Abstract
The receptor tyrosine kinase c-Met and its ligand, hepatocyte growth factor/scatter factor (HGF/SF) modulate signaling cascades implicated in cellular proliferation, survival, migration, invasion, and angiogenesis. Therefore, dysregulation of HGF/c-Met signaling can compromise the cellular capacity to moderate these activities, and lead to tumorigenesis, metastasis, and therapeutic resistance in various human malignancies. To facilitate studies investigating HGF/cMet receptor coupling or c-Met signaling events in real time and in living cells and animals, we here describe a genetically engineered reporter wherein bioluminescence can be used as a surrogate for c-Met tyrosine kinase activity. C-Met kinase activity in cultured cells and tumor xenografts was monitored quantitatively and dynamically in response to the activation or inhibition of the HGF/c-Met signaling pathway. Treatment of tumor bearing animals with a c-Met inhibitor and the HGF neutralizing antibody stimulated the reporter’s bioluminescence activity in a dose dependent manner and led to a regression of U-87 MG tumor xenografts. Results obtained from these studies provide unique insights into the pharmacokinetics and pharmacodynamics of agents that modulate c-Met activity and validate c-Met as a target for human glioblastoma therapy.
Keywords: c-Met, non-invasive molecular imaging, bioluminescence, kinase activity
Introduction
c-Met is a prototypic member of the receptor tyrosine kinase family and is the only known high-affinity receptor for hepatocyte growth factor (HGF)/ scatter factor (SF)[1]. The importance of the HGF/c-Met pathway in normal mammalian development is exemplified by the fact that c-Met and HGF knockout mice are embryonic lethal partly due to a failure to undergo epithelial–mesenchymal transition during organ morphogenesis [2]. c-Met signaling also modulates cell migration, invasion, angiogenesis, and organization of three-dimensional (3D) tubular structures during embryogenesis and tissue repair[3].
HGF/SF is believed to be a mesenchymal cell-derived cytokine while its receptor, c-Met is predominantly seen in epithelial cells. However, in Glioblastoma multiforme (GBM), cancer cells concomitantly express both the ligand (HGF/SF) and the receptor (c-Met) resulting in an autocrine signaling loop [4]. It has also been reported that expression of HGF/SF and c-Met correlates with the histological grade of the tumor. A majority of our current understanding of c-Met mediated signaling at various stages of tumor progression suggests that c-Met may be an important target for anticancer therapy.
Although non-invasive imaging of receptor activation has been described previously [3; 5; 6; 7; 8; 9; 10; 11; 12], we in the current studies describe engineering of a unique reporter wherein c-Met tyrosine kinase activity can be monitored non-invasively and dynamically in real time in cell culture systems as well as in live mouse models. Using this reporter we investigated drug-target interaction and phamacodynamics/pharmacokinetics of HGF/c-Met inhibitors in cultured cells as well as in a U87 glioma mouse model. We further demonstrated that administration of a HGF neutralizing antibody resulted in inhibition of c-Met activity in tumor xenografts as well as tumor growth delay, thus validating the use of ligand neutralizing antibodies as a viable therapeutic strategy.
Materials and Methods
Plasmid construction
The gene for the Bioluminescent MET reporter (BMRwt) was generated using our previously described bioluminescent Akt reporter (BAR)[3] as backbone. METpep domain (derived from pyk2, residues 391-411) and the Met binding domain (MBD, residues 486-498 of Gab1) were amplified with the appropriate linkers (GGSGG) using two primers (Primer1:5′-CGTTGTCTAGAGGAGGAAGTGGAGGACTCTCAGAGAGCTGCAGCATAGAGTCAGACATCTACGCAGAGATTCCCGACGAAACCCTGCGAGGAGGAAGTGG and Primer 2: CGTACCCCGGGTCCTCCACTTCCTCCCCTGAAGCCCATATGAGCAGGAGGAGGAACTTGCATTCCTCCTCCACTTCCTCCTCGCAGGGTTTCGTCGGG) to yield the XbaI-Linker-MET substrate-Linker-MBD-Linker-XmaI coding fragment that was cloned into the Akt reporter as a XbaI-XmaI fragment in place of the Akt substrate peptide. The SH2 domain (derived from mouse shc2, residue 374 to 465) was amplified on a NotI-XbaI fragment using two PCR primers, Primer 1: CCCATAGGCGGCCGCTGGTTCCACGGGAAGC and Primer 2: CGCCTCTAGACACGGGTTGCTGTAGG. This fragment was cloned into the resulting plasmid (to replace the FHA2 domain). The complete plasmid, SalI-Kozak-Nluc-NotI-SH2-XbaI-Linker-MET substrate-Linker-MBD-Linker-XmaI-Cluc-EcoRI, was generated as a SalI-EcoRI fragment in vector pEF. BMRmut (Y530A) was constructed using the appropriate primers and the QuickChange kit (Stratagene, CA).
