S, Hilditch BA, Austin RC. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2005;111:1814–1821. doi: 10.1161/01.CIR.0000160864.31351.C1. [DOI] [PubMed] [Google Scholar]
- 94.Civelek M, Manduchi E, Riley RJ, Stoeckert CJ, Jr, Davies PF. Chronic endoplasmic reticulum stress activates unfolded protein response in arterial endothelium in regions of susceptibility to atherosclerosis. Circ Res. 2009;105:453–461. doi: 10.1161/CIRCRESAHA.109.203711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, Asada Y, Okada K, Ishibashi-Ueda H, Gabbiani G, Bochaton-Piallat ML, Mochizuki N, Kitakaze M. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 96.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe−/− and Ldlr−/− mice lacking CHOP. Cell Metab. 2009;9:474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsukano H, Gotoh T, Endo M, Miyata K, Tazume H, Kadomatsu T, Yano M, Iwawaki T, Kohno K, Araki K, Mizuta H, Oike Y. The endoplasmic reticulum stress-C/EBP homologous protein pathway-mediated apoptosis in macrophages contributes to the instability of atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2010;30:1925–1932. doi: 10.1161/ATVBAHA.110.206094. [DOI] [PubMed] [Google Scholar]
- 98.Tabas I. The role of endoplasmic reticulum stress in the progression of atherosclerosis. Circ Res. 2010;107:839–850. doi: 10.1161/CIRCRESAHA.110.224766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park SH, Choi HJ, Yang H, Do KH, Kim J, Lee DW, Moon Y. Endoplasmic reticulum stress-activated C/EBP homologous protein enhances nuclear factor-kappaB signals via repression of peroxisome proliferator-activated receptor gamma. J Biol Chem. 2010;285:35330–35339. doi: 10.1074/jbc.M110.136259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Haberzettl P, Vladykovskaya E, Srivastava S, Bhatnagar A. Role of endoplasmic reticulum stress in acrolein-induced endothelial activation. Toxicol Appl Pharmacol. 2009;234:14–24. doi: 10.1016/j.taap.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95:858–66. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 103.Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kanematsu Y, Kanematsu M, Kurihara C, Tada Y, Tsou TL, van Rooijen N, Lawton MT, Young WL, Liang EI, Nuki Y, Hashimoto T. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke. 2011;42:173–178. doi: 10.1161/STROKEAHA.110.590976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- 106.Marfella R, D’Amico M, Di Filippo C, Baldi A, Siniscalchi M, Sasso FC, Portoghese M, Carbonara O, Crescenzi B, Sangiuolo P, Nicoletti GF, Rossiello R, Ferraraccio F, Cacciapuoti F, Verza M, Coppola L, Rossi F, Paolisso G. Increased activity of the ubiquitin-proteasome system in patients with symptomatic carotid disease is associated with enhanced inflammation and may destabilize the atherosclerotic plaque: effects of rosiglitazone treatment. J Am Coll Cardiol. 2006;47:2444–2455. doi: 10.1016/j.jacc.2006.01.073. [DOI] [PubMed] [Google Scholar]
- 107.Frangogiannis NG, Dewald O, Xia Y, Ren G, Haudek S, Leucker T, Kraemer D, Taffet G, Rollins BJ, Entman ML. Critical role of monocyte chemoattractant protein-1/CC chemokine ligand 2 in the pathogenesis of ischemic cardiomyopathy. Circulation. 2007;115:584–592. doi: 10.1161/CIRCULATIONAHA.106.646091. [DOI] [PubMed] [Google Scholar]
- 108.Hayashidani S, Tsutsui H, Shiomi T, Ikeuchi M, Matsusaka H, Suematsu N, Wen J, Egashira K, Takeshita A. Anti-monocyte chemoattractant protein-1 gene therapy attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2003;108:2134–140. doi: 10.1161/01.CIR.0000092890.29552.22. [DOI] [PubMed] [Google Scholar]
