Abstract
The piloneural collar in mammalian hairy skin comprises an intricate pattern of circumferential and longitudinal sensory afferents that innervate primary and secondary pelage hairs. The longitudinal afferents tightly associate with terminal Schwann cell processes to form encapsulated lanceolate nerve endings of rapidly adapting mechanoreceptors. The molecular basis for piloneural development, maintenance and function is poorly understood. Here, we show that Nefh-expressing glutamatergic neurons represent a major population of longitudinal and circumferential sensory afferents innervating the piloneural collar. Our findings using a VGLUT2 conditional-null mouse model indicate that glutamate is essential for innervation, patterning and differentiation of NMDAR+ terminal Schwann cells during piloneural collar development. Similarly, treatment of adult mice with a selective NMDAR antagonist severely perturbed piloneural collar structure and reduced excitability of these mechanosensory neurons. Collectively, these results show that DRG-derived glutamate is essential for the proper development, maintenance and sensory function of the piloneural mechanoreceptor.
Keywords: Skin, Hair follicle, NMDAR, Schwann cell, VGLUT2, Mouse
INTRODUCTION
The somatosensory system is essential for perception of external stimuli as well as for determining physical positioning within our environment (Chalfie, 2009). Mammalian skin is home to a host of somatosensory terminal nerve endings encoding a variety of touch receptors that decipher mechanical forces including light touch, vibration, stretch and displacement of hair and skin (Lumpkin et al., 2010). In the hairy skin, putative touch receptors have been localized to the piloneural collar in primary and secondary hair follicles, which consists of up to five distinct populations of terminating sensory afferents (Millard and Woolf, 1988). The termini of inner piloneural afferent populations are reported to align with the neck of the hair follicle in a longitudinal direction forming palisades of lanceolate nerve endings, and outer populations innervate in a circumferential pattern (Munger et al., 1989; Halata, 1993). Many of these sensory afferents are derived from early Ret-positive myelinated neurons in the dorsal root ganglion (DRG) and are thought to function as rapidly adapting (RA) mechanoreceptors (Luo et al., 2009; Bourane et al., 2009; Rice et al., 1998; Johnson, 2001) that detect hair displacement (Lumpkin et al., 2010). Recent studies have shown that cell-autonomous expression of the receptor tyrosine kinase Ret and the transcription factor MafA are required for proper development and/or maintenance of myelinated piloneural afferents (Luo et al., 2009; Bourane et al., 2009); however, cellular and molecular determinants in the periphery that specify the development, maintenance and function of these somatosensory end organs remain largely unknown.
The palisade patterning of terminal nerve endings are a unique feature of the piloneural collar receptor, which appears to be influenced, in part, by the presence of type II terminal Schwann cells (tIISCs). These tIISCs express nestin (Li et al., 2003) and S100 and display long finger-like processes that extend upwards from tIISC cell bodies and interdigitate with terminating longitudinal fibers so that each nerve ending is tightly juxtaposed on either side with tIISC processes (supplementary material Fig. S1). Electron microscopy studies have shown that N-cadherin-mediated adherens junctions are formed between outer root sheath (ORS) keratinocytes in the hair follicle and either tIISC processes or the terminal nerve endings themselves (Kaidoh and Inoue, 2000; Kaidoh and Inoue, 2008), suggesting that the maintenance of this receptor might rely on communication between all three cellular components. Some precedence for this concept has been demonstrated in the central nervous system where the excitatory neurotransmitter glutamate represents a major form of communication between neurons and glial cells (Alix and Domingues, 2011). However, a role for glutamate in the regulation of peripheral glial cells is unknown. Finally, these anatomical studies collectively imply that, in addition to the myelination of sensory afferents, terminal Schwann cells might have a key role in the function of somatosensory receptors by facilitating the positioning of terminating sensory afferents. In this study, we aimed to identify the molecular basis for the development and maintenance of piloneural mechanoreceptors in the hairy skin. In this paper, we report that perpetual glutamate release is required to maintain intact mechanosensory capacity in pelage hairs.
MATERIALS AND METHODS
Mice
Mice used include Wnt1Cre (Danielian et al., 1998) (Jackson Laboratories), Krt14Cre (Dassule et al., 2000) (Jackson Laboratories), R26REYFP (Srinivas et al., 2001) (Jackson Laboratories), VGLUT2fl/fl (Wallen-Mackenzie et al., 2006) (Jackson Laboratories), FVB (Taconic Farms) and C57Bl/6 (Taconic Farms). Wnt1Cre mice were crossed with VGLUT2fl/fl mice to generate Wnt1Cre;VGLUT2–/Wt heterozygous conditional null mice. Wnt1Cre;VGLUT2–/Wt mice were crossed with Wnt1Cre;VGLUT2–/Wt mice to generate Wnt1Cre;VGLUT2–/– (VGLUT2cKO) mice. Mice were maintained according to Institute of Comparative Medicine (ICM) guidelines with Institutional Animal Care and Use Committee (IACUC) approval.
Antibodies
The following primary antibodies were used in this study: cytokeratin Krt14 (Covance), Krt10 (Covance), hard acid keratin Ha1 (Acris Antibodies), mGlur1α (R&D Systems), mGlur5 (Abcam), mGlur5 (Millipore), AMPAR (Glur1 subunit, Abcam), Glur6/7 (AnaSpec), GFP-FITC (Rockland Immunochemicals), NMDAR1 (GenScript), Nefh (Aves Labs), nestin (Aves Labs), nestin (Covance) and VGLUT2 (Invitrogen).