Cell culture and transfections
D54 (human glioma) and U87 (human glioma) cells were maintained in RPMI (Gibco, MD) supplemented with 10% fetal bovine serum (Gibco, MD). To construct stable cell lines, bioluminescent Met reporters (BMRwt and BMRmut) and bioluminescent Akt reporter (BARwt) plasmids were stably transfected into D54 and U87 cells using Lipofectamine 2000 (Invitrogen, CA), and the resulting stable clones were selected using 200μg/ml G418 (Invitrogen, CA) for D54 cells and 500 μg/ml G418 for U87 cells. Resulting cell lines were isolated and determined by western blots for expression level of the recombinant plasmids.
Antibodies and chemicals
Rabbit polyclonal Met (pYpYpY1230/1234/1235) and mouse polyclonal Met antibodies were purchased from Invitrogen (Carlsbad, CA). Rabbit polyclonal antibodies to Akt, phospho-Akt (Ser473), phospho-EGFR (Y845), phospho-tyrosine and phospho-pyk2 (402) were purchased from Cell Signaling Technology (Beverly, MA). Rabbit EGFR (SC-03) antibody was bought from Santa Cruz biotechnology (Santa Cruz, CA). SU11274, an inhibitor of c-Met, was purchased from Sigma-Aldrich (St. Louis, MO). Luciferin was obtained from Biosynth (Naperville, IL). Hepatocyte growth factors (HGF) were purchased from US Biological (Swampscott, MA); epidermal growth factors (EGF) were purchased from Invitrogen (Carlsbad, CA). HGF neutralizing antibody was a gift from Amgen (Thousand Oaks, CA).
Western blot analysis
Cells were washed with PBS and lysed with NP40 lysis buffer (1% NP40, 150mM NaCl, 25mM Tris pH 8.0) supplemented with protease inhibitors (Calbiochem, CA) and phosphatase inhibitors (Sigma, MO). Proteins were estimated using detergent-compatible protein assay kit from Bio-Rad (Hercules, CA), then resolved by SDS/PAGE and analyzed by western blotting using appropriate antibodies. Detection of bound antibody was with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence (Amersham Pharmacia, Uppsala, Sweden).
Immunoprecipitation
Immunoprecipitation was done as described [3]. Briefly, cells were washed with PBS and lysed with NP 40 lysis buffer. Cell extracts were incubated with the luciferase specific antibody for 1 hour. Immune complexes were captured using protein G–Sepharose (Amersham Biosciences, NJ), and washed using NP40 lysis buffer 3 times. The resulting pellet was boiled for 5 minutes in sample buffer and resolved by SDS/PAGE. Protein expression was detected using phospho-tyrosine or phopsho-pyk2 (402) specific antibody followed by horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence.
Small interfering RNA (SiRNA) transfection
U87-BMRwt cells were plated onto 6-well plates at a density of 2.5 × 105 cells/ml and incubated for 24 hr in culture medium. The cells were then transfected with Met or scrambled siRNAs duplexes using Oligofectamine (Invitrogen) according to the manufacturer suggested protocol. Briefly, Met siRNA or scrambled SiRNA (at a final concentration of 50 nM) was added to the mixture of 6 μl of Oligofectamine and 144 μl of OptiMEM medium, and incubated at room temperature for 20 min. The siRNA complex was then added to cells. The medium was replaced 4 h later with fresh complete medium. After 72 h transfection, live cells were imaged for bioluminescence activity and lysed with NP40 lysis buffer for western blotting of total c-Met or phospho-c-Met.
In vitro bioluminescence imaging
Live cell bioluminescence imaging was achieved by supplementing D-luciferin (100 μg/ml final concentration) in the growth medium. Serial BLI images were acquired for each condition 5 minutes post-incubation with D-luciferin using an IVIS imaging system (Xenogen, CA).
In vivo studies
Subcutaneous tumors expressing BMRwt were established by implanting 8 × 106 stably transfected U87-BMRwt glioma cells in the flank of 4 weeks old male athymic mice (CD-1 nu/nu, Charles River Laboratory, MA). When tumors reached approximately 40-60mm3 in volume, bioluminescence imaging was done before treatment as well as at various times after treatment with HGF neutralizing antibody (i.p. injection) or control antibody (i.p. injection) twice per week for 3 weeks. For the trochar implantation, the tumors were chopped into pieces and washed by growth medium. Tumor pieces of similar size were implanted subcutaneously into the flank of male nude mice with a trochar needle.
In vivo bioluminescence imaging
Animals were anesthetized using 2% isofluorane/air mixture and given a single i.p. dose of 150 mg/kg luciferin in normal saline. Image acquisition was initiated approximately 8 minutes post luciferin injection. Serial BLI images were acquired prior to treatment and followed at different times until completion of the experiment.
MR imaging
The imaging was done twice a week, each day after treatment for 3 weeks, using a Varian Unity Inova MRI system (company name and location) equipped with 9.4T, 16cm horizontal bore system with a linear rathead coil. During the procedures, animals were anesthetized with 1.25% isoflurane and placed inside the RF coil. During imaging, body temperature was maintained by heated air flowing through the coil.
