Abstract
Near-infrared (NIR) absorbing dyes represent an intriguing avenue for extracting biological information from living subjects since they can be monitored with noninvasive optical imaging techniques. We designed and synthesized an imaging agent which contains a NIR fluorochrome (IR780) and peptidyl fluoromethyl ketone (FMK) for caspase-9 imaging of cells undergoing apoptosis. The IR780-FMK fluorescent probe had a Strokes shift of 79 nm and quantum yield 0.75. Prostate cancer DU145 cells undergoing apoptosis were successfully imaged using as little as 0.1 μM of IR780-FMK.
Keywords: Cell death, cancer, screening, apoptosis, fluoromethyl ketone
1. Introduction
Apoptosis is the process of programmed cell death by which multicellular organisms regulate cell number and maintain homeostasis. Within the series of biochemical events involved in apoptosis, the activation of caspases is recognized a critical marker. Apoptosis can be triggered by extrinsic or intrinsic signals such as physiological activators (TNF family, neurotransmitters, calcium, glucocorticoids), damage-related inducers (heat shock, viral infection, tumor suppressors p53, oxidants, free radicals), therapy-associated agents (chemotherapeutic agents, gamma radiation and UV radiation) and toxins (ethanol, β-amyloid peptide) (1-5). Defective apoptosis processes can lead to severe pathological disorders, for example, downregulated apoptosis is involved in autoimmune diseases, cancer and viral infections (6,7); abnormal upregulation of apoptosis is associated with AIDS, neurodegenerative disorders and ischemic injury (7,8). Therefore, the development of caspase inhibitors could be novel treatments for a variety apoptosis associated diseases.
A number of peptidyl caspase inhibitors have been developed including peptidyl chloromethyl ketones, peptidyl fluoromethyl ketones and peptidyl aldehydes. The chloromethyl ketones have strong electrophilicity and are not stable to high concentrations of thiol which limits their use in vivo (9). The aldehyde based inhibitors are poorly cell permeable and are not effective caspase inhibitors under concentrations of 1 μM (10). The fluoromethyl ketone (FMK) inhibitors, which are more stable in vivo and cell permeable (10,11), act as broad-spectrum, irreversible caspase inhibitors (12) with no added cytotoxic effects. Inhibitors synthesized with a benzyloxycarbonyl group (such as Boc- or Z-) at the N-terminus and O-methyl side chains such as Z-Val-Ala-Asp(OMe)-FMK display improved cellular permeability facilitating their use in both in vitro cell culture and in vivo animal studies (13,14).
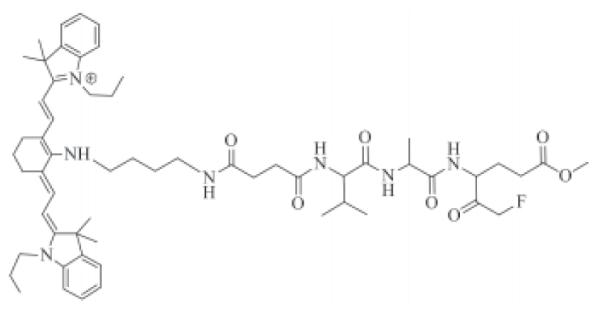
In this work, we synthesized an imaging agent containing a NIR fluorochrome IR780 with high quantum yield and the cell permeable fluoromethyl ketone Z-Val-Ala-Glu(OMe). The structure of it is shown in Figure 1. The agent irreversibly binds caspase-9 in cells undergoing apoptosis and can be used to monitor live cells undergoing apoptosis. Fluorochromes with absorption and emission maxima between 650 and 900 nm are within the NIR range and are ideally suited for imaging in tissue due to the minimal optical absorption from hemoglobin, water, and lipids over this range (15-17). This is a significant benefit over current commerical cell caspase imaging agents, such as FLIVO™, which use fluorophores wavelengths less than 600 nm, e.g. fluorescein and rhodamine, where there is significantly more tissue autofluorescence and optical attenuation.
Figure 1. Structure of cell permeable fluoromethyl ketone Z-Val-Ala-Glu (OMe) (IR780-linker-Val-Ala-Glu(OMe)-FMK).
2. Materials and Methods
2.1. Chemicals
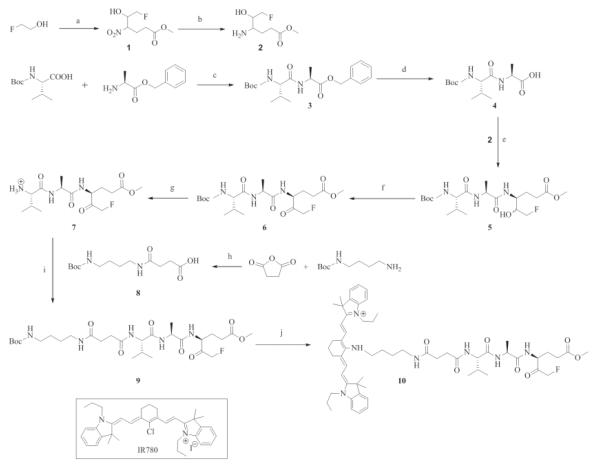
All chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA) and used as received unless stated otherwise. Solvents were distilled under argon immediately before use. NMR spectra were taken on a 400-MHz Bruker with the TMS peak as internal reference. Mass spectra were run in the electrospray ionization mass spectrometry (ESI-MS) mode or atmospheric pressure chemical ionization (APCI-MS) mode. Reactions were carried out under dry argon with flame-dried glassware. Dichloromethane (DCM), N,N-dimethylformamide (DMF) and triethylamine (TEA) were freshly distilled from CaH2, and tetrahydrafuran (THF) was freshly distilled from sodium benzophenone. The NIR fluorescent imaging agent 4′ C-[4-[2-(fluoromethylketone-Ala-Val-NH)carbonyl]ethyl]carbonyl]amino]butyl]amino-IR780 [IR780-linker-Val-Ala-Glu(OMe)-FMK] (compound 10) was synthesized in 11 steps (Figure 2; Appendix).
