Abstract
Objective:
This analysis was performed to assess whether antiepileptic drugs (AEDs) modulate the effectiveness of temozolomide radiochemotherapy in patients with newly diagnosed glioblastoma.
Methods:
The European Organization for Research and Treatment of Cancer (EORTC) 26981–22981/National Cancer Institute of Canada (NCIC) CE.3 clinical trial database of radiotherapy (RT) with or without temozolomide (TMZ) for newly diagnosed glioblastoma was examined to assess the impact of the interaction between AED use and chemoradiotherapy on survival. Data were adjusted for known prognostic factors.
Results:
When treatment began, 175 patients (30.5%) were AED-free, 277 (48.3%) were taking any enzyme-inducing AED (EIAED) and 135 (23.4%) were taking any non-EIAED. Patients receiving valproic acid (VPA) only had more grade 3/4 thrombopenia and leukopenia than patients without an AED or patients taking an EIAED only. The overall survival (OS) of patients who were receiving an AED at baseline vs not receiving any AED was similar. Patients receiving VPA alone (97 [16.9%]) appeared to derive more survival benefit from TMZ/RT (hazard ratio [HR] 0.39, 95% confidence interval [CI] 0.24–0.63) than patients receiving an EIAED only (252 [44%]) (HR 0.69, 95% CI 0.53–0.90) or patients not receiving any AED (HR 0.67, 95% CI 0.49–0.93).
Conclusions:
VPA may be preferred over an EIAED in patients with glioblastoma who require an AED during TMZ-based chemoradiotherapy. Future studies are needed to determine whether VPA increases TMZ bioavailability or acts as an inhibitor of histone deacetylases and thereby sensitizes for radiochemotherapy in vivo.
The life-time risk of patients with glioblastoma to experience epileptic seizures is in the range of 30%–50%.1 Many considerations support thoughtful use of antiepileptic drugs (AEDs) in patients with brain tumors, including the resistance of the seizure disorder, drug-to-drug interactions, and side effects.2,3 Rash, drowsiness, or other cognitive alterations may affect the patients‘ quality of life. Drug interaction with chemotherapy is of concern by overlapping hematologic toxicity and by hepatic enzyme induction or inhibition. Notably, the older AEDs such as phenytoin, phenobarbital, or carbamazepine will induce a number of coenzymes of the cytochrome P450 system and may thus enhance the metabolism of many commonly administered chemotherapy agents as well as corticosteroids. Conversely, the enzyme-inhibiting effect of valproic acid (VPA) appears clinically of lesser importance, although increased myelosuppression may develop in patients receiving nitrosoureas or cisplatinum-based chemotherapy concomitantly.4 There is also concern that VPA increases the risk of perioperative bleeding complications, although this is not supported by the literature.5,6
Intrinsic antitumor activity of certain AEDs and synergy with chemotherapy or radiotherapy (RT) have been suggested in some, but not all, studies.7 For instance, phenytoin has been attributed antimitotic and anti-invasive properties, and VPA has been suggested to induce cell differentiation, growth arrest, and apoptosis mediated by its histone deacetylase (HDAC)–inhibiting properties.8,9
In a recent retrospective analysis of 3 trials performed by the North Central Cancer Treatment Group, a possible association of enzyme-inducing antiepileptic drug (EIAED) use with a favorable outcome in patients with glioblastoma was reported.10 This observation prompted us to assess a potential predictive value on outcome of the AED used within the pivotal trial of concomitant and adjuvant temozolomide (TMZ) and radiotherapy (TMZ/RT→TMZ), the current standard of care.11–13
METHODS
Patients.