To determine the tumor volume within the cortex, a fast spin echo sequence (TR= 4000ms, TE=40ms) was performed to acquire 15 axial slices with a slice thickness of 1.0 mm, a matrix size of 256 × 128, and a field of view of 3.0 × 3.0 cm2. The volume of each tumor was calculated based on the number of voxels the tumor occupied in the images using an “in-house” region drawing tool developed in Matlab.
Data analysis
Fold induction in signal intensity was calculated using pretreatment values as baseline and plotted as means ± SEM for each of the groups. Statistical comparisons were made by using the unpaired Student’s t test with a value of p< 0.05 being the cut off for significance.
Results
Engineering and validation of a bioluminescent c-Met reporter (BMR)
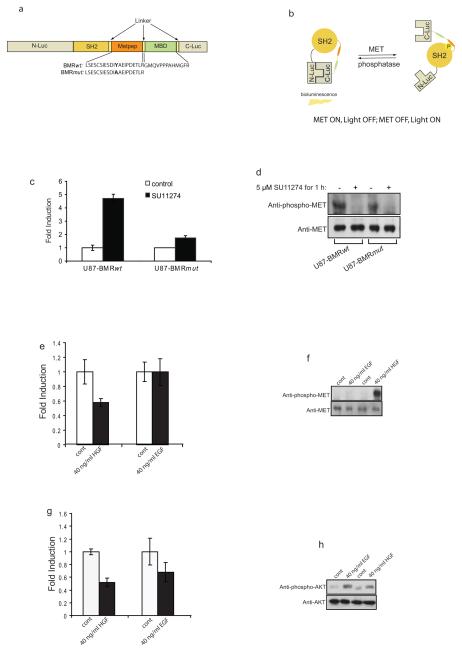
To detect c-Met kinase activity, we constructed a hybrid molecule (Fig. 1a) consisting of an 11 amino acid peptide sequence (derived from Pyk2)[13; 14] which is a substrate for c-Met. The bioluminescent c-Met reporter (BMR) consisted of the sh2 phospho-tyrosine binding domain (residues 374 to 465)[15], flanked by N-Luc (residues 1 to 416 of firefly luciferase) and C-Luc (residues 398 to 550 of firefly luciferase)[16]. In addition to the wild type reporter, a mutant version as control was also constructed (Fig. 1a) in which tyrosine (Tyr530) of BMR was mutated to alanine (BMRmut). The functional basis (Fig. 1b) of the reporter was that when c-Met was active, phosphorylation of the substrate peptide would result in the binding of the phospho-tyrosine residue to the sh2 domain, thus preventing reconstitution of N-Luc with C-Luc due to stearic hindrance. Inhibition of c-Met kinase activity would result in loss of phosphorylation of the substrate peptide, thus releasing it from interaction with the sh2 phospho-tyrosine binding domain. In this situation, N-Luc and C-Luc association is facilitated thus reconstituting luciferase activity, which can be detected by bioluminescence imaging. The Met binding domain (MBD)[17] from Gab1 was included adjacent to the Pyk2 target sequence to enhance the specificity and sensitivity of the reporter.
Figure 1. Bioluminescence Met reporter (BMR).
a. The domain structure of the BMR. N-Luc and C-Luc are the amino- and carboxyl- terminal domains of firefly luciferase that were fused to animal- and carboxyl- terminal ends of the reporter. METpep domain which constitutes a consensus c-Met substrate sequence was fused to the Met binding domain (MBD). At the amino-terminal of the c-Met substrate sequence, a tyrosine phospho-tyrosine binding domain (SH2) was added. Flexible linker sequence (GGSGG) was included between SH2 and Metpep, Metpep and MBD as well as MBD and C-Luc. Two versions of the c-Met reporter were constructed. The BMRwt molecule contains the wild-type Metpep sequence, and the BMRmut molecule contains a Tyr to Ala substitution at the primary phosphrylation site.
b. BMR functions in a manner of c-Met-dependent phosphorylation of the Metpep substrate. In the presence of c-Met (Met-ON), the phosphorylation of Metpep results in its interaction with the SH2 domain, which prevents the binding of split luciferase domains and generates minimal bioluminescence activity. In the absence of c-Met activation (Met-OFF), the reconstitution of N-terminal and C-terminal luciferase domains restores the bioluminescent activity.
c. U87-BMRwt cells as well as U87-BMRmut cells were treated with SU11274 at 5 μM for 1 h. The changes in bioluminescence activity over control vehicle treated levels were determined and reported as fold induction. The data were generated from a minimum of 5 experiments.
d. Cells from one experiment of c was collected and lysed for western blotting analysis with phospho-c-Met or total c-Met specific antibody.
e. U87-BMRwt cells were serum starved overnight and treated with 40 ng/ml HGF or 40 ng/ml EGF. The bioluminescence imaging was observed within 30 min of treatment. The changes in bioluminescence activity over pre-treated levels were determined as fold induction.
f. Western blotting analysis of samples from e using phospho-c-Met or total c-Met specific antibody.
g. U87-BARwt cells were serum starved overnight, treated with 40 ng/ml HGF or 40 ng/ml EGF, and imaged at 30 min. The changes in bioluminescence activity over pre-treated levels were determined as fold induction.
h. Cell extracts from h were used for western blotting analysis with phospho-Akt as well as total Akt antibody.
