Abstract
BACKGROUND
Hemophilia B, an X-linked disorder, is ideally suited for gene therapy. We investigated the use of a new gene therapy in patients with the disorder.
METHODS
We infused a single dose of a serotype-8–pseudotyped, self-complementary adenovirus-associated virus (AAV) vector expressing a codon-optimized human factor IX (FIX) transgene (scAAV2/8-LP1-hFIXco) in a peripheral vein in six patients with severe hemophilia B (FIX activity, <1% of normal values). Study participants were enrolled sequentially in one of three cohorts (given a high, intermediate, or low dose of vector), with two participants in each group. Vector was administered without immunosuppressive therapy, and participants were followed for 6 to 16 months.
RESULTS
AAV-mediated expression of FIX at 2 to 11% of normal levels was observed in all participants. Four of the six discontinued FIX prophylaxis and remained free of spontaneous hemorrhage; in the other two, the interval between prophylactic injections was increased. Of the two participants who received the high dose of vector, one had a transient, asymptomatic elevation of serum aminotransferase levels, which was associated with the detection of AAV8-capsid–specific T cells in the peripheral blood; the other had a slight increase in liver-enzyme levels, the cause of which was less clear. Each of these two participants received a short course of glucocorticoid therapy, which rapidly normalized aminotransferase levels and maintained FIX levels in the range of 3 to 11% of normal values.
CONCLUSIONS
Peripheral-vein infusion of scAAV2/8-LP1-hFIXco resulted in FIX transgene expression at levels sufficient to improve the bleeding phenotype, with few side effects. Although immune-mediated clearance of AAV-transduced hepatocytes remains a concern, this process may be controlled with a short course of glucocorticoids without loss of transgene expression. (Funded by the Medical Research Council and others; ClinicalTrials.gov number, NCT00979238.)
Hemophilia B is an X-linked bleeding disorder that results from a defect in the gene encoding coagulation factor IX (FIX), a serine protease that is critical for blood clotting. Persons with severe hemophilia B have functional FIX levels that are less than 1% of normal values and have frequent bleeding episodes, which are associated with crippling arthropathy and early death.1,2 Current treatment involves frequent intravenous injections of FIX protein concentrate (i.e., two to three times a week). However, this treatment is prophylactic rather than curative, is extremely expensive, and is associated with inhibitor formation. Somatic gene therapy for hemophilia B offers the potential for a cure through continuous endogenous production of FIX after a single administration of vector, especially since a small rise in circulating FIX to at least 1% of normal levels can substantially ameliorate the bleeding phenotype.
At present, gene transfer mediated by an adenovirus-associated virus (AAV) vector shows the greatest promise for long-term correction of hemophilia B in the preclinical setting.3–7 However, a combined phase 1 and 2 study that involved serotype 2–based AAV vectors (AAV2) showed only transient expression of FIX and suggested that stable expression of therapeutic levels of FIX may be limited by a capsid-specific cytotoxic T-cell response against the transduced hepatocytes.8,9
We have tested an approach to treating patients with severe hemophilia B that is distinct from the approaches used in previous clinical trials of AAV-mediated gene transfer in three important respects. First, we developed a codon-optimized FIX (FIXco) expression cassette that is packaged as complementary dimers within a single virion. These self-complementary AAV (scAAV) vectors mediate transgene expression at substantially higher levels than do single-stranded AAV vectors.6,10 Second, to circumvent the possibility of humoral immunity to AAV, we pseudotyped these vectors with a capsid of serotype 8 (AAV8), which has a lower seroprevalence in humans than does AAV2.11,12 Finally, since AAV8 has a strong tropism for the liver, we were able to administer the vector in the peripheral vein — a simple, noninvasive approach that is safe for patients with a bleeding diathesis. On the basis of our preclinical safety and efficacy data, we conducted a combined phase 1 and 2 clinical trial of scAAV2/8-LP1-hFIXco–mediated gene transfer in patients with hemophilia B.6,7,10
METHODS
STUDY DESIGN
Patients who met the entry criteria and did not have neutralizing antibodies to AAV8, as determined by an in vivo transduction-inhibition assay (see Table 1 and the Methods section in the Supplementary Appendix, available with the full text of this article at NEJM.org), were enrolled after providing written informed consent. Participants 1 through 5 were recruited in 2010 and Participant 6 was recruited early in 2011. All were admitted to the Royal Free Hospital for vector administration and subsequent observation overnight. Participants were enrolled sequentially into one of three cohorts according to dose level: low (2×1011 vector genomes [vg] per kilogram of body weight), intermediate (6×1011 vg per kilogram), and high (2×1012 vg per kilogram).