Tissue harvesting and immunolabeling
Dorsal skin specimens were harvested from postnatal and adult mice and whole embryos [embryonic day (E) 12.5-18.5], and DRG (T11-L2) specimens were harvested from adult mice. Skin and DRG specimens were cryopreserved in OCT medium. In some cases, postnatal and adult skin specimens were fixed in 4% paraformaldehyde prior to cryopreservation to preserve EYFP detection. Tissue sections were probed with primary antibodies, which were detected with species-specific Alexa Fluor-488, -594 or -647 conjugated secondary antibodies (Invitrogen). DAPI was used to visualize nuclei. Bright-field and fluorescence imaging of thin (6-7 μm) tissue sections was conducted using a Zeiss Axioplan 2 microscope. For thick (40-50 μm) dorsal skin sections, confocal images were acquired using a Zeiss LSM 5 Exciter microscope with 488 nm and 543 nm laser excitation. Color merging and three-dimensional reconstruction of confocal images were conducted using NIH ImageJ software.
NMDAR antagonist treatment
Eight-week-old C57Bl/6 mice received subcutaneous injections of the NMDAR antagonist CGP 39551 (100 nmol; Tocris Bioscience) once a day for up to 5 days or vehicle (water); control and dorsal skins were harvested after day 1, 4 and 5 for phenotypic analysis. Antagonist-treated mice exhibited no signs of morbidity or weight loss (data not shown).
RA afferent recordings
Ex vivo skin-saphenous nerve recordings were performed from female mice (8-10 weeks of age) as previously described (Maricich et al., 2009; Wellnitz et al., 2010). Mice were injected with CGP 39551 or water as described above and all recordings were performed blind to treatment group. The intact skin and saphenous nerve were dissected, placed epidermis-side-up in a custom recording chamber, perfused with carbogen-buffered synthetic interstitial fluid at 32°C and viewed with a stereomicroscope. Extracellular recordings were performed with reference and recording electrodes connected to a differential amplifier. Afferents were teased apart, placed onto a recording electrode and individual units were distinguished with multi-dimensional cluster analysis (SciWorks; Datawave Technologies). Mechanical threshold was estimated with calibrated von Frey filaments and conduction velocity was measured by electrically stimulating the receptive field. To measure stimulus-response relationships, a custom indenter was used to deliver families of axial displacements (1.0-1.4 mm) to the epidermal surface. RA myelinated (Aβ) afferents were identified by their transient responses during the dynamic phase of ramp-and-hold stimuli and conduction velocity >10 m/s. Data were analyzed with MatLab and GraphPad Prism. Statistical significance was assessed using Student’s t-test and two-way ANOVA. Electrophysiological results are expressed as mean ± s.e.m.
RESULTS
VGLUT2+ vesicular loading in the termini of cutaneous Nefh+ sensory afferents
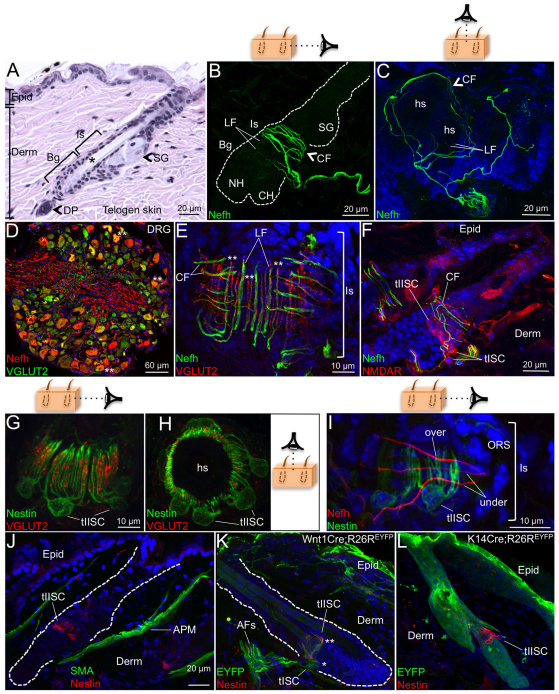
The piloneural collar comprises a variety of sensory afferents and terminal Schwann cells that innervate a region of the hair follicle below the sebaceous gland in the hairy skin; however, the phenotype and function of these fiber subsets is poorly described. To characterize the piloneural collar further, sections of murine skin were probed with antibodies to neurofilament, heavy polypeptide (Nefh), a filament protein that marks myelinated fibers innervating mammalian skin (Christianson et al., 2007; Axelsson et al., 2009), including the piloneural collar (Stucky et al., 1998). We found Nefh to mark multiple fiber populations innervating pelage hairs in both circumferential and longitudinal patterns (Fig. 1A-C). In addition, Nefh+ afferents typically branched to connect multiple follicles (Fig. 1C). Innervation by Nefh+ afferents extended upwards from the border between the bulge and isthmus regions in telogen hair follicles (Fig. 1B; see 1A for follicle compartments) with the majority of circumferential fibers wrapping ORS keratinocytes residing in the isthmus region (Fig. 1B).
Fig. 1.
VGLUT2+ vesicles in the end organs of cutaneous Nefh+ sensory afferents. (A-C,E-L) Mouse skin sections stained with Hematoxylin and Eosin (H&E) (A) or antibodies against Nefh (B,C,E,F,I), VGLUT2 (E,G,H), NMDAR (F), nestin (G-L), SMA (J) or EGFP (K-L). (D) Section of DRG co-labeled with antibodies against Nefh and VGLUT2. Asterisks indicate Nefh+VGLUT2+ DRG neuron somata (**, D) and afferents (**, E), nestin+EYFP+ Schwann cells (**, K), location of palisade ending complex in the isthmus of the hair follicle (*, A), or nestin-EYFP+ Schwann cells (*, K). Cartoons illustrate side (B,G,I) or bird’s eye (C,H) viewing angles. Dashed line demarcates the border between the dermis and hair follicle ORS cells (B,J,K). AFs, afferent fibers; APM, arrector pili muscle; Bg, bulge; CF, circumferential fiber; CH, club hair; Derm, dermis; DP, dermal papilla; Epid, epidermis; hs, hair shaft; Is, isthmus; LF, lanceolate fiber; NH, new hair; SG, sebaceous gland.