To test the ability of BMR to sense c-Met activity in live cells, the expression vectors for wild-type and mutant reporters were stably transfected into human glioma cells (U87 and D54). Treatment of U87-BMRwt cells with a c-Met inhibitor (SU11274)[18] resulted in a 5 fold induction in bioluminescence activity compared to control DMSO treated cells. In contrast, U87-BMRmut cells had no significant change in bioluminescence activity in response to SU11274 treatment (Fig. 1c). Western blot analysis with either stable cell line upon SU11274 treatment revealed a decrease in phospho-c-Met levels, but not total c-Met levels (Fig. 1d).
To investigate if the BMR was able to sense activation of c-Met activity (rather than inhibition) in response to hepatocyte growth factor (HGF) treatment, U87-BMRwt cells were serum starved overnight and treated with HGF or epidermal growth factor (EGF) as control. Within 30 min of treatment, cells treated with HGF underwent a 40% decrease in bioluminescence activity compared to cells treated with vehicle control, which correlated with an increase in the levels of phospho-c-Met. In contrast, treatment of U87-BMRwt cells with EGF did not result in a substantial change in bioluminescence activity nor in the levels of phospho-c-Met (Fig. 1e and 1f). The impact of HGF-mediated activation of c-Met on downstream signaling was evaluated using U87 glioma cells expressing a previously described bioluminescent Akt reporter (BAR)[3]. U87-BARwt cells were serum starved overnight and treated for 30 min with HGF or EGF, and changes in bioluminescence activity were evaluated. Treatment with either growth factor resulted in a 40% decrease in bioluminescence activity and a correlative increase in levels of phosphorylated Akt but not total Akt (Fig. 1g and 1h).
Imaging c-Met activity in vitro
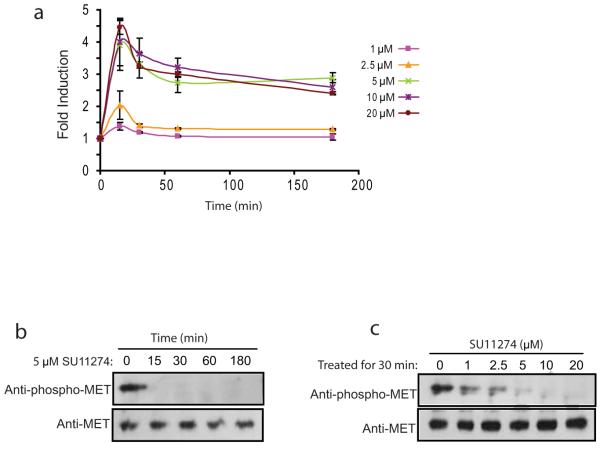
To test the ability of BMR to detect changes in c-Met in a quantitative and dynamic manner, U87-BMRwt cells were treated with increasing doses of c-Met inhibitor SU11274 and bioluminescence activity was monitored at various times. In all cases, bioluminescence activity increased with time and reached a peak within 15 min and plateaued thereafter (Fig. 2a). Cells treated with lower doses of SU11274 (1 μM and 2.5 μM) had a significant but small increase in bioluminescence activity. In contrast, cells treated with higher doses of SU11274 (5 μM, 10 μM and 20 μM) resulted in a much greater increase in bioluminescence activity. Western blotting analysis showed a considerable decrease in the levels of phospho-c-Met with treatment of higher doses of SU11274 (5 μM, 10 μM and 20 μM) compared to lower doses (1 μM and 2.5 μM) (Fig. 2b and 2c), thus validating the changes in bioluminescence activity of the reporter.
Figure 2. Imaging of c-Met activity.
a. U87-BMRwt cells treated with various doses (1, 2.5, 5, 10, and 20 μM) of SU11274 were imaged at different times (5, 15, 30, 60 min). The changes in bioluminescence activity were plotted as fold induction over pretreatment values. Data were derived from a minimum of five independent experiments.
b. Western blotting analysis of samples treated with 5 μM SU11274 at various times from a was conducted using antibodies specific for phospho-c-Met or total c-Met.
c. Cells treated with SU11274 at various concentrations for 30 min were collected and lysed for western blotting analysis using antibodies specific for phospho-c-Met or total c-Met.