Table 1.
Characteristics of the Six Study Participants at Baseline and after Gene Transfer, According to Dose of Vector.*
| Characteristic | Vector Dose, 2×1011 vg/kg | Vector Dose, 6×1011 vg/kg | Vector Dose, 2×1012 vg/kg | |||
|---|---|---|---|---|---|---|
| Participant 1 | Participant 2 | Participant 3 | Participant 4 | Participant 5 | Participant 6 | |
|
At baseline
| ||||||
| Age (yr) | 31 | 64 | 43 | 29 | 32 | 27 |
|
| ||||||
| Mutation in FIX gene | 31280G→A E387K | 2bp deletion, frame shift | 30097G→T W215C | 31290G→A A309T | 20518C→T R180W | –52 del C |
|
| ||||||
| FIX prophylaxis | Twice weekly | Twice weekly | Twice weekly | Targeted (average, weekly) | Twice weekly | Thrice weekly |
|
| ||||||
| Hepatitis C status | Negative | Positive, spontaneous clearance | Positive, clearance with interferon plus anti-viral therapy | Positive, spontaneous clearance | Positive, clearance with interferon plus anti-viral therapy | Negative |
|
| ||||||
| Antibody titer (relative units)†
| ||||||
| AAV2 IgG | 5 | 20 | 77 | 12 | 10 | 22 |
|
| ||||||
| AAV8 IgG | 1 | 12 | 37 | 1 | 5 | 8 |
|
| ||||||
|
After gene transfer
| ||||||
| Maximum FIX level (IU/dl)‡ | 2 | 2 | 3 | 4 | 8 | 12 |
|
| ||||||
| Duration of FIX expression (mo) | >15 | >11 | >9 | >8 | >6 | >5 |
|
| ||||||
| FIX expression on in vivo transduction-inhibition assay (% of control value)§ | 138 | 116 | 42 | 109 | 92 | 93 |
|
| ||||||
| Peak alanine aminotransferase value (IU/liter)¶ | 36 | 20 | 39 | 34 | 202 | 36 |
FIX denotes factor IX, and vg vector genomes.
The lower limit of detection for serotype-2 adenovirus-associated virus (AAV2) IgG antibodies was 3 relative units; the lower limit of detection for serotype-8 adenovirus-associated virus (AAV8) IgG antibodies was 1 relative unit.
The lower limit of detection was 1%.
The lower limit of detection was 0.7%.
The upper limit of the normal range is 41 IU per liter.
The study was sponsored by St. Jude Children’s Research Hospital and was conducted independently by the authors in accordance with the protocol, which is available at NEJM.org, and with the provisions of the Good Clinical Practice guidelines.
VECTOR PRODUCTION AND FORMULATION
The viral vector, scAAV2/8-LP1-hFIXco, has been described previously.6,10 It was manufactured and formulated in phosphate-buffered saline (PBS) supplemented with 0.25% human serum albumin by the Children’s GMP, in Memphis, Tennessee, in accordance with Good Manufacturing Practice and regulatory requirements.13 The vector titer initially reported was determined by means of a validated, quantitative real-time polymerase-chain-reaction (qPCR) assay.14 Subsequent studies have shown that with the qPCR assay, the presence of the covalently closed hairpin at one end of the scAAV-vector genome results in an underestimate of the titer by a factor of almost 10; therefore, the doses reported here are based on titers determined by a method that circumvents this issue and results in more accurate and reliable vector-genome titration of the scAAV vector (see Methods in the Supplementary Appendix).15 Preparation and infusion of the vector are described in the protocol.
SAFETY AND EFFICACY ASSESSMENTS
Assessments performed after vector infusion included vital signs, as well as plasma FIX activity, anti-capsid and anti-FIX antibody levels, vector shedding, and cellular immune response.8–10,16 Efficacy was defined as the persistence of biologically active FIX at 3% or more of normal levels.
