Abstract
Purpose
The combination of gemcitabine plus erlotinib has shown a small but statistically significant survival advantage when compared to gemcitabine alone in patients with advanced pancreatic cancer. However, the overall survival rate with the erlotinib and gemcitabine combination is still low. In this study we sought to identify gene targets that, when inhibited, would enhance the activity of EGFR-targeted therapies in pancreatic cancer cells.
Experimental Design
A high-throughput RNAi screen was carried out to identify candidate genes. Selected gene hits were further confirmed and mechanisms of action were further investigated using various assays.
Results
Six gene hits from siRNA screening were confirmed to significantly sensitize BxPC-3 pancreatic cancer cells to erlotinib. One of the hits, MAPK1, was selected for further mechanistic studies. Combination treatments of erlotinib plus two MAP kinase kinase (MEK) inhibitors, RDEA119 and AZD6244, showed significant synergistic effect for both combinations (RDEA119-erlotinib and AZD6244-erlotinib) compared to the corresponding single drug treatments in pancreatic cancer cell lines with wild-type KRAS (BxPC-3 and Hs 700T) but not in cell lines with mutant KRAS (MIA PaCa-2 and PANC-1). The enhanced antitumor activity of the combination treatment was further verified in the BxPC-3 and MIA PaCa-2 mouse xenograft model. Examination of the MAPK signaling pathway by Western blotting indicated effective inhibition of the EGFR signaling by the drug combination in KRAS wildtype cells but not in KRAS mutant cells.
Conclusions
Overall, our results suggest that combination therapy of an EGFR and MEK inhibitors may have enhanced efficacy in patients with pancreatic cancer.
Keywords: pancreatic cancer, EGFR, MEK, KRAS, combination chemotherapy
Introduction
With a 5-year survival rate of less than 5%, pancreatic cancer remains the most deadly of all major cancer types (1, 2). Gemcitabine has been the standard first line therapy for patients with advanced pancreatic cancer since 1996. Since then, multiple clinical trials combining gemcitabine with other chemotherapeutics have failed to show improvement in overall survival compared to gemcitabine alone (3-5). Until recently in 2005, the FDA approved the regimen of gemcitabine plus erlotinib (Tarceva®), an EGFR (epidermal growth factor receptor) inhibitor based upon increased survival.
The EGFR tyrosine kinase signaling pathway has been implicated in several cellular processes pertinent to cancer progression including cell survival, proliferation, invasion, and metastasis. Dysregulation of EGFR signaling occurs in as much as one-half of all pancreatic cancers (6). Erlotinib is a small molecular weight inhibitor of the EGFR tyrosine kinase that has shown clinical activity in patients with advanced non-small cell lung or pancreatic cancer. Patients with advanced pancreatic cancer treated with the combination of erlotinib plus gemcitabine have shown statistical significant survival advantage over patients treated with gemcitabine alone. However, the overall response and survival rate with the erlotinib plus gemcitabine combination is still low with the survival rate at one year increased from 17% to 23% with the combination (7).
The RAS-RAF-mitogen-activated protein kinase (MAPK) pathway is one of the downstream effector pathways of the EGFR tyrosine kinase signaling. It is constitutively activated in multiple human tumors due to gain-of-function mutations in RAS or RAF. Of the RAS family, KRAS is more often mutated with approximately 20% of all human tumors possessing an activating mutation in codon 12, 13, and more rarely 61 (8). KRAS mutations are the most prevalent in pancreatic ductal adenocarcinomas (70-90%) (9-11), followed by colorectal adenocarcinomas (35%) and lung carcinomas (17%) (12). Of the RAF family, mutations only in BRAF have been reported, but are mutually exclusive from a KRAS mutation. It is a rare exception that a tumor possesses both KRAS and BRAF mutations (13).
Downstream of RAS and RAF is MEK1/2, which is critical to transmitting signals to ERK and has become an attractive pharmaceutical target in tumors harboring aberrant signaling in the MAPK pathway. AZD6244 (ARRY-142886) and RDEA119 (BAY 869766) are two potent MEK1/2 inhibitors that are currently in clinical development. AZD6244 is a selective ATP-uncompetitive inhibitor of MEK1/2, which has been reported to inhibit cellular growth and induce apoptosis in vitro and reduce tumor growth in the BxPC-3 and HT-29 xenograft models (14). RDEA119 is an allosteric, selective inhibitor of MEK1/2, which has been reported to inhibit cell proliferation in vitro and reduce tumor growth in various in vivo models (15). Clinically, RDEA119 is currently being evaluated in at least three studies: a Phase I dose-escalation study, a Phase I monotherapy in Japanese patients, and a Phase 1/2 study in combination with sorafenib in advanced cancer patients (http://www.clinicaltrials.gov).
