Abstract
Lepidium sativum is widely used in folk medicine for treatment of hyperactive airways disorders, such as asthma, bronchitis and cough. The crude extract of Lepidium sativum (Ls.Cr) inhibited carbachol (CCh, 1 μM-) and K+ (80 mM-) induced contractions in a pattern similar to that of dicyclomine. Ls.Cr at 0.03 mg/mL produced a rightward parallel shift of CCh curves, followed by nonparallel shift at higher concentration (0.1 mg/mL), suppressing maximum response, similar to that caused by dicyclomine. Pretreatment of tissues with Ls.Cr (0.1–0.3 mg/mL) shifted Ca++ concentration-response curves (CRCs) to right, as produced by verapamil. Ls.Cr at low concentrations (0.03–0.1 mg/mL) caused leftward shift of isoprenaline-induced inhibitory CRCs, like that caused by rolipram, a phosphodiesterase (PDE) inhibitor. These results indicate that bronchodilatory effect of Lepidium sativum is mediated through a combination of anticholinergic, Ca++ antagonist and PDE inhibitory pathways, which provides sound mechanistic background for its medicinal use in the overactive airways disorders.
1. Introduction
Most of the disorders of respiratory system results from the hyperactivity of airways. Asthma is a major congestive respiratory disorder, characterized by episodic wheezing, cough, and chest tightness, associated with airflow obstruction. The worldwide prevalence of asthma has been increasing particularly in children. According to the World Health Organization, it affects about 5–10% of adults and 10% of children globally [1]. Cough is a spasmodic contraction of the thoracic cavity that results in abrupt release of air from the lungs. Excessive cough is one of the most common symptoms for which the patients seek medical care and may represent up to one-third of a pulmonologist's outpatient practice referrals [2].
The conventional therapy for these congestive airways disorders particularly asthma is not always safe, efficacious and is beyond the access and/or affordability of large proportion of world population, who look for alternative therapeutic measures. Phytotherapy is the most popular alternative remedy, and a number of traditional systems of medicine are heavily based on the use of herbs as medicine [3, 4]. At the same time, there is a global revival of interest in the use of botanicals, and the physicians of the modern medicine are now beginning to accept the traditional remedies once they are scientifically validated [5, 6].
Lepidium sativum Linn. belongs to family Cruciferae (cabbage family) and is commonly known as “Common cress,” “Garden cress,” or “Halim.” The plant is called “Hab el Rashaad” or “Thufa” in Saudi Arabia and is a popular herbal plant grown in many regions of Saudi Arabia, such as Hijaz, AL-Qaseem, and the Eastern Province [7, 8]. In Europe and America, the leaves are used in salad. In various countries of Africa, Lepidium sativum seeds are thought to be an effective medicinal remedy to cure respiratory disorders, like bronchitis and asthma [9]. The plant is cultivated as culinary vegetable all over Asia [10]. In South Asia, it is used in traditional medicine to treat asthma, bronchitis, and cough and is considered useful as abortifacient, antibacterial, aphrodisiac, diuretic, expectorant, gastrointestinal stimulant, gastroprotective, laxative, and stomachic [11, 12].
The plant is known to contain imidazole, lepidine, semilepidinoside A and B [13], β-carotenes, ascorbic acid, linoleic acid, oleic acid, palmitic acid, stearic acid [14], sinapic acid and sinapin [15]. Lepidium sativum is reported to exhibit antihypertensive [16], diuretic [17], anti-inflammatory, analgesic, anticoagulant [18], antirheumatic [19], hypoglycemic [20], laxative, prokinetic [21], antidiarrheal, and antispasmodic [22] properties. It has been shown to possess antiasthmatic [23] and bronchodilatory [24] potential in preliminary studies, but there is no report available in the literature on the pharmacological basis for its medicinal use in airways disorders. In this study, we explored the possible mechanisms underlying its airways relaxant effect, mediated through multiple pathways, including inhibitory effect on muscarinic receptors, Ca++ channels and phosphodiesterase (PDE) enzyme; thus, this study provides sound pharmacological rational for its effectiveness in the airways hyperactive disorders.