Figure 2. Synthetic scheme of IR780-linker-Val-Ala-Glu(OMe)-FMK imaging agent.
Reagents: a) i, Swern oxidation; ii, methyl 4-nitrobutyrate, TEA; b) 10% Pd/C, H2, in MeOH; c) TEA, HBTU, DMF; d) 10% Pd/C, H2, in THF; e) EDCI, DMAP, THF; f) Dess-Martin periodinane, in DCM; g) 4M HCl/EtOAc; h) 1,4-dioxan, DMAP, reflux; i) EDCI, HOBt, DMAP, THF; j) i, 4M HCl/EtOAc; ii, IR 780, TEA, DMF.
2.2. Spectral properties of IR780-linker-Val-Ala-Glu(OMe)-FMK
The fluorescent spectrum of a 1-μM solution of IR780-linker-Val-Ala-Glu(OMe)-FMK in DMSO was recorded using a Shimadzu RF-5301 PC spectrofluorophotometer. The Stokes shift was determined by the difference in wavelength between excitation and emission maxima. The quantum yield was measured according to a reported protocol (18,19) using cresyl violet as a reference.
2.3. Cell imaging for apoptosis
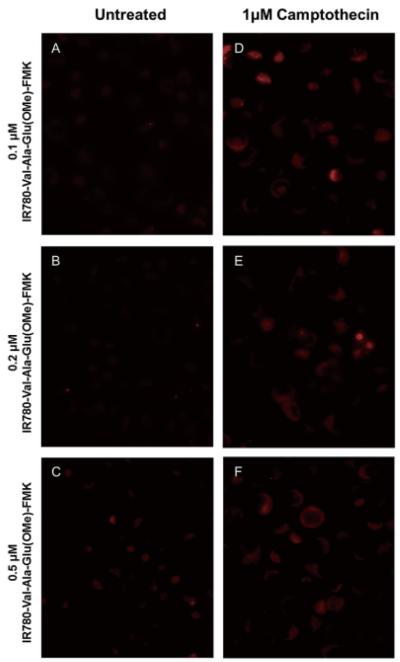
The DU145 cell line was cultured in Eagle’s Minimum Essential Medium (MEM) supplemented with 10% fetal bovine serum and 1% l-glutamine. Cells were seeded into an 8-chamber culture slide at a density of 40,000 per well, and after overnight incubation, they were treated with or without 1 μM of camptothecin. After 24 h, IR780-linker-Val-Ala-Glu(OMe)-FMK (compound 10) or IR780 iodide at different concentrations (0.1, 0.2, 0.5 and 1.0 μM) was applied, and the cells were incubated at 37°C for 30 min. Fresh MEM medium was exchanged once every hour for 3 h. The cells were washed once with sterile phosphate buffered saline (PBS) for fluorescent imaging using a Nikon Eclipse 80i microscope coupled with a Hamamatsu ORCA-ER digital camera. The fluorescent images were analyzed using the MetaMorph software.
For the nuclear counterstain, DU145 cells were seeded onto a 12-mm coverslip in 6-well plate at a density of 1.5 × 105 cells per well for overnight. After cells were treated with or without 1 μM of camptothecin for 24 h, IR780-linker-Val-Ala-Glu(OMe)-FMK at concentrations of 0.1 or 0.5 μM was incubated with cells for 30 min at 37°C. Then the cells were washed twice with sterile PBS and subsequently incubated with 5-μg/mL DAPI in MEM for at 37°C for 5 min. Finally, the cells were washed with sterile PBS twice and imaged using the system described above.
The acute toxicity of the IR780-linker-Val-Ala-Glu(OMe)-FMK in cell culture was determined by incubating the cells with the imaging agent for 30 min and then measuring the ratio of dead to live (attached cells). DU145 cells had been seeded at 1.5 × 105 cells/well in a 6-well plate and allowed to attach overnight before addition of the imaging agent.