RESULTS
CHARACTERISTICS OF THE STUDY PARTICIPANTS
Six men with severe hemophilia B (FIX activity, <1% of normal values) were enrolled under a protocol approved by the relevant ethics boards and regulatory agencies, after providing written informed consent. All except one participant received regular prophylaxis with FIX concentrates (two or three times per week) before gene transfer (Table 1). Participant 4 was receiving targeted prophylaxis (about once weekly), which was tailored to his sports activities and the associated risk of traumatic injury. Participant 2 had a null mutation in the FIX gene, and Participant 6 had a promoter mutation; consequently, neither had FIX protein expression. The other four participants had mis–sense mutations, which resulted in normal plasma levels of FIX antigen but less than 1% clotting activity. All participants had modest levels (>5 relative units) of anti-AAV2 IgG antibodies before gene transfer.
SAFETY AND EFFICACY OF PERIPHERAL-VEIN INFUSION OF VECTOR
No immediate changes in vital signs were noted during or after vector infusion in any participant, despite the persistence of the vector in bodily fluids (including plasma) for up to 15 days after gene transfer (Fig. 1 in the Supplementary Appendix). There were no clinically significant changes in serum chemical values (including liver enzyme levels) in any participant for the first 6 weeks after vector infusion (Fig. 2 in the Supplementary Appendix). Three adverse events were reported: anemia developed in Participants 1 and 2 at 5 and 7 weeks, respectively, after gene transfer, and Participant 3 had a transient period of bradycardia at 16 weeks after gene transfer, when he was being prepared for surgery on his left knee (see the Supplementary Appendix for details).
Low-Dose Group
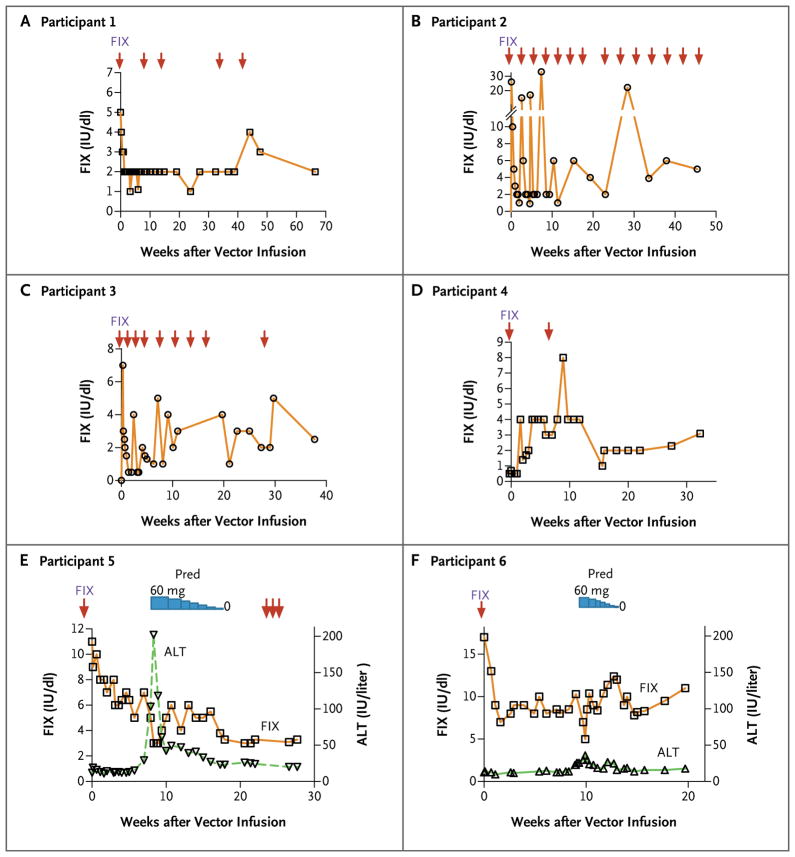
In Participant 1, after the initial clearance of exogenously administered FIX protein, plasma FIX activity stabilized at 2% of normal levels on day 14 after the infusion of 2×1011 vg of scAAV2/8-LP1-hFIXco per kilogram. FIX activity remained relatively stable at this level for more than 16 months after gene transfer, despite the discontinuation of twice-weekly FIX prophylaxis (Fig. 1A). This level was substantially higher than his baseline FIX activity level (<1% of normal levels) and is consistent with endogenous synthesis of FIX. He had no spontaneous hemorrhages during this period, but FIX prophylaxis was required on five occasions to provide protection in the event of accidental injuries and during elective surgery.