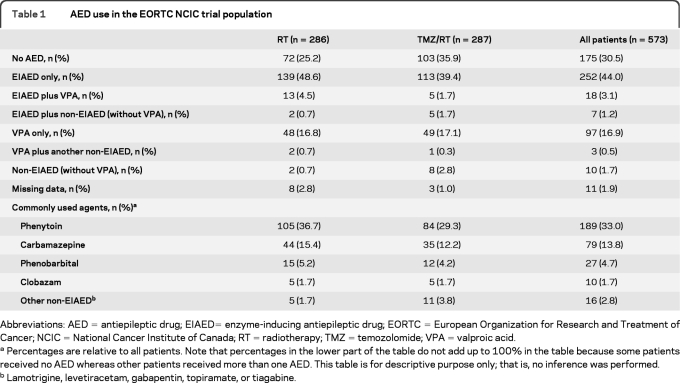
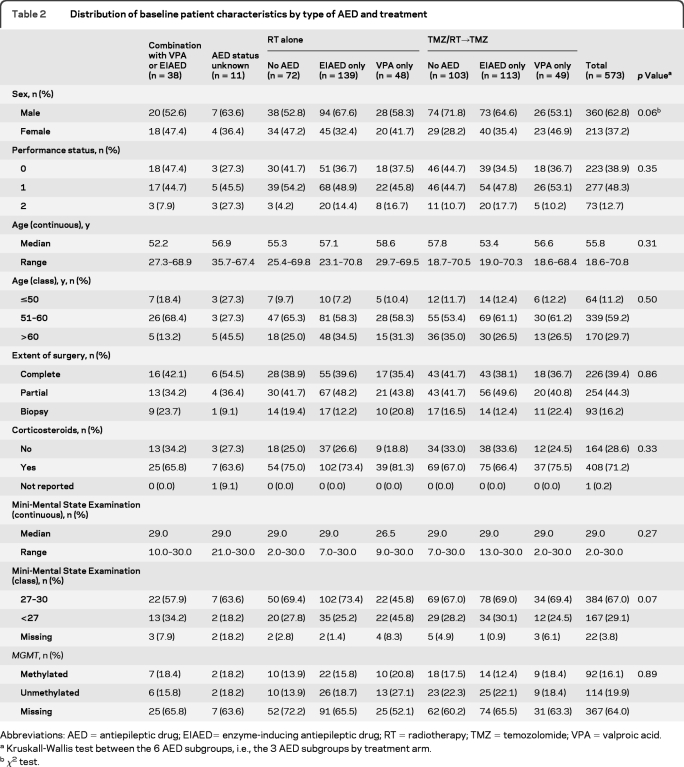
We retrospectively analyzed the subgroup of patients who received an AED while being treated within a pivotal large randomized clinical chemotherapy trial. In that randomized trial, 573 patients with newly diagnosed glioblastoma were treated between 2000 and 2002 with either initial RT alone or RT with concomitant and adjuvant TMZ chemotherapy (TMZ/RT→TMZ).11 Eligible patients had histologically confirmed glioblastoma, were aged 18–70 years, and had a World Health Organization performance status of 0–2. National Cancer Institute Common Toxicity Criteria were used to grade toxicity. Baseline medication was recorded in all patients, and AED use for the purpose of this analysis refers to baseline use, that is, at any time during concomitant radiochemotherapy, unless specified otherwise. The reason for the prescription of an AED was not recorded; thus, patients may have been receiving an AED because of a seizure at disease presentation or for prophylaxis during the perioperative and postoperative phase. We identified 387 patients (68% of all patients) who received any AED; 110 patients (28% of those receiving an AED) were prescribed a non-EIAED, mostly VPA, exclusively, whereas the others received at least one EIAED, either phenytoin, carbamazepine, oxcarbazepine, or phenobarbital. Patient characteristics and details of the assigned oncologic therapy and type of AED used are summarized in tables 1 and 2.
Table 1.
AED use in the EORTC NCIC trial population
Abbreviations: AED=antiepileptic drug; EIAED= enzyme-inducing antiepileptic drug; EORTC=European Organization for Research and Treatment of Cancer; NCIC=National Cancer Institute of Canada; RT=radiotherapy; TMZ=temozolomide; VPA=valproic acid.
Percentages are relative to all patients. Note that percentages in the lower part of the table do not add up to 100% in the table because some patients received no AED whereas other patients received more than one AED. This table is for descriptive purpose only; that is, no inference was performed.
Lamotrigine, levetiracetam, gabapentin, topiramate, or tiagabine.
Table 2.
Distribution of baseline patient characteristics by type of AED and treatment
Abbreviations: AED=antiepileptic drug; EIAED= enzyme-inducing antiepileptic drug; RT=radiotherapy; TMZ=temozolomide; VPA=valproic acid.
Kruskall-Wallis test between the 6 AED subgroups, i.e., the 3 AED subgroups by treatment arm.
χ2 test.
Standard protocol approvals, registrations, and patient consents.
The trial was conducted by the European Organization for Research and Treatment of Cancer (EORTC) and the National Cancer Institute of Canada (NCIC) Clinical Trials Group. All patients provided written informed consent, and the study was approved by the ethics committees and competent authorities of all participating centers.
Statistical analysis.
A χ2 test was used for binary and categorical factors and a Kruskal-Wallis test for continuous variables and scores to assess the significance of observed imbalances in baseline characteristics. The treatment effect on outcome (progression-free survival [PFS] or overall survival [OS]) was analyzed by univariate analysis in the 3 major subgroups (table 1) defined by the administration of an AED: no AED vs VPA only vs EIAED only. OS was defined as the time between randomization and death. Patients alive at the time of analysis were censored at the last visit. PFS was defined as the time between randomization and progression or death, which ever occurred first. Patients who did not progress were censored at the last patient visit.
