Abstract
Objective:
Data on long-term use of secondary prevention medications following stroke are limited. The Adherence eValuation After Ischemic stroke–Longitudinal (AVAIL) Registry assessed patient, provider, and system-level factors influencing continuation of prevention medications for 1 year following stroke hospitalization discharge.
Methods:
Patients with ischemic stroke or TIA discharged from 106 hospitals participating in the American Heart Association Get With The Guidelines–Stroke program were surveyed to determine their use of warfarin, antiplatelet, antihypertensive, lipid-lowering, and diabetes medications from discharge to 12 months. Reasons for stopping medications were ascertained. Persistence was defined as continuation of all secondary preventive medications prescribed at hospital discharge, and adherence as continuation of prescribed medications except those stopped according to health care provider instructions.
Results:
Of the 2,880 patients enrolled in AVAIL, 88.4% (2,457 patients) completed 1-year interviews. Of these, 65.9% were regimen persistent and 86.6% were regimen adherent. Independent predictors of 1-year medication persistence included fewer medications prescribed at discharge, having an adequate income, having an appointment with a primary care provider, and greater understanding of why medications were prescribed and their side effects. Independent predictors of adherence were similar to those for persistence.
Conclusions:
Although up to one-third of stroke patients discontinued one or more secondary prevention medications within 1 year of hospital discharge, self-discontinuation of these medications is uncommon. Several potentially modifiable patient, provider, and system-level factors associated with persistence and adherence may be targets for future interventions.
Nearly 700,000 persons have ischemic strokes in the United States each year, and about 160,000 of these events are recurrent.1 Several classes of medications are effective in modifying stroke risk factors and preventing stroke recurrence.1,2 Although continuing prescribed medications is key to improving patient outcomes, medication use commonly declines over time,3 leading to potentially avoidable stroke recurrence, disability, and death.4 Prior studies have identified multiple barriers to long-term continuation of medications including inadequate care transitions, side effects, poor patient–provider communication, suboptimal patient resources, and medication affordability, as well as inadequate provider knowledge of drug costs and insurance coverage.5 Information on longitudinal medication use and the reasons for medication discontinuation in stroke patients is limited.
The aim of the Adherence eValuation After Ischemic stroke–Longitudinal (AVAIL) Registry was to measure secondary prevention medication regimen persistence from discharge to 12 months in a nationwide sample of patients from hospitals participating in the American Heart Association/American Stroke Association–administered Get With The GuidelinesSM–Stroke program (GWTG-Stroke). This study sought to determine 1) rates of medication use at 12 months, 2) reasons for medication discontinuation, and 3) patient, provider, and system-level factors associated with medication use or nonuse.
METHODS
The study design and methods of the AVAIL registry program has been published previously.6 Participants were eligible for this observational cohort study if they were age 18 years or older, hospitalized for a primary diagnosis of acute ischemic stroke or TIA, directly admitted based on physician evaluation or arrival through the emergency department, provided consent to participate, and their data were collected as part of the GWTG-Stroke™ Program. The recruitment period for this study was July 2006 through July 2008, and follow-up was completed October 2009. Sample size was estimated at 3,000 and the number was based on feasibility of enrollment in this multicenter study and not a power calculation.
Trained multilingual interviewers from the Duke Clinical Research Institute (DCRI) contacted AVAIL participants 3 and 12 months after hospital discharge. For patients who could not respond because of illness severity, speech or language deficits, or death, interviewers attempted to speak with an informed proxy, such as a family member or caregiver. Proxy respondents were not asked to provide subjective data on the patient's perceived functional status, medication knowledge, or satisfaction with provider communications. A patient was classified as lost to follow-up only after multiple contact attempts were unsuccessful and the time from discharge was more than 639 days (or 274 days after 12-month anniversary of discharge), or the subject/proxy refused.
Medication continuation was ascertained by comparing hospital discharge medications (faxed to the coordination center) with the current medications reported by the patient/proxy. Patients who reported discontinuing a medication were asked whether they chose to stop the medication or were instructed to do so by a health care provider. If discontinuation was self-initiated, patients were asked to select a response that most closely reflected the reason: side-effects, cost, medication not helping, or other.
Persistence is generally defined as duration of therapy, whereas adherence refers to the extent at which patients take medications as prescribed by their providers.7,8 In AVAIL, 12-month persistence was defined as continuation of all secondary preventive medications prescribed at hospital discharge, and adherence as continuation of prescribed medications except those stopped according to health care provider instructions.7 Patients were considered nonpersistent if they discontinued a medication regardless of the reason, and nonadherent if they discontinued a medication for reasons other than provider recommendation. The primary outcome, regimen persistence, was analyzed as an all-or-none variable (i.e., subjects who remained on all discharge medication classes at the 12-month follow-up were considered persistent, regimen persistence = 1, whereas subjects who stopped at least one class of medication prescribed at discharge were nonpersistent, regimen persistence = 0). Composite persistence was defined as the percentage (0% to 100%) of discharge medication classes that subjects reported taking at 12 months.
Outcome Sciences, Inc., serves as the data collection and coordination center for the initial in-hospital data collection via GWTG. The DCRI serves as the data analysis center for both GWTG-Stroke and AVAIL and has an agreement to analyze the aggregate deidentified data for research purposes.
Standard protocol approvals, registrations, and patient consents.
Each participating site obtained institutional review board approval before screening subjects for AVAIL. Written informed consent was obtained from all patients (or guardians of patients) participating in the study.
Statistical analysis.