In this study, we employed high throughput RNA interference (RNAi) screening approach to identify targets that would enhance the activity of erlotinib in pancreatic cancer cells. We determined that the combination of a MEK inhibitor and erlotinib has significant anti-tumor activity in a subset of pancreatic cancer cells that harbor wildtype KRAS in in vitro and in vivo models.
Materials and Methods
Cell Line Culture
The pancreatic cancer cell lines BxPC-3, Hs 700T, MIA PaCa-2, and PANC-1 were obtained from the American Type Culture Collection (ATCC, Manassas, VA). All cell lines were maintained in RPMI 1640 supplemented with 10% fetal bovine serum, penicillin (100 U/ml) and streptomycin (100 mg/ml). Cells were cultivated in a humidified incubator at 37°C and 5% CO2. Cells were harvested with 0.05% trypsin at 70-80% cell density.
Cell line identities were verified by STR profiling (16) using the AmpFISTR Identifiler PCR amplification kit (Applied Biosystems, Foster City, CA). This method simultaneously amplifies 15 STR loci and Amelogenin in a single tube, using 5 dyes, 6-FAM™, JOE™, NED™, PET™, and LIZ™ which are then separated on a 3100 Genetic Analyzer (Applied Biosystems). GeneMapper ID v3.2 software was used for analysis (Applied Biosystems). AmpFISTR control DNA and the AmpFISTR allelic ladder were run concurrently. Results were compared to published STR sequences from the ATCC. The STR profiling is repeated once a cell line has been passaged more than 6 months after previous STR profiling.
siRNA library screening and hit selection
An RNAi screen using a library of short interfering (siRNA) duplex oligonucleotides targeting 588 known human kinase genes (2 siRNAs/gene, Qiagen, Germantown, MD) was performed to identify sensitizing targets for erlotinib using a reverse transfection protocol as described previously (17). Two non-silencing siRNAs were used as negative controls, while the AllStars Hs Cell Death Control (Qiagen) was used as a positive control. The siRNAs were first arrayed into 384-well plates for a final assay concentration of 20 nM in duplicates. The arrayed siRNAs was then incubated with 20 μl serum-free RPMI 1640 cell culture media (Invitrogen, Carlsbad, CA) containing 0.04 μl siLentfect lipid reagent (Bio-Rad, Hercules, CA) at room temperature for 30 minutes. Next, BxPC-3 cells were plated to the siRNA-transfection reagent mix at 1,200 cells/well and serum-supplemented at a final concentration of 5%. The plates were incubated in a humidified incubator at 37°C for 24 hours. Afterwards, a serial dilution of erlotinib (6 concentrations between 0-100 μM) was added to the wells and incubated for 96 hours. Cell viability was determined by CellTiter-Glo Luminescent Assay (Promega, Madison, WI) and the luminescence was recorded with the Synergy HT microplate reader (BioTek, Winooski, VT).
The percent cell survival of the siRNA-erlotinib combination was normalized to the percent cell survival of corresponding siRNA alone control. The IC50 values for each siRNA-erlotinib treatment were calculated by fitting the data to a sigmoid dose-response model using nonlinear regression with the Matlab software (2007a, The MathWorks Inc). Each siRNA was evaluated and ranked by the following qualifiers as potential hits: i.) the R2 for the sigmoid fitting curve was greater than 0.9, ii.) the effect on cell viability of the siRNA itself was less than 50%, and iii.) two-fold or higher decrease in the IC50 value compared to the Neg siRNA control. To further validate the positive gene hits, an additional 2 siRNA sequences (4 total) were obtained for each gene and subjected to a confirmation screen with 8 erlotinib concentrations using the same procedures described above. Genes that have at least 2 out of 4 siRNA sequences showing 2-fold or higher reduction in erlotinib IC50 value compared to the Neg siRNA control were selected as confirmed hits.
Verification of siRNA mediated mRNA and protein expression knock-down using RT-PCR and Western blotting
To evaluate the gene specific knock-down of mRNA expression by siRNA, cancer cells (250,000 cells/well) were reverse transfected with 20 nM of the MAPK1 siRNAs (Qiagen) for 72 hours in 6-well plates. The cells were then washed with DPBS twice and harvested with trypsinization. For RNA extraction, the RNeasy Mini Kit (Qiagen) was utilized following the manufacturer’s recommended protocol. One microgram of total RNA was used in a 20 μl cDNA synthesis reaction (Quanta Biosciences, Gaithersburg, MD). One microliter of the cDNA reaction mix, 10 μl of FastStart SYBR Green Master mix (Roche), and 4 μl of MAPK1 primer mix (5′-TCATCCTCGGAAAACAGACC-3 ′ and 5 ′-TCAATGCTGTGTGGACCTTC-3′, final concentration 0.4 μM each) were combined. A separate reaction for GAPDH was included as a control, and the primers used were 5′-ATTGCCCTCAACGACCACTT-3′ and 5′-GGTCCACCACCCTGTTGC-3′. The real-time qPCR was performed using the MyIQ real-time PCR detection system (Bio-Rad). The cycling program used was 95°C for 10 min, 95°C for 30 sec, 58°C for 30 sec, and 72°C for 30 sec for 30 cycles. Each sample was run in duplicates, normalized to GADPH, and analyzed by the comparative CT method (18).