To characterize these Nefh+ sensory afferents further, we co-labeled DRG sections with antibodies against Nefh and vesicular glutamate transporter protein VGLUT2 (Slc17a6 – Mouse Genome Informatics), which labels the majority of large and small diameter DRG neurons (Scherrer et al., 2010). A subset (60%; n=531 neurons from five mice) of VGLUT2+ DRG neurons co-expressed Nefh and virtually all Nefh+ neurons co-expressed VGLUT2 (Fig. 1D). A subset of Nefh+VGLUT2+ neurons were large or medium diameter (Fig. 1D) consistent with an Aβ or Aδ cutaneous mechanoreceptor phenotype, suggesting that glutamate might be present in cutaneous piloneural collars. Indeed, VGLUT2+ vesicles were concentrated in the terminal endings of both circumferential and lanceolate Nefh+ sensory afferents (Fig. 1E; supplementary material Fig. S2A) and in the lanceolate endings of Nefh– longitudinal fibers indicating the existence of at least two VGLUT2+ fiber types, Nefh+VGLUT2+ and Nefh–VGLUT2+, innervating pelage hairs (Fig. 1E). The loading of VGLUT2+ vesicles in lanceolate and circumferential fibers suggests that glutamate might be released by these terminal nerve endings and regulate piloneural collar structure or function. To address this idea, we examined skin tissue sections for cells expressing metabotropic and ionotropic glutamate receptors in the vicinity of VGLUT2+ terminal nerve endings. We found the n-methyl-d-aspartate receptor (NMDAR) to be highly expressed in type I (tISC) and type II (tIISC) terminal Schwann cells (Fig. 1F); however, no labeling for metabotropic mGlur1α (Grm1 – Mouse Genome Informatics) and mGlur5 (Grm5 – Mouse Genome Informatics), kainate iGlur6 (Grik2 – Mouse Genome Informatics) or iGlur7 (Grik3 – Mouse Genome Informatics) or alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid receptor (AMPAR; Gria) classes was observed in the piloneural complex or in other regions of the hair follicle, including ORS keratinocytes (data not shown).
Co-labeling skin sections with VGLUT2 and the tIISC marker nestin demonstrated that the tIISC processes are tightly associated with longitudinal VGLUT2+ sensory afferents as the VGLUT2+ fibers and tIISC processes interdigitate in a circumferential pattern around hair follicle ORS cells (Fig. 1G,H). In addition, tIISC processes were intertwined with Nefh+ circumferential fibers forming a weave pattern (Fig. 1I). These results show that tIISC processes maintain a tightly regulated interaction with longitudinal and circumferential fibers in the piloneural collar of adult skin, suggesting that tIISCs might be crucial for the structural maintenance of this mechanoreceptor.
To confirm the lineage of cells comprising the piloneural collar, we examined the localization of EYFP in skin sections from Wnt1Cre;R26REYFP (targeting neural crest stem cells) and Krt14Cre;R26REYFP (targeting keratinocyte stem cells) mice. In Wnt1Cre;R26REYFP skin, EYFP labeling was found in tISCs (nestin–) and tIISCs (nestin+) in the hair follicle isthmus, Schwann cells underlying the epidermis, and sensory afferents innervating the epidermis and hair follicles (Fig. 1K; supplementary material Fig. S2B,C) but not in keratinocyte lineages (Fig. 1K). In Krt14Cre;R26REYFP skin, EYFP labeling was exclusive to keratinocyte lineages in the epidermis, hair follicle and sebaceous gland and was absent in nestin+ tIISCs (Fig. 1L). Finally, the piloneural complex does not appear to directly associate with arrector pili muscle (APM) cells attached to bulge region cells (Fig. 1J).
Innervation of the epidermis and hair follicles during skin development
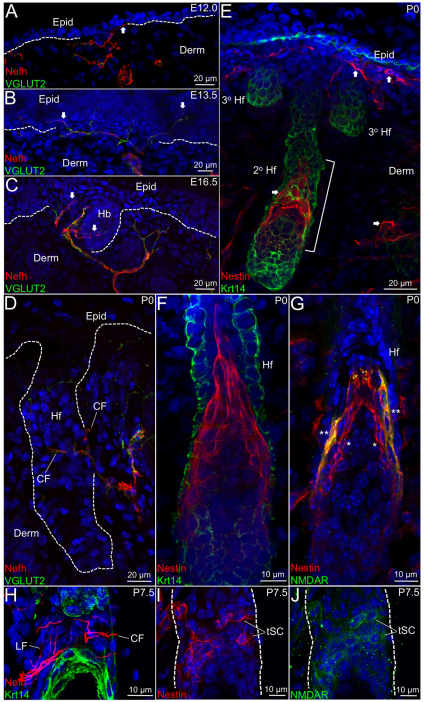
Nefh+ afferents were present in E12.0 dorsal skin and targeted the earliest embryonic epidermal keratinocyte layer (Fig. 2A). Trace levels of VGLUT2+ vesicles were detected in Nefh+ afferents in E12.0 skin (Fig. 2A). In E13.5 skin, these levels were dramatically increased in these afferents, which now extended into the differentiated epidermal layers (Fig. 2B; supplementary material Fig. S2D-F). In E16.5 skin, developing hair buds appeared to be closely associated with Nefh+ afferents that contained high levels of VGLUT2+ vesicles (Fig. 2C), and in P0 skin the initiation of circumferential patterning of secondary hairs by Nefh+ afferents was apparent (Fig. 2D). From E13.5 to P0, most cutaneous Nefh+ fibers contained high levels of VGLUT2+ vesicles; however, it is unclear whether direct contact was occurring between Nefh+ afferents and hair follicle cells during this time frame.