Log-rank tests were used to compare PFS and OS curves. For both PFS and OS, 3 Cox proportional hazards models were fit to assess the treatment effect in each AED subgroup. Three separate Cox models with an interaction term were fit to compare treatment efficacy between AED subgroups and to estimate their predictive values for treatment efficacy on PFS and OS. All models were adjusted for possible confounding effects of 4 previously identified prognostic factors: age (score: ≤50, >50–≤60, and >60 years), extent of tumor resection (score: biopsy, partial resection, or complete resection), administration of corticosteroids (yes/no), and Mini-Mental State Examination (MMSE) (score: 27–30 or <27). Forest plots with Peto (unadjusted) heterogeneity tests were computed. Hazard ratios (HRs) are presented with 95% confidence intervals (CI). No adjustment for multiple comparisons was performed in this exploratory analysis. Instead, a 5% significance level was used for all analyses. A significance level of 5%–10% was considered borderline nonsignificant. The size of subgroups does not allow for sufficient power for these exploratory analyses. p values are presented to point out what the main effects are but not to provide definitive conclusions. SAS version 9.2 (SAS Institute Inc., Cary, NC) was used for all statistical analyses.
RESULTS
AED use in the EORTC/NCIC trial.
The pattern of AED use by patients treated in the EORTC/NCIC trial at baseline is summarized in table 1. Table 2 shows that baseline patient characteristics were similar among patients not receiving any AED, VPA only-treated patients, and EIAED only-treated patients. Patients treated in the TMZ/RT arm were less often treated with an AED than patients in the RT only arm (61% vs 72%, p =0.007). There was no significant imbalance in VPA only-treated patients: 30% in the TMZ/RT arm vs 26% in the RT only arm (p =0.34). There was a slight imbalance in gender distribution (p =0.06), and more patients treated with VPA in the RT only arm had a lower MMSE score (<27) compared with other subgroups (p =0.07). We also considered the possibility of a bias introduced by parallel changes in patterns of care in terms of tumor treatment and AED use. To this end, we assessed the prescription of AED by year of accrual (2000, 2001, or 2002) and by split into cohorts (first half of patients vs second half of patients), but there was no significant change of AED prescription within this short time period (data not shown).
Information on AED use as concomitant medication was available for the duration of adjuvant TMZ administration but was not available after the end of RT for patients in the RT alone arm. Among the 287 patients in the TMZ/RT→TMZ arm, 223 started adjuvant TMZ; of these, 206 patients belonged to one of the 3 main subgroups (table 1): 79 no AED, 88 EIAED only, and 39 VPA only. Overall, 957 cycles of TMZ were administered to the 223 patients. Of 340 adjuvant cycles given to patients without an AED at baseline (36%), 301 cycles remained without an AED (88.5%), 18 were with an EIAED (5.3%), and 5 were with VPA (1.5%). Of 375 cycles given to patients with an EIAED only at baseline (39%), 320 remained with an EIAED only (85.3%), 33 were without an AED (8.8%), and 6 were with VPA only (1.6%). Of 172 cycles given to baseline VPA only patients (18%), 128 remained with VPA (74.4%), 27 without an AED (15.7%), and 10 with an EIAED only (5.8%). When all switches, e.g., no AED to AED and vice versa, VPA to EIAED, or VPA to a combination, were counted as a change in AED status, there was no significant difference in the pattern of switch between the 3 baseline groups (p =0.2). In patients treated with an AED at baseline, the frequency of changing AED type during chemoradiotherapy was 11% with EIAED only and 18% with VPA only (p =0.4).
AED use and hematologic toxicity.
To identify differential hematologic toxicity from TMZ chemoradiotherapy, we focused on the 3 well-defined largest groups of patients: patients without any AED, patients treated with VPA only, and patients treated with an EIAED only. There was no difference in the number of adjuvant TMZ cycles across these 3 baseline AED groups (p =0.90). There was also no difference in the rate of grade 3/4 hematologic toxicity during concomitant TMZ among these groups (data not shown). However, patients starting adjuvant TMZ while taking VPA had thrombocytopenia (p =0.002), neutropenia (p =0.004), and leukopenia (p =0.03) more often than patients without an AED or patients with an EIAED, whereas no significant difference was found for anemia (table e-1 on the Neurology® Web site at www.neurology.org). These differences persisted when the patients who switched AED treatment were removed from the analysis (data not shown). Moreover, VPA only–treated patients had 30% of the adjuvant cycles delayed compared with 16% in EIAED only–treated patients and 17% in no AED patients (p =0.0001). However, there was no difference with regard to the number of cycles with dose reduction.
AED use and outcome.
The overall outcome of patients receiving an AED at baseline was similar to that of the patients not receiving an AED (PFS: p =0.19, HR 1.13, 95% CI 0.94–1.35; OS: p =0.69, HR 1.04, 95% CI 0.86–1.25) and was independent of allocated treatment arm (RT arm, PFS: p =0.84, HR 1.03, 95% CI 0.78–1.34; OS: p =0.85, HR 1.03, 95% CI 0.78–1.35; TMZ/RT arm, PFS: p =0.58, HR 1.07, 95% CI 0.84–1.37; OS: p =0.89, HR 0.98, 95% CI 0.76–1.27).