- 109.Kaikita K, Hayasaki T, Okuma T, Kuziel WA, Ogawa H, Takeya M. Targeted deletion of CC chemokine receptor 2 attenuates left ventricular remodeling after experimental myocardial infarction. Am J Pathol. 2004;165:439–447. doi: 10.1016/S0002-9440(10)63309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kolattukudy PE, Quach T, Bergese S, Breckenridge S, Hensley J, Altschuld R, Gordillo G, Klenotic S, Orosz C, Parker-Thornburg J. Myocarditis induced by targeted expression of the MCP-1 gene in murine cardiac muscle. Am J Pathol. 1998;152:101–111. [PMC free article] [PubMed] [Google Scholar]
- 111.Niu J, Azfer A, Rogers LM, Wang X, Kolattukudy PE. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovasc Res. 2007;73:549–559. doi: 10.1016/j.cardiores.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Younce CW, Wang K, Kolattukudy PE. Hyperglycaemia-induced cardiomyocyte death is mediated via MCP-1 production and induction of a novel zinc-finger protein MCPIP. Cardiovasc Res. 2010;87:665–674. doi: 10.1093/cvr/cvq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Martire A, Fernandez B, Buehler A, Strohm C, Schaper J, Zimmermann R, Kolattukudy PE, Schaper W. Cardiac overexpression of monocyte chemoattractant protein-1 in transgenic mice mimics ischemic preconditioning through SAPK/JNK1/2 activation. Cardiovasc Res. 2003;57:523–534. doi: 10.1016/s0008-6363(02)00697-1. [DOI] [PubMed] [Google Scholar]
- 114.Morimoto H, Takahashi M, Izawa A, Ise H, Hongo M, Kolattukudy PE, Ikeda U. Cardiac overexpression of monocyte chemoattractant protein-1 in transgenic mice prevents cardiac dysfunction and remodeling after myocardial infarction. Circ Res. 2006;99:891–899. doi: 10.1161/01.RES.0000246113.82111.2d. [DOI] [PubMed] [Google Scholar]
- 115.Shintani-Ishida K, Nakajima M, Uemura K, Yoshida K. Ischemic preconditioning protects cardiomyocytes against ischemic injury by inducing GRP78. Biochem Biophys Res Commun. 2006;345:1600–1605. doi: 10.1016/j.bbrc.2006.05.077. [DOI] [PubMed] [Google Scholar]
- 116.Liu XH, Zhang ZY, Sun S, Wu XD. Ischemic postconditioning protects myocardium from ischemia/reperfusion injury through attenuating endoplasmic reticulum stress. Shock. 2008;30:422–427. doi: 10.1097/SHK.0b013e318164ca29. [DOI] [PubMed] [Google Scholar]
- 117.Morimoto H, Hirose M, Takahashi M, Kawaguchi M, Ise H, Kolattukudy PE, Yamada M, Ikeda U. MCP-1 induces cardioprotection against ischaemia/reperfusion injury: role of reactive oxygen species. Cardiovasc Res. 2008;78:554–562. doi: 10.1093/cvr/cvn035. [DOI] [PubMed] [Google Scholar]
- 118.Vitadello M, Penzo D, Petronilli V, Michieli G, Gomirato S, Menabò R, Di Lisa F, Gorza L. Overexpression of the stress protein Grp94 reduces cardiomyocyte necrosis due to calcium overload and simulated ischemia. FASEB J. 2003;17:923–925. doi: 10.1096/fj.02-0644fje. [DOI] [PubMed] [Google Scholar]
- 119.Zhang PL, Lun M, Teng J, Huang J, Blasick TM, Yin L, Herrera GA, Cheung JY. Preinduced molecular chaperones in the endoplasmic reticulum protect cardiomyocytes from lethal injury. Ann Clin Lab Sci. 2004;34:449–457. [PubMed] [Google Scholar]
- 120.Toldo S, Severino A, Abbate A, Baldi A. The role of PDI as a survival factor in cardiomyocyte ischemia. Methods Enzymol. 2011;489:47–65. doi: 10.1016/B978-0-12-385116-1.00003-0. [DOI] [PubMed] [Google Scholar]
- 121.Severino A, Campioni M, Straino S, Salloum FN, Schmidt N, Herbrand U, Frede S, Toietta G, Di Rocco G, Bussani R, Silvestri F, Piro M, Liuzzo G, Biasucci LM, Mellone P, Feroce F, Capogrossi M, Baldi F, Fandrey J, Ehrmann M, Crea F, Abbate A, Baldi A. Identification of protein disulfide isomerase as a cardiomyocyte survival factor in ischemic cardiomyopathy. J Am Coll Cardiol. 2007;50:1029–1037. doi: 10.1016/j.jacc.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 122.Li J, Wang JJ, Zhang SX. Preconditioning with endoplasmic reticulum stress mitigates retinal endothelial inflammation via activation of X-box binding protein 1. J Biol Chem. 2011;286:4912–4921. doi: 10.1074/jbc.M110.199729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hayakawa K, Hiramatsu N, Okamura M, Yamazaki H, Nakajima S, Yao J, Paton AW, Paton JC, Kitamura M. Acquisition of anergy to proinflammatory cytokines in nonimmune cells through endoplasmic reticulum stress response: a mechanism for subsidence of inflammation. J Immunol. 2009;182:1182–1191. doi: 10.4049/jimmunol.182.2.1182. [DOI] [PubMed] [Google Scholar]