Fig. 2.
Innervation of epidermis and hair follicles in developing skin. (A-J) Histological sections of E12.0 (A), E13.5 (B), E16.5 (C), P0 (D-G) and P7.5 (H-J) mouse skin were co-labeled with antibodies against Nefh and VGLUT2 (A-D), Nestin and Krt14 (E,F), nestin and NMDAR (G,I,J) or Nefh and Krt14 (H). Single color panels are shown for nestin (I) and NMDAR (J) co-labeled P7 hair follicles. Arrows indicate innervation sites in the epidermis and follicles (A-C,E). Bracket delineates the triangular organization of Nestin+ hair follicle cells (E). Asterisks indicate outer track nestin+NMDAR+ cells (**) or inner track nestin+NMDAR– cells (*). Dashed line demarcates the epidermal-dermal border (A-D) and hair follicle ORS cells (C,D,I,J). 2° hf, secondary hair follicle; 3° hf, tertiary hair follicle; CF, circumferential fiber; Derm, dermis; Epid, epidermis; LF, longitudinal fiber; Hb, hair bud; Hf, hair follicle; tSC, putative terminal Schwann cells.
Nestin+ cells were also present in E12.0-16.5 embryonic skin (data not shown), and in E18.5-P0 skin nestin+ cells were localized underneath the epidermal basement membrane, throughout the dermis and tightly associated with secondary pelage hair follicles (Fig. 2E; supplementary material Fig. S3A-C). Nestin+ cells were also present on primary guard (data not shown) but not tertiary follicles in P0 skin (Fig. 2E). Nestin+ cells localized to hair follicles were patterned in a triangular structure in the middle region of developing follicles (Fig. 2E) comprising two rows of cells (Fig. 2F). Hair follicle-associated nestin+ cells did not show immunoreactivity for cytokeratin Krt14 (Fig. 2F; supplementary material Fig. S3A-C), indicating that these cells are not part of the outer root sheath layer of the hair follicle and suggesting that these cells might not be derived from Krt14+ progenitors. The outer row of nestin+ cells co-expressed NMDAR (Fig. 2G) matching the phenotype of nestin+NMDAR+ tSCs in adult skin (Fig. 1F,G), whereas the inner row did not express NMDAR (Fig. 2G), indicating two populations of follicular nestin+ cells in postnatal skin. One possibility is that hair follicle-associated nestin+NMDAR+ cells in P0 skin might serve as precursors for tIISCs in the fully developed piloneural collar. To address this idea, we analyzed these cells at later time points of postnatal skin development. In P4.5 skin, the nestin+ triangular structure appeared to split into upper and lower populations of nestin+NMDAR+ cells (supplementary material Fig. S3D-F), and between P5.5 and P7.5, the upper population began to disperse in a circumferential pattern (Fig. 2I,J) around the same follicular region innervated by Nefh+ fibers (Fig. 2H), although very few of these nestin+NMDAR+ cells exhibited upward processes. These results suggest that nestin+NMDAR+ tIISCs are derived from nestin+ precursors associated with hair follicles in P0 skin but do not exclude the possibility that nestin+ dermal cells also contribute to this early Schwann cell collar in P7.5 skin.
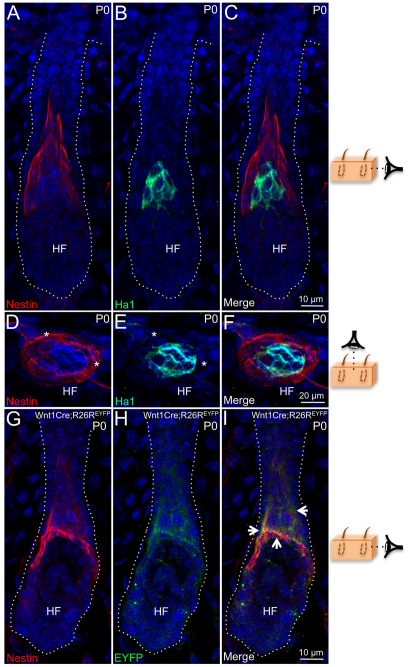
To assess the derivation of nestin+NMDAR+ cells further, P0 skin sections were co-labeled with antibodies against nestin and Ha1 (Krt31 – Mouse Genome Informatics), a type I keratin that selectively marks cortex cells in the hair follicle (Langbein et al., 1999). A small compartment of Ha1+ cortex cells was present in P0 secondary hair follicles (Fig. 3B,E); however, no overlap was observed between nestin+ and Ha1+ zones in P0 follicles (Fig. 3C,F). Collectively, our results distinguish nestin+ cells from both K14+ outer root sheath (Fig. 2) and Ha1+ cortex populations in the hair follicle (Fig. 3). When P0 skin sections from Wnt1Cre;R26REYFP mice were co-labeled with nestin and EYFP antibodies, EYFP labeling was observed in the nestin+ compartment (Fig. 3I) confirming that nestin+NMDAR+ cells are derived from Wnt1+ progenitors, thereby excluding them from the keratinocyte lineage.
Fig. 3.
Derivation of nestin+NMDAR+ hair follicle cells. (A-I) Histological sections of P0 Wt mouse skin were co-labeled with antibodies against Nestin and Ha1 (A-F), and P0 Wnt1Cre;R26REYFP mouse skin sections were co-labeled with nestin and EYFP antibodies (G-I). Single color panels are shown for nestin (A,D,G), Ha1 (B,E) and EYFP (H). Asterisks label the nestin+ layer surrounding the inner Ha1+ compartment in axial sections (D-F). Arrowheads identify nestin+EYFP+ hair follicle cells (I). Dotted line demarcates the border between the dermis and hair follicle ORS cells (A-C,G-I). Cartoons illustrate side (A-C,G-I) or bird’s eye (D-F) viewing angles. HF, hair follicle.
