Summary
Interictal spikes have been implicated in epileptogenesis and cognitive dysfunction in epilepsy. Unfortunately, antiepileptic drugs have shown poor efficacy in suppressing interictal discharges and novel therapies are needed. Surface charge on neuronal membranes provides a novel target for abolishing interictal spikes. This property can be modulated through the use of neuraminidase, an enzyme which decreases the amount of negatively charged sialic acid. In the present report we determined whether applying neuraminidase to brains of rats with a prior history of status epilepticus would reduce number of interictal discharges. Following pilocarpine-induced status epilepticus rats received intrahippocampal injections of neuraminidase, which significantly decreased the number of interictal spikes recorded in the CA1 region. This study provides evidence that sialic acid degradation can reduce the number of interictal spikes. Furthermore, the results suggest that modifying surface charge created by negatively charged sialic acid may provide new opportunities for reducing aberrant epileptiform events in epilepsy.
Keywords: neuraminidase, epilepsy, seizures, electroencephalography
Introduction
Interictal spikes (IIS), focal neural discharges seen on electroencephalogram (EEG) recordings, are a result of synchronous, paroxysmal depolarization of neurons producing a rapid succession of action potentials (APs). In addition to delineating the seizure focus, IIS are also felt to play an important role in epileptogenesis (Staley et al., 2005; Staley & Dudek, 2006) and are associated with transitory memory impairment in humans {Krauss, 1997 11988/id} and rats {Kleen, 2010 12185/id}. However, attempts to suppress IIS with standard antiepileptic drugs (AEDS) have not been effective (Spencer et al., 2008; D’Antuono et al., 2010) and new approaches to reducing IIS are needed.
A potential new target for suppressing synchronization of epileptiform activity is the modulation of surface charge. Surface charge refers to the charge associated with the channel protein or adjacent membrane, which contributes significantly to the field near the channel voltage sensor (Frankenhaeuser & Hodgkin, 1957; Hille et al., 1975; Campbell & Hille, 1976; Green & Andersen, 1991). By neutralization of the negative surface charge, the activation of voltage-gated channels shifts to more positive values, thus reducing neuronal net activity (Isaev et al., 2007).
Neuraminidase (NEU) is a key enzyme which cleaves sialic acid residuals and regulates the amount of negative charge on the cellular membrane. NEU treatment causes a large depolarizing shift of voltage-gated sodium channel activation/inactivation and AP threshold without any change in the resting membrane potential of hippocampal CA3 pyramidal neurons (Isaev et al., 2007). By neutralizing surface charge created by sialics acid, the amount of depolarizing input required for generating APs increases, thus lowering the likelihood of neuronal cell firing and synchronization. Thus, desialylation may be a powerful approach to reducing neuronal excitability and even treating seizures. In the present work we used the pilocarpine model of chronic epilepsy to study the effect of NEU on IIS. We show that NEU significantly reduces the number of IIS and suggest that surface charge created by sialic acid compounds is an attractive target for suppression of IIS.
Methods
Male Sprague-Dawley rats (n = 45; postnatal age of 40–120 days) were used for all experiments and treated accordingly to the animal procedures approved by the Dartmouth Institutional Animal Care and Use Committee in accordance with the National Institutes of Health guidelines.
We used two approaches to elicit status epilepticus (SE): i) Systemic (intraperitoneal [IP]) injection of pilocarpine following lithium (n = 12); and ii) Intrahippocampal (IH) pilocarpine injection (n = 27). In the systemic model, fifteen rats were injected with lithium chloride (127 mg/kg) IP at P40–50, followed 24 hours later by IP pilocarpine as a bolus injection (30 mg/kg). SE was terminated after 2 hours with isoflurane anesthesia. Rats underwent surgery for EEG and cannula placement two weeks after SE (coordinates with respect to bregma: CA1: 3.8 mm posterior, 2.5 mm lateral, and 2.0 mm below dura) and 7–10 days before EEG monitoring was conducted. In the IH model, rats had electrode and cannula implantation in the hippocampus prior to pilocarpine injection at the age of P50–60. One week after surgery rats were administered pilocarpine (0.5 mg/ml in 0.9% saline) into the hippocampus at a rate of 0.05 μl/min, while the EEG was monitored from electrodes adjacent to the infusion site. The infusion was terminated when the EEG showed sustained rhythmic sharp waves or spikes lasting greater than 2 minutes, regardless of the behavioral state of the rat. This method ensured that only the minimum amount of pilocarpine necessary was used to induce EEG seizures, minimizing mortality {Kleen, 2010 12185/id}. Control animals (n = 6) received 0.9% saline as an IH (n = 3) or IP administration (n = 3) using volumes comparable to their pilocarpine-treated counterparts.