- 124.Zhou L, Azfer A, Niu J, Graham S, Choudhury M, Adamski FM, Younce C, Binkley PF, Kolattukudy PE. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ Res. 2006;98:1177–1185. doi: 10.1161/01.RES.0000220106.64661.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liang J, Song W, Tromp G, Kolattukudy PE, Fu M. Genome-wide survey and expression profiling of CCCH-zinc finger family reveals a functional module in macrophage activation. PLoS One. 2008;3:e2880. doi: 10.1371/journal.pone.0002880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Voskuil M, van Royen N, Hoefer IE, Seidler R, Guth BD, Bode C, Schaper W, Piek JJ, Buschmann IR. Modulation of collateral artery growth in a porcine hindlimb ligation model using MCP-1. Am J Physiol Heart Circ Physiol. 2003;284:H1422–428. doi: 10.1152/ajpheart.00506.2002. [DOI] [PubMed] [Google Scholar]
- 127.Niiyama H, Kai H, Yamamoto T, Shimada T, Sasaki K, Murohara T, Egashira K, Imaizumi T. Roles of endogenous monocyte chemoattractant protein-1 in ischemia-induced neovascularization. J Am Coll Cardiol. 2004;44:661–666. doi: 10.1016/j.jacc.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 128.Moldovan NI, Goldschmidt-Clermont PJ, Parker-Thornburg J, Shapiro SD, Kolattukudy PE. Contribution of monocytes/macrophages to compensatory neovascularization: the drilling of metalloelastase-positive tunnels in ischemic myocardium. Circ Res. 2000;87:378–384. doi: 10.1161/01.res.87.5.378. [DOI] [PubMed] [Google Scholar]
- 129.Kamei N, Tobe K, Suzuki R, Ohsugi M, Watanabe T, Kubota N, Ohtsuka-Kowatari N, Kumagai K, Sakamoto K, Kobayashi M, Yamauchi T, Ueki K, Oishi Y, Nishimura S, Manabe I, Hashimoto H, Ohnishi Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Nagai R, Kadowaki T. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J Biol Chem. 2006;281:26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 130.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tateya S, Tamori Y, Kawaguchi T, Kanda H, Kasuga M. An increase in the circulating concentration of monocyte chemoattractant protein-1 elicits systemic insulin resistance irrespective of adipose tissue inflammation in mice. Endocrinology. 2010;151(3):971–979. doi: 10.1210/en.2009-0926. [DOI] [PubMed] [Google Scholar]
- 132.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tamura Y, Sugimoto M, Murayama T, Ueda Y, Kanamori H, Ono K, Ariyasu H, Akamizu T, Kita T, Yokode M, Arai H. Inhibition of CCR2 ameliorates insulin resistance and hepatic steatosis in db/db mice. Arterioscler Thromb Vasc Biol. 2008;28:2195–201. doi: 10.1161/ATVBAHA.108.168633. [DOI] [PubMed] [Google Scholar]
- 134.Tamura Y, Sugimoto M, Murayama T, Minami M, Nishikaze Y, Ariyasu H, Akamizu T, Kita T, Yokode M, Arai H. C-C chemokine receptor 2 inhibitor improves diet-induced development of insulin resistance and hepatic steatosis in mice. J Atheroscler Thromb. 2010;17:219–228. doi: 10.5551/jat.3368. [DOI] [PubMed] [Google Scholar]
- 135.Ezenwaka CE, Nwagbara E, Seales D, Okali F, Sell H, Eckel J. Insulin resistance, leptin and monocyte chemotactic protein-1 levels in diabetic and non-diabetic Afro-Caribbean subjects. Arch Physiol Biochem. 2009;115:22–27. doi: 10.1080/13813450802676343. [DOI] [PubMed] [Google Scholar]