2.4. Western blotting
DU145 cells were treated with 1 μM of camptothecin as described in the previous section, and cell lysates were prepared with a solution of 1% Nonidet P-40, 50-mM Tris, 150-mM NaCl, APL protease inhibitors, and PMSF adjusted to pH 7.4. After treatment, floating cells were collected by aspiration, and attached cells were collected by trypsinization followed by centrifugation at 350 × g for 5 min. Cell pellets were incubated with lysis buffer on ice for 20 min and then centrifuged at 3,000 × g for 20 min at 4°C. The protein concentration was determined by BCA assay. Samples (40 μg of protein/sample) were separated by 10% polyacrylaminde gel and then transferred onto 0.45-μm nitrocellulose membrane, which was then blocked with 5% (w/v) non-fat milk in TBS and 0.1% Tween 20 (TBS/T). After washing with TBS/T, the nitrocellulose membrane was incubated with the anti-caspase-9 polyclonal antibody (diluted 1:1,000, Cell Signaling Technology #9502, Danvers, MA, USA) overnight at 4°C, followed by horseradish peroxidase-conjugated secondary antibody (diluted 1:2,000, Santa Cruz Biotechnology #sc-2004, Santa Cruz, CA, USA) for 1 h at ambient temperature. Proteins were visualized with the Western Lightening® ECL detection system from Perkin Elmer.
2.5. TUNEL assay
DU145 cells were treated with 1 μM of camptothecin as described in section 2.3 for 24 h and then were fixed in freshly prepared 4% paraformaldehyde in PBS, pH 7.4, for 1 h at ambient temperature. After washing with PBS, cells were permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice. Cells were incubated with TUNEL reaction mixture (Roche Diagnostics, Indianapolis, IN, USA) at 37°C in a humidified atmosphere for 1 h. Samples were directly imaged under a Nikon Eclipse 80i microscope coupled with a Hamamatsu ORCA-ER digital camera at 465-495 nm excitation and 515-555 nm emission.
3. Results and Discussion
3.1. Synthesis of the NIR fluorescent imaging agent [IR780-linker-Val-Ala-Glu(OMe)-FMK]
We successfully synthesized the NIR fluorescent imaging agent [IR780-linker-Val-Ala-Glu(OMe)-FMK] in 11 steps with an overall yield 0.75%. The structure of each compound was determined by 1H-NMR or together with APCI-MS (ESI-MS). Optically pure starting materials were used in the synthesis; however, isomers may have been introduced during the synthesis at the three chiral centers. During the purification of compound 5, only the major compound was collected, a pair of enantiomers, which resulted in the low yield of 32%. The enantiomers were not separated further before proceeding.
3.2. Absorption and emission spectrum of IR780-linker-Val-Ala-Glu(OMe)-FMK
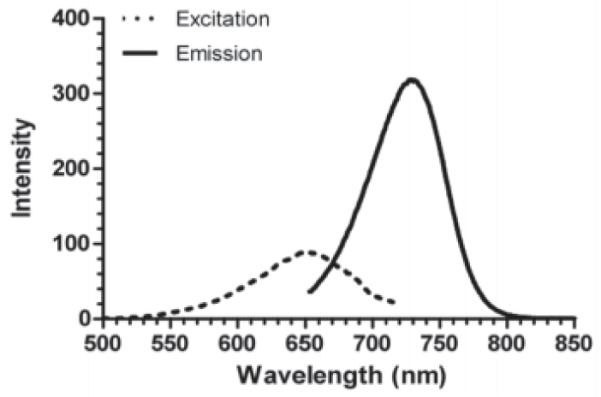
The structure of IR780-linker-Val-Ala-Glu(OMe)-FMK consists of three parts: the IR780 fluorophore, the linker, and the fluoromethyl ketone of the tripeptides valine, alanine, and O-methyle-glutamic acid [Val-Ala-Glu(OMe)-FMK] as the reactive part to the caspase-9. IR780-linker-Val-Ala-Glu(OMe)-FMK had a maximum excitation at 650 nm (Figure 3) and emission at 729 nm respectively. By contrast, the λmax of unconjugated IR780 dye was at 685 and 760 nm for excitation and emission, respectively. The fluorescence quantum yield (Φ) was calculated as 0.75, which was determined in methanol with reference compound cresyl violet (Φ = 0.54 in methanol, (20)). The quantum yield is high enough to be employed as a fluorescent label in cell imaging studies.
Figure 3. Excitation (dashed) and emission (solid) spectrum of IR780-linker-Val-Ala-Glu(OMe)-FMK.
3.3. Camptothecin induces apoptosis via activation of caspases-9
The caspase proteases can be activate either though the death signal-induced or stress-induced pathways. Caspase-9 is an activator of apoptosis in the mitochondrion-mediated or stress-induced pathway, wherein it subsequently activates caspases-3/6/7. Caspase-9 can be bypassed in the death signal-induced pathway when death receptors (e.g. TNF receptor) activate caspases-3/6/7 directly via caspase-10 (21). Camptothecin inhibits DNA synthesis and was expected to induce apoptosis via the caspase-9 pathway. After treatment with camptothecin, TUNEL assay indicated DNA fragmentation characteristic of apoptosis (Figure 4) and immunoblotting found cleaved caspase-9 fragments (Figure 5), which are the target of the IR780-linker-Val-Ala-Glu(OMe)-FMK imaging agent.
Figure 4. TUNEL assay.