Disruption of the piloneural collar in VGLUT2cKO mice
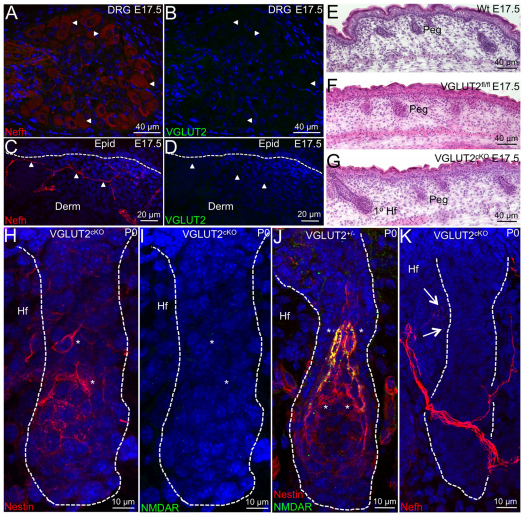
To assess the functional significance of glutamate in the piloneural collar, we utilized VGLUT2fl/fl mice to ablate glutamate in the terminal endings of cutaneous sensory afferents because conditional deletion of VGLUT2 has previously been shown to be an adequate way of abolishing glutamate signaling in DRG neurons (Lagerstrom et al., 2010; Scherrer et al., 2010). We crossed Wnt1Cre mice (Fig. 1K) with VGLUT2fl/fl mice to generate VGLUT2cKO mice to ablate VGLUT2 expression in neural crest stem cell lineages. As expected, no labeling for VGLUT2 was observed in DRG neurons in VGLUT2cKO mice (Fig. 4A,B compared with Fig. 1D); however, the development of Nefh+ skin afferents remained intact (Fig. 4C,D) compared with wild-type (Wt) mice (Fig. 2C). VGLUT2cKO mice suffer from the same postnatal lethality as reported for VGLUT2 complete knockout mice and perish soon after birth presumably owing to respiratory failure. However, given the establishment of early innervation by Nefh+VGLUT2+ afferents and nestin+NMDAR+ cells in P0 skin (Fig. 2), VGLUT2cKO mice still represent an effective tool for ascertaining whether excitatory glutamate is necessary for development of the piloneural collar. No differences in the development of epidermal and hair follicle keratinocyte lineages were observed in VGLUT2cKO skin compared with Wt and VGLUT2fl/fl skin (Fig. 4E-G), indicating that DRG-derived glutamate is not essential for epithelial development or skin homeostasis at early postnatal stages.
Fig. 4.
VGLUT2 ablation disrupts piloneural collar development. (A-K) Histological sections of DRG (A,B) or skin (C-K) from E17.5 (A-G) or P0 (H-K) Wt (E), VGLUT2cKO (A-D,G,H,I,K), VGLUT2fl/fl (F) or VGLUT2+/– (J) mice were stained with Hematoxylin and Eosin (H&E) (E-G), co-labeled with antibodies against Nefh and VGLUT2 (A-D) or nestin and NMDAR (H-J) or Nefh alone (K). Panels A,B, C,D and H,I represent the same field of view. Arrowheads identify the location of selected Nefh+ DRG neurons (A,B) and associated afferents (C,D) under both fields of view. Asterisks indicate nestin+NMDAR+ cells (**) or nestin+NMDAR- cells (*). Arrows indicate approaching Nefh+ fibers that fail to innervate hair follicles (K). Dashed line demarcates the border between the epidermis and dermis (C,D) or hair follicle ORS cells (H-K). 1° Hf, primary guard hair follicle; Derm, dermis; Epid, epidermis; Hf, hair follicle; Peg, secondary hair peg.
Although nestin+ cells were present and associated with hair follicles in VGLUT2cKO postnatal skin, the frequency and organization of these cells was severely perturbed (Fig. 4H; supplementary material Fig. S4A). In most hair follicles, nestin+ cells failed to organize in inner or outer tracks (Fig. 4H; supplementary material Fig. S4A), as was observed in Wt follicles (Fig. 2F,G), but appeared instead to be randomly dispersed around the lower half of developing follicles (Fig. 4H) (VGLUT2cKO: 1.7% follicles with organized nestin+ cells, n=300 follicles examined from five P0 mice; Wt: 100% follicles with organized nestin+ cells, n=150 follicles examined from five P0 littermate mice). In addition, these disorganized nestin+ cells in VGLUT2cKO mice did not express NMDAR (Fig. 4I); however, the inner and outer rows of nestin+ cells were intact in P0 skin from VGLUT2+/– littermates (Fig. 4J). In the few cases in which nestin+ cells were organized, only the inner row of cells was present in VGLUT2cKO postnatal skin (supplementary material Fig. S4B) resembling nestin+NMDAR– cells in Wt skin (Fig. 2). Although Nefh+ fibers were present in VGLUT2cKO postnatal skin, little to no circumferential innervation was observed in hair follicles (Fig. 4K) suggesting that patterning of the outer track of nestin+NMDAR+ cells does not influence the recruitment of Nefh+ fibers but might be essential for initiation of their circumferential innervation. However, our results cannot address the extent of any potential defect in circumferential innervation or whether circumferential innervation of Nefh+ fibers might occur at later time points during hair follicle morphogenesis in VGLUT2cKO postnatal skin.