To be eligible for determination of persistence and adherence, documentation of being on a specific medication or class of medication at discharge was required. Persistence was determined for the following 5 medication classes: warfarin, antiplatelet, antihypertensive, lipid-lowering, and diabetes medications. Both regimen and composite persistence scores were used to summarize class-level persistence. Subjects for whom data were missing for one or more medication classes were not included in this analysis (n = 14).
Contingency tables were generated to explore the relationship between 12-month regimen persistence and patient characteristics, provider factors, and hospital-level characteristics. For these analyses, data were analyzed for all patients (including proxy responders) and repeated for self-responders only. Continuous variables are presented as medians with interquartile ranges and categorical variables are expressed as frequencies with percentages. The Wilcoxon rank sum test was used for continuous variables and Pearson χ2 test was used for categorical variables to evaluate the associations between regimen persistence and patient and hospital characteristics.
A multivariable logistic regression model was used to further evaluate the influence or confounding of demographic, clinical, and other factors on 12-month regimen persistence and included the following prespecified covariates: patient baseline characteristics including age, gender, race/ethnicity, marital status, living situation, education level, work status, household income, medical history, discharge destination, ambulatory status, and number of discharge medications. Additional potential covariates included whether patients had received medical instruction, if and how they tracked medication use, medication cost, insurance coverage, whether they had a follow-up appointment with a provider, modified Rankin Scale (mRS) score, and rehabilitation utilization. Hospital characteristics included size, type, and geographic region. The same covariates were used in a logistic regression model to evaluate factors affecting adherence.
We also determined whether medication regimen persistence varied based on whether the survey responses were provided by the subject or a proxy. The self-responders were then analyzed as a subpopulation separate from the proxy responders. In addition to the previous list, the “subject only” information included understanding of medication purpose and side effects, provider communication perceptions, EuroQOL-5D (EQ5D)9 quality of life scale, and Patient Health Questionnaire-8 (PHQ8)10 depression scale. The PHQ-8 excludes a question about suicidality, but nearly identical scoring thresholds for depression severity can be used for the PHQ-9 and PHQ-8.10
Missing data were imputed to the most popular category or based on clinical perspective (except for hospital characteristics for which missing data were excluded). A backward selection procedure was used to eliminate insignificant factors. The final models included potential predictors with a p value of <0.10. Likelihood ratios were used to determine the different model results based on a full model vs the backward elimination model. The Generalized Estimating Equation method with exchangeable working correlation structure was used to account for within-hospital clustering. Odds ratios and 95% confidence intervals were calculated. All p values are 2-sided tests. Due to the exploratory nature of our analysis, the p values for the primary outcome were not adjusted for multiple testing. All statistical analyses were performed by the DCRI using SAS software (version 9.2, SAS Institute, Cary, NC).
RESULTS
A total of 3,068 stroke patients from 106 sites were assessed for eligibility for the AVAIL Registry. A total of 188 (6.1%) were excluded because they did not meet inclusion criteria, leaving 2,880 patients. Reasons for exclusions from the analysis are listed in the figure, leaving a final sample size of 2,457 patients. Eight percent were lost to follow-up at 12 months (229/2,880), and the median time to the 12-month interview was 396 days.
Figure. Flow diagram of enrollment, follow-up, and analysis for Adherence eValuation After Ischemic stroke–Longitudinal Registry (AVAIL) subjects.
GWTG-Stroke = Get With The GuidelinesSM–Stroke.
Regimen persistence for secondary prevention medications at 12 months was 65.6%. Self-responders reported higher persistence than patients for whom a proxy was the respondent. Of the 365 questionnaires completed by a proxy at 12 months, 195 (53.4%) reported persistence for the subject at 12 months vs 68.1% self-reported regimen persistence (p < 0.0001).
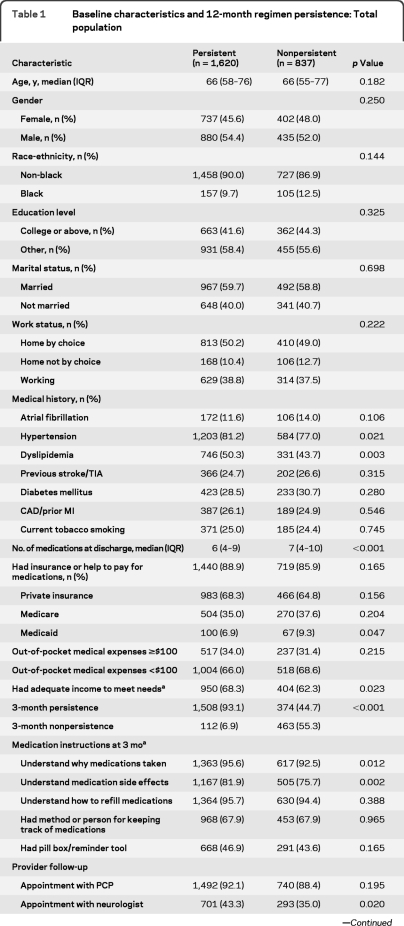
Patient-level variables associated with 12-month persistence are shown in table 1. Factors associated with 12-month persistence included a history of hypertension or dyslipidemia, fewer discharge medications, having an adequate income, 3-month persistence, a follow-up appointment with a neurologist, and overall satisfaction with provider communications.
Table 1.