For protein expression, the cells were washed with DPBS twice and subsequently lysed with RIPA buffer (Cell Signaling Technology, Danvers, MA) supplemented with Complete Mini protease cocktail and PhoSTOP (Roche). The lysates were incubated on ice for 30 minutes prior to centrifugation at 14,000 x g for 10 minutes at 4°C. Protein concentration was determined using the bicinchoninic acid protein assay (Pierce Biotechnology, Rockford, IL). A total of 30 μg of protein/lane was separated by 10% SDS-PAGE electrophoresis (Invitrogen) and transferred to nitrocellulose membrane blots. The membranes were blocked for 1 hr with 5% fat-free milk at room temperature and then incubated with a rabbit polyclonal p42 MAP Kinase (Erk2) antibody (1:1000 dilution, Cell Signaling) overnight at 4°C. The blots were washed with Tris Buffered Saline containing 0.1% Tween-20 (TBST) buffer for 3 times and then exposed to anti-rabbit IgG HRP-linked antibody (1:4000 dilution, Cell Signaling) at room temperature for 1 hour. The protein bands were visualized using the chemilumeniscence detection method (Millipore, Billerica, MA).
Drug combination treatment and immunoblotting analysis
Erlotinib, AZD6244, and RDEA119 were purchased from ChemieTek (Indianapolis, IN) and dissolved in DMSO to make 50 mM (erlotinib) or 20 mM (AZD6244 and RDEA119) stock solutions. Cells were seeded into 384-well plates at 1,200 cells/well, allowed to grow for 24 hours, and then treated with drugs for another 96 hours. Ten concentrations of AZD6244 and RDEA119 were made in 1:3 serial dilutions in serum-free medium. Three drug concentrations of erlotinib were added in combination to the MEK inhibitors at 1:1 ratio concurrently. At the end of treatment, cell viability was determined by the CellTiter-Glo assay. The percent cell survival of erlotinib alone at the indicated drug concentration was normalized to the percent cell survival of the respective drug combination. The growth curves were plotted in the GraphPad Prism® v5 software and the IC50 was determined by the software using the nonlinear regression (curve fit) analysis.
For Western blot analysis, 1 × 106 cells were seeded in T25 flasks for overnight growth. The cells were treated with drugs in serum conditions similar to the 384-well assays. The drugs at concentrations indicated in the figure legends were added directly to the medium for incubation at hours indicated. Cell lysates were prepared as described previously. The cell lysates (30 μg per lane, except 15 μg for AKT) were separated on NuPAGE protein pre-cast gels (Invitrogen), transferred to nitrocellulose (Bio-Rad) and then probed with specific antibodies using manufacturer’s recommended dilutions. The antibodies, phospho-MAPK (Thr202/Tyr204) (pMAPK), MAPK, phospho-EGFR (Tyr1068) (pEGFR), EGFR, phospho-AKT (Ser473) (pAKT), AKT, BIM, PARP, cyclin D1, p27, and β-actin were purchased from Cell Signaling.
Apoptosis and cell cycle analysis
Apoptosis analysis was performed using the Annexin V: FITC Apoptosis Detection Kit II (BD Biosciences, San Jose, CA) following the manufacturer’s protocol. Briefly, cells were seeded and treated with the drugs as described previously for the immunoblotting studies. Afterwards, the cells were washed twice with DPBS and 1 × 106 cells were resuspended in 1 ml of 1x Annexin V Binding Buffer. Cells undergoing apoptotic cell death was analyzed by counting the cells that stained positive for Annexin V FITC and negative for propidium iodide, and late stage of apoptosis, necrosis, or already dead as Annexin V FITC and propidium iodide positive using FACSCalibur (BD Biosciences). Differences of the drug combination group compared to the single drug group was confirmed using an independent two-tailed t-Test, and considered statistically significant when p < 0.05.