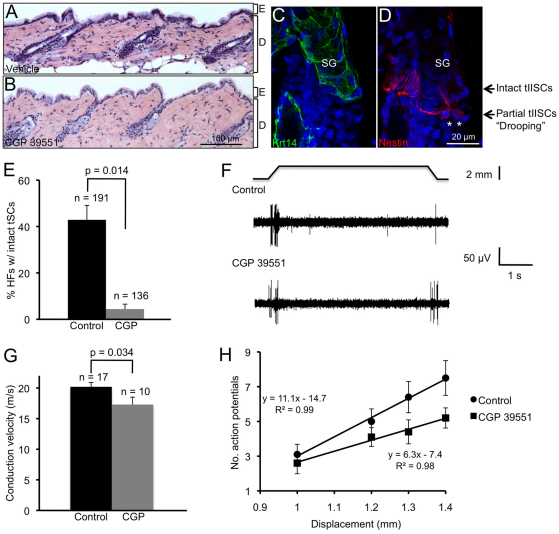
To determine whether glutamatergic signaling regulates the maintenance of the piloneural mechanoreceptor in developed skin, adult mice were treated with the competitive NMDAR antagonist CGP 39551 (an AP5 analog) (Fagg et al., 1990; Chapman et al., 1991). Histological skin sections of control and CGP 39551-treated mice were indistinguishable (Fig. 5A,B), and no changes in epithelial proliferation or differentiation were observed (data not shown). However, compared with intact tIISCs, CGP 39551 treatment severely compromised the structure of tIISC processes as well as the position of tIISCs along the hair follicle (Fig. 5C,D). In most cases, affected tIISCs appeared to retain only one or two processes that were positioned in a horizontal direction (Fig. 5D). In addition, affected tIISCs appeared at a lower or ‘drooped’ position along the follicle relative to intact tIISCs (Fig. 5D). Overall, we observed a seven- to eightfold reduction in the frequency of hair follicles exhibiting intact tIISCs in CGP 39551-treated mice compared with control skin (P=0.014) (Fig. 5E).
Fig. 5.
Effects of NMDAR antagonist on piloneural collar structure and response to touch stimuli. (A-D) Histological sections of dorsal skin from mice treated five times with CGP 39551 or vehicle were stained with Hematoxylin and Eosin (H&E) (A,B). Representative images of dorsal skin sections from CGP 39551-treated (five times) mice co-labeled with Krt14 and nestin antibodies (C,D). Asterisks indicate partially intact tIISCs (D) and arrows indicate the orientation of intact (upper) and ‘drooping’ partially intact (lower) tIISCs along the hair follicle (D). Panels C and D represent the same field of view. SG, sebaceous gland. (E) Percentage of hair follicles (HFs) exhibiting intact tIISCs in skin from mice treated five times with CGP 39551. Error bars represent s.d. (three mice per treatment group). n, number of hair follicles counted in each treatment group; Student’s t-test. (F) Representative RA responses from a control (top) and a CGP 39551-treated mouse (bottom). (G) Mean conduction velocities of RA afferents (n=10 RA afferents from six CGP 39551 treated mice and n=17 RA afferents from eight water-injected control mice; Student’s t-test). Error bars represent s.e.m. (H) Displacement-response relations for the RA afferents shown in G. Squares denote responses from CGP 39551-treated mice and circles denote controls. Responses differed significantly across displacement (P=0.0005) and treatment group (P=0.018; two-way ANOVA). Error bars represent s.e.m.
To investigate whether glutamatergic signaling modulates the excitability of piloneural mechanosensory receptors in the skin, we compared touch-evoked responses of cutaneous afferents from mice injected with CGP 39551 or vehicle (water). To do so, we recorded extracellular action potentials from RA afferents innervating hairy skin using an ex vivo skin-nerve preparation (Fig. 5F) (Maricich et al., 2009). We focused on RA afferents because these predominantly innervate hair follicles in mouse hairy skin (Luo et al., 2009). Overall, the proportion and von Frey thresholds of RA afferents did not differ between treatment groups; however, conduction velocities of RA afferents were significantly lower in mice treated with CGP 39551 (Fig. 5G; P=0.03, Student’s t-test). Moreover, the mechanical sensitivity of RA afferents, defined as the number of spikes elicited by suprathreshold stimuli, was reduced in mice treated with CGP 39551 compared with control afferents (Fig. 5H; P=0.018 for treatment groups, two-way ANOVA). Collectively, these results indicate that glutamatergic signaling through NMDARs increases the excitability of RA afferents in hairy skin.
DISCUSSION
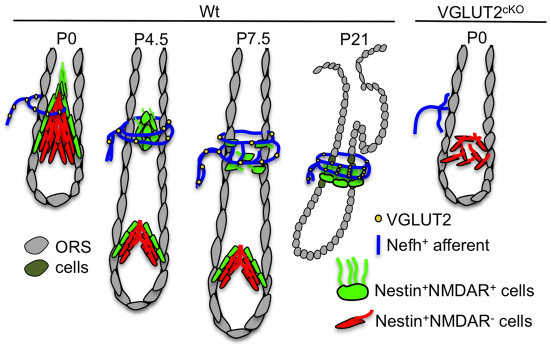
Here, we show that the piloneural collar, comprising tIISCs and Nefh+VGLUT2+ and Nefh–VGLUT2+ longitudinal and circumferential innervating fibers, is localized to the border of the upper bulge region extending into and covering a majority of ORS keratinocytes in the isthmus region of pelage hair follicles. NMDAR+ tIISCs appear to originate from a pool of nestin+Krt14– hair follicle cells present in developing skin. Finally, the proper development and maintenance of this putative mechanoreceptor is dependent on glutamate derived from DRG neurons that might serve to activate NMDAR signaling in tIISCs (Fig. 6).
Fig. 6.