Figure 1. Factor IX (FIX) Activity after Peripheral-Vein Infusion of the Adenovirus-Associated Virus (AAV) Vector in the Six Study Participants.
A one-stage clotting assay was used to determine FIX coagulation activity at prespecified time points (arrows) after the administration of the AAV vector (scAAV2/8-LP1-hFIXco). Panels A and B show FIX levels in Participants 1 and 2, respectively, who received the low dose of vector (2×1011 vector genomes [vg] per kilogram of body weight). Panels C and D show FIX levels in Participants 3 and 4, respectively, who received the intermediate dose of vector (6×1011 vg per kilogram). Panels E and F show both FIX levels and alanine aminotransferase (ALT) levels in Participants 5 and 6, respectively, who received the high dose of vector (2×1012 vg per kilogram). The duration of treatment with prednisolone (Pred), which was initiated at a dose of 60 mg per day and then gradually tapered, is also shown.
In Participant 2, the plasma FIX level was 1% of normal levels 18 days after gene transfer, which was 3 weeks after his last injection of FIX concentrate. Although this level was consistent with endogenous synthesis of transgenic FIX, he continued to have spontaneous hemorrhages and resumed regular FIX prophylaxis. Over time, he gradually increased the interval between prophylactic FIX injections to 2 weeks, with no spontaneous hemorrhages. At 23 weeks after gene transfer and 3 weeks after the last FIX concentrate injection, his plasma FIX level was 2% of normal values, which suggested ongoing endogenous FIX synthesis (Fig. 1B).
Intermediate-Dose Group
In Participant 3, the level of FIX activity in plasma consistently drifted downward to below 1% of normal values in the absence of FIX concentrate during the first 5 weeks after the infusion of 6×1011 vg of scAAV2/8-LP1-hFIXco per kilogram. This change raised the possibility that the transduction of hepatocytes was blocked because the level of anti-AAV8 antibody before gene transfer was higher in this participant than in the other participants (Table 1). However, over time, Participant 3 extended the period between prophylactic FIX injections to 2 weeks. Between weeks 20 and 33, he did not receive FIX prophylaxis and remained free of spontaneous bleeding. During this period, his plasma FIX level was between 1 and 3% of normal values, which is consistent with endogenous synthesis of transgenic protein (Fig. 1C).
Plasma FIX levels in Participant 4 increased from a baseline value of less than 1% of normal values to a peak of 4% 4 weeks after gene transfer. FIX expression remained at this level for almost 3 months before declining to 2 to 3% of normal values (Fig. 1D). The reason for this decline is unclear, since the results of liver-function tests remained in the normal range, and assays for neutralizing anti-FIX antibodies were consistently negative. Despite this drop in FIX activity, he had no bleeding episodes, even though he engaged in physical activities that had previously provoked such episodes (e.g., playing soccer and cricket without having received targeted prophylaxis). Approximately 9 weeks after gene transfer, however, he did receive a bolus of FIX concentrate to prevent severe bleeding after a fall.
High-Dose Group
Fourteen days after peripheral-vein infusion of 2×1012 vg of scAAV2/8-LP1-hFIXco per kilogram (17 days after the last infusion of FIX concentrate), the plasma FIX level in Participant 5 was 7% of normal values, and this level was maintained until week 7 without the administration of concentrate. On day 49 after the vector infusion, aspartate aminotransferase and alanine aminotransferase levels were elevated, reaching peaks of 143 and 202 IU per liter, respectively, at 58 days (upper limit of the normal range, 37 and 41 IU per liter, respectively). He was asymptomatic, with normal bilirubin levels and prothrombin time, but the plasma FIX level dropped to 3% of normal values (Fig. 1E). After ruling out possible causes of this reduction, such as autoimmune responses, toxic factors (alcohol, drugs, or toxins), or infectious viral causes (hepatitis A, B, C, or E virus, herpes simplex virus, Epstein–Barr virus, cytomegalovirus, human immunodeficiency virus, or human T-lymphotrophic virus type I or II), we considered an immunologic response to scAAV2/8-LP1-hFIX as a potential explanation for the liver injury. This was reported as a grade 3 adverse event related to the study agent. Treatment with prednisolone was begun on day 58 at a dose of 60 mg per day, with subsequent tapering of the dose, rapidly reducing aminotransferase levels. Nine weeks after glucocorticoid therapy was discontinued, transgene expression persisted at the 3% level, and the results of liver-function tests remained in the normal range. The patient was free of spontaneous hemorrhage for more than 6 months after the gene transfer but required three boluses of FIX concentrate to prevent bleeding due to traumatic injuries sustained during a recent geologic field trip.