- 136.Mine S, Okada Y, Tanikawa T, Kawahara C, Tabata T, Tanaka Y. Increased expression levels of monocyte CCR2 and monocyte chemoattractant protein-1 in patients with diabetes mellitus. Biochem Biophys Res Commun. 2006;344:780–785. doi: 10.1016/j.bbrc.2006.03.197. [DOI] [PubMed] [Google Scholar]
- 137.Niu J, Azfer A, Zhelyabovska O, Fatma S, Kolattukudy PE. Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP) J Biol Chem. 2008;283:14542–14551. doi: 10.1074/jbc.M802139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ghosh R, Lipson KL, Sargent KE, Mercurio AM, Hunt JS, Ron D, Urano F. Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS One. 2010;5:e9575. doi: 10.1371/journal.pone.0009575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Younce CW, Azfer A, Kolattukudy PE. MCP-1 (monocyte chemotactic protein-1)-induced protein, a recently identified zinc finger protein, induces adipogenesis in 3T3-L1 pre-adipocytes without peroxisome proliferator-activated receptor gamma. J Biol Chem. 2009;284:27620–27628. doi: 10.1074/jbc.M109.025320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, Czaja MJ. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest. 2009;119:3329–3339. doi: 10.1172/JCI39228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Baerga R, Zhang Y, Chen PH, Goldman S, Jin S. Targeted deletion of autophagy-related 5 (atg5) impairs adipogenesis in a cellular model and in mice. Autophagy. 2009;5:1118–1130. doi: 10.4161/auto.5.8.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang Y, Goldman S, Baerga R, Zhao Y, Komatsu M, Jin S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc Natl Acad Sci USA. 2009;106:19860–19865. doi: 10.1073/pnas.0906048106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Baker-LePain JC, Nakamura MC, Lane NE. Effects of inflammation on bone: an update. Curr Opin Rheumatol. 2011;23:389–395. doi: 10.1097/BOR.0b013e3283474dbe. [DOI] [PubMed] [Google Scholar]
- 144.Abu-Amer Y. Inflammation, cancer, and bone loss. Curr Opin Pharmacol. 2009;9:427–433. doi: 10.1016/j.coph.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kim MS, Day CJ, Selinger CI, Magno CL, Stephens SR, Morrison NA. MCP-1-induced human osteoclast-like cells are tartrate-resistant acid phosphatase, NFATc1, and calcitonin receptor-positive but require receptor activator of NFkappaB ligand for bone resorption. J Biol Chem. 2006;281:1274–1285. doi: 10.1074/jbc.M510156200. [DOI] [PubMed] [Google Scholar]
- 146.Sul OJ, Ke K, Kim WK, Kim SH, Lee SC, Kim HJ, Kim SY, Suh JH, Choi HS. Absence of MCP-1 leads to elevated bone mass via impaired actin ring formation. J Cell Physiol. 2011 doi: 10.1002/jcp.22879. [DOI] [PubMed] [Google Scholar]
- 147.Wang K, Niu J, Kim HB, Kolattukudy PE. Osteoclast precursor differentiation by MCPIP via oxidative stress, endoplasmic reticulum stress and autophagy. J Mol Cell Biol. 2011 doi: 10.1093/jmcb/mjr021. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhao Y, Chen G, Zhang W, Xu N, Zhu JY, Jia J, Sun ZJ, Wang YN, Zhao YF. Autophagy regulates hypoxia-induced osteoclastogenesis through the HIF-1α/BNIP3 signaling pathway. J Cell Physiol. 2011 doi: 10.1002/jcp.22768. [DOI] [PubMed] [Google Scholar]
- 149.Younce CW, Kolattukudy PE. MCP-1 causes cardiomyoblast death via autophagy resulting from ER stress caused by oxidative stress generated by inducing a novel zinc-finger protein, MCPIP. Biochem J. 2010;426:43–53. doi: 10.1042/BJ20090976. [DOI] [PubMed] [Google Scholar]