2. Materials and Methods
2.1. Plant Material and Extraction
Seeds of Lepidium sativum were purchased from herbal store (Bin Menqash, Riyadh, Saudi Arabia) in March, 2010. The plant was authenticated by Dr. Mohammed Yusuf, King Saud University, and the specimen has been preserved at the herbarium of the College of Pharmacy, King Saud University, Riyadh, Saudi Arabia and also at Natural Product Research Unit, Department of Biological and Biomedical Sciences, Aga Khan University, Karachi with voucher no. Ls-SE-04-10-98. The Lepidium sativum seeds were soaked in 70% methanol for three days [25] and then filtered through muslin cloth and Whatman filter paper (Maidstone, UK). This procedure was repeated three times, and all the filtrates were pooled and evaporated on rotary evaporator (model RE-111, Buchi, Flawil, Switzerland) under reduced pressure (−760 mm Hg) to obtain the crude extract of Lepidium sativum (Ls.Cr), yielding approximatly 15%.
2.2. Chemicals and Animals
Atropine sulphate, carbachol (CCh), dicyclomine, isoprenaline, verapamil, and rolipram were purchased from Sigma Chemicals Company, St. Louis, MO, USA. Guinea-pigs (500–550 g) of either sex and local breed were kept at the Animal House of the Aga Khan University, maintained at 23–25°C, and were given standard diet and tap water. Experiments were performed in compliance with the rulings of the Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council [26] and approved by Ethical Committee of the Aga Khan University.
2.3. Guinea-Pig Trachea
Tracheal ring strips obtained from guinea-pigs sacrificed by cervical dislocation were mounted individually in 20 mL tissue bath containing Kreb's solution, at 37°C and aerated with carbogen. A tension of 1 g was applied to each of the tissues and equilibrated for 1 hr before the addition of any drug. Carbachol (1 μM) and K+0020(80 mM) were used to induce sustained contractions, and relaxant activity of crude extract was assessed by adding in cumulative fashion. High K+ (>30 mM) is known to cause smooth muscle contractions through opening of voltage-dependent L-type Ca++ channels, thus allowing influx of extracellular Ca++ causing a contractile effect and the substance causing inhibition of high K+-induced contraction is considered as inhibitor of Ca++ influx [27, 28]. The antimuscarinic effect of plant extract was investigated through constructing CCh-concentration-response curves (CRCs) in its presence [29]. The Ca++ channel blockade (CCB) effect of test substance was confirmed via building Ca++-CRCs in the Ca++-free medium [28]. The presence of PDE inhibitory effect was evaluated indirectly through constructing isoprenaline-induced inhibitory CRCs against CCh-induced contractions in absence and presence of plant material, as described previously [30]. Isometric responses were recorded on a Grass model 7 Polygraph (Grass Instrument Company, Quincy, MA, USA).
2.4. Statistical Analysis
The median effective concentrations (EC50) with 95% confidence intervals (CIs) are provided. CRCs were analyzed by nonlinear regression using GraphPad program (GraphPAD, San Diego, CA, USA).
3. Results
3.1. Inhibitory Effect of Ls.Cr on CCh and High K+-Induced Contractions
When tested against CCh (1 μM-) and K+ (80 mM-) induced contractions, Ls.Cr inhibited the CCh-induced contractions at lower concentration with EC50 value of 0.32 mg/mL (0.30–0.44, 95% CI), compared to its effect against K+ (80 mM) with EC50 value of 5.4 mg/mL (4.3–7.2) as shown in Figure 1(a). Dicyclomine also showed a similar pattern of inhibitory effect (Figure 1(b)) with respective EC50 values of 0.28 μM (0.20–0.40) and 4.6 μM (3.0–7.2), whereas verapamil was more potent against K+-induced contractions with EC50 value of 0.14 μM (0.10–0.20), when compared with CCh-induced contractions (2.7 μM (1.7–3.5)) as shown in Figure 1(c). Atropine only relaxed the CCh (1 μM-) induced contraction, with EC50 value of 0.006 μM (0.002–0.01), without any effect on K+ (80 mM-) induced contractions (Figure 1(d)), as expected.
Figure 1.