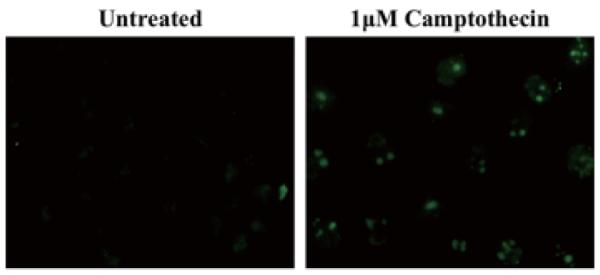
Apoptotic DU145 cells that have been treated with 1 μM camptothecin for 24 h were labeled by TUNEL staining of the DNA fragments. In comparison, untreated cells did not undergo apoptosis.
Figure 5. Western blot analysis of caspase-9 cleavage in untreated DU145 cells, or cells treated with 1-μM camptothecin for 24 h.

a, full length; b, cleaved caspase-9 (37 kDa); c, cleaved caspase-9 (35 kDa).
The normal DU145 cells had no uptake of IR780 dye in the tested concentration range (0.1-1 μM). The unconjugated IR780 dye is highly polar and cell membrane impregnable, and we did not observe nonspecific uptake of the unconjugated IR780 dye in normal or apoptotic DU145 cells (images not shown). DU145 cells undergoing apoptosis showed fluorescent signals, because IR780-linker-Val-Ala-Glu(OMe)-FMK bound to the cleaved caspase-9 induced by camptothecin, which caused it to be retained within the cells (Figures 6D, 6E, and 6F). Furthermore, counter-staining of the cells with DAPI confirmed that the IR780-linker-Val-Ala-Glu(OMe)-FMK was confined to the cytoplasm (Figure 7). The inhibition and binding of IR780-linker-Val-Ala-Glu(OMe)-FMK to cleaved caspase-9 is a result of the peptide sequence recognition and the nucleophilic substitution of the fluoro- by a sulfhydryl group of the cysteine protease (22). By contrast, after untreated DU145 cells (~ 90% cell confluence) were incubated with IR780-linker-Val-Ala-Glu(OMe)-FMK, there was no accumulation of fluorescence either on the cell membrane or inside the cells at 0.1- or 0.2-μM IR780-Val-Ala-Glu(OMe)-FMK concentrations (Figures 6A and 6B). However, there was a small amount of fluorescence detected when the cells were incubated with 0.5-μM IR780-linker-Val-Ala-Glu(OMe)-FMK (Figure 6C). This was probably due to the high cell confluence (~ 90%) used for the imaging, as this non-specific binding was not observed in the cells at 60% confluency used for the DAPI counterstaining (Figure 7), and the imaging agent alone was not toxic. When the cells were incubated with the imaging agent for 30 min, there were no statistically significant differences in cell death between 0, 0.1 and 0.5 μM of agent, which resulted in 3.17 ± 0.85, 3.01 ± 1.17, and 3.34 ± 1.01% dead cells, respectively.
Figure 6. DU145 cell imaging after treatment with IR780-linker-Val-Ala-Glu(OMe)-FMK at concentrations of: A) 0.1 μM; B) 0.2 μM; C) 0.5 μM; IR780-linker-Val-Ala-Glu(OMe)-FMK with 1 μM of camptothecin at concentrations of: D) 0.1 μM; E) 0.2 μM; F) 0.5 μM.
Figure 7. Fluorescent microscopic images of IR780-linker-Val-Ala-Glu(OMe)-FMK-stained DU145 cells; DAPI counter stain.
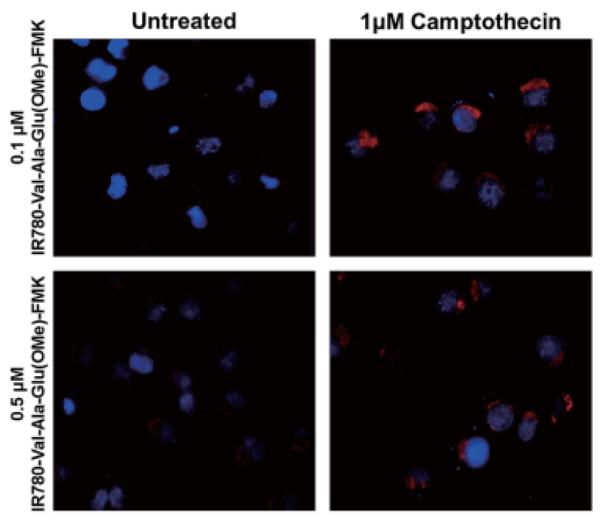
The IR780-linker-Val-Ala-Glu(OMe)-FMK fluorescent label (red) is distributed within the cytoplasm of the cells.
4. Conclusion
The synthesis of a new NIR fluorescent imaging agent [IR780-Val-Ala-Glu(OMe)-FMK] for caspase-9 was successfully accomplished in 11 steps (0.75% overall yield), which has a maxima excitation at 650 nm and emission at 729 nm. The in vitro cell imaging demonstrated the sensitivity of this imaging agent for caspase-9-mediated cell apoptosis. At high confluences and dye concentrations, the dye uptake lost specificity. The NIR fluorescent probe could be ideally suited for in vivo imaging to monitor tumor cell progress and cell apoptosis induced by chemotherapeutics.