Further, the overall benefit from the addition of TMZ was similar in patients without an AED (PFS: p < 0.0001, HR 0.50, 95% CI 0.36–0.69; OS: p =0.016, HR 0.67, 95% CI 0.49–0.93 and with an AED (PFS: p < 0.0001, HR 0.57, 95% CI 0.46–0.70; OS: p < 0.0001, HR 0.61, 95% CI 0.49–0.76; interaction tests, PFS: p =0.50; OS: p =0.86).
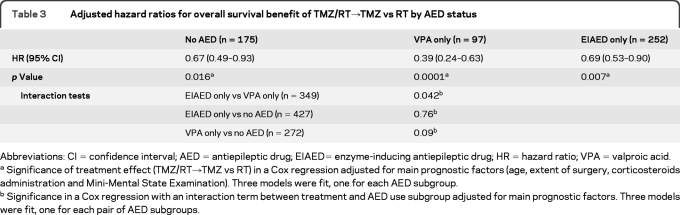
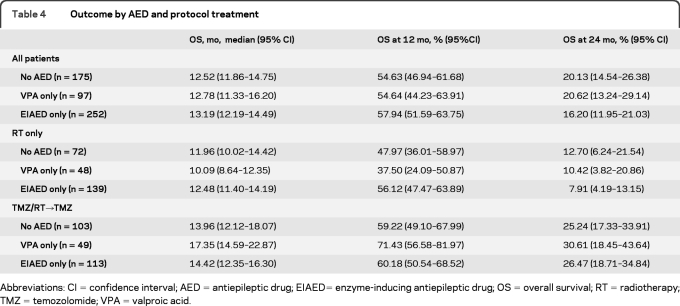
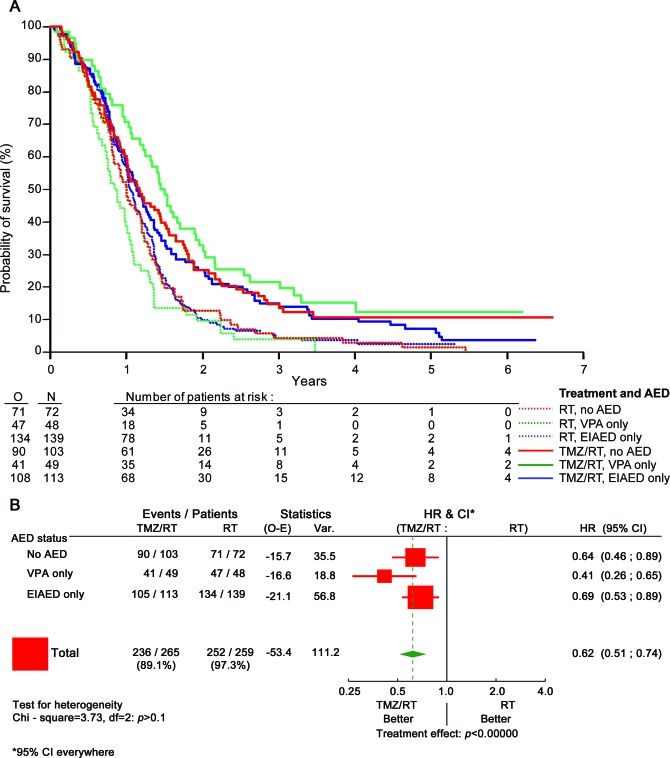
Further analyses were again restricted to the 3 major groups of patients as detailed in table 1: patients without any AED, patients treated with VPA only, and patients treated with an EIAED only. Table 3 shows that patients treated with VPA alone (97 [16.9%]) had a superior survival benefit from TMZ/RT (HR 0.39, 95% CI 0.24–0.63) compared with patients treated with an EIAED only (252 [44%]) (HR 0.69, 95% CI 0.53–0.90) or patients without any AED (HR 0.67, 95% CI 0.49–0.93). Interaction tests were significant for the VPA only vs EIAED only comparison (p =0.042), borderline nonsignificant for VPA only vs no AED (p =0.09), and not significant for EIAED only vs no AED (p =0.76). Corresponding OS data are provided in table 4 and figure 1A. No such effect was seen for PFS (table e-2, figure e-1). This discrepancy between PFS and OS could not be attributed to different salvage treatments administered at recurrence across the 3 groups of patients (data not shown). Similar analyses were also separately conducted for the 2 most commonly used EIAEDs, phenytoin and carbamazepine, but neither drug had a statistically significant impact either (data not shown).
Table 3.