BMR is a substrate for c-Met
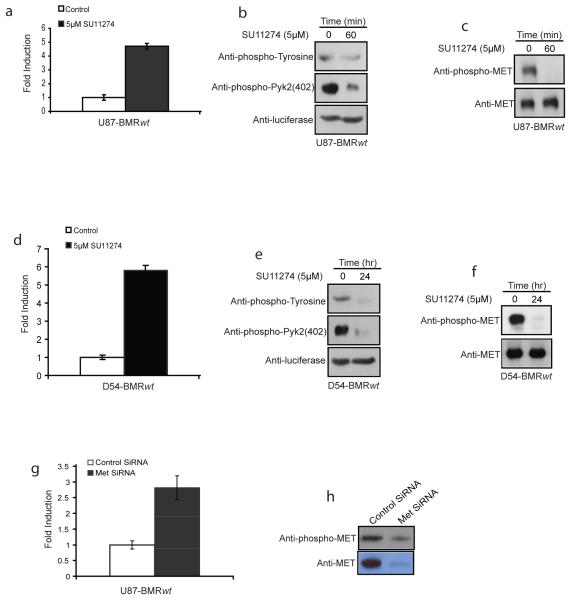
Since BMR function was predicated on it being phosphorylated in a c-Met dependent manner, we treated U87 cells stably expressing BMR (U87-BMRwt) with SU11274 or saline as a control and observed an approximately five fold increase in bioluminescence (Fig. 3a). Immunoprecipitation of BMR from these cells using a luciferase specific antibody followed by western blot analysis using a phospho-tyrosine or phospho-pyk2 antibody revealed a decrease in tyrosine phosphorylation of BMR in response to SU11274 treatment (Fig. 3b). Inhibition of c-Met in treated but not control cells was also observed upon western blot analysis of cell extracts using antibodies specific for total and phospho-c-Met (Fig. 3c). Analogous studies performed using D54-BMRwt cells further validated that inhibition of c-Met activity resulted in an increase in reporter activity due to a decrease in phosphorylation of BMR (Fig. 3d, 3e and 3f). Additional proof that BMR is a specific indicator for c-Met activity was obtained from experiments in which c-Met activity was inhibited in response to siRNA mediated down-regulation of the receptor. Targeted down-regulation of c-Met expression resulted in a 3 fold induction of the bioluminescence activity compared to a control non-silencing siRNA in U87-BMRwt cells (Fig. 3g). This result was consistent with a substantial decrease in levels of total c-Met and phosphorylated c-Met as determined by western blotting analysis (Fig. 3h).
Figure 3. C-Met dependent phosphorylation of BMR.
a. U87-BMRwt cells were treated with SU11274 (5 μM) or control vehicle for 1 hr, and changes in bioluminescence activity were plotted as fold induction over control vehicle treatment values.
b. U87-BMRwt cells treated with Su11274 (5 μM) or vehicle control were collected and lysed. Luciferase specific antibody was used to immunoprecipitate BMR protein, and western blotting analysis was then performed using phospho-tyrosine, phospho-pyk2 (402) specific antibody, as well as luciferase antibody as control.
c. Cell extracts from a were used for western blotting analysis using phospho-c-Met as well as total c-Met antibodies.
d. D54-BMRwt stably transfected cells were treated with SU11274 (5 μM) or control vehicle, and imaged at 24 h time point. Changes in bioluminescence activity were plotted as fold induction using control treatment level as baseline.
e. D54-BMRwt cells treated with SU11274 or control vehicle were used to prepare extracts for subsequent analysis. BMR molecule was immunoprecipitated using luciferase antibody and western blotting analysis was conducted using antibodies specific for phospho-tyrosine, phospho-Pyk2 (402) or luciferase as a control.
f. Extracts from c were analyzed by western blotting using antibodies specific for phospho-c-Met and total c-Met.
g. U87-BMRwt cells were transfected with c-Met SiRNA or scrambled SiRNA, and bioluminescence activity was monitored after 72 h. Changes in bioluminescence activity were plotted as fold induction using scrambled SiRNA treatment level as baseline.
h. Cell lysates from g were collected and analyzed by western blotting analysis using antibodies specific for phospho-c-Met and total c-Met.
C-Met is a valid target for brain cancer therapy
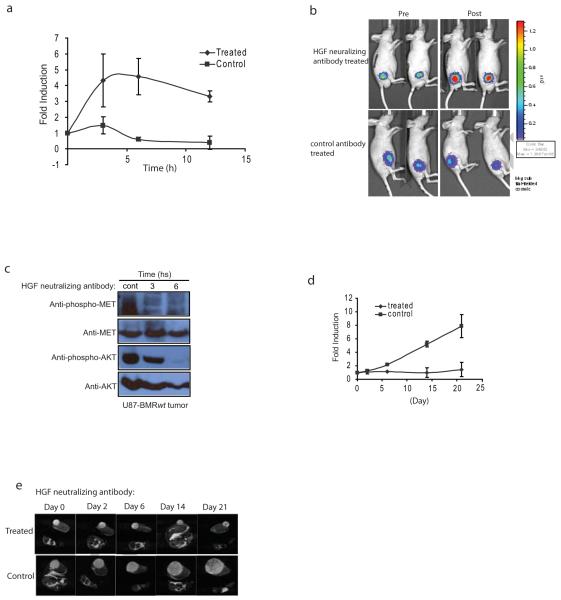
To evaluate the utility of BMR in the evaluation of c-Met targeted cancer therapies, U87-BMRwt cells were implanted subcutaneously in the flank of nude mice to establish tumors. When tumors reached ~50 mm3, mice were divided randomly into two groups with 8-10 mice per group and treated with control immunoglobulin or with HGF neutralizing antibody[19] twice a week for 3 weeks. Tumor specific bioluminescence was measured pre- and at various times post-treatment. Tumors in control antibody treated animals had no significant increase in bioluminescence activity post drug administration. In contrast, HGF neutralizing antibody treated tumors had a 4-5 fold increase in bioluminescence activity at 3h, which was sustained for 10h (Fig. 4a). Beyond 15h, a significant decline in reporter activity was observed (data not shown). Representative images of mice in each treatment group are shown in Fig. 4b. To confirm that the changes in bioluminescence activity in these animals were due to inhibition of c-Met activity, tumors were resected and analyzed by Western blotting. A sustained inhibition of c-Met phosphorylation as well as a decrease in phospho-Akt levels in response to drug administration was observed (Fig. 4c). Further, a remarkable tumor growth delay was monitored in animals treated with the HGF neutralizing antibody compared to control antibody treated animals (Fig. 4d and 4e). At the end of the treatment, tumors of animals treated with control antibody underwent an 8 fold increase in initial tumor volume while tumors in treated animals exhibited a complete growth delay (Fig. 4d).