Baseline characteristics and 12-month regimen persistence: Total population
Abbreviations: CAD = coronary artery disease; EQ5D = EuroQOL5D; MI = myocardial infarction; IQR = interquartile ratio; PCP = primary care provider; PHQ-8 = Patient Health Questionnaire-8 (suicide question omitted).
Variables collected from subject responders only and not proxy.
Variables collected at 3-month interview.
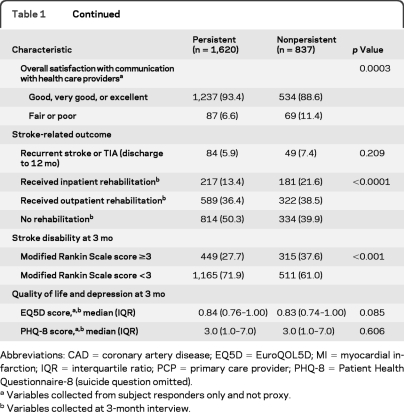
The percentage of patients prescribed medications by class and by type at discharge, 12-month persistence, and percentage discontinued for each (physician's recommendation, self-discontinued, or reason not otherwise specified) are given in table 2. By medication class, 12-month persistence was highest for antihypertensive medications (87.9%), followed by antiplatelet (87.1%), diabetes (82.3%), lipid-lowering (77.6%), and warfarin (68.2%) medications. The most common reason for nonpersistence was discontinuation by the health care provider, although percentages varied widely by drug class.
Table 2.
Twelve-month persistence by drug class and drug type
Abbreviations: ACE = angiotensin-converting enzyme; ARB = angiotensin-receptor blocker; ASA = aspirin; NOS = not otherwise specified.
Includes subjects where data on class level persistence were provided. The denominator for persistence and nonpersistence for each drug or drug class is use at discharge.
The all-or-none adherence at 1 year was 86.6%. By medication category, adherence ranged from 94.8% for antiplatelet therapy to 90.7% for lipid-lowering medications (table 2).
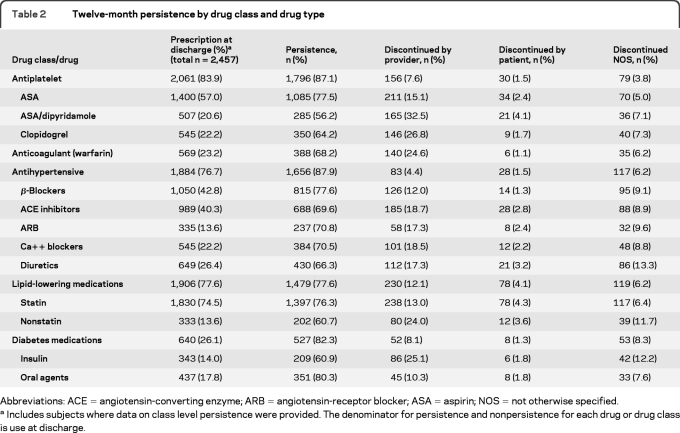
The percentage of missing covariates was low (<2%) for both logistic regression models. The multivariable predictors of persistence and adherence are given in table 3. Patient age, history of hypertension, history of dyslipidemia, those with less than a college level of education, and mRS <3 were independent predictors of 12-month persistence, with a trend toward persistence for those with a history of smoking (p = 0.066) and pillbox use to keep track of medications (p = 0.062). Additional factors associated with 12-month persistence included having an appointment with a primary care provider (PCP) or a neurologist before the 3-month follow-up, having an adequate income to meet the needs of the household, receiving either inpatient (odds ratio [OR] 0.57, 95% confidence interval [CI] 0.43–0.76) or outpatient (OR 0.76, 95% CI 0.65–0.89) rehabilitation, and discharge from a nonacademic (vs academic) hospital. Findings were similar if the analysis was restricted to self-responders (table e-1 on the Neurology® Web site at www.neurology.org).
Table 3.
Multiple regression models of 12-month persistence and 12-month adherencea
Abbreviations: CI = confidence interval; OR = odds ratio; PCP = primary care provider.
Multiple regression models of 12-month persistence (for total population of subject and proxy responder, n = 2,457; C index = 0.64), and 12-month adherence, defined as persistent if physician discontinued (n = 2,457; C index 0.69). Models adjusted for age, sex, race: black vs other, stroke type, previous stroke/TIA, coronary artery disease/prior myocardial infarction, carotid stenosis, diabetes, peripheral vascular disease, hypertension, dyslipidemia, and smoking.
The multivariable model of adherence was similar to persistence (table 3), including associations with fewer medications at discharge, having an adequate income, mRS, and having an appointment with a PCP. Factors associated with adherence but not persistence included pillbox use, medication insurance, having received medication instructions, being married, and being discharge to home.
DISCUSSION
The risk of recurrent stroke is 15% over 5 years and highest in the first 6 months after the index stroke, emphasizing the need for early initiation of appropriate prevention therapies.11 We found that although up to one-third of stroke patients discontinued evidence-based medications prescribed at hospital discharge within 1 year, the majority did so based on postdischarge health care provider recommendations. We cannot determine the appropriateness of these recommendations as there are a variety of justifiable reasons prompting a provider to stop a medication or class of medications, and providers were not interviewed. We did, however, identify several provider- and system-related factors that were associated with long-term medication persistence that might be amenable to modification.