In Participant 6, plasma FIX expression remained between 8 and 12% of normal levels during an 8-week period after vector administration. He was able to stop FIX prophylaxis even while he was training for a marathon. Clinical and laboratory measures remained within the normal range until week 9 (day 62 after gene transfer), when aspartate aminotransferase and alanine aminotransferase values roughly doubled, as compared with baseline levels (with both reaching 36 IU per liter). This change followed a weekend of intensive physical activity, which included participation in a half-marathon, and was associated with a rise in the level of lactate dehydrogenase (to 523 IU per liter; normal range, 240 to 480). When FIX activity declined to 5% of normal levels, Participant 6 also began a short (4-week) course of prednisolone for suspected immune-mediated hepatocyte clearance after other possible causes had been ruled out. Aminotransferase levels subsequently returned to baseline values, and FIX expression remained at a level that was between 8 and 12% of normal values (Fig. 1F). However, even at their peak, liver enzyme levels in Participant 6 remained below the upper limit of the normal range and therefore did not meet the criteria for an adverse event.
IMMUNE RESPONSES TO VECTOR AND TRANSGENE
Neutralizing antibodies to FIX were not detected at any time point in any participant. The kinetic characteristics of the humoral immune response to the AAV8 capsid were similar in all six participants and were consistent with a primary immune response to AAV8 (Fig. 2 and Table 1, and Results in the Supplementary Appendix).
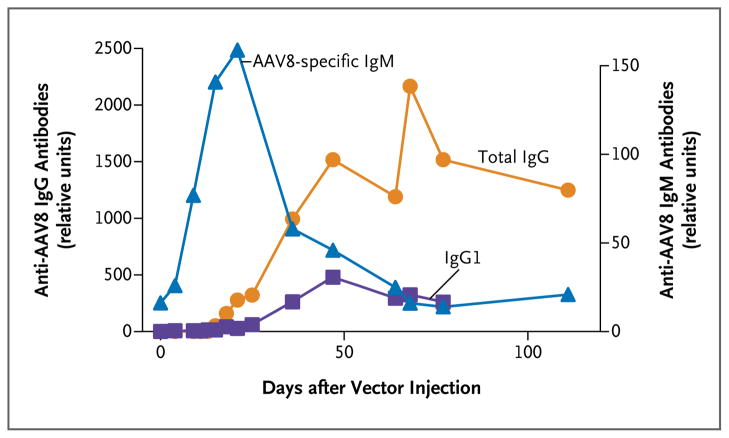
Figure 2. Humoral Immune Response in Participant 1.
The profile of the humoral immune response in Participant 1 is representative of the responses seen in the other participants. Plasma samples obtained after peripheral-vein infusion of the scAAV2/8-LP1-hFIXco vector were analyzed with the use of an enzyme-linked immunosorbent assay for the presence of AAV8-specific IgM antibodies, total IgG antibodies, and IgG isotypes. Data for the IgG1 isotype are shown. IgG2 and IgG4 levels did not increase above baseline (data not shown).