Figure 4. C-Met as a target for brain cancer treatment.
a. U87-BMRwt stably transfected cells were implanted subcutaneously into nude mice. The developed tumors were taken out, chopped into pieces and implanted subcutaneously into male nude mice. When tumor volume reached ~50 mm3, mice were separated into two groups. Bioluminescence activity before treatment and in response to treatment with control antibody or HGF neutralizing antibody (300 μg/mouse, ip) was monitored at various times (0, 3, 6, 12 h). Fold induction of signal intensity over pretreatment values was plotted as mean ± s.e.m. for each of the groups. 8-10 animals were used for each treatment group.
b. Tumor bearing mice were treated with control antibody or HGF neutralizing antibody. Images of representative mice are presented before treatment (Pre) and 6 h after treatment (post).
c. At various time points (0, 3, 6h) after administration of HGF neutralizing antibody, mice were euthanized, tumor samples were collected and lysates were prepared for western blotting analysis using specific antibodies for phospho-c-Met and total c-Met, phospho-Akt and total Akt.
d. Tumor bearing mice were treated with control antibody or HGF neutralizing antibody (300 μg/mouse, i.p.) twice per week for three weeks. Tumor volumes obtained form serial MR images of individual mice are displayed versus time. Changes in tumor volume were calculated and expressed as fold induction based on pretreated levels.
e. A series of T2-weighted MR images of a mouse bearing a U87 tumor on the flank before treatment and at 2, 6, 14 and 21 days post-treatment, respectively. Each displayed image is from approximately a similar slice within each tumor.
Discussion
Molecular imaging has enabled non-invasive, real time, dynamic and quantitative imaging of kinase activity in living cells and subjects. In our previous study, quantitative, dynamic imaging of the Akt serine-threonine kinase activity was accomplished using a luciferase complementation assay[3]. In the present report, we have adapted the previously described platform to enable imaging c-Met tyrosine kinase activity. Our initial efforts wherein a c-Met target was incorporated adjacent to a phospho-tyrosine binding domain failed due to a lack of specificity for c-Met (data not shown). Since the specificity of many kinases is influenced by factors such as subcellular compartmentalization, co-localization via anchoring proteins and scaffolds, substrate capture by non-catalytic interaction domains (e.g., SH2 domains) and kinase-docking motifs within substrates and regulatory subunits[20; 21; 22; 23; 24; 25; 26; 27], we adapted the reporter to harbor c-Met binding domain (MBD) in addition to the target sequence. This addition of a c-Met docking site from Gab1 (MBD) onto the reporter significantly improved the specificity of the reporter. We have previously demonstrated that modification of the reporter can result in enhanced sensitivity and/or specificity of the reporter. For example, modification of the Akt reporter (BAR) such that it was targeted to the plasma membrane enhanced the sensitivity of the bioluminescence reporter [11].
Time and dose dependent inhibition of c-Met in response to SU11274 were sensed by BMR and resulted in corresponding changes in bioluminescence activity (Fig. 2). Doses beyond 5 μM of SU11274 resulted in a significantly greater induction of bioluminescence activity which correlated with a reduction in of levels of phospho-c-Met in response to SU11274 treatment at these doses as detected by western blot analysis (Fig 2). SU11274 mediated inhibition c-Met in both U87 and D54 cells resulted in a concomitant decrease in the phosphorylation status of the BMR (Fig 3). This observation confirmed that changes in the BMR bioluminescence activity were due to changes in phosphorylation status of the reporter, which in turn were mediated by c-Met tyrosine kinase activity. In support of the notion that BMR was a substrate for c-Met, siRNA-mediated targeted down-regulation of c-Met expression resulted in a corresponding increase in bioluminescence activity.
Since c-Met is actively being pursued as a target for anticancer therapies, the ability to non-invasively and quantitatively image c-Met activity in live animals would significantly enhance our understanding of pharmacokinetics and bioavailability of novel c-Met specific agents. Treatment of mice bearing U87 xenografts with AMG 102[28], an HGF neutralizing antibody, resulted in an increase in bioluminescence activity, indicating inhibition of c-Met activity and continued treatment eventually led to a delay in tumor growth. These results suggest a key role for molecular imaging reporters (BMR) in validating targets (such as c-Met) for cancer therapy. Further, inferences from our data can also be made for non-invasive and real time determination of dose and schedule of therapies and also regarding the efficacy of a therapy. These studies would greatly benefit future clinical trials.