Adjusted hazard ratios for overall survival benefit of TMZ/RT→TMZ vs RT by AED status
Abbreviations: CI=confidence interval; AED=antiepileptic drug; EIAED= enzyme-inducing antiepileptic drug; HR=hazard ratio; VPA=valproic acid.
Significance of treatment effect (TMZ/RT→TMZ vs RT) in a Cox regression adjusted for main prognostic factors (age, extent of surgery, corticosteroids administration and Mini-Mental State Examination). Three models were fit, one for each AED subgroup.
Significance in a Cox regression with an interaction term between treatment and AED use subgroup adjusted for main prognostic factors. Three models were fit, one for each pair of AED subgroups.
Table 4.
Outcome by AED and protocol treatment
Abbreviations: CI=confidence interval; AED=antiepileptic drug; EIAED= enzyme-inducing antiepileptic drug; OS=overall survival; RT=radiotherapy; TMZ=temozolomide; VPA=valproic acid.
Figure 1. Survival plots.
(A) Overall survival: Kaplan-Meier curve per tumor treatment and antiepileptic drug (AED) status. Red curves, patients not receiving AED. Green curves, valproic acid (VPA)–treated patients. Blue curves, enzyme-inducing antiepileptic drug (EIAED)–treated patients. Solid curves (top curves), patients treated with temozolomide (TMZ)/radiotherapy (RT). Dashed curves, patients treated with RT. (B) Overall survival: Forest plot of interaction between treatment and AED. CI=confidence interval; HR=hazard ratio; E=events expected; N=number of patients treated with either RT or TMZ/RT and respective subgroups; O=events observed.
Methylation of the O6-methylguanine methyltransferase (MGMT) gene has been identified as a predictor of benefit from TMZ in this trial.12 Thus, we explored the possibility that an uneven distribution of patients with methylated vs unmethylated tumors accounted for the association of VPA use and outcome. However, this was not the case: the rates of MGMT promoter methylation in the 3 groups by treatment arm were 10 of 20 (50.0%) for no AED, 10 of 23 (43%) for VPA only, and 22 of 48 (46%) for EIAED only in the RT arm and 18 of 41 (44%) for no AED, 9 of 18 (50.0%) for VPA only, and 14 of 39 (36%) for EIAED in the TMZ/TR arm (Kruskall-Wallis test, p =0.89).
DISCUSSION
This retrospective analysis of the dataset from the pivotal trial for TMZ in newly diagnosed glioblastoma11–13 suggests a predictive impact on survival of AED choice in patients with glioblastoma treated according to current standards of care. Patients who were treated with TMZ/RT→TMZ and received VPA appeared to have a better outcome than patients treated with an EIAED and even patients not receiving any AED. This effect could not be attributed to a differential distribution of patients with tumors with vs without MGMT promoter methylation within the subgroups analyzed.
At least 2 mechanisms underlying this interesting observation may be considered. TMZ is a prodrug converted to 3-methyl-(triazen-1-yl)imidazole-4-carboxamide, which is either hydrolyzed or unchanged before excretion. No effect of phenytoin, carbamazepine, or barbituric acid on TMZ clearance has been observed, whereas VPA decreased its clearance by 5% (www.temodar.com). To some extent, the increased hematologic toxicity during adjuvant TMZ with VPA (table e-1) may thus be related to increased bioavailability of TMZ. However, thrombocytopenia is not an uncommon side effect in patients treated with VPA alone.
Alternatively, our observation may lend support to the notion that the HDAC inhibitory properties of VPA mediate the superior benefit derived from radiochemotherapy. Improved survival for patients with a number of tumors has been reported if HDAC-inhibitory agents including VPA are combined with one or more chemotherapeutic agents.8,14,15 Previously, a prolonged survival of 14 months vs 11 months was observed in patients with glioblastoma treated with adjuvant CCNU who received antiepileptic therapy with a non-EIAED, mainly VPA, compared with patients who received an EIAED, mainly carbamazepine.16 Exploratory trials using HDAC inhibitors more potent than VPA such as vorinostat in combination with TMZ radiochemotherapy are ongoing and may provide further support for this hypothesis. Selective HDAC inhibitors might also have a preferred tolerability profile compared with VPA, which induces weight gain, alopecia, and tremor in some patients.
Our results must be interpreted with caution. They were generated from an unplanned and insufficiently powered retrospective analysis. The indication for and the choice of an AED were decided according to local practice before enrollment into the clinical trial. Although we have no evidence for a bias—the decisions for prescription and type of AED were at the investigators' discretion, and randomization was stratified by participating center—the observed differences may be the result of patient selection. This potential flaw might be tempered by the balancing effect of the treatment randomization and adjustment for known prognostic factors in all analyses. However, there was an imbalance in that more patients with a lower MMSE score (<27) were treated with VPA than with an EIAED in the RT arm (table 2), enhancing the TMZ treatment effect observed. The indication for the prescription of an AED and the duration of use were not recorded in detail, and we suspect that several patients may have received primary antiseizure prophylaxis in the perioperative and postoperative period, which was common practice in many centers in 2000–2002. Today, more and more neurooncology centers restrict the indication for an AED to secondary prophylaxis and prefer newer AEDs such as levetiracetam over either an EIAED or VPA.