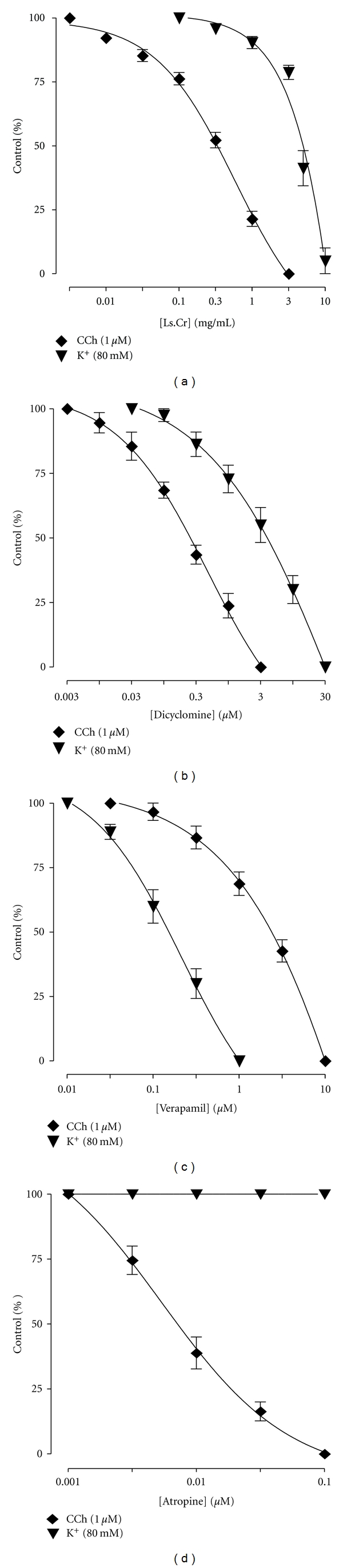
Concentration-response curves showing comparison (a) crude extract of Lepidium sativum (Ls.Cr), (b) dicyclomine, (c) verapamil, and (d) atropine for the inhibitory effect against carbachol (CCh) and high K+-induced contractions in isolated guinea-pig tracheal preparations, n = 3–5.
3.2. Effect of Ls.Cr on CCh Curves
Pretreatment of the tissues with Ls.Cr at 0.03 mg/mL caused rightward parallel shift of CCh curves, without suppression of maximum contractile response, followed by a non-parallel shift with the suppression of maximum response at next higher concentration, 0.1 mg/mL (Figure 2(a)). Dicyclomine (0.03-0.1 μM) also showed a similar pattern of shift (Figure 2(b)), while verapamil (0.1–0.3 μM) produced a nonparallel rightward shift at both tested concentration with suppression of the maximum response (Figure 2(c)). Atropine (0.01–0.03 μM) caused rightward parallel shift without the suppression of the maximum contractile effect (Figure 2(d)).
Figure 2.
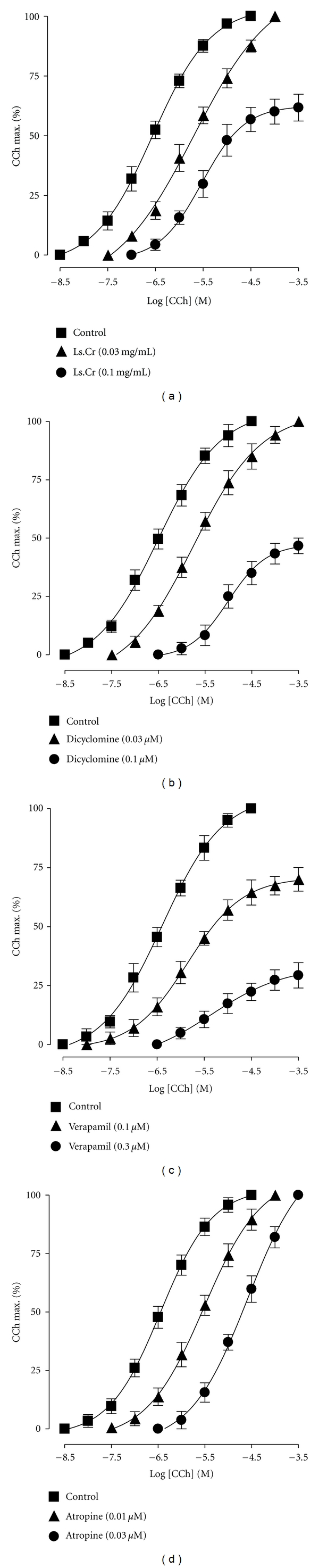
Concentration-response curves of carbachol (CCh) in the absence and presence of different concentrations of (a) crude extract of Lepidium sativum (Ls.Cr), (b) dicyclomine, (c) verapamil, and (d) atropine in isolated guinea-pig tracheal preparations, n = 3-4.
3.3. Effect of Ls.Cr on Ca++ Curves
When tested for the possible interaction with Ca++ channels, Ls.Cr (0.1–0.3 mg/mL) produced rightward shift in the Ca++ curves (Figure 3(a)), similar to that caused by verapamil (Figure 3(b)).