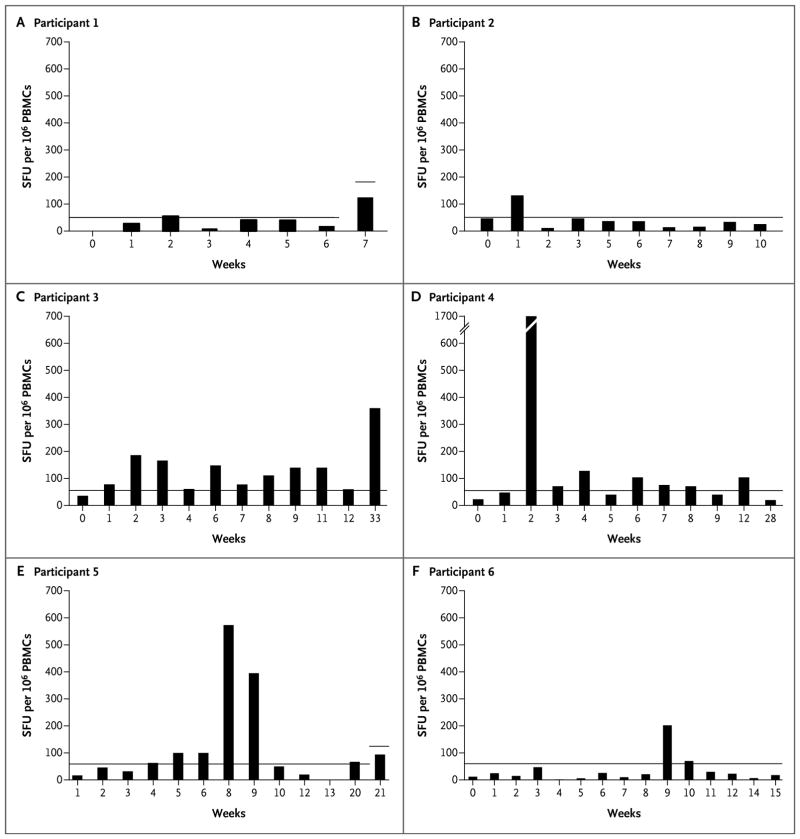
T-cell–mediated immune responses to the FIX transgene or putative products of translation from alternative open reading frames in the codon-optimized FIX complementary DNA17 were not detectable in any of the participants (data not shown), as determined with the use of an interferon-γ enzyme-linked immunosorbent spot (ELISPOT) assay. The same assay showed that participants in the low-dose cohort did not have significant AAV8-capsid–specific T-cell responses after gene transfer. At the intermediate-dose level, a significant increase in the frequency of AAV8-capsid–specific T cells was observed. In particular, Participant 3 had T-cell reactivity to the AAV capsid until week 33 after the gene transfer (Fig. 3), and Participant 4 had a very strong capsid-specific T-cell response at week 2 (about 1700 spot-forming units [SFU] per 1 million peripheral-blood mononuclear cells [PBMCs]). At the highest dose level, an increase in circulating AAV8-capsid–specific T cells was observed in Participant 5 starting at week 5 after transduction and reaching a peak of more than 500 SFU per 1 million PBMCs by week 8, which was concomitant with the increase in liver enzyme levels. In this participant, broad reactivity was observed across the six capsid peptide pools assessed (Fig. 3 in the Supplementary Appendix). By week 10, capsid-specific T cells were once again undetectable. No T-cell reactivity to the AAV capsid was detectable in PBMCs from Participant 6 until week 8 after the gene transfer, although this may have been due in part to reduced cell recovery and viability. At weeks 9 and 10, capsid-specific T cells became detectable, with levels of up to approximately 200 SFU per 1 million PBMCs, a finding that was concomitant with the minor increase in liver enzyme levels.
Figure 3. Cellular Immune Response in the Six Study Participants.
Results of the interferon-γ enzyme-linked immunosorbent spot assay for capsid-specific T-cell responses are shown as the maximum number of spot-forming units (SFU) per 1 million peripheral-blood mononuclear cells (PBMCs) in response to AAV8-capsid peptide pools. The horizontal lines in each panel represent the threshold for positivity, defined as three times the T-cell response for the negative control (medium only) and as at least 50 SFU per 1 million PBMCs. Liver-enzyme levels were elevated in Participant 5 at weeks 8 and 9 and in Participant 6 at week 9. In Participant 6, poor cell recovery and viability were noted at weeks 4 through 8.