No significant difference in outcome was observed for PFS in any of the analyses, but this secondary study endpoint is subject to many additional limitations, including the occurrence of pseudoprogression and is also subject to variations in medical practice, such as frequency of imaging, which may be summarized as information bias. Moreover, the date of progression was not centrally reviewed in this trial. Importantly, if VPA truly has radiochemosensitizing effects, it may also enhance the incidence of pseudoprogression, possibly diluting a PFS effect in a trial lacking a central radiology review for PFS determination.
Our study and this analysis are unique and have some distinguishing strengths compared with other reports.10,17,18 Our analysis is contemporary and evaluates patients treated with the current standard of care with concomitant chemoradiotherapy,11,13 whereas a previous report evaluated a pooled database of clinical trials with negative results.10 In addition, by analyzing the HR for treatment benefit within a randomized trial rather than analyzing prognostic values, we counterbalance potential inhomogeneities of hidden prognostic factors.
Our data suggest that combined modality therapy may be more effective in patients with newly diagnosed glioblastoma treated with VPA than in patients treated with an EIAED. A more intriguing finding is that VPA-treated patients may even have a better outcome than patients receiving no AED at all. One explanation for the observed interaction between VPA and TMZ/RT is the inhibition of HDAC by VPA,19,20 and autophagy of glioma cells may possibly also be induced by VPA.21 The presence of better acetylation status of histones may allow greater efficacy of chemotherapy and may explain the longer survival in patients receiving VPA in combination with another chemotherapeutic agent, i.e., TMZ, and not with the use of EIAED or without any AED.8,9 The full potential of VPA may not have been exploited because VPA was not given during the full course of TMZ chemoradiotherapy in some of the patients included in the VPA group.
Despite the limitations of this retrospective analysis, these results suggest that the choice of AED in patients with brain tumors should be carefully considered because it may affect survival. The present observation also further supports a recent trend to favor an non-EIAED for patients with a malignancy to allow administration of modern chemotherapy and targeted agents that often show increased hepatic metabolism if patients are given an EIAED.
Supplementary Material
GLOSSARY
- AED
antiepileptic drug
- EIAED
enzyme-inducing antiepileptic drug
- EORTC
European Organization for Research and Treatment of Cancer
- HDAC
histone deacetylase
- MMSE
Mini-Mental State Examination
- NCIC
National Cancer Institute of Canada
- OS
overall survival
- PFS
progression-free survival
- RT
radiotherapy
- TMZ
temozolomide
- VPA
valproic acid
Footnotes
Editorial, page 1114
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
The trial was designed by R. Stupp, R. Mirimanoff, D. Lacombe, and many other members of the EORTC Brain Tumour and Radiation Oncology Groups. The concept for this analysis was headed by M. Weller and R. Stupp. The statistical analysis was done by T. Gorlia. The manuscript was written by M. Weller, R. Stupp, and T. Gorlia, with substantial input and advice from J.G. Cairncross, C. Vecht, A. Rossetti, and M.J. van den Bent. M. Weller, J.G. Cairncross, M.J. van den Bent, W. Mason, A. Brandes, K. Belanger, U. Bogdahn, D. Macdonald, P. Forsyth, R.-O. Mirimanoff, C. Vecht, and R. Stupp recruited patients into the original study and provided clinical data. All authors have seen, reviewed, commented on, and approved the manuscript.
ACKNOWLEDGMENT
The authors thank the patients treated at 85 centers, the local investigators, nurses, and data managers for participation and care and colleagues, and collaborators at the EORTC headquarters for their support, data, and database management and acknowledge editorial support by Frances Godson, administrative secretary to the EORTC Brain Tumor and Radiation Oncology Groups.
Study Funding
The original clinical trial was supported by an unrestricted educational grant and drug supply by Schering-Plough, Kenilworth, NJ. The EORTC-NCIC trial was an academic trial conducted under EORTC leadership with Dr. Stupp as the Principal Investigator. This publication was supported by the National Cancer Institute (grants 5U10 CA011488-26 through 5U10 CA11488-32). This analysis was conducted with financial support from grants by the Schweizerische Krebsliga (Switzerland) to the EORTC Charitable Trust. The content of this article is solely the responsibility of the authors and does not necessarily reflect the official views of the National Cancer Institute.