Figure 3.
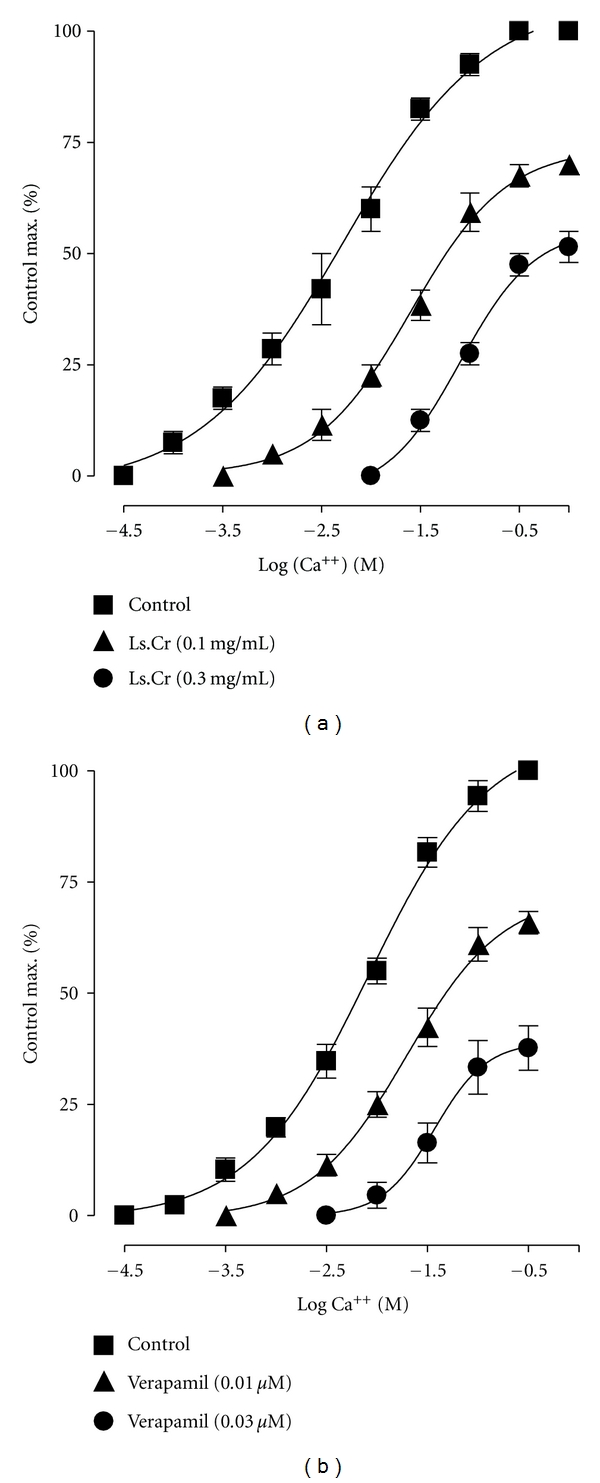
Concentration-response curves of Ca++ in the absence and presence of the increasing concentrations of (a) crude extract of Lepidium sativum (Ls.Cr) and (b) verapamil in isolated guinea-pig tracheal preparations, n = 3-4.
3.4. Effect of Ls.Cr on Isoprenaline Curves
Pretreatment of tissues with Ls.Cr at low concentrations (0.03–0.1 mg/mL) shifted the isoprenaline-induced inhibitory CRCs to the left (Figure 4(a)), showing potentiating effect. Rolipram (0.3–1.0 μM) also caused similar leftward shift of the isoprenaline curves, as shown in Figure 4(b). Dicyclomine, verapamil, and atropine were found devoid of such effect (data not shown).
Figure 4.