We here demonstrate that BMR is reversible, in that it allowed for bioluminescence to be a surrogate for c-Met activation as well as inhibition. For example, activation of c-Met by exogenous HGF (Fig. 1e and 1f) was readily detected by a decrease in bioluminescence activity. In addition, the impact of c-Met activation and inhibition on downstream signaling pathways was also demonstrated using a bioluminescent Akt reporter in the presented results (Fig. 1g and 1h). These results indicated that activation of the c-Met receptor tyrosine kinase as well as its downstream effectors in response to a mitogenic signal can be non-invasively monitored using bioluminescence activity.
Since mouse HGF/SF is not a potent agonist of the human c-Met receptor, growth of the U87 glioma in athymic nude mice must have been supported by autocrine c-Met activation. Activation of the c-met receptor in vitro in long term cultures but not in short term cultures (presumable due to a lack of HGF accumulation, data not shown) confirms the presence of an HGF/cmet autocrine loop in U87 cells. Autocrine activation of c-Met is common in glioblastoma tumors and has been previously documented in U87 cells[29]. The results of these studies indicate that interrupting paracrine HGF:c-Met signaling with AMG 102 may provide a potent intervention strategy to treat patients with glioblastoma.
In summary, bioluminescent imaging as a surrogate for receptor tyrosine kinase activity is an important tool and provides novel insights into the role of c-Met in oncogenesis. Real time, dynamic, non-invasive and quantitative surrogates for tyrosine kinase activity will greatly impact the process of drug discovery by enabling the validation of drug-target interaction (i.e. that HGF neutralization results in inhibition of c-Met) as well as in the preclinical determination of optimal drug dosage, schedule and optimization of therapies.
Acknowledgements
Dr. Rehemtulla acknowledges constructive discussions with all members of the Center for Molecular Imaging and Dr Judith Connett for editorial assistance. This work was supported by the US National Institue of Health research grants R01CA129623 (A.R.), U24CA083099 (B.D.R.), P50CA093990 (B.D.R.) and P01CA085878 (B.D.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, Aaronson SA. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–4. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- [2].Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768–71. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- [3].Zhang L, Lee KC, Bhojani MS, Khan AP, Shilman A, Holland EC, Ross BD, Rehemtulla A. Molecular imaging of Akt kinase activity. Nat Med. 2007;13:1114–9. doi: 10.1038/nm1608. [DOI] [PubMed] [Google Scholar]
- [4].Nabeshima K, Shimao Y, Sato S, Kataoka H, Moriyama T, Kawano H, Wakisaka S, Koono M. Expression of c-Met correlates with grade of malignancy in human astrocytic tumours: an immunohistochemical study. Histopathology. 1997;31:436–43. doi: 10.1046/j.1365-2559.1997.3010889.x. [DOI] [PubMed] [Google Scholar]
- [5].Kunkel MT, Ni Q, Tsien RY, Zhang J, Newton AC. Spatio-temporal dynamics of protein kinase B/Akt signaling revealed by a genetically encoded fluorescent reporter. J Biol Chem. 2005;280:5581–7. doi: 10.1074/jbc.M411534200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kunkel MT, Toker A, Tsien RY, Newton AC. Calcium-dependent regulation of protein kinase D revealed by a genetically encoded kinase activity reporter. J Biol Chem. 2007;282:6733–42. doi: 10.1074/jbc.M608086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Violin JD, Zhang J, Tsien RY, Newton AC. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase C. J Cell Biol. 2003;161:899–909. doi: 10.1083/jcb.200302125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, Chien S. Visualizing the mechanical activation of Src. Nature. 2005;434:1040–5. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- [9].Sasaki K, Sato M, Umezawa Y. Fluorescent indicators for Akt/protein kinase B and dynamics of Akt activity visualized in living cells. J Biol Chem. 2003;278:30945–51. doi: 10.1074/jbc.M212167200. [DOI] [PubMed] [Google Scholar]
- [10].Zhang GJ, Safran M, Wei W, Sorensen E, Lassota P, Zhelev N, Neuberg DS, Shapiro G, Kaelin WG., Jr. Bioluminescent imaging of Cdk2 inhibition in vivo. Nat Med. 2004;10:643–8. doi: 10.1038/nm1047. [DOI] [PubMed] [Google Scholar]