Acknowledgements
This work was supported by awards from the National Institutes of Health (R21 CA132033) and the American Cancer Society (RSG-08-133-01-CDD). In addition, YL was partially supported by a generous exchange fellowship from the government of the Peopleșs Republic of China. QY and SD contributed equally to this work.
Appendix
Steps of synthesis of IR780-linker-Val-Ala-Glu(OMe)-FMK
6-Fluoro-5-hydroxy-4-nitrohexanoic acid methyl ester (compound 1)
Anhydrous DMSO (1.4 mL, 19 mmol) was added dropwise to the solution of oxalyl chloride (0.9 mL, 9.6 mmol) in DCM (5 mL) at −78°C. To this solution was added 2-fluoroethanol (0.44 mL, 7.5 mmol) in DCM (2 mL). Fifteen minutes later, the reaction mixture was diluted with DCM (60 mL), followed by addition of TEA (4.4 mL, 31 mmol). The mixture was allowed to warm up to 0°C and stirred for 2 h followed by the addition of methyl 4-nitrobutyrate (0.93 g, 6.3 mmol) in DCM (5 mL). The mixture was stirred at 0°C for 3 h and then ambient temperature (ca. 20°C) overnight (23). The solution was concentrated and washed with ethyl acetate (EtOAc). Removal of the solvent followed by purification by silica gel column (hexanes:EtOAc = 5:1) gave the desired compound (1.0 g, 76%) as a yellow viscous oil. 1H-NMR (400 MHz, CDCl3): 2.40-2.45 (m, 4H), 3.69 (s, 3H), 4.20 (brs) and 4.25 (brs, 1H), 4.40-4.80 (m, 3H), 5.02 (brs) and 5.19 (brs, 1H).
4-Amino-6-fluoro-5-hydroxyhexanoic acid methyl ester (compound 2)
A solution of 6-fluoro-5-hydroxy-4-nitrohexanoic acid methyl ester (compound 1, 1.15 g, 5.5 mmol) in methanol (20 mL) and acetic acid (0.5 mL) was hydrogenated with H2 (40-45 psi) at ambient temperature for 5 h using 10% Pd/C (0.5 g) catalyst. The Pd/C was filtered off and the solvent was evaporated under reduced pressure. The desired compound was obtained as colorless viscous oil (0.92 g, yield 87%), which was used for the next step without further purification. 1H-NMR (400 MHz, MeOD): 1.65-2.00 (m, 2H), 2.90-3.42 (m, 2H), 3.65 (s, 3H), 3.66-3.3.95 (m, 2H), 4.41-4.55 (m, 2H).
Boc-Val-Ala-OBn (compound 3)
Boc-Val (3.0 g, 13.8 mmol), alanine benzyl ester hydrochloride salt (3.3 g, 15.3 mmol) and O-benzotriazole-N,N,N’,N’-tetramethyl-uronium-hexafluoro-phosphate (HBTU, 5.76 g, 15.3 mmol) were dissolved in DMF (100 mL) followed by addition of TEA (4.3 mL). The reaction was stirred at ambient temperature for 24 h and then diluted with saturated citric acid (100 mL). Then the mixture was washed with EtOAc (100 mL × 2), and the combined organic layers were washed with brine, saturated NaHCO3 and brine, respectively, and then dried over Na2SO4. Removal of the solvent under reduced pressure followed by purification on silica gel (EtOAc:hexanes = 1:3) gave the desired compound (4.48 g, 86%) as a white solid. 1H-NMR (400 MHz, CDCl3): 0.93 (d, J = 7.0 Hz, 3H), 0.97 (d, J = 6.8 Hz, 3H), 1.44 (d, J = 7.2 Hz, 3H), 1.46 (s, 9H), 2.08-2.20 (m, 1H), 3.94 (t, J = 7.6 Hz, 1H), 4.62-4.69 (m, 1H), 5.08 (d, J = 8.8, 1H), 5.19 (dd, J = 12.3, 8.2 Hz, 2H), 6.44 (d, J = 5.8 Hz, 1H), 7.32-7.44 (m, 5H).
Boc-Val-Ala-COOH (compound 4)
Boc-Val-Ala-OBn (Compound 3, 5.0 g, 12.7 mmol) was dissolved in THF (100 mL), and the solution was hydrogenated with H2 (1 atm) using 10% Pd/C catalyst (0.50 g) for 24 h. The solid Pd/C was removed by filtration, and the solvent was evaporated under reduced pressure. The desired compound was obtained as a white solid (3.4 g, 82%). 1H-NMR (400 MHz, CDCl3): 0.92 (d, J = 6.7 Hz, 3H), 0.96 (d, J = 6.7 Hz, 3H), 1.42-1.47 (m, 12H, overlap), 2.00-2.11 (m, 1H), 4.01 (t, J = 8.0 Hz, 1H), 4.57 (t, J = 7.0 Hz, 1H), 5.51 (brs, 1H), 7.04 (brs, 1H), 9.66 (brs, 1H).