NEU from Arthrobacter ureafaciens (Roche) was dissolved in 0.9% NaCl solution at a concentration 0.4 U/ml. The 30 μl solution was manually injected in one or both hemispheres over 3–4 min using microsyringes. In the control animals 30 μl of 0.9% NaCl solution was injected through each cannula.
Following the SE at P80–90 we administered NEU IH bilaterally or unilaterally (ipsilateral to the side receiving the pilocarpine in the IH model) and assessed outcome by quantifying IIS number. Based on preliminary results we anticipated that the maximum number of IIS would be two weeks after IH pilocarpine and four weeks after systemic pilocarpine. We used a within-subjects experimental design and our primary outcome variable was the change in IIS frequency following NEU. IIS were counted manually by an investigator blinded to treatment group. Spikes were identified by morphology and amplitude (> 0.1mV). To compare IIS frequency in IH and systemically- treated groups we used the unpaired Mann-Whitney test. Mean IIS frequency between groups before and after NEU was compared using a Wilcoxon matched-pairs signed rank test. All data are presented as a mean±SD.
Results
Injection of pilocarpine using either IH or IP protocols produced severe seizures which progressed into SE in all rats, corresponding to Stage III–V behavioral seizures as described by Racine et al. (Racine, 1972). Recording of baseline EEG was began two weeks after IH pilocarpine infusion and one month following IP pilocarpine administration. IIS were always present during baseline EEG monitoring in the hippocampal CA1 region of IH- and IP-treated animals. The IIS frequency varied considerably from animal to animal (from 84 to 856 IIS/hour for IH and from 128 to 781 IIS/hour for IP-treated rats). However the day-by-day analysis shows that the IIS frequency remained stable with no more than a 12% fluctuation from the mean value. We did not find a significant difference between mean IIS frequency recorded in IH- and IP-treated rats (380.7 ± 211.5 [IH, n = 16] vs 448.4±176.4 [IP, n = 12] IIS/hour, p = 0.38, Mann-Whitney test).
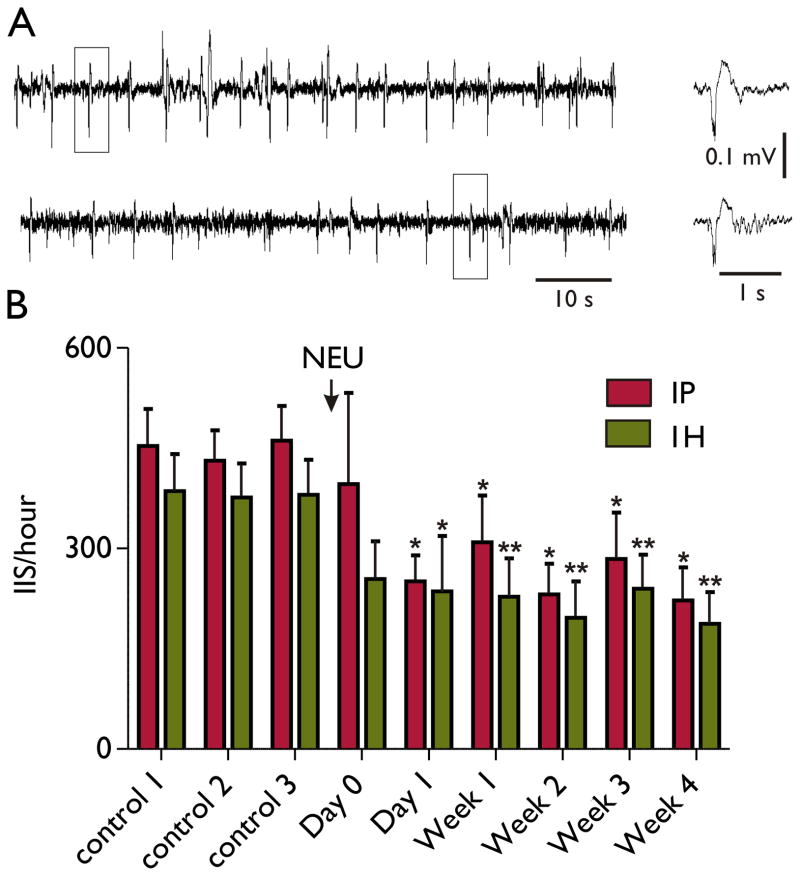
After a single IH injection of NEU we monitored the EEG for 2–4 hours during the first day after injection and then once a week for four weeks. As shown in the Figure, infusion of NEU did not produce a significant effect on IIS frequency on the day of injection (p = 0.43 for IP group and p = 0.15 for IH group, Wilcoxon matched-pairs signed rank test). However, 24 hours after the NEU there was a pronounced suppression of IIS (p < 0.05, Wilcoxon matched-pairs signed rank test). Injection of a NaCl solution as a control did not change the IIS frequency during the monitoring period (N = 6, data not shown). Other than for mild lethargy and decreased activity during the first 24 hours after injection, the NEU did not lead to any observable adverse behavioral side effects.