- 150.Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- 151.Cao DJ, Wang ZV, Battiprolu PK, Jiang N, Morales CR, Kong Y, Rothermel BA, Gillette TG, Hill JA. Histone deacetylase (HDAC) inhibitors attenuate cardiac hypertrophy by suppressing autophagy. Proc Natl Acad Sci U S A. 2011;108:4123–4128. doi: 10.1073/pnas.1015081108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lamkanfi M, Festjens N, Declercq W, Vanden Berghe T, Vandenabeele P. Caspases in cell survival, proliferation and differentiation. Cell Death Differ. 2007;14:44–55. doi: 10.1038/sj.cdd.4402047. [DOI] [PubMed] [Google Scholar]
- 153.Liang J, Wang J, Azfer A, Song W, Tromp G, Kolattukudy PE, Fu M. A novel CCCH-zinc finger protein family regulates proinflammatory activation of macrophages. J Biol Chem. 2008;283:6337–6346. doi: 10.1074/jbc.M707861200. [DOI] [PubMed] [Google Scholar]
- 154.Liang J, Saad Y, Lei T, Wang J, Qi D, Yang Q, Kolattukudy PE, Fu M. MCP-induced protein 1 deubiquitinates TRAF proteins and negatively regulates JNK and NF-kappaB signaling. J Exp Med. 2010;207:2959–2973. doi: 10.1084/jem.20092641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Matsushita K, Takeuchi O, Standley DM, Kumagai Y, Kawagoe T, Miyake T, Satoh T, Kato H, Tsujimura T, Nakamura H, Akira S. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature. 2009;458:1185–1190. doi: 10.1038/nature07924. [DOI] [PubMed] [Google Scholar]
- 156.Niu J, Wang K, Graham S, Azfer A, Kolattukudy PE. MCP-1-induced protein attenuates endotoxin-induced myocardial dysfunction by suppressing cardiac NF-κB activation via inhibition of IκB kinase activation. J Mol Cell Cardiol. 2011;51:177–186. doi: 10.1016/j.yjmcc.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 157.Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430– 437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 158.Xia ZP, Sun L, Chen X, Pineda G, Jiang X, Adhikari A, Zeng W, Chen ZJ. Direct activation of protein kinases by unanchored polyubiquitin chains. Nature. 2009;461:114–119. doi: 10.1038/nature08247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tokunaga F, Sakata S, Saeki Y, Satomi Y, Kirisako T, Kamei K, Nakagawa T, Kato M, Murata S, Yamaoka S, Yamamoto M, Akira S, Takao T, Tanaka K, Iwai K. Involvement of linear polyubiquitylation of NEMO in NF-kappaB activation. Nat Cell Biol. 2009;11:123–132. doi: 10.1038/ncb1821. [DOI] [PubMed] [Google Scholar]
- 160.Harhaj EW, Dixit VM. Deubiquitinases in the regulation of NF-κB signaling. Cell Res. 2011;21:22–39. doi: 10.1038/cr.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Coornaert B, Carpentier I, Beyaert R. A20: central gatekeeper in inflammation and immunity. J Biol Chem. 2009;284:8217–21. doi: 10.1074/jbc.R800032200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Courtois G. Tumor suppressor CYLD: negative regulation of NF-kappaB signaling and more. Cell Mol Life Sci. 2008;65:1123–1132. doi: 10.1007/s00018-007-7465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Takami Y, Nakagami H, Morishita R, Katsuya T, Hayashi H, Mori M, Koriyama H, Baba Y, Yasuda O, Rakugi H, Ogihara T, Kaneda Y. Potential role of CYLD (Cylindromatosis) as a deubiquitinating enzyme in vascular cells. Am J Pathol. 2008;172:818–829. doi: 10.2353/ajpath.2008.070312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li HL, Zhuo ML, Wang D, Wang AB, Cai H, Sun LH, Yang Q, Huang Y, Wei YS, Liu PP, Liu DP, Liang CC. Targeted cardiac overexpression of A20 improves left ventricular performance and reduces compensatory hypertrophy after myocardial infarction. Circulation. 2007;115:1885–1894. doi: 10.1161/CIRCULATIONAHA.106.656835. [DOI] [PubMed] [Google Scholar]