The results of previous studies evaluating poststroke secondary prevention medication persistence vary widely. The Riks-Stroke Register in Sweden found persistence by medication categories at 2 years postdischarge (e.g., 56% for statins, 74% for antihypertensive drugs) that was in the range of the AVAIL 1-year regimen persistence.3 Because the Swedish registry assessed persistence based on pharmacy refills, and because of differences in health care delivery systems, factors associated with persistence differed from those found in AVAIL and included advanced age, institutional living at follow-up, absence of low mood, treatment in a stroke unit, presence of diabetes and atrial fibrillation, and good self-perceived health.3 Other studies, such as the Preventing Recurrence Of Thromboembolic Events through Coordinated Treatment (PROTECT) study, report higher persistence rates.12 PROTECT, however, was a single-center quality improvement initiative that focused on evidence-based tools and algorithms for stroke prevention strategies. At 1 year of follow-up of 128 patients, antithrombotic use was maintained in 98%, statins in 99%, angiotensin converting enzyme inhibitors/angiotensin receptor blockers in 89%, and thiazide diuretics in 82%.12 In another study, stroke patients in Nova Scotia, Canada, had a self-reported persistence of >90% for all categories of stroke prevention medications.13
In AVAIL, longitudinal assessment of prevention regimen all-or-none persistence decreased from 76% at 3 months14 to 66% at 1 year. This decline is consistent with the findings of other studies.3 For individual drug classes in AVAIL, 1-year persistence was 68.2% (warfarin) to 87.9% (antihypertensive medications) and adherence was 85% to 95% for these medication classes.
Several baseline factors, such as age, medical history, and education level, predicted long-term persistence. The presence of a cardiovascular diagnosis prestroke may mean fewer changes in medications at discharge and it is possible that familiarity with the medications may aid in longer-term persistence. There was an inverse relationship between college education and long-term persistence, although this was not associated with adherence. One possible explanation for this observation is that more educated stroke patients may research their medications and provide reasons to their provider that mutually led to discontinuation of a medication.
In the AVAIL cohort, understanding why medications are taken as well as a reported understanding of potential side effects were significant predictors of persistence. This may be because these patients are better able to weigh the risks vs benefits of a medication as compared with patients who have a poorer understanding of their treatments. Using a pillbox or other tool to track medications was associated with better persistence and adherence, highlighting the importance of reminders and organized pill-taking. Receiving instructions about their medications was associated with better adherence but not persistence. This is expected as persistence does not reflect health care provider instructions to stop a medication after the initial prescription.
Self-reported financial variables were associated with both long-term persistence and adherence. AVAIL participants were asked if their household income adequately met their needs in general; those who responded “adequately” or “more than adequately” were 30% more likely to be persistent and nearly 50% more likely to be adherent (table 3). Having insurance coverage for medication costs was also associated with about a 60% higher likelihood of adherence.
Health care provider discontinuation was the most common reason for nonpersistence in AVAIL. The rate of nonadherence was 14% as compared to 34% for nonpersistence. Based on the number of patients reclassified, it seems that provider discontinuation occurred across multiple medication classes in different patients. Because we did not contact providers, we cannot determine the reasons these providers discontinued a specific medication. A study of evidence-based cardiovascular medication persistence in patients with acute coronary syndromes reported that provider discontinuation of medications increased with patient age.15 Further examination of the reasons for medication nonpersistence from both the provider and patient perspectives in stroke patients is needed.
In the AVAIL cohort, an appointment with a PCP (as opposed to merely having a PCP) was associated with a 47% increase in 1-year persistence; an appointment with a neurologist was associated with persistence to a lesser degree (table 3). Contact with a physician provides both the opportunity to obtain prescription refills and for patients to communicate information about their condition and any medication concerns. This is not only important for medication persistence; in a study of Medicaid patients in Tennessee, ambulatory care visits were associated with decreased mortality in patients with hypertension.16
Another provider-level influence on long-term persistence is a lower number of medications at discharge. Persistence increased by 4% for each decrease in the number of prescribed medications. This is consistent with a study of Medicare enrollees and cardiovascular disease medication refill adherence, which reported that a regimen of more than 2 medications was independently predictive of poor refill adherence as compared to less than 3 medications.17 For hypertension, the use of simplified antihypertensive medications (such as fixed-dose combinations of drugs in a single pill) was associated with greater adherence in a high-risk Medicaid population.18
Stroke patients participating in rehabilitation programs may have more changes in their medications because rehabilitation requires frequent contact with health care providers. The inpatient rehabilitation setting, in particular, is an opportunity to change medications prescribed at hospital discharge to different ones that may be better tolerated while on the rehabilitation unit. Because medication changes during inpatient are made based on health care provider orders, rehabilitation was not independently associated with adherence (table 3).
The AVAIL Registry has several strengths: it is one of the largest registries outside of a clinical trial of medication use focused on stroke patients. The AVAIL Registry includes assessments of known barriers related to patient, provider, and system-level factors affecting medication persistence/adherence.
AVAIL has several limitations. AVAIL subjects were hospitalized in GWTG-Stroke sites having ongoing quality improvement programs; AVAIL results may not be generalizable to patients hospitalized in non-GWTG hospitals. These analyses were exploratory, and because of the backward selection method of model building, the interpretation of the individual variable's association with the outcome is limited. The C indices reflect relatively poor model performance. In addition, persistence and adherence were obtained through self-report and not independently audited by pill counts or otherwise validated. Other large studies, such as the Prospective Registry Evaluating Myocardial Infarction: Events and Recovery (PREMIER),19 utilized self-reported adherence. In other settings, self-report generally agrees with pharmacy claims data,20 and is the best method to ascertain reasons for nonpersistence.21 We did not independently review medical records or measure prevention treatment endpoints and therefore cannot determine adequate risk factor control. Although we might assume an association between medication persistence/adherence and recurrent stroke, we were unable to analyze this association because events may have occurred prior to the assessment of the 3- and 12-month medication use.