DISCLOSURE
Dr. Weller serves on scientific advisory boards for Roche, Merck Serono, Merck & Co., Inc., OncoMethylome Sciences, Exelixis Inc., and Bristol-Myers Squibb; received funding for travel or speaker honoraria from Roche, Merck Serono, Schering Plough Corp., and Merck & Co., Inc.; serves as European Editor for Neuro-Oncology and on the editorial boards of Glia, Journal of Neuro-Oncology, Journal of Neurochemistry, and Cellular Physiology & Biochemistry; and receives research support from Roche, Merck Serono, Schering-Plough Corp., and Merck & Co., Inc. Dr. Gorlia reports no disclosures. Dr. Cairncross served on scientific advisory boards for Schering-Plough Corp. and Merck Serono; has received funding for travel from Schering-Plough Corp.; serves on the editorial board of Annals of Neurology; receives publishing royalties for Neurological Therapeutics: Principles and Practice (Informa Healthcare, 2006); and receives research support from Alberta Cancer Foundation Research, Alberta Cancer Research Institute, and Grant in Aid Brain Tumor Foundation of Canada. Dr. van den Bent served on scientific advisory boards for Bristol-Myers Squibb, Exelixis, Merck & Co, Inc., Merck Serono, Antisense Therapeutics Limited, Exelixis Inc., OncoMethylome Sciences, Roche, Schering-Plough Corp., Siena Biotech S.p.A., and Ark Therapeutics; received funding for travel from Merck & Co, Inc.; serves on the editorial boards of the European Journal of Cancer, Neuro-Oncology, and Lancet Oncology; receives publishing royalties from Up-To-Date; serves on the speakers' bureau of Schering-Plough Corp.; and receives research support from Roche, Novartis, Schering-Plough Corp., and the Dutch Cancer Society. Dr. Mason serves on a scientific advisory board for Roche; serves on the speakers' bureau for Merck & Co., Inc.; has received speaker honoraria from Schering-Plough Corp. and Merck & Co., Inc.; and served as a consultant for Schering-Plough Corp., Merck & Co., Inc., Roche, AstraZeneca and Exelixis Inc. Dr. Bélanger served on a scientific advisory board for Schering-Plough Corp. Dr. Brandes serves on scientific advisory boards for Roche and Schering-Plough Corp.; has received funding for travel and speaker honoraria from GlaxoSmithKline and Schering-Plough Corp.; and served as a consultant for Bristol-Myers Squibb and OncoMethylome Sciences. Dr. Bogdahn serves on scientific advisory boards for Antisense Therapeutics Limited, Schering-Plough Corp., Merck Serono, and Roche; has received funding for travel or speaker honoraria from Merck & Co., Inc. and Novartis; is author on a patent re: Melanoma Inhibiting Activity (licensed to Roche); treats patients with brain tumors (10% clinical effort) for the Brain Tumor Therapy Center, University of Regensburg, Germany; receives research support from Siemens AG; and has provided expert legal advice to Schering-Plough Corp. Dr. Macdonald has served on scientific advisory boards for Schering-Plough Corp., Roche, AstraZeneca, Merck Serono, and Boehringer Ingelheim; has received speaker honoraria from Merck Serono and Schering-Plough Corp. and funding for travel from Merck Serono, Schering-Plough Corp., Roche, and EMD Serono, Inc.; served as a consultant for Merck Serono, Schering-Plough Corp., Roche, and EMD Serono, Inc.; serves on speakers' bureaus for Merck Serono and Schering-Plough Corp.; receives research support from Roche, EMD Serono, Inc., Schering-Plough Corp., the National Cancer Institute of Canada (NCIC), and Radiation Therapy Oncology Group (RTOG). Dr. Forsyth served on scientific advisory boards for Merck Serono, Schering-Plough Corp., and Jennerex Inc. and receives research support from ACF, #25023, CCSRI, CIHR, Ivy Foundation, the National Cancer Institute of Canada, and the Ben and Catherine Ivy Foundation. Dr. Rossetti served on scientific advisory boards for Eisai Inc., Janssen, GlaxoSmithKline, and UCB and receives research support from Pfizer Inc and UCB. Dr. Lacombe reports no disclosures. Dr. Mirimanoff served on scientific advisory boards for Merck & Co., Inc., Schering-Plough Corp., and Think Tank Accuray. Dr. Vecht has served on scientific advisory boards for and received speaker honoraria from Eisai Inc., Janssen, GlaxoSmithKline, and UCB; serves on the editorial board of Clinical Neurology and Neurosurgery; and receives research support from Pfizer Inc, GlaxoSmithKline, and UCB. Dr. Stupp served on scientific advisory boards for Roche, Schering-Plough Corp, OncoMethylome Sciences, Exelixis Inc., Merck Serono, Merck & Co., Inc., Bristol-Myers Squibb; and serves on the editorial boards of Neuro-Oncology, the Journal of Clinical Oncology, and the European Journal of Oncology.