- 165.Huang H, Tang QZ, Wang AB, Chen M, Yan L, Liu C, Jiang H, Yang Q, Bian ZY, Bai X, Zhu LH, Wang L, Li H. Tumor suppressor A20 protects against cardiac hypertrophy and fibrosis by blocking transforming growth factor-beta-activated kinase 1-dependent signaling. Hypertension. 2010;56:232–239. doi: 10.1161/HYPERTENSIONAHA.110.149963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Komander D, Reyes-Turcu F, Licchesi JD, Odenwaelder P, Wilkinson KD, Barford D. Molecular discrimination of structurally equivalent Lys 63-linked and linear polyubiquitin chains. EMBO Rep. 2009;10:466–473. doi: 10.1038/embor.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mizgalska D, Wegrzyn P, Murzyn K, Kasza A, Koj A, Jura J, Jarzab B, Jura J. Interleukin-1-inducible MCPIP protein has structural and functional properties of RNase and participates in degradation of IL-1beta mRNA. FEBS J. 2009;276:7386–7399. doi: 10.1111/j.1742-4658.2009.07452.x. [DOI] [PubMed] [Google Scholar]
- 168.Deleault KM, Skinner SJ, Brooks SA. Tristetraprolin regulates TNF-alpha mRNA stability via a proteasome dependent mechanism involving the combined action of the ERK and p38 pathways. Mol Immunol. 2008;45:13–24. doi: 10.1016/j.molimm.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 169.Dinarello CA. Anti-inflammatory Agents: Present and Future. Cell. 2010;140:935–950. doi: 10.1016/j.cell.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ridker PM. The JUPITER trial: results, controversies, and implications for prevention. Circ Cardiovasc Qual Outcomes. 2009;2:279–285. doi: 10.1161/CIRCOUTCOMES.109.868299. [DOI] [PubMed] [Google Scholar]
- 171.Steffens S, Mach F. Drug insight: Immunomodulatory effects of statins--potential benefits for renal patients? Nat Clin Pract Nephrol. 2006;2:378–387. doi: 10.1038/ncpneph0217. [DOI] [PubMed] [Google Scholar]
- 172.Morrow DA, de Lemos JA, Sabatine MS, Wiviott SD, Blazing MA, Shui A, Rifai N, Califf RM, Braunwald E. Clinical relevance of C-reactive protein during follow-up of patients with acute coronary syndromes in the Aggrastat-to-Zocor Trial. Circulation. 2006;114:281–288. doi: 10.1161/CIRCULATIONAHA.106.628909. [DOI] [PubMed] [Google Scholar]
- 173.Brodmerkel CM, Huber R, Covington M, Diamond S, Hall L, Collins R, Leffet L, Gallagher K, Feldman P, Collier P, Stow M, Gu X, Baribaud F, Shin N, Thomas B, Burn T, Hollis G, Yeleswaram S, Solomon K, Friedman S, Wang A, Xue CB, Newton RC, Scherle P, Vaddi K. Discovery and pharmacological characterization of a novel rodent-active CCR2 antagonist, INCB3344. J Immunol. 2005;175:5370–5378. doi: 10.4049/jimmunol.175.8.5370. [DOI] [PubMed] [Google Scholar]
- 174.Kang YS, Cha JJ, Hyun YY, Cha DR. Novel C-C chemokine receptor 2 antagonists in metabolic disease: a review of recent developments. Expert Opin Investig Drugs. 2011;20:745–756. doi: 10.1517/13543784.2011.575359. [DOI] [PubMed] [Google Scholar]
- 175.Sayyed SG, Ryu M, Kulkarni OP, Schmid H, Lichtnekert J, Grüner S, Green L, Mattei P, Hartmann G, Anders HJ. An orally active chemokine receptor CCR2 antagonist prevents glomerulosclerosis and renal failure in type 2 diabetes. Kidney Int. 2011;80:68–78. doi: 10.1038/ki.2011.102. [DOI] [PubMed] [Google Scholar]
- 176.Gilbert J, Lekstrom-Himes J, Donaldson D, Lee Y, Hu M, Xu J, Wyant T, Davidson M MLN1202 Study Group. Effect of CC chemokine receptor 2 CCR2 blockade on serum C-reactive protein in individuals at atherosclerotic risk and with a single nucleotide polymorphism of the monocyte chemoattractant protein-1 promoter region. Am J Cardiol. 2011;107:906–911. doi: 10.1016/j.amjcard.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 177.Arefieva TI, Krasnikova TL, Potekhina AV, Ruleva NU, Nikitin PI, Ksenevich TI, Gorshkov BG, Sidorova MV, Bespalova ZD, Kukhtina NB, Provatorov SI, Noeva EA, Chazov EI. Synthetic peptide fragment (65–76) of monocyte chemotactic protein-1 (MCP-1) inhibits MCP-1 binding to heparin and possesses anti-inflammatory activity in stable angina patients after coronary stenting. Inflamm Res. 2011;60:955–964. doi: 10.1007/s00011-011-0356-z. [DOI] [PubMed] [Google Scholar]