4-(Boc-Val-Ala-amido)-6-fluoro-5-hydroxylhexa-noic acid methyl ester (compound 5)
Boc-Val-Ala-COOH (compound 4, 1.0 g, 3.5 mmol), 4-dimethyla-minopyridine (DMAP, 0.25 g, 1.9 mmol), and N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydro-chloride (EDAC, 0.74 g, 3.9 mmol) were dissolved in anhydrous THF (10 mL) at ambient temperature. After 10 min a solution of 4-amino-6-fluoro-5-hydroxyhexanoic acid methyl ester (compound 2, 0.69 g, 3.9 mmol) in anhydrous THF (10 mL) was added directly to the above solution, and the reaction was stirred at ambient temperature overnight. The solvent was removed under reduced pressure, and the residue was dissolved in EtOAc, washed with saturated NaHCO3, brine, and citric acid, respectively. The organic layer was dried with Na2SO4, and the product was purified over silica gel (EtOAc:hexanes = 3:1) to give the desired compound as a white solid (0.5 g, 32%). APCI -MS: [M + 1] = 450.3, [M + 1-C4 H8] = 394.2, [M-Boc] = 350.2. H-NMR (400 MHz, CDCl3): 0.95 (d, J = 7.0 Hz, 3H), 0.98 (d, J = 6.6 Hz, 3H), 1.40 (d, J = 7.6 Hz, 3H), 1.45 (s, 9H), 1.85-1.97 (m, 2H), 2.08-2.18 (m, 1H), 2.41 (t, J = 7.2 Hz, 2H), 3.69 (s, 3H), 3.83-3.95 (m, 1H), 3.96-4.10 (m, 1H), 4.29-4.55 (m, 3H), 6.54 (brs, 1H), 6.58 (brs, 1H).
4-(Boc-Val-Ala-amido)-6-fluoro-5-oxohexanoic acid methyl ester (compound 6)
A solution of compound 5 (0.898 g, 2.0 mmol) in DCM (20 mL) was treated with Dess-Martin periodinane solution (20 mL, 0.3 M in DCM). The reaction mixture was stirred at ambient temperature for 12 h. The solvent was removed under reduced pressure, and the crude product was purified by silica gel chromatography (EtOAc:hexanes = 1:1) to give the desired compound as a white solid (0.749 g, 81%). APCI-MS: [M + 1] = 448.3, [M + 1-C4H8] = 392.2, [M-Boc] = 348.2. 1H-NMR (400 MHz, CDCl3): 0.95 (d, J = 7.0 Hz, 3H), 0.99 (d, J = 6.6 Hz, 3H), 1.40 (d, J = 7.6 Hz, 3H), 1.45 (s, 9H), 1.88-1.96 (m, 2H), 2.12-2.22 (m, 1H), 2.43 (t, J = 7.2 Hz, 2H), 3.68 (s, 3H), 3.87-3.99 (m, 1H), 4.46-4.59 (m, 1H), 5.09-5.21 (m, 2H), 6.76 (brs, 1H), 7.37 (brs, 1H).
4-(Val-Ala-amido)-6-fluoro-5-oxohexanoic acid methyl ester [Val-Ala-Glu(OMe), compound 7]
A solution of 4-M HCl in anhydrous EtOAc (20 mL) was cooled to 0°C, 4-(Boc-Val-Ala-amido)-6-fluoro-5-oxohexanoic acid methyl ester (compound 6, 0.749 g, 1.62 mmol) was added, and the mixture was stirred at ambient temperature overnight. Collection of the precipitated solid followed by washing with EtOAc (50 mL) gave a pale yellow solid (0.564 g, quantitative yield), which was used for the next step without further purification.
4-(4-(Tert-butoxycarbonyl)ethylamino)-4-oxobutanoic acid (Boc-linker, compound 8)
Mono-Boc-protected butane-1,4-diamine (2.82 g, 15 mmol; prepared according to previous reports (24,25)) in 10 mL of dioxane was added slowly to a solution of succinic anhydride (1.5 g, 15 mmol) in 10 mL of dioxane and then stirred at 80°C for 3 h. Removal of the solvent followed by purification of the residue through silica gel chromatography (EtOAc:hexanes:acetic acid = 50:5:1) gave the desired compound as a white solid (2.64 g, 63%). 1H-NMR (400 MHz, MeOD): 1.46 (s, 9H), 1.46-1.57 (m, 4H), 2.39-2.50 (m, 2H), 2.55-2.67 (m, 2H), 3.06 (t, J = 4.6 Hz, 2H), 3.19 (t, J = 6.5 Hz, 2H), 6.53 (brs, 1H), 7.90 (brs, 1H), 12.10 (brs, 1H).