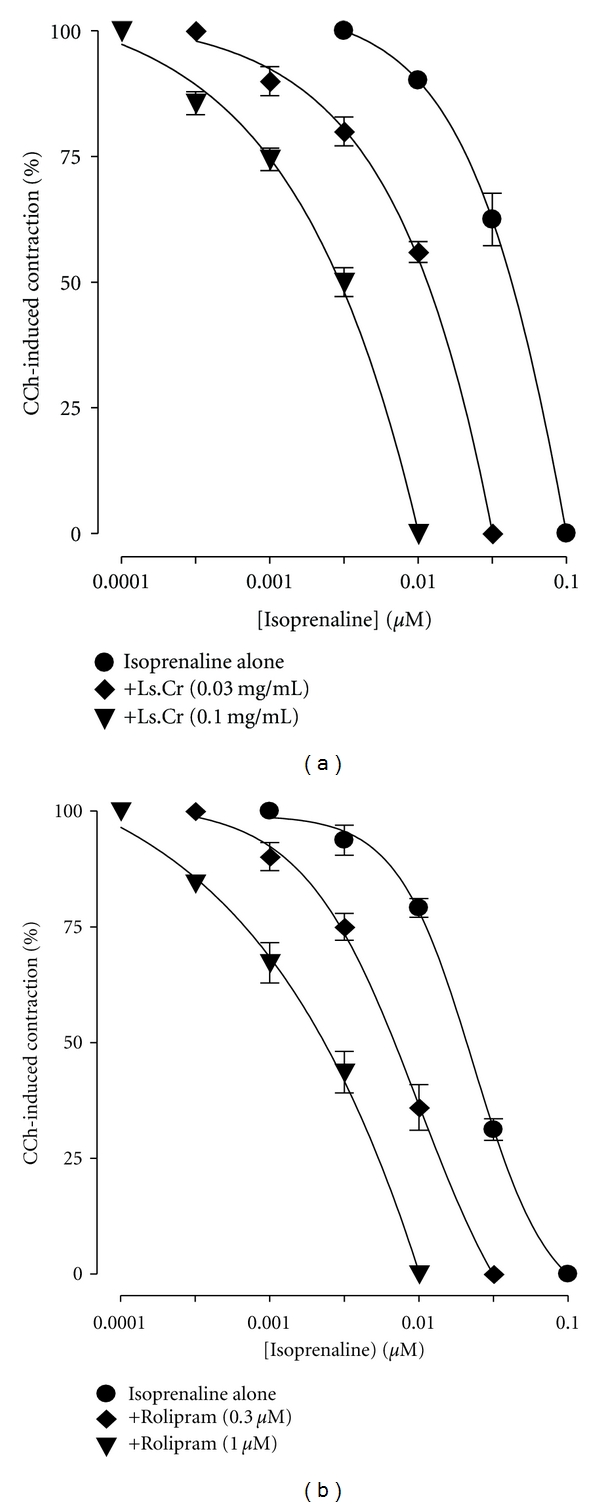
Inhibitory concentration-response curves of isoprenaline against carbachol (CCh)-induced contractions in the absence and presence of different concentrations of (a) crude extract of Lepidium sativum (Ls.Cr) and (b) rolipram in isolated guinea-pig tracheal preparations, n = 3. The curves obtained by pretreatment of tissues with Ls.Cr and rolipram are significantly different from the respective isoprenaline control curves (P < 0.05), Student's t-test.
4. Discussion
In view of the medicinal use of Lepidium sativum in the hyperactive airways disorders, its aqueous-methanol extract was tested for the possible bronchodilatory effect. In guinea-pig tracheal preparations, plant extract inhibited the CCh- and high K+-induced contractions, being more potent against CCh. Dicylomine, a dual blocker of muscarinic receptors and Ca++ influx [31], showed a similar pattern of inhibition, while verapamil, a standard Ca++ antagonist [32], was more potent against the K+-induced contractions than those induced by CCh, while atropine, a muscarinic receptor antagonist [33], relaxed the CCh-induced contractions only. It is thereby suggesting the inhibitory effect on muscarinic receptor and Ca++ channels. The presence of anticholinergic and CCB actions was further confirmed, respectively, through constructing the CCh and Ca++ CRCs in the presence of different concentrations of the plant extract. A parallel displacement of CCh curves without suppression of the maximum effect was observed at the lower concentration of Ls.Cr, a characteristic of a competitive or specific antagonist, like atropine [34], followed by non-parallel shift with suppression of the maximum effect at higher concentration, pointing towards the presence of nonspecific inhibition, like known with Ca++ antagonists [35]. Dicyclomine also shifted the CCh curves to the right, similar to that of the crude extract, while verapamil resulted in rightward but non-parallel shift with suppression of the maximum effect at both the concentrations used. Atropine caused a rightward parallel shift of the CCh curves without suppression of maximum response. Pretreatment of tissues with Ls.Cr shifted the Ca++ curves to the right accompanied by the suppression of maximum response, similar to that caused by verapamil, confirming the Ca++ antagonistic effect.