Figure.
Single injection of neuraminidase decreased frequency of hippocampal IIS in pilocarpine model of epilepsy. (A) IIS recording from CA1 region of hippocampus from IP pilocarpine treated rat before (upper panel) and 3 days after (lower panel) of intrahippocampal neuraminidase (NEU) injection. IIS marked with squares shown in expanded scales in the right panel. (B) Summary plot shows effect of NEU on IIS frequency recorded from IP (n = 12) and IH (n = 16) pilocarpine-treated rats. All data and statistics are shown relatively to the mean control obtained from three day recordings before NEU injection. Controls were pooled from two hours recordings obtained for each rat three times during the week before NEU injection. Day 0 to Week 4 columns represent relative decreases in IIS frequency over the first day and 4 subsequent weeks post-NEU injection. All columns are mean±SD. Statistics were done using Wilcoxon matched-pairs signed rank test, *p < 0.05, ** p < 0.01 versus control.
Histological analyses of the brains showed electrode and catheter placement in the hippocampus with gliosis along the electrode tracks. In rats with either IH or IP pilocarpine-induced status epilepticus variable cell loss was seen primarily in CA1 with some cell loss in CA3 and the hilus. In no rats did the NEU injections result in apparent tissue destruction. Examples of the histology are shown in the supplementary figure.
Discussion
In this work we demonstrate a long lasting effect of desialylation on hippocampal IIS in the pilocarpine model of epilepsy. As expected, the effect of NEU was not acute and no changes in IIS were seen immediately following the NEU injection. However, by the following day a significant reduction in IIS occurred. This finding corresponds well with our previous observation that enzymatic reduction of sialic acid required 12 hours to show a significant effect on kindling (Isaev et al., 2007). The persistent effect of NEU on IIS frequency also corresponds well with a previous study showing that NEU injection into the lateral ventricle of mice showed no detectable polysialic acid after 14 days (Seki & Rutishauser, 1998).
The likely mechanism responsible for suppressing the IIS was reduction of the negative surface charge through enzymatic degradation of sialic acid. Desialylation results in a depolarizing shift in the gating kinetics of the Na+ channels, leading to an increase of the AP threshold in the NEU-treated neurons. This mechanism differs from that occurring with the so-called sodium channel drugs. For example carbamazepine, which does not reduce IIS (D’Antuono et al., 2010), targets the sodium channel by prolonging the inactivated state and preventing reopening of the channel. Conversely, altering surface charge does not change the chemical or morphological structure of the channel. Reducing surface charge by desialylation decreases the possibility of the channel opening under conditions of low intensity excitation input, thus lowering the likelihood of synchronization of epileptic discharges, though not interfering with the kinetics of sodium channels once they are activated.
This study provides proof of principle that reduction of sialic acid residuals which are carriers of surface charge on the neuron membrane can reduce number of IIS and lays the groundwork for additional studies in which dose titration will be used to find the most effective and safe dosage. While it is unlikely that enzyme degradation of sialic acid will become a mainstream treatment option for suppressing IIS, targeting surface charge may provide a novel therapeutic direction.
Supplementary Material
Supplementary Figure. Examples of histology from a rat with intrahippocampal (A) and intraperitoneal (B) pilocarpine. At the end of the experiment, rats were sacrificed with a lethal dose of sodium pentobarbital (65 mg/kg) and perfused transcardially with: 1) 200 ml of normal saline; and 2) 200 ml of 4% PFA. The brains were removed, postfixed in 4% PFA for 24 hours and placed in 30% sucrose for 24 hours or longer until the brains sank. Coronal sections along the entire extent of the hippocampus were cut at 20 μM on a freezing microtome and sections were stored in phosphate buffered saline (pH 7.3). Every fourth section was stained with thionin. Electrode/cannula placement and gross cell loss was evaluated in each animal. Variable cell loss was seen primarily in CA1, CA3 and the hilus. In the photomicrograph from the rat with intrahippocampal pilocarpine (A) cell loss is apparent in CA1 (arrowhead). In the example from the rat with intraperitonal pilocarpine (B) little cell loss is seen. A remnant of the tract of the cannula to CA1 is shown by the arrow. In none of the rats did the NEU result in apparent tissue destruction.