Schematic of glutamate-dependent Nestin+/NMDAR+ cell organization in the development of the piloneural collar in postnatal and developed mouse skin. A representation of the dissemination of nestin+NMDAR+ and nestin+NMDAR– cells and innervation of Nefh+ sensory afferents during hair follicle development in postnatal day (P) 0, P4.5, P7.5 and P21 wild-type mouse skin is shown. The disruption of piloneural collar development observed in VGLUT2cKO skin (right) indicates that the presence and intact patterning of nestin+NMDAR+ cells are dependent on glutamate.
Although glutamate release regulates astrocyte physiology in the CNS (Alix and Domingues, 2011), to our knowledge, this is the first evidence that excitatory glutamate derived from the DRG can regulate the differentiation of peripheral glial cells in the skin. Glutamate release can be induced in the periphery following stimulation of A or C sensory afferents (deGroot et al., 2000), and glutamate release from synaptic vesicles has been observed following stretch activation of mechanosensory nerve terminals innervating muscle spindles (Banks et al., 2002; Bewick et al., 2005). Our results strongly suggest that glutamate is crucial for maintaining the sensory capability of the piloneural collar. In doing so, our results confer an efferent functionality to this population of sensory afferents in the skin. Although it is possible that VGLUT2 ablation could be compensated for by other family members, such as VGLUT1 (Slc17a7 – Mouse Genome Informatics) or VGLUT3 (Slc17a8 – Mouse Genome Informatics), previous studies have demonstrated that conditional VGLUT2 ablation using a DRG-targeted Cre recombinase was sufficient to erase glutamate function in peripherin-expressing sensory neurons (Scherrer et al., 2010).
We previously identified a unique population of ORS keratinocyte progenitors residing in the isthmus of pelage hairs (Jensen et al., 2008), the same region that is contained by the piloneural collar. The adhesion events between ORS cells, presumably in the isthmus, and terminal endings in the piloneural collar identified by electron microscopy studies (Kaidoh and Inoue, 2008) allows for the intriguing possibility that, in addition to peripheral glial cells, ORS progenitors might be influenced by communication events with sensory afferents (Brownell et al., 2011). However, we did not observe any perturbations in hair follicle or epidermal homeostasis in VGLUT2cKO skin or in NMDAR antagonist-treated mice, consistent with previous studies showing that anagen hair follicle induction was not dependent on intact innervation by sensory afferents (Maurer et al., 1998).
Supplementary Material
Acknowledgments
We thank the Yan Lu and the Skin Phenotyping Core Facility within the Department of Dermatology Skin Diseases Research Center (P30 AR044535) for technical assistance. We thank Dr Angela Christiano for providing the Ha1 antibody and Ms Kara Marshall for discussions.
Footnotes
Funding
This work was funded by grants from the National Cancer Institute (NCI) [R01CA114014 to D.M.O. and S.H.W.]; New York State Stem Cell Science (NYSTEM) [N08G-335 to D.M.O., A.M.F. and S.H.W.]; the National Institute of Arthritis, and Musculoskeletal and Skin Diseases (NIAMS) [R01AR051219 to E.A.L. and Y.B.]; and the National Institute of Neurological Disorders and Stroke (NINDS) [R01073119 to E.A.L.]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.070847/-/DC1
References
- Alix J. J., Domingues A. M. (2011). White matter synapse: form, function, and dysfunction. Neurology 76, 397–404 [DOI] [PubMed] [Google Scholar]
- Axelsson H. E., Mindle J. K., Sonesson A., Toolanen G., Hogestatt E. D., Zygmunt P. M. (2009). Transient receptor potential vanilloid 1, vanilloid 2 and melastatin 8 immunoreactive nerve fibers in human skin from individuals with and without Norrbottnian congenital insensitivity to pain. Neuroscience 162, 1322–1332 [DOI] [PubMed] [Google Scholar]
- Banks R. W., Bewick G. S., Reid B., Richardson C. (2002). Evidence for activity-dependent modulation of sensory-terminal excitability in spindles by glutamate release from synaptic-like vesicles. Adv. Exp. Med. Biol. 508, 13–18 [DOI] [PubMed] [Google Scholar]
- Bewick G. S., Reid B., Richardson C., Banks R. W. (2005). Autogenic modulation of mechanorecptor excitability by glutamate release from synaptic-like vesicles: evidence from the rat muscle spindle primary sensory ending. J. Physiol. 562, 381–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourane S., Garces A., Venteo S., Pattyn A., Hubert T., Fichard A., Puech S., Boukhaddaoui H., Baudet C., Takahashi S., Valmier J., Carroll P. (2009). Low-threshold mechanoreceptor subtypes selectively express MafA and are specified by Ret signaling. Neuron 64, 857–870 [DOI] [PubMed] [Google Scholar]
- Brownell I., Guevara E., Bai C. B., Loomis C. A., Joyner A. L. (2011). Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 8, 552–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M. (2009). Neurosensory mechanotransduction. Nat. Rev. Mol. Cell. Biol. 10, 44–52 [DOI] [PubMed] [Google Scholar]
- Chapman A. G., Graham J. L., Patel S., Meldrum B. S. (1991). Anticonvulsant activity of two orally active competitive N-methyl-D-aspartate antagonists, CGP 37849 and CGP 39551, against sound-induced seizures in DBA/2 mice and photically induced myoclonus in Papio papio. Epilepsia 32, 578–587 [DOI] [PubMed] [Google Scholar]
- Christianson J. A., Ryals J. M., Johnson M. S., Dobrowsky R. T., Wright D. E. (2007). Neurotrophic modulation of myelinated cutaneous innervation and mechanical sensory loss in diabetic mice. Neuroscience 145, 303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielian P. S., Muccino D., Rowitch D. H., Michael S. K., McMahon A. P. (1998). Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr. Biol. 8, 1323–1326 [DOI] [PubMed] [Google Scholar]