We have experienced that plants with medicinal use in the overactive airways disorders usually possess PDE inhibitory effect, which usually coexists with Ca++ antagonist activity [30, 36, 37]. To investigate whether Lepidium sativum also exhibits PDE enzyme inhibition component(s), isoprenaline inhibitory CRCs were constructed against CCh-induced contractions by pretreatment of tissues with Ls.Cr, as PDE inhibitors are known to potentiate the isoprenaline effect [38]. The presence of PDE inhibitory effect in Lepidium sativum was confirmed when the plant extract potentiated isoprenaline relaxant effect, by causing leftward shift of isoprenaline-induced inhibitory curves, similar to that caused by rolipram, a PDE4 inhibitor, predominant enzyme in airways [39]. These findings indicate that bronchodilator effect of Lepidium sativum is mediated through combined anticholinergic, CCB, and PDE inhibitory pathways; thus, this study provides sound mechanistic basis for its application in airways hyperactivity disorders. The usefulness of anticholinergics and PDE inhibitors in asthma is well established [40], though major limitation is cardiac stimulation, as a side-effect when applied orally [41, 42]. Interestingly, Ca++ antagonists are also useful in bronchoconstriction [43] and are known to exhibit cardiosuppressant effect [44]. The coexistence of CCB constituent(s) with antimuscarinic and PDE inhibitors in Lepidium sativum is perhaps meant by nature to offset the tachycardia, usually associated with anticholinergics or PDE inhibitors when used alone. This scenario strengthens the concept that natural remedies possess “effect enhancing and/or side-effects neutralizing” potential in addition to cost-effectiveness and offers merit in evidence-based studies [6, 45].
In conclusion, these results show that Lepidium sativum offers a unique combination of bronchodilator activities (anticholinergic, Ca++ antagonist and PDE inhibitory effects), which may explain its medicinal use in the hyperactive airways disorders, such as cough and asthma. However, further in-depth studies are required to probe nature of chemicals constituents and molecular base of biological activities.
Acknowledgments
This study was initiated during the visit of A. H. Gilani to King Saud University as a part of Visiting Professor Program and partially supported by the Higher Education Commission, Government of Pakistan, as an indigenous Ph.D. scholarship to Mr. Najeeb-ur-Rehman. The authors would express their appreciation to the Deanship of Scientific Research at King Saud University for the provision of funding for publication through the College of Pharmacy Research Center.
References
- 1.Malhotra S. Managing asthma in children-the valair study. Pakistan Journal of Chest Medicine. 2000;6:27–30. [Google Scholar]
- 2.Irwin RS, Madison JM. The diagnosis and treatment of cough. New England Journal of Medicine. 2000;343(23):1715–1721. doi: 10.1056/NEJM200012073432308. [DOI] [PubMed] [Google Scholar]
- 3.Saad B, Azaizeh H, Said O. Tradition and perspectives of Arab herbal medicine: a review. Evidence-based Complementary and Alternative Medicine. 2005;2(4):475–479. doi: 10.1093/ecam/neh133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azaizeh H, Saad B, Cooper E, Said O. Traditional Arabic and Islamic medicine, a re-emerging health aid. Evidence-based Complementary and Alternative Medicine. 2010;7(4):419–424. doi: 10.1093/ecam/nen039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azaizeh H, Saad B, Khalil K, Said O. The state of the art of traditional Arab herbal medicine in the Eastern region of the Mediterranean: a review. Evidence-based Complementary and Alternative Medicine. 2006;3(2):229–235. doi: 10.1093/ecam/nel034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilani AH, Atta-ur-Rahman Trends in ethnopharmacology. Journal of Ethnopharmacology. 2005;100(1-2):43–49. doi: 10.1016/j.jep.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Ageel AM, Tariq M, Mossa JS, Al-Yahya MA, Al-Said MS. Plant Used in Saudi Folk Medicine: Experimental Report Submitted to the King Abdul Aziz City for Science and Technology. Riyadh, Saudi Arabia: King Saud University Press; 1987. [Google Scholar]