REFERENCES
- 1. Van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol 2007;6:421–430 [DOI] [PubMed] [Google Scholar]
- 2. Wick W, Menn O, Meisner C, et al. Pharmacotherapy of epileptic seizures in glioma patients: who, when, why and how long? Onkologie 2005;28:391–396 [DOI] [PubMed] [Google Scholar]
- 3. Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000;54:1886–1893 [DOI] [PubMed] [Google Scholar]
- 4. Bourg V, Lebrun C, Chichmanian RM, Thomas P, Frenay M. Nitroso-urea-cisplatin-based chemotherapy associated with valproate: increase of haematologic toxicity. Ann Oncol 2001;12:217–219 [DOI] [PubMed] [Google Scholar]
- 5. Ward MM, Barbaro NM, Laxer KD, Rampil IJ. Preoperative valproate administration does not increase blood loss during temporal lobectomy. Epilepsia 1996;37:98–101 [DOI] [PubMed] [Google Scholar]
- 6. Anderson GD, Lin YX, Berge C, Ojemann GA. Absence of bleeding complications in patients undergoing cortical surgery while receiving valproate treatment. J Neurosurg 1997;87:252–256 [DOI] [PubMed] [Google Scholar]
- 7. Ständer M, Dichgans J, Weller M. Anticonvulsant drugs fail to modulate chemotherapy-induced cytotoxicity and growth inhibition of human malignant glioma cells. J Neurooncol 1998;37:191–198 [DOI] [PubMed] [Google Scholar]
- 8. Eyal S, Yagen B, Sobol E, Altschuler Y, Shmuel M, Bialer M. The activity of antiepileptic drugs as histone deacetylase inhibitors. Epilepsia 2004;45:737–744 [DOI] [PubMed] [Google Scholar]
- 9. Li XN, Shu Q, Su JM, Perlaky L, Blaney SM, Lau CC. Valproic acid induces growth arrest, apoptosis, and senescence in medulloblastomas by increasing histone hyperacetylation and regulating expression of p21Cip1, CDK4, and CMYC. Mol Cancer Ther 2005;4:1912–1922 [DOI] [PubMed] [Google Scholar]
- 10. Jaeckle KA, Ballman K, Furth A, Buckner JC. Correlation of enzyme-inducing anticonvulsant use with outcome of patients with glioblastoma. Neurology 2009;73:1207–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–996 [DOI] [PubMed] [Google Scholar]
- 12. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and response to temozolomide in glioblastoma. N Engl J Med 2005;352:997–1003 [DOI] [PubMed] [Google Scholar]
- 13. Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459–466 [DOI] [PubMed] [Google Scholar]
- 14. Munster P, Marchion D, Bicaku E, et al. Clinical and biological effects of valproic acid as a histone deacetylase inhibitor on tumor and surrogate tissues: phase I/II trial of valproic acid and epirubicin/FEC. Clin Cancer Res 2009;15:2488–2496 [DOI] [PubMed] [Google Scholar]
- 15. Rocca A, Minucci S, Tosti G, et al. A phase I–II study of the histone deacetylase inhibitor valproic acid plus chemoimmunotherapy in patients with advanced melanoma. Br J Cancer 2009;100:28–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oberndorfer S, Piribauer M, Marosi C, Lahrmann H, Hitzenberger P, Grisold W. P450 enzyme inducing and non-enzyme inducing antiepileptics in glioblastoma patients treated with standard chemotherapy. J Neurooncol 2005;72:255–260 [DOI] [PubMed] [Google Scholar]
- 17. Rossetti AO, Stupp R. Correlation of enzyme-inducing anticonvulsant use with outcome of patients with glioblastoma. Neurology 2010;74:1329–1330 [DOI] [PubMed] [Google Scholar]
- 18. Rosenow F, Reif PS, Haag A, Schmidt K, Strik H. Correlation of enzyme-inducing anticonvulsant use with outcome of patients with glioblastoma. Neurology 2010;74:1330–1331 [DOI] [PubMed] [Google Scholar]
- 19. Ecke I, Petry F, Rosenberger A, et al. Antitumor effects of a combined 5-aza-2′deoxycytidine and valproic acid treatment on rhabdomyosarcoma and medulloblastoma in Ptch mutant mice. Cancer Res 2009;69:887–895 [DOI] [PubMed] [Google Scholar]
- 20. Oi S, Natsume A, Ito M, et al. Synergistic induction of NY-ESO-1 antigen expression by a novel histone deacetylase inhibitor, valproic acid, with 5-aza-2′-deoxycytidine in glioma cells. J Neurooncol 2009;92:15–22 [DOI] [PubMed] [Google Scholar]
- 21. Fu J, Shao CJ, Chen FR, Ng HK, Chen ZP. Autophagy induced by valproic acid is associated with oxidative stress in glioma cell lines. Neuro Oncol 2010;12:328–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.