Acknowledgments
Supported by the Christopher Donalty and Kyle Coggins Memorial grant from Citizens United for Research in Epilepsy (CURE).
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with these guidelines.
Footnotes
Disclosures
Dr. Holmes has served as a consultant for Eisai Pharmaceuticals, Pfizer, Sepracor, Questcor Pharmaceuticals, National Institutes of Health, and the Food & Drug Administration. The remaining authors have no conflicts of interest.
Reference List
- Campbell DT, Hille B. Kinetic and pharmacological properties of the sodium channel of frog skeletal muscle. J Gen Physiol. 1976;67:309–323. doi: 10.1085/jgp.67.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Antuono M, Kohling R, Ricalzone S, Gotman J, Biagini G, Avoli M. Antiepileptic drugs abolish ictal but not interictal epileptiform discharges in vitro. Epilepsia. 2010;51:423–431. doi: 10.1111/j.1528-1167.2009.02273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankenhaeser B, Hodgkin AL. The action of calcium on the electrical properties of squid axons. J Physiol. 1957;137:218–244. doi: 10.1113/jphysiol.1957.sp005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green WN, Andersen OS. Surface charges and ion channel function. Annu Rev Physiol. 1991;53:341–359. doi: 10.1146/annurev.ph.53.030191.002013. [DOI] [PubMed] [Google Scholar]
- Hille B, Woodhull AM, Shapiro BI. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975;270:301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Lenck-Santini PP. Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav. 2006;8:504–515. doi: 10.1016/j.yebeh.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Isaev D, Isaeva E, Shatskih T, Zhao Q, Smits NC, Shworak NW, Khazipov R, Holmes GL. Role of extracellular sialic acid in regulation of neuronal and network excitability in the rat hippocampus. J Neurosci. 2007;27:11587–11594. doi: 10.1523/JNEUROSCI.2033-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleen JK, Scott RC, Holmes GL, Lenck-Santini PP. Hippocampal interictal spikes disrupt cognition in rats. Ann Neurol. 2010;67(2):250–7. doi: 10.1002/ana.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation - II. Motor seizures. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Seki T, Rutishauser U. Removal of polysialic acid-neural cell adhesion molecule induces aberrant mossy fiber innervation and ectopic synaptogenesis in the hippocampus. J Neurosci. 1998;18:3757–3766. doi: 10.1523/JNEUROSCI.18-10-03757.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SS, Goncharova II, Duckrow RB, Novotny EJ, Zaveri HP. Interictal spikes on intracranial recording: behavior, physiology, and implications. Epilepsia. 2008;49:1881–1892. doi: 10.1111/j.1528-1167.2008.01641.x. [DOI] [PubMed] [Google Scholar]
- Staley K, Hellier JL, Dudek FE. Do interictal spikes drive epileptogenesis? Neuroscientist. 2005;11:272–276. doi: 10.1177/1073858405278239. [DOI] [PubMed] [Google Scholar]
- Staley KJ, Dudek FE. Interictal spikes and epileptogenesis. Epilepsy Curr. 2006;6:199–202. doi: 10.1111/j.1535-7511.2006.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure. Examples of histology from a rat with intrahippocampal (A) and intraperitoneal (B) pilocarpine. At the end of the experiment, rats were sacrificed with a lethal dose of sodium pentobarbital (65 mg/kg) and perfused transcardially with: 1) 200 ml of normal saline; and 2) 200 ml of 4% PFA. The brains were removed, postfixed in 4% PFA for 24 hours and placed in 30% sucrose for 24 hours or longer until the brains sank. Coronal sections along the entire extent of the hippocampus were cut at 20 μM on a freezing microtome and sections were stored in phosphate buffered saline (pH 7.3). Every fourth section was stained with thionin. Electrode/cannula placement and gross cell loss was evaluated in each animal. Variable cell loss was seen primarily in CA1, CA3 and the hilus. In the photomicrograph from the rat with intrahippocampal pilocarpine (A) cell loss is apparent in CA1 (arrowhead). In the example from the rat with intraperitonal pilocarpine (B) little cell loss is seen. A remnant of the tract of the cannula to CA1 is shown by the arrow. In none of the rats did the NEU result in apparent tissue destruction.