- 8.Rahman MA, Mossa JS, Al-Said MS, Al-Yahya MA. Medicinal plant diversity in the flora of Saudi Arabia 1: a report on seven plant families. Fitoterapia. 2004;75(2):149–161. doi: 10.1016/j.fitote.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Kloos H. Preliminary studies of medicinal plants and plant products in Ethiopian markets. Journal of Ethiopian Pharmaceutical Association. 1976;2:18–28. [Google Scholar]
- 10.Nadkarni KM. Indian Materia Medica. Bombay, India: Popular Prakashan; 1976. [Google Scholar]
- 11.Baquar SR. Medicinal and Poisonous Plants of Pakistan. Karachi, Pakistan: Printas; 1989. [Google Scholar]
- 12.Duke JA, Bogenschutz-Godwin MJ, Ducelliar J, Duke PAK. Handbook of Medicinal Herbs. Boca Raton, Fla, USA: CRC Press; 2002. [Google Scholar]
- 13.Maier UH, Gundlach H, Zenk MH. Seven imidazole alkaloids from Lepidium sativum . Phytochemistry. 1998;49(6):1791–1795. doi: 10.1016/s0031-9422(98)00275-1. [DOI] [PubMed] [Google Scholar]
- 14.Duke JA. Handbook of Phytochemical Constituents of GRAS Herbs and Other Economical Plants. London, UK: CRC Press; 1992. [Google Scholar]
- 15.Schultz OE, Gmelin R. Purification of glycoside from Lepidum sativum by chromatography on a cellulose powder column. Arzneimittel-Forschung. 1952;2(12):568–569. [PubMed] [Google Scholar]
- 16.Maghrani M, Zeggwagh NA, Michel JB, Eddouks M. Antihypertensive effect of Lepidium sativum L. in spontaneously hypertensive rats. Journal of Ethnopharmacology. 2005;100(1-2):193–197. doi: 10.1016/j.jep.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 17.Patel U, Kulkarni M, Undale V, Bhosale A. Evaluation of diuretic activity of aqueous and methanol extracts of Lepidium sativum garden cress (Cruciferae) in rats. Tropical Journal of Pharmaceutical Research. 2009;8(3):215–219. [Google Scholar]
- 18.Al-Yahya MA, Mossa JS, Ageel AM, Rafatullah S. Pharmacological and safety evaluation studies on Lepidium sativum L. seeds. Phytomedicine. 1994;1:155–159. doi: 10.1016/S0944-7113(11)80035-8. [DOI] [PubMed] [Google Scholar]
- 19.Ahsan SK, Tariq M, Ageel M, Al-Yahya MA, Shah AH. Studies on some herbal drugs used in fracture healing. International Journal of Crude Drug Research. 1989;27(4):235–239. [Google Scholar]
- 20.Patole AP. Effect of mucilaginous seeds on in vitro rate of starch hydrolysis and blood glucose levels of NIDDM subjects with special reference to garden cress seeds. Journal of Medicinal and Aromatic Plant Sciences. 1998;20:1005–1008. [Google Scholar]
- 21.Rehman N, Mehmood MH, Alkharfy KM, Gilani AH. Prokinetic and laxative activities of Lepidium sativum seed extract with species and tissue selective gut stimulatory actions. Journal of Ethnopharmacology. 2011;134:878–883. doi: 10.1016/j.jep.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 22.Rehman N, Mehmood MH, Alkharfy KM, Gilani AH. Studies on antidiarrhoel and antispasmodic activities of Lepidium sativum crude extract in rats. doi: 10.1002/ptr.3642. Phytotherapy Research. In press. [DOI] [PubMed] [Google Scholar]
- 23.Paranjape AN, Mehta AA. A study on clinical efficacy of Lepidium sativum seeds in treatment of bronchial asthma. Iranian Journal of Pharmacology and Therapeutics. 2006;5(1):55–59. [Google Scholar]
- 24.Mali RG, Mahajan SG, Mehta AA. Studies on bronchodilatory effect of Lepidium sativum against allergen induced bronchospasm in guinea pigs. Pharmacognosy Magazine. 2008;4(15):189–192. [Google Scholar]
- 25.Kaur M, Goel RK. Anti-convulsant activity of Boerhaavia diffusa: plausible role of calcium channel antagonism. Evidence-Based Complementary and Alternative Medicine. 2011;2011:7 pages. doi: 10.1093/ecam/nep192. Article ID 310420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC, USA: National Academy Press; 1996. [Google Scholar]
- 27.Godfraind T, Miller R, Wibo M. Calcium antagonism and calcium entry blockade. Pharmacological Reviews. 1986;38(4):321–416. [PubMed] [Google Scholar]