The AVAIL study showed that there are several factors predictive of long-term stroke prevention medication persistence and adherence including patient, provider, and system level factors. Given the complex aspects of medication-taking behavior, future interventions will likely require multifaceted approaches with involvement of the patient, caregiver, hospital and primary care providers, pharmacists, and insurers in order to effectively improve medication persistence and adherence.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Laura Drew and Judith A. Stafford from the AVAIL coordinating team; the site investigators and coordinators for their work on the study; and Andrea Davis, Leslie Wilson, Tatiana Meteleva, and Vicky Pena for their expertise in patient interviewing and data collection.
GLOSSARY
- AVAIL
Adherence eValuation After Ischemic stroke–Longitudinal Registry
- CI
confidence interval
- DCRI
Duke Clinical Research Institute
- EQ5D
EuroQOL-5D
- GWTG-Stroke
Get With The GuidelinesSM–Stroke
- mRS
modified Rankin Scale
- OR
odds ratio
- PCP
primary care provider
- PHQ8
Patient Health Questionnaire-8
- PROTECT
Preventing Recurrence Of Thromboembolic Events through Coordinated Treatment.
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
C.D.B. planned the study design, analysis, and interpretation of the data, and wrote the manuscript. D.M.O. planned the analysis and provided comments on drafts of the manuscript. L.O.Z. aided in the literature search, wrote pieces of the manuscript, and provided comment on all drafts. W.P. and X.Z. wrote the statistical analysis plan, performed the analyses, and provided input on the methods and results. M.A., S.F., G.F., S.J., C.K., L.S., B.O., K.A.L., L.B.G., and E.D.P. provided comments on all drafts of the manuscript.
COINVESTIGATORS
The AVAIL Publications Committee: Mark Alberts, MD, Northwestern University; Bruce Coull, MD, University of Arizona; Pamela Duncan, PT, PhD, Duke University; Susan C. Fagan, PharmD, University of Georgia; Michael Frankel, MD, Emory University; Larry Goldstein, MD, Duke University; Philip Gorelick, MD, MPH, University of Illinois Chicago; S. Claiborne Johnston, MD, PhD, University of California at San Francisco; Chelsea Kidwell, MD, Georgetown University; Kenneth A. LaBresh, MD, Research Triangle International; Pamela Mitchell, RN, PhD, University of Washington; Bruce Ovbiagele, MD, University of California at Los Angeles; Ralph Sacco, MD, MS, University of Miami; Lee Schwamm, MD, Massachusetts General Hospital; Linda Williams, MD, Roudebush VA Medical Center and Indiana University; Richard Zorowitz, MD, Johns Hopkins Bayview Medical Center. AVAIL participating sites, PIs, and coordinators: B. Franklin Diamond, MD, Jenny McGowan (Abington Memorial Hospital, PI and Coordinator), Gary Bernardini, MD, Linda Graca (Albany Medical Center, PI and Coordinator), Wende Fedder, RN, MBA, Mona Olges, RN (Alexian Brothers Medical Center, PI and Coordinator), Terri Nielsen, RN (Alta Bates Summit Medical Center, Coordinator), John Andrefsky, MD, Judy Werstler, RN (Aultman Hospital, PI and Coordinator), John Wulff, MD, Sherry Simmonds (Ball Memorial Hospital, PI and Coordinator), David Bear, MD, Tammie Stefanko (Baptist Hospital, PI and Coordinator), Doreen Donohue, RN, Michelle Silver (Baptist Medical Center, PI and Coordinator), John Gannon, RN, MBA, Andrea Hrin, ARNP (Bayfront Medical Center, PI and Coordinator), Sandy Nylu (Benefis Healthcare, Coordinator), Alec Kloman, MD, Deborah Jewell, NP (Berkshire Medical Center, PI and Coordinator), Thomas Kelly, MSN, RN (Beverly Hospital, Coordinator), Dan Rodriguez, MD, Bobbie Nolan (Billings Clinic, PI and Coordinator), Vicki Hanselman, (Bluffton Regional Medical Center, PI), James Bobenhouse, MD, Kathy Ware (Bryan LGH Medical Center, PI and Coordinator), Donna Falwell (Centra Health, Coordinator), Henry Echiverri, MD, Susan Strickland (Central Dupage Hospital, PI and Coordinator), Elias Gizaw, MD, Wanda Shaver (Central Florida Regional Hospital, PI and Coordinator), Harry Reahl, MD, Deborah Rectenwald, CNRN (Charleston Area Medical Center, PI and Coordinator), Karen McCuaig, RN (Columbia-St. Mary's, Carter, Coordinator), Debbie Ferguson, MSN, RN (Community Hospital Indianapolis, Coordinator), Alfred Bowles, MD, Monica Updyke (Conemaugh Memorial Medical Center, PI and Coordinator), Gina Briscoe, RN, MSN, Amy Foster, RN, MSN (Decatur General, PI and Coordinator), Cindy Wagner, RN (Doctors Hospital, Coordinator), Donna Seif, RN (Doylestown Hospital, Coordinator), Tang Kejan, MD, Rose Watroba (Ellis Hospital, PI and Coordinator), Michael O'Brien, MD, PhD, Sherry Wirt (Enloe Medical Center, PI and Coordinator), Kathy Sciarappa (Firelands Regional Medical Center, Coordinator), Pamela Petry, Betty Brown (Flagler Hospital, PI and Coordinator), Chere Chase, MD, Gwen Ainsworth (Forsyth Medical Center-Stroke & Vascular Center, PI and Coordinator), James DeMatteis, MD, Patty Henry, RN (Hamot Medical Center, PI and Coordinator), Sheri Muska, RN (Harris Methodist Fort Worth Hospital, Coordinator), Scott Hitchcock, DO, Mary Brethour (Huntsville Hospital, PI and Coordinator), Jaci Phillips (Indiana