- Dassule H. R., Lewis P., Bei M., Maas R., McMahon A. P. (2000). Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 127, 4775–4785 [DOI] [PubMed] [Google Scholar]
- deGroot J., Zhou S., Carlton S. M. (2000). Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. NeuroReport 11, 497–502 [DOI] [PubMed] [Google Scholar]
- Fagg G. E., Olpe H.-R., Pozza M. F., Baud J., Steinmann M., Schmutz M., Portet C., Baumann P., Thedinga K., Bittiger H., Allgeier H., Heckendorn R., Angst C., Brundish C., Dingwall J. G. (1990). CGP 37849 and CGP 39551: novel and potent competitive N-methyl-D-aspartate receptor antagonists with oral activity. Br. J. Pharmacol. 99, 791–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halata Z. (1993). Sensory innervation of the hairy skin (light and electron microscopic study). J. Invest. Dermatol. 101, 75S–81S [DOI] [PubMed] [Google Scholar]
- Jensen U. B., Yan X., Triel C., Woo S.-H., Christensen R., Owens D. M. (2008). A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. J. Cell Sci. 121, 609–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. O. (2001). The roles and functions of cutaneous mechanoreceptors. Curr. Opin. Neurobiol. 11, 455–461 [DOI] [PubMed] [Google Scholar]
- Kaidoh T., Inoue T. (2000). Intercellular junctions between palisade nerve endings and outer root sheath cells of rat vellus hairs. J. Comp. Neurol. 420, 419–427 [DOI] [PubMed] [Google Scholar]
- Kaidoh T., Inoue T. (2008). N-cadherin expression in palisade nerve endings of rat vellus hairs. J. Comp. Neurol. 506, 525–534 [DOI] [PubMed] [Google Scholar]
- Lagerstrom M. C., Rogoz K., Abrahamsen B., Persson E., Reinius B., Nordenankar K., Olund C., Smith C., Mendez J. A., Chen Z.-F., Wood J. N., Wallen-Mackenzie A., Kullander K. (2010). VGLUT2-dependent sensory neurons in the TRPV1 population regulate pain and itch. Neuron 68, 529–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbein L., Rogers M. A., Winter H., Praetzel S., Beckhaus U., Rackwitz H.-R., Schweizer J. (1999). The catalog of human hair keratins. I. Expression of the nine type I members in the hair follicle. J. Biol. Chem. 274, 19874–19884 [DOI] [PubMed] [Google Scholar]
- Li L., Mignone J., Yang M., Matic M., Pennman S., Enikolopov G., Hoffman R. M. (2003). Nestin expression in hair follicle sheath progenitor cells. Proc. Natl. Acad. Sci. USA 100, 9958–9961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumpkin E. A., Marshall K. L., Nelson A. M. (2010). The cell biology of touch. J. Cell Biol. 191, 237–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Enomoto H., Rice F. L., Milbrandt J., Ginty D. D. (2009). Molecular identification of rapidly adapting mechanorecpetors and their developmental dependence on ret signaling. Neuron 64, 841–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maricich S. M., Wellnitz S. A., Nelson A. M., Lesniak D. R., Gerling G. J., Lumpkin E. A., Zoghbi H. Y. (2009). Merkel cells are essential for light touch responses. Science 324, 1580–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M., Peters E. M. J., Botchkarev V. A., Paus R. (1998). Intact hair follicle innervation is not essential for anagen induction. Arch. Dermatol. Res. 290, 574–578 [DOI] [PubMed] [Google Scholar]
- Millard C. L., Woolf C. J. (1988). Sensory innervation of the hairs of the rat hind limb: a light microscopic analysis. J. Comp. Neurol. 277, 183–194 [DOI] [PubMed] [Google Scholar]
- Munger B. L., Renehan W. E. (1989). Degeneration and regeneration of peripheral nerve in the rat trigeminal system: III. Abnormal sensory reinnervation of rat guard hairs following nerve transection and crush. J. Comp. Neurol. 283, 169–176 [DOI] [PubMed] [Google Scholar]
- Rice F. L., Albers K. M., Davis B. M., Silos-Santiago I., Wilkinson G. A., LeMaster A. M., Ernfors P., Smeyne R. J., Aldskogius H., Phillips H. S., Barbacid M., DeChiara T. M., Yancopoulos G. D., Dunne C. E., Fundin B. T. (1998). Differential dependency of unmyelinated and A delta epidermal and upper dermal innervation on neurotrophins, trk receptors, and p75LNGFR. Dev. Biol. 198, 57–81 [PubMed] [Google Scholar]
- Scherrer G., Low S. A., Wang X., Zhang J., Yamanaka H., Urban R., Solorzano C., Harper B., Hnasko T. S., Edwards R. H., Basbaum A. I. (2010). VGLUT2 expression in primary afferent neurons is essential for normal acute pain and injury-induced heat hypersensitivity. Proc. Natl. Acad. Sci. USA 107, 22296–22301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S., Watanbe T., Lin C. S., William C. M., Tanabe Y., Jessell T. M., Costantini F. (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 1, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucky C. L., DeChiara T., Lindsay R. M., Yancopoulos G. D., Koltzenburg M. (1998). Neurotrophin 4 is required for the survival of a subclass of hair follicle receptors. J. Neurosci. 18, 7040–7046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen-Mackenzie A., Gezelius H., Thoby-Brisson M., Nygard A., Enjin A., Fujiyama F., Fortin G., Kullander K. (2006). Vesicular glutamate transporter 2 is required for central respiratory rhythm generation but not for locomotor central pattern generation. J. Neurosci. 26, 12294–12307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellnitz S. A., Lesniak D. R., Gerling G. J., Lumpkin E. A. (2010). The regularity of sustained firing levels reveals two populations of slowly adapting touch receptors in mouse hairy skin. J. Neurophysiol. 103, 3378–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.