- 28.Bashir S, Memon R, Gilani AH. Antispasmodic and antidiarrheal activities of Valeriana hardwickii rhizome are putatively mediated through calcium channel blockade. Evidence-Based Complementary and Alternative Medicine. 2011;2011:6 pages. doi: 10.1155/2011/304960. Article ID 304960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah AJ, Gilani AH. Bronchodilatory effect of Acorus calamus (Linn.) is mediated through multiple pathways. Journal of Ethnopharmacology. 2010;131(2):471–477. doi: 10.1016/j.jep.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Gilani AH, Khan AU, Subhan F, Khan M. Antispasmodic and bronchodilator activities of St John’s wort are putatively mediated through dual inhibition of calcium influx and phosphodiesterase. Fundamental and Clinical Pharmacology. 2005;19(6):695–705. doi: 10.1111/j.1472-8206.2005.00378.x. [DOI] [PubMed] [Google Scholar]
- 31.Downie JW, Twiddy DAS, Awad SA. Antimuscarinic and noncompetitive antagonist properties of dicyclomine hydrochloride in isolated human and rabbit bladder muscle. Journal of Pharmacology and Experimental Therapeutics. 1977;201(3):662–668. [PubMed] [Google Scholar]
- 32.Fleckenstein A. Specific pharmacology of Ca++ in myocardium, cardiac pacemakers, and vascular smooth muscle. Annual Review of Pharmacology and Toxicology. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- 33.Delmendo RE, Michel AD, Whiting RL. Affinity of muscarinic receptor antagonists for three putative muscarinic receptor binding sites. British Journal of Pharmacology. 1989;96(2):457–464. doi: 10.1111/j.1476-5381.1989.tb11838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arunlakhshana O, Schild HO. Some quantitative uses of drug antagonists. British Journal of Pharmacology and Chemotherapy. 1959;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilani AH, Khan AU, Ali T, Ajmal S. Mechanisms underlying the antispasmodic and bronchodilatory properties of Terminalia bellerica fruit. Journal of Ethnopharmacology. 2008;116(3):528–538. doi: 10.1016/j.jep.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Gilani AH, Shah AJ, Zubair A, et al. Chemical composition and mechanisms underlying the spasmolytic and bronchodilatory properties of the essential oil of Nepeta cataria L. Journal of Ethnopharmacology. 2009;121(3):405–411. doi: 10.1016/j.jep.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Khan M, Khan A, Rehman N, Gilani AH. Pharmacological explanation for the use of Juniperus excelsa in hyperactive gastrointestinal and respiratory disorders. doi: 10.1007/s11418-011-0605-z. Journal of Natural Medicines. In press. [DOI] [PubMed] [Google Scholar]
- 38.Lorenz KL, Wells JN. Potentiation of the effects of sodium nitroprusside and of isoproterenol by selective phosphodiesterase inhibitors. Molecular Pharmacology. 1983;23(2):424–430. [PubMed] [Google Scholar]
- 39.Lipworth BJ. Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. The Lancet. 2005;365(9454):167–175. doi: 10.1016/S0140-6736(05)17708-3. [DOI] [PubMed] [Google Scholar]
- 40.Barnes PJ. Drugs for asthma. British Journal of Pharmacology. 2006;147(supplement 1):S297–S303. doi: 10.1038/sj.bjp.0706437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicholas JG. Anticholinergic agents in asthma and COPD. European Journal of Pharmacology. 2006;533(1–3):36–39. doi: 10.1016/j.ejphar.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 42.Nawrath H. Action potential, membrane currents and force of contraction in cat ventricular heart muscle treated with papaverine. Journal of Pharmacology and Experimental Therapeutics. 1981;218(2):544–549. [PubMed] [Google Scholar]
- 43.Twiss MA, Harman E, Chesrown S, Hendeles L. Efficacy of calcium channel blockers as maintenance therapy for asthma. British Journal of Clinical Pharmacology. 2002;53(3):243–249. doi: 10.1046/j.0306-5251.2001.01560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Billman GE. The antiarrythmic effects of the calcium antagonists. In: Epstein M, editor. Calcium Antagonists in Clinical Medicine. Philadelphia, Pa, USA: Hanley and Belfus; 1992. pp. 183–212. [Google Scholar]
- 45.Ernst E. The efficacy of herbal medicine-an overview. Fundamental and Clinical Pharmacology. 2005;19(4):405–409. doi: 10.1111/j.1472-8206.2005.00335.x. [DOI] [PubMed] [Google Scholar]