Neuroscience Institute, Coordinator), Stephen J. Martino, MD, Alison Trembly (Jersey Shore University Medical Center, PI and Coordinator), Christopher Sinclair, MD, Lori Blom (John T Mather Hospital, PI and Coordinator), Kent Taub, MD, Allison Rico (JPS Health Network, PI and Coordinator), Eileen Allosso, MS, Claudia Fitzgerald (Lahey Clinic Medical Center, PI and Coordinator), Judy Erb, Joan Vance (Lancaster General Hospital, PI and Coordinator), Paul Ash, MD, Jean Carlton (Legacy Emanuel Hospital, PI and Coordinator), Paul Ash, MD, Jean Carlton (Legacy-Meridian Park Hospital, PI and Coordinator), Randolph Shey, MD, Angie West (Long Beach Memorial Medical Center, PI and Coordinator), Michael Schneck, MD, Linda Chadwick, BSN, RN (Loyola University Medical Center, PI and Coordinator), Lisa Guyll, BSN, RN (Lutheran Hospital of Indiana, Coordinator), Maryland Stroke & Brain Attack Team, Karen Yarbrough, MS, CRNP (University of Maryland Medical System, Coordinator), Lee Schwamm, MD, Ahsan Pervez, MD (Massachusetts General Hospital, PI and Coordinator), Brenda Hogan, RN, BS, Sandra Studebaker, RN, MS, ACM (Maury Regional Hospital, PI and Coordinator), Patricia Farrell, RN, Victoria Lindemann, RN (Mease Dunedin, PI and Coordinator), Nicki Roderman, RN, MSN (Medical Center of Plano, Coordinator), Michel Torbey, MD, Erin Brandenburg, BA (Medical College of Wisconsin/Froedtert Memorial Lutheran Hospital, PI and Coordinator), Gretchen Tietjen, MD, Andrea Korsnack (Medical University of Ohio, PI and Coordinator), Jennifer Pary, MD, Deb Motz, RN (Mercy Medical Center, PI and Coordinator), Jennifer Pary, MD, Diane Handler (Mercy Medical Center, PI and Coordinator), Mark Young, MD, Deb Motz, RN (Mercy Medical Center, PI and Coordinator), Mark Young, MD, Diane Handler (Mercy Medical Center, PI and Coordinator), Joseph Hanna, MD, Dana Cook, BSN (Metro Health Medical Center, PI and Coordinator), Ajay Arora, MD, Teresa Jones, RN, CCRC (Morton Plant Hospital, PI and Coordinator), Janice Ulmer, RN, PhD (Munroe Regional Medical Center, Coordinator), Tricia Westbrook, RN, MSN, Amanda Grindle (Northeast Georgia Medical Center, PI and Coordinator), David Hart, MD, Christine Ball (Northeast Health-Albany Memorial, PI and Coordinator), David Hart, MD, Christine Ball (Northeast Health-Samaritan Hospital, PI and Coordinator), Roger Gietzen, MD, Elaine Siwiec (Northern Michigan Hospital, PI and Coordinator), Stephen Martino, MD, Jane Kaiser (Ocean Medical Center, PI and Coordinator), Fen-Lei Chang, PhD, Lynn Wilton (Parkview Hospital, PI and Coordinator), Raymond Reichwein, MD, Judy Dillon, MSN, RN (Penn State Hershey Medical Center, PI and Coordinator), Marie Welch (Pitt County Memorial Hospital, PI), Aris Chaconas, MD, Amy Holland, RN (Presbyterian Hospital, PI and Coordinator), Julie McDonald, RN, Stephanie Abbott (Providence Everett Medical Center, PI and Coordinator), Walter Bohnenblust, MD, Ruth Bailey, RN, BSN (Reading Hospital, PI and Coordinator), Paul Katz, MD, Nicholas Green, RN, BSN (Renown Regional Medical Center, PI and Coordinator), Noah Gilson, MD, Rebecca Graboso, RN, MSN (Riverview Medical Center, PI and Coordinator), W. Scott Burgin, MD, Cheryl Weber, MS, RN (Rochester General Hospital, PI and Coordinator), Shyam Prabhakaran, MD, MS, Susan Dvojack, MS (Rush University Medical Center, PI and Coordinator), Richard Smith, MD, Christine Potzer (Saint Anthony's Central Hospital, PI and Coordinator), Teri Kozik, MSN, Judith Brady (Saint Mary's Regional Medical Center, PI and Coordinator), Michael Kaminski, MD, Deb Pitts (Saint Thomas Hospital, PI and Coordinator), Michelle VanDemark, MS, CNS, CNR (Sanford USD Medical Center, Coordinator), Adrian Goldszmidt, MD, Kathleen Birnbaum (Sinai Hospital of Baltimore, PI and Coordinator), Margaret Tremwel, MD, Cheryl Hyde (Sparks Regional Medical Center, PI and Coordinator), Wende Fedder, RN, MBA, Mona Olges, RN (St. Alexius Medical Center, PI and Coordinator), Susan Hickenbottom, MD, MS, Kimberly Gray, RN (St. Joseph Mercy Hospital, PI and Coordinator), Carl McComas, MD, Jennifer Edwards (St. Mary's Medical Center, PI and Coordinator), Steve Erlemeier, MD, Jennifer Gottschald, LPN (St. Mary's Duluth Clinic Health System, PI and Coordinator), Lee Vanderburgh, MD, Nancy J. Newkirk (St. Peter's Hospital, PI and Coordinator), Srinath Kadimi, MD, Jennifer Nascimento, RN (St. Vincent's Medical Center, PI and Coordinator), Robert Lada, MD, Jan Weinhardt (Summa Health System, PI and Coordinator), Kyle Malone, MS (Sunrise Hospital and Medical Center, Coordinator), William Likosky, MD, Jeannie Bush (Swedish Medical Center, PI and Coordinator), Pierre Fayad, MD, Mary Phillips, RN, BSN (The Nebraska Medical Center, PI and Coordinator), Deborah Green, MD, Lyle Oshita (The Queen's Medical Center, PI and Coordinator), Deborah Green, MD, Tracy Stern (The Queen's Medical Center, PI and Coordinator), Bruce Ovbiagele, MD, Sandra Pineda (UCLA Medical Center, PI and Coordinator), Mehari Gebreyohanns, MD, Cindy Hoff, RNC, BSN (United Regional Healthcare System, PI and Coordinator), Kerri Remmel, MD, Elizabeth Wise, ARNP (University of Louisville Hospital, PI and Coordinator), Larry Wechsler, MD, Sharon DeCesare (UPMC Stroke Institute, PI and Coordinator), Jeanne Robinson, MSN, RN, CNRN, Nancy Wiech (Wallace Kettering Neuroscience Institute at Kettering Medical Center, PI and Coordinator), Ash Jain, MD, Douglas Van Houten, RN (Washington Hospital Healthcare System, PI and Coordinator), Stephen Marks, MD, Kathryn D'Aquila, RN (Westchester Medical Center, PI and Coordinator), David Wheeler, MD, Melissa Tapp (Wyoming Medical Center, PI and Coordinator), Marta Jimenez, MD, Brenda Chapman, RN, BSN (York Hospital/Wellspan Health, PI and Coordinator).