DISCUSSION
The development and widespread use of clotting factor concentrates for the treatment of hemophilia in the early 1970s dramatically improved the life expectancy for patients with the disease. Subsequent development of recombinant clotting factor concentrates has improved their safety profile, but there remains a strong interest in treatment strategies that would eliminate the need for long-term intravenous infusions and that would be available to the hemophilia population throughout the world. This study documents a critical step toward that goal and shows that sustained therapeutic expression of a transferred factor IX gene can be achieved in humans. The increase in FIX levels in our study participants was roughly dose-dependent, with the high dose of the vector scAAV2/8-LP1-hFIX (2×1012 vg per kilogram) mediating peak expression at 8 to 12% of normal levels. After peripheral-vein administration of scAAV2/8-LP1-hFIXco, four of the six participants were able to stop using prophylaxis with FIX concentrate without having spontaneous hemorrhage, even when they undertook activities that had provoked bleeding in the past. For the other two participants, the interval between prophylactic FIX concentrate injections was extended, but prophylaxis was not completely discontinued. These two participants had severe preexisting hemophilic arthropathy. Maximal protection from bleeding in this subgroup may require higher endogenous expression of FIX.18
None of the participants had an immunologic response to the FIX transgene product. As reported in previous studies, the kinetics and magnitude of capsid-specific T-cell responses in PBMCs were highly variable in our study, with the highest number of capsid-specific T cells detected in Participant 4 at 2 weeks after gene transfer.8,19,20 There was no evidence of hepatocellular injury in this participant, suggesting that the T-cell repertoire in peripheral blood may be distinct from that in the liver or that levels of AAV8-capsid–antigen presentation on major-histocompatibility-complex class I hepatocytes were insufficient to drive the immune-mediated clearance of transduced cells. A scenario consistent with AAV8-capsid–specific T-cell–mediated destruction of transduced hepatocytes was observed 7 weeks after gene transfer in Participant 5. It is less certain whether the small increase in liver enzyme levels 9 weeks after gene transfer in Participant 6 was due to immune-mediated destruction of transduced hepatocytes. This increase occurred after a period of intense physical exertion, which is known to increase aminotransferase and lactate dehydrogenase levels.21,22 In both Participant 5 and Participant 6, the initiation of prednisolone therapy was followed by a return of the aminotransferase levels to baseline values and the disappearance of capsid-specific T cells from peripheral blood. Arguably, this could have happened without glucocorticoid treatment, since at 7 weeks after gene transfer, many of the transduced hepatocytes will have cleared the provoking AAV8 capsid peptide through natural proteolytic degradation and would therefore escape elimination by effector T cells.
The different outcomes of gene transfer in the participants in this study and in the previous trial of AAV2 hemophilia B gene therapy8 suggest that in addition to AAV vector dose, other factors such as individual variations in antigen processing and presentation, as well as exposure to wild-type AAV, seem to influence the type and magnitude of the immunological response to AAV-vector particles.23 It is very important to identify these immuno-modifying factors, as well as to determine the frequency of clinically significant elevations in aminotransferase levels after gene transfer. However, this will require a larger number of participants treated at the high-dose level. Currently, we do not think that routine use of a course of prophylactic glucocorticoids is indicated, since the incidence and timing of immune-mediated elevations in aminotransferase levels are uncertain, whereas early intervention, when needed, appears to be effective at curbing the immune response to transduced hepatocytes.
In summary, we have found that a single peripheral-vein infusion of our scAAV2/8-LP1-hFIXco vector consistently leads to long-term expression of the FIX transgene at therapeutic levels, without acute or long-lasting toxicity in patients with severe hemophilia B. Immune-mediated, AAV-capsid–induced elevations in aminotransferase levels remain a concern, but our data suggest that this process may be controlled by a short course of glucocorticoids, without loss of transgene expression. Follow-up of larger numbers of patients for longer periods of time is necessary to fully define the benefits and risks and to optimize dosing. However, this gene-therapy approach, even with the associated risk of transient hepatic dysfunction, has the potential to convert the severe bleeding phenotype into a mild form of the disease or to reverse it entirely.
Supplementary Material
Acknowledgments
Supported by grants from the NIHR (RP-PG-0310-1001), the Medical Research Council, the Katharine Dormandy Trust, the U.K. Department of Health, NHS Blood and Transplant, the NIHR Biomedical Research Centers (to University College London Hospital and University College London), the ASSISI Foundation of Memphis, the American Lebanese Syrian Associated Charities, the Howard Hughes Medical Institute, the National Heart, Lung, and Blood Institute (HL094396), the Royal Free Hospital Charity Special Trustees Fund 35, the Royal Free Hospital NHS Trust, and St. Jude Children’s Research Hospital.
We thank all the study participants for their interest and participation in this study and Patricia Lilley, Anja Griffioen, Cheng-Hock Toh, Paul Eddlemon, Alison Evans, Paul Lloyd-Evans, and Keith Williams for their help with trial-related activities, including vector importation and qualification, data entry, and regulatory affairs.