- [11].Zhang L, Bhojani MS, Ross BD, Rehemtulla A. Enhancing Akt imaging through targeted reporter expression. Mol Imaging. 2008;7:168–74. [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang L, Bhojani MS, Ross BD, Rehemtulla A. Molecular imaging of protein kinases. Cell Cycle. 2008;7:314–7. doi: 10.4161/cc.7.3.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Maulik G, Kijima T, Ma PC, Ghosh SK, Lin J, Shapiro GI, Schaefer E, Tibaldi E, Johnson BE, Salgia R. Modulation of the c-Met/hepatocyte growth factor pathway in small cell lung cancer. Clin Cancer Res. 2002;8:620–7. [PubMed] [Google Scholar]
- [14].Ma PC, Maulik G, Christensen J, Salgia R. c-Met: structure, functions and potential for therapeutic inhibition. Cancer Metastasis Rev. 2003;22:309–25. doi: 10.1023/a:1023768811842. [DOI] [PubMed] [Google Scholar]
- [15].Ting AY, Kain KH, Klemke RL, Tsien RY. Genetically encoded fluorescent reporters of protein tyrosine kinase activities in living cells. Proc Natl Acad Sci U S A. 2001;98:15003–8. doi: 10.1073/pnas.211564598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Luker KE, Smith MC, Luker GD, Gammon ST, Piwnica-Worms H, Piwnica-Worms D. Kinetics of regulated protein-protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. Proc Natl Acad Sci U S A. 2004;101:12288–93. doi: 10.1073/pnas.0404041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Weidner KM, Di Cesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–6. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- [18].Sattler M, Pride YB, Ma P, Gramlich JL, Chu SC, Quinnan LA, Shirazian S, Liang C, Podar K, Christensen JG, Salgia R. A novel small molecule met inhibitor induces apoptosis in cells transformed by the oncogenic TPR-MET tyrosine kinase. Cancer Res. 2003;63:5462–9. [PubMed] [Google Scholar]
- [19].Burgess T, Coxon A, Meyer S, Sun J, Rex K, Tsuruda T, Chen Q, Ho SY, Li L, Kaufman S, McDorman K, Cattley RC, Sun J, Elliott G, Zhang K, Feng X, Jia XC, Green L, Radinsky R, Kendall R. Fully human monoclonal antibodies to hepatocyte growth factor with therapeutic potential against hepatocyte growth factor/c-Met-dependent human tumors. Cancer Res. 2006;66:1721–9. doi: 10.1158/0008-5472.CAN-05-3329. [DOI] [PubMed] [Google Scholar]
- [20].Linding R, Jensen LJ, Pasculescu A, Olhovsky M, Colwill K, Bork P, Yaffe MB, Pawson T. NetworKIN: a resource for exploring cellular phosphorylation networks. Nucleic Acids Res. 2008;36:D695–9. doi: 10.1093/nar/gkm902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Remenyi A, Good MC, Bhattacharyya RP, Lim WA. The role of docking interactions in mediating signaling input, output, and discrimination in the yeast MAPK network. Mol Cell. 2005;20:951–62. doi: 10.1016/j.molcel.2005.10.030. [DOI] [PubMed] [Google Scholar]
- [22].Bhattacharyya RP, Remenyi A, Good MC, Bashor CJ, Falick AM, Lim WA. The Ste5 scaffold allosterically modulates signaling output of the yeast mating pathway. Science. 2006;311:822–6. doi: 10.1126/science.1120941. [DOI] [PubMed] [Google Scholar]
- [23].Barsyte-Lovejoy D, Galanis A, Sharrocks AD. Specificity determinants in MAPK signaling to transcription factors. J Biol Chem. 2002;277:9896–903. doi: 10.1074/jbc.M108145200. [DOI] [PubMed] [Google Scholar]
- [24].Tanoue T, Adachi M, Moriguchi T, Nishida E. A conserved docking motif in MAP kinases common to substrates, activators and regulators. Nat Cell Biol. 2000;2:110–6. doi: 10.1038/35000065. [DOI] [PubMed] [Google Scholar]
- [25].Vinciguerra M, Vivacqua A, Fasanella G, Gallo A, Cuozzo C, Morano A, Maggiolini M, Musti AM. Differential phosphorylation of c-Jun and JunD in response to the epidermal growth factor is determined by the structure of MAPK targeting sequences. J Biol Chem. 2004;279:9634–41. doi: 10.1074/jbc.M308721200. [DOI] [PubMed] [Google Scholar]
- [26].Biondi RM, Nebreda AR. Signalling specificity of Ser/Thr protein kinases through docking-site-mediated interactions. Biochem J. 2003;372:1–13. doi: 10.1042/BJ20021641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tanoue T, Nishida E. Molecular recognitions in the MAP kinase cascades. Cell Signal. 2003;15:455–62. doi: 10.1016/s0898-6568(02)00112-2. [DOI] [PubMed] [Google Scholar]
- [28].Jun HT, Sun J, Rex K, Radinsky R, Kendall R, Coxon A, Burgess TL. AMG 102, a fully human anti-hepatocyte growth factor/scatter factor neutralizing antibody, enhances the efficacy of temozolomide or docetaxel in U-87 MG cells and xenografts. Clin Cancer Res. 2007;13:6735–42. doi: 10.1158/1078-0432.CCR-06-2969. [DOI] [PubMed] [Google Scholar]
- [29].Chattopadhyay N, Butters RR, Brown EM. Agonists of the retinoic acid- and retinoid X-receptors inhibit hepatocyte growth factor secretion and expression in U87 human astrocytoma cells. Brain Res Mol Brain Res. 2001;87:100–8. doi: 10.1016/s0165-3806(00)00154-1. [DOI] [PubMed] [Google Scholar]