Boc-linker-Val-Ala-Glu(OMe)-FMK (compound 9)
To a solution of compound 8 (1.44 g, 5.0 mmol) in THF (30 mL) was added N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride (EDCI, 0.96 g, 5.0 mmol), 1-hydroxybenzotriazole (HOBt; 0.68 g, 5.0 mmol), and 4-dimethylaminopyridine (DMAP) (0.31 g, 2.5 mmol). The mixture was stirred for 10 min at ambient temperature followed by the addition of compound 7 (2.24 g, 5.0 mmol) in THF (15 mL). The mixture was stirred at ambient temperature overnight. After removing the solvent under reduced pressure, the residue was diluted with EtOAc (100 mL) and washed with brine (50 mL). The organic layer was dried over sodium sulfate the solvent was removed under reduced pressure. Purification of the residue by silica gel chromatography (EtOAc:MeOH = 20:1) gave the desired compound as a yellow solid (1.17 g, 38%). APCI-MS: [M + 1] = 618.7, [M-Boc] = 518.5. 1H-NMR (400 MHz, CDCl3): 0.95 (d, J = 7.0 Hz, 3H), 0.99 (d, J = 6.6 Hz, 3H), 1.40 (d, J = 7.6 Hz, 3H), 1.46 (s, 9H), 1.47-1.55 (m, 4H), 1.67-1.78 (m, 2H), 2.14-2.26 (m, 1H), 2.39-2.50 (m, 4H), 2.55-2.68 (m, 2H), 3.07 (t, J = 4.7 Hz, 2H), 3.20 (t, J = 6.4, 2H), 3.68 (s, 3H), 4.08-4.20 (m, 1H), 4.45-4.58 (m, 1H), 4.48-4.59 (m, 1H), 4.99-5.30 (m, 2H), 6.19 (brs, 1H), 6.50 (brs, 1H), 7.45 (brs, 1H), 7.53 (brs, 1H), 7.56 (brs, 1H).
IR780-linker-Val-Ala-Glu(OMe)-FMK (compound 10)
To a solution of Boc-linker-Val-Ala-Glu(OMe)-FMK (compound 9, 0.46 g, 0.75 mmol) in dry DMF (5 mL) was added a solution of 4-M HCl/EtOAc (10 mL) at 0°C. The mixture was stirred for 12 h at ambient temperature, followed by the addition of TEA (55 μL, 0.75 mmol) and IR780 iodide (0.1 g, 0.15 mmol) in dry DMF (5 mL) (26,27). The mixture was then heated to 85°C and stirred continuously for 24 h in the dark. Removal of the solvent followed by purification of the obtained residue through silica gel chromatography (CHCl3:MeOH = 50:1) gave the desired compound as a blue solid (0.038 g, 25%). ESI-MS: [M+ + 1] = 1022.3. 1H-NMR (400 MHz, DMSO-d6): 0.95 (d, J = 7.0 Hz, 3H), 0.98 (d, J = 6.6 Hz, 3H), 1.03-1.07 (m, 6H), 1.32-1.41 (m, 2H), 1.42-1.51 (m, 5H), 1.52-1.57 (m, 4H), 1.59-1.70 (m, 15H), 1.71-1.76 (m, 4H), 2.07-2.15 (m, 2H), 2.18-2.27 (m, 1H), 2.34 (t, J = 7.2 Hz, 2H), 2.49 (t, J = 6.8 Hz, 2H), 2.57 (t, J = 6.8 Hz, 2H), 2.83 (t, J = 7.5 Hz, 4H), 2.89 (t, J = 6.8 Hz, 2H), 3.05 (t, J = 6.8 Hz, 2H), 3.64 (s, 3H), 3.79 (t, J = 6.8, 2H), 3.94 (t, J = 7.2 Hz, 2H), 4.10-4.20 (m, 1H), 4.26-4.37(m, 1H), 4.48-4.56 (m, 1H), 5.13-5.25 (m, 2H), 5.86 (d, J = 13.0 Hz, 2H), 7.06-7.15 (m, 4H), 7.29-7.36 (m, 1H), 7.62-7.71 (m, 1H), 7.77 (d, J = 13.0 Hz, 2H), 8.13-8.25 (m, 2H).
References
- 1.Bosman FT, Visser BC, van Oeveren J. Apoptosis: Pathophysiology of programmed cell death. Pathol Res Pract. 1996;192:676–683. doi: 10.1016/S0344-0338(96)80089-6. [DOI] [PubMed] [Google Scholar]
- 2.Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994;15:7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 3.Reed JC. Bcl-2 and the regulation of programmed cell-death. J Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sachs L, Lotem J. Control of programmed cell-death in normal and leukemic-cells: New implications for therapy. Blood. 1993;82:15–21. [PubMed] [Google Scholar]
- 5.Vaux DL, Weissman IL, Kim SK. Prevention of programmed cell-death in caenorhabditis-elegans by human bcl-2. Science. 1992;258:1955–1957. doi: 10.1126/science.1470921. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 7.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 8.Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- 9.Smith RE, Rasnick D, Burdick CO, Cho K, Rose JC, Vahratian A. Visualization of time-dependent inactivation of human-tumor cathepsin-b isozymes by a peptidyl fluoromethyl ketone using a fluorescent print technique. Anticancer Res. 1988;8:525–529. [PubMed] [Google Scholar]
- 10.Ekert PG, Silke J, Vaux DL. Caspase inhibitors. Cell Death Differ. 1999;6:1081–1086. doi: 10.1038/sj.cdd.4400594. [DOI] [PubMed] [Google Scholar]