STUDY FUNDING
Adherence eValuation After Ischemic stroke—Longitudinal (AVAIL) was supported by unrestricted funds from Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership and conducted through collaboration with the GWTG-Stroke program. AVAIL analyses were also supported in part by the Agency for Healthcare Research and Quality (AHRQ) cooperative agreement U18HS016964. DCRI developed the protocol, owns the data, and is responsible for study oversight, materials development and data collection, site communications, and all regulatory and clinical questions related to the AVAIL Registry study.
DISCLOSURE
Dr. Bushnell serves on a data safety monitoring board for Boehringer Ingelheim; and received research support from the Bristol-Myers Squibb/Sanofi Partnership, the NIH/NINDS, the American Heart Association/Bugher Foundation, and the Hazel K Goddess Fund for Research on Stroke in Women. Dr. Olson serves on speakers' bureaus for and has received speaker honoraria from Zoll Medical, Integra Neuroscience, and The Medicines Company; and has received research support from the Bristol-Myers Squibb/Sanofi Partnership. X. Zhao and Dr. Pan report no disclosures. L. Zimmer receives research support from AHRQ and holds stock in Abbott. Dr. Goldstein serves on a scientific advisory board for Allergan; has received funding for travel and speaker honoraria from Bayer Schering Pharma; serves on the editorial boards of Neurology®, Emergency Medicine, Stroke, Cerebrovascular Diseases, and Circulation: Cardiovascular Quality and Outcomes and as an Associate Editor for Continuum; receives publishing royalties from UpToDate and Henry Stewart Talks; serves as a consultant for Pfizer Inc, Boehringer Ingelheim, Johnson & Johnson, and Merck Serono; receives research support to his institution from Pfizer Inc, AGA Medical Corporation, and Abbott; and receives research support from the NIH, the American Heart Association, and El Centro Hispano. Dr. Alberts serves on a scientific advisory board, as a consultant, and on the speakers' bureau for and has received funding for travel and speaker honoraria from the Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership; receives publishing royalties from Blackwell Publishing; and has received royalties from Athena Diagnostics, Inc. re: apoE genetic testing. Dr. Fagan serves on scientific advisory boards for Pfizer Inc and Genentech, Inc.; serves on the editorial board of Pharmacotherapy; serves as a consultant for Pfizer Inc, Genentech, Inc, and Ferrer Group; and receives research support from the NIH/NINDS and the US Veterans Administration. Dr. Fonarow serves as a consultant for Novartis and Pfizer Inc. Dr. Johnston is co-holder of patent re: the RNA panel to identify TIA and risk stratify; and receives research support from sanofi-aventis, Strkyer Neurovascular, Boston Scientific, the NIH (NCRR, NINDS), Kaiser-Permanente, and the AHA/ASA, Bugher Award. Dr. Kidwell serves on the editorial boards of Neurocritical Care, the Journal of Neuroimaging, and Stroke Research and Treatment; serves/served as a consultant for Embrella Cardiovascular, Inc., and Simcere Pharmaceutical Group; and receives research support from Baxter International Inc. and the NIH/NINDS. Dr. LaBresh reports no disclosures. Dr. Ovbiagele serves on a scientific advisory board for Avanir Pharmaceuticals and serves as an Assistant Editor of Stroke, an Associate Editor of Journal Watch Neurology and BMC Public Health, and on the editorial boards of the Journal of Stroke and Cerebrovascular Diseases and Stroke Research and Treatment. Dr. Schwamm serves as chair of the American Heart Association GWTG Steering Committee; serves on scientific advisory boards for CoAxia, Inc., Phreesia, and Lundbeck Inc.; serves on the editorial boards of Neurocritical Care and Stroke; may accrue revenue on a patent re: Imaging system for obtaining quantitative perfusion indices; his wife receives royalties from publishing Obstetric Anesthesia (Cambridge Pocket Clinicians, 2007); serves/has served as a consultant to CryoCath® Technologies Inc., Research Triangle Inc., Massachusetts Department of Public Health, and the Canadian Stroke Network; receives research support from Lundbeck Inc., the NIH (NINDS, NCRR), and the Department of Health and Human Services (CDC); and has provided expert medical opinions in malpractice lawsuits (re: stroke treatment and prevention). Dr. Peterson serves on the editorial board of the Journal of the American Medical Association and receives research support from Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership and Merck Serono.