Footnotes
The views expressed in this article are those of the authors and not necessarily those of the National Health Service (NHS), the National Institute of Health Research (NIHR), or the Department of Health in the United Kingdom.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Nathwani AC, Tuddenham EG. Epidemiology of coagulation disorders. Baillieres Clin Haematol. 1992;5:383–439. doi: 10.1016/s0950-3536(11)80025-9. [DOI] [PubMed] [Google Scholar]
- 2.Darby SC, Kan SW, Spooner RJ, et al. Mortality rates, life expectancy, and causes of death in people with hemophilia A or B in the United Kingdom who were not infected with HIV. Blood. 2007;110:815–25. doi: 10.1182/blood-2006-10-050435. [DOI] [PubMed] [Google Scholar]
- 3.Nathwani AC, Davidoff AM, Tuddenham EG. Prospects for gene therapy of haemophilia. Haemophilia. 2004;10:309–18. doi: 10.1111/j.1365-2516.2004.00926.x. [DOI] [PubMed] [Google Scholar]
- 4.Herzog RW, Yang EY, Couto LB, et al. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999;5:56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- 5.Snyder RO, Miao C, Meuse L, et al. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat Med. 1999;5:64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- 6.Nathwani AC, Gray JT, Ng CY, et al. Self complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107:2653–61. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nathwani AC, Rosales C, McIntosh J, et al. Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins. Mol Ther. 2011;19:876–85. doi: 10.1038/mt.2010.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manno CS, Arruda VR, Pierce GF, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–7. doi: 10.1038/nm1358. [Erratum, Nat Med 2006;12:592.] [DOI] [PubMed] [Google Scholar]
- 9.Mingozzi F, Maus MV, Hui DJ, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13:419–22. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 10.Nathwani AC, Gray JT, McIntosh J, et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–21. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:11854–9. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381–90. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allay JA, Sleep S, Long S, et al. Good Manufacturing Practice production of self-complementary serotype 8 adeno-associated viral vector for a hemophilia B clinical trial. Hum Gene Ther. 2011;22:595–604. doi: 10.1089/hum.2010.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nathwani AC, Tuddenham E, Rosales C, et al. Early clinical trial results following administration of a low dose of a novel self complementary adeno-associated viral vector encoding human Factor IX in two subjects with severe hemophilia B. Blood. 2010;116:114. abstract. [Google Scholar]
- 15.Fagone P, Wright JF, Nathwani AC, Nienhuis AW, Davidoff AM, Gray JT. Systemic errors in quantitative polymerase chain reaction titration of self-complementary adeno-associated viral vectors and improved alternative methods. Hum Gene Ther. 2011 September 23; doi: 10.1089/hgtb.2011.104. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasper CK, Pool JG. Letter: measurement of mild factor VIII inhibitors in Bethesda units. Thromb Diath Haemorrh. 1975;34:875–6. [PubMed] [Google Scholar]
- 17.Li C, Goudy K, Hirsch M, et al. Cellular immune response to cryptic epitopes during therapeutic gene transfer. Proc Natl Acad Sci U S A. 2009;106:10770–4. doi: 10.1073/pnas.0902269106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.den Uijl IE, Fischer K, Van Der Bom JG, Grobbee DE, Rosendaal FR, Plug I. Analysis of low frequency bleeding data: the association of joint bleeds according to baseline FVIII activity levels. Haemophilia. 2011;17:41–4. doi: 10.1111/j.1365-2516.2010.02383.x. [DOI] [PubMed] [Google Scholar]
- 19.Brantly ML, Chulay JD, Wang L, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci U S A. 2009;106:16363–8. doi: 10.1073/pnas.0904514106. [Erratum, Proc Natl Acad Sci U S A 2009;106: 17606.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mendell JR, Campbell K, Rodino- Klapac L, et al. Dystrophin immunity in Duchenne’s muscular dystrophy. N Engl J Med. 2010;363:1429–37. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith JE, Garbutt G, Lopes P, Pedoe DT. Effects of prolonged strenuous exercise (marathon running) on biochemical and haematological markers used in the investigation of patients in the emergency department. Br J Sports Med. 2004;38:292–4. doi: 10.1136/bjsm.2002.002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pettersson J, Hindorf U, Persson P, et al. Muscular exercise can cause highly pathological liver function tests in healthy men. Br J Clin Pharmacol. 2008;65:253–9. doi: 10.1111/j.1365-2125.2007.03001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mingozzi F, Meulenberg JJ, Hui DJ, et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114:2077–86. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.