- 11.Yang W, Guastella J, Huang JC, Wang Y, Zhang L, Xue D, Tran M, Woodward R, Kasibhatla S, Tseng B, Drewe J, Cai SX. MX1013, a dipeptide caspase inhibitor with potent in vivo antiapoptotic activity. Br J Pharmacol. 2003;140:402–412. doi: 10.1038/sj.bjp.0705450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadhukhan R, Leone JW, Lull J, Wang Z, Kletzien RF, Heinrikson RL, Tomasselli AG. An efficient method to express and refold a truncated human procaspase-9: A caspase with activity toward Glu-X bonds. Protein Expr Purif. 2006;46:299–308. doi: 10.1016/j.pep.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Guo YP, Kyprianou N. Restoration of transforming growth factor beta signaling pathway in human prostate cancer cells suppresses tumorigenicity via induction of caspase-1-mediated apoptosis. Cancer Research. 1999;59:1366–1371. [PubMed] [Google Scholar]
- 14.Schlegel J, Peters I, Orrenius S, Miller DK, Thornberry NA, Yamin TT, Nicholson DW. CPP32/apopain is a key interleukin 1 beta converting enzyme-like protease involved in Fas-mediated apoptosis. J Biol Chem. 1996;271:1841–1844. doi: 10.1074/jbc.271.4.1841. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Ke S, Kwon S, Yallampalli S, Cameron AG, Adams KE, Mawad ME, Sevick-Muraca EM. A new optical and nuclear dual-labeled imaging agent targeting interleukin 11 receptor alpha-chain. Bioconjug Chem. 2007;18:397–402. doi: 10.1021/bc0602679. [DOI] [PubMed] [Google Scholar]
- 16.Hilderbrand SA, Kelly KA, Weissleder R, Tung CH. Monofunctional near-infrared fluorochromes for imaging applications. Bioconjug Chem. 2005;16:1275–1281. doi: 10.1021/bc0501799. [DOI] [PubMed] [Google Scholar]
- 17.Bouteiller C, Clavé G, Bernardin A, Chipon B, Massonneau M, Renard PY, Romieu A. Novel water-soluble near-infrared cyanine dyes: Synthesis, spectral properties, and use in the preparation of internally quenched fluorescent probes. Bioconjug Chem. 2007;18:1303–1317. doi: 10.1021/bc0700281. [DOI] [PubMed] [Google Scholar]
- 18.Williams ATR, Winfield SA, Miller JN. Relative fluorescence quantum yields using a computer-controlled luminescence spectrometer. Analyst. 1983;108:1067–1071. [Google Scholar]
- 19.Dhami S, De Mello AJ, Rumbles G, Bishop SM, Phillips D, Beeby A. Phthalocyanine fluorescence at high-concentration - dimers or reabsorption effect? Photochem Photobiol. 1995;61:341–346. [Google Scholar]
- 20.Magde D, Brannon JH, Cremers TL, Olmsted J. Absolute luminescence yield of cresyl violet. A standard for the red. J Phys Chem. 1979;83:696–699. [Google Scholar]
- 21.Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai) 2005;37:719–727. doi: 10.1111/j.1745-7270.2005.00108.x. [DOI] [PubMed] [Google Scholar]
- 22.Haberkorn U, Kinscherf R, Krammer PH, Mier W, Eisenhut M. Investigation of a potential scintigraphic marker of apoptosis: radioiodinated Z-Val-Ala-DL-Asp(O-methyl)-fluoromethyl ketone. Nucl Med Biol. 2001;28:793–798. doi: 10.1016/s0969-8051(01)00247-5. [DOI] [PubMed] [Google Scholar]
- 23.Zhang HZ, Zhang H, Kemnitzer W, Tseng B, Cinatl J, Michaelis M, Doerr HW, Cai SX. Design and synthesis of dipeptidyl glutaminyl fluoromethyl ketones as potent severe acute respiratory syndrome coronovirus (SARS-CoV) inhibitors. J Med Chem. 2006;49:1198–1201. doi: 10.1021/jm0507678. [DOI] [PubMed] [Google Scholar]
- 24.Montero A, Goya P, Jagerovic N, Callado LF, Meana JJ, Girón R, Goicoechea C, Martín MI. Guanidinium and aminoimidazolinium derivatives of N-(4-piperidyl)propanamides as potential ligands for mu opioid and I2-imidazoline receptors: Synthesis and pharmacological screening. Bioorg Med Chem. 2002;10:1009–1018. doi: 10.1016/s0968-0896(01)00356-x. [DOI] [PubMed] [Google Scholar]
- 25.Unciti-Broceta A, Diezmann F, Ou-Yang CY, Fara MA, Bradley M. Synthesis, penetrability and intracellular targeting of fluorescein-tagged peptoids and peptide-peptoid hybrids. Bioorg Med Chem. 2009;17:959–966. doi: 10.1016/j.bmc.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 26.Strekowski L, Lipowska M, Patonay G. Substitution-reactions of a nucleofugal group in heptamethine cyanine dyes. Synthesis of an isothiocyanato derivative for labeling of proteins with a near-infrared chromophore. J Org Chem. 1992;57:4578–4580. [Google Scholar]
- 27.Masotti A, Vicennati P, Boschi F, Calderan L, Sbarbati A, Ortaggi G. A novel near-infrared indocyanine dye-polyethylenimine conjugate allows DNA delivery imaging in vivo. Bioconjug Chem. 2008;19:983–987. doi: 10.1021/bc700356f. [DOI] [PubMed] [Google Scholar]