REFERENCES
- 1. Roger V, Go A, Lloyd-Jones D, et al. Heart disease and stroke statistics 2011 update: a report from the American Heart Association. In: Circulation [serial online] 2010. Available at: http://circ.ahajournals.org Accessed December 23, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sacco R, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke. Stroke 2006;37:577–617 [DOI] [PubMed] [Google Scholar]
- 3. Glader E-V, Sjolander M, Eriksson M, Lundberg M. Persistent use of secondary preventive drugs declines rapidly during the first 2 years after stroke. Stroke 2010;41:2552–2558 [DOI] [PubMed] [Google Scholar]
- 4. Shaya F, El Khoury A, Mullins C, et al. Drug therapy persistence and stroke recurrence. Am J Manag Care 2006;12:313–319 [PubMed] [Google Scholar]
- 5. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487–497 [DOI] [PubMed] [Google Scholar]
- 6. Bushnell C, Zimmer L, Schwamm L, et al. The Adherence eValuation After Ischemic Stroke Longitudinal (AVAIL) Registry: design, rationale, and baseline patient characteristics. Am Heart J 2009;157:428–435 [DOI] [PubMed] [Google Scholar]
- 7. Cramer J, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health 2008;11:44–47 [DOI] [PubMed] [Google Scholar]
- 8. Benner J, Glynn R, Mogun H, et al. Long-term persistence in use of statin therapy in elderly patients. JAMA 2002;288:455–461 [DOI] [PubMed] [Google Scholar]
- 9. The EuroQol Group EuroQol*: a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208 [DOI] [PubMed] [Google Scholar]
- 10. Kroenke K, Spitzer R. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann 2002;32:1–7 [Google Scholar]
- 11. Hankey G, Jamrozik K, Broadhurst R, et al. Long-term risk of first recurrent stroke in the Perth Community Stroke Study. Stroke 1998;29:2491–2500 [DOI] [PubMed] [Google Scholar]
- 12. Ovbiagele B, Kidwell C, Selco S, Razinia T, Saver J. Treatment adherence rates one year after initiation of a systematic hospital-based stroke prevention program. Cerebrovasc Dis 2005;20:280–282 [DOI] [PubMed] [Google Scholar]
- 13. Lummis H, Sketris I, Gubitz G, Joffres M, Flowerdew G. Medication persistence rates and factors associated with persistence in patients following stroke: a cohort study. In: BMC Neurol [serial online] 2008. Available at: http://www.biomedcentral.com Accessed July 1, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bushnell C, Zimmer L, Pan W, et al. Persistence with stroke prevention medications 3 months after hospitalization. Arch Neurol 2010;67:1456–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ali R, Melloni C, Ou F-S, et al. Age and persistent use of cardiovascular medication after acute coronary syndrome: results from the Medication Applied and Sustained Over Time. J Am Geriatr Soc 2009;57:1990–1996 [DOI] [PubMed] [Google Scholar]
- 16. Bailey J, Wan J, Tang J, Ghani M, Cushman W. Antihypertensive medication adherence, ambulatory visits, and risk of stroke and death. J Gen Intern Med 2010;25:495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gazmararian J, Kripalani S, Miller M, et al. Factors associated with medication refill adherence in cardiovascular-related diseases: a focus on health literacy. J Gen Intern Med 2006;21:1215–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shaya F, Du D, Gbarayor C, et al. Predictors of compliance with antihypertensive therapy in a high-risk Medicaid population. J Natl Med Assoc 2009;101:34–39 [DOI] [PubMed] [Google Scholar]
- 19. Spertus J, Kettelkamp R, Vance C, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER Registry. Circulation 2006;113:2803–2809 [DOI] [PubMed] [Google Scholar]
- 20. Pit S, Byles J, Cockburn J. Accuracy of telephone self-report of drug use in older people and agreement with pharmaceutical claims data. Drugs Aging 2008;25:71–80 [DOI] [PubMed] [Google Scholar]
- 21. Otsuki M, Clerisme-Beaty E, Rand C, Riekert K. Measuring adherence to medication regimens in clinical care and research. In: Shumaker S, Ockene J, Riekert K. eds. The Handbook of Health Behavior Change, 3rd ed. New York: Springer; 2009:309–325 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.