For cell cycle analysis, cells were treated with erlotinib (3 μM and 12.5 μM) and RDEA119 (100nM) alone or in combination as described above and harvested by trypsinization. The cells were resuspended and stained with propidium iodide (Sigma-Aldrich, St. Louis, MO) in a modified Krishan buffer (19) for one hour at 4°C. The propidium iodide stained samples were then analyzed with a FACScan flow cytometer (BD Immunocytometry systems, San Jose, CA). Histograms were analyzed for cell cycle compartments and the percentage of cells at each phase of the cell cycle was calculated using FlowJo (Tree Star, Inc, Ashland, OR) analysis software.
In vivo studies
Animal studies were performed at the Translational Genomics Research Institute Drug Development Services (TD2) under IACUC approved protocols. Female ICR-SCID mice (IcrTac: ICR-Prkdcscid) (Taconic, Hudson, NY) were inoculated subcutaneously in the right flank with 0.1 ml of a 50% RPMI/50% Matrigel™ (BD Biosciences) mixture containing a suspension of BxPC-3 (1 × 107 cells/mouse) or MIA PaCa-2 (5 × 106 cells/mouse) tumor cells. Four days following inoculation, tumors were measured using calipers and tumor weight was calculated using the Study Director V.1.6.80a software (20). Tumor bearing mice were pair-matched into the six groups (8 mice/group) by random equilibration using Study Director (Day 1). Body weights were recorded when the mice were pair-matched and were taken twice weekly thereafter in conjunction with tumor measurements. On Day 1, RDEA119 (6 mg/kg), vehicle control (0.4% CMC), and erlotinib (50 mg/kg) were administered orally, while gemcitabine (BxPC-3, 40 mg/kg; MIAPaCa-2, 20 mg/kg) was administered by intraperitoneal injection. RDEA119 and vehicle control were administered twice daily for eleven days. Erlotinib was dosed daily for 21 days. Gemcitabine was dosed three times per day for four days. The study was terminated when tumor burden exceeded 1000 mg in the Vehicle control group.
Mean tumor growth inhibition (TGI) was calculated utilizing the following formula, with X as the mean tumor weight:
All statistical analyses in the xenograft study were performed with GraphPad Prism v4 software. Differences in final tumor weights were confirmed using an independent one-tailed t-Test, and considered statistically significant when p<0.05.
Results
Inhibition of MAPK1 expression by siRNA sensitizes pancreatic cancer cells to erlotinib
To identify gene targets that, when inhibited, sensitize pancreatic cancer cells to the treatment of erlotinib, we performed siRNA screening in the presence of a serial dilution of erlotinib in BxPC-3 cells using a kinase-focused siRNA library (See Material and Methods). A total of six genes were confirmed (Table 1). Of the six genes, the siRNA silencing of GCK and MAPK1 (ERK2) had the highest sensitizing effects on erlotinib (6-16 and 6-8 fold reduction in IC50, respectively) (Fig. S1A and S1B), whereas BMPR2, BRAF, FLT3, and PRKAB2 had moderate effects (ranging from 2- to 7-fold reduction in IC50) (Fig. S1C-F).
Table 1.
Top ranked gene hits identified from siRNA screening
| siRNA sequences* | Erlotinib IC50 (μM) |
IC50 fold difference to Neg siRNA |
|---|---|---|
| Neg siRNA | 37.9 | – |
| BMPR2_4 | 9.2 | 4.1 |
| BMPR2_6 | 5.3 | 7.2 |
| BRAF_3 | 6.7 | 5.7 |
| BRAF_4 | 6.2 | 6.1 |
| FLT_3 | 1.4 | 2.7 |
| FLT_6 | 8.9 | 4.3 |
| GCK_3 | 5.6 | 6.8 |
| GCK_4 | 2.3 | 16.5 |
| MAPK1_1 | 4.5 | 8.4 |
| MAPK1_2 | 6.2 | 6.1 |
| PRKAB2_5 | 8.4 | 4.5 |
| PRKAB2_6 | 7.1 | 5.4 |
Number after underscore indicates the siRNA sequence number designated by Qiagen.
Due to the high sensitizing effect of its siRNAs and its well known involvement in cancer, MAPK1 was selected for further validation. The specific knock-down of MAPK1 expression by the siRNAs was evaluated by qPCR and Western blotting analysis. Two MAPK1 siRNA sequences (MAPK1_1 and MAPK1_2) were able to achieve more than 80% efficiency in reducing mRNA transcript expression (data not shown) and protein expression levels at 72 hrs in all four cell lines (Fig. 1A and S3). The MAPK1 siRNA and erlotinib treatment in BxPC-3 cells were further repeated with 10 serial dilutions of erlotinib, mock-transfected control (Neg siRNA), and a drug alone control. The MAPK1 siRNA-erlotinib combinations yielded an IC50 of a 6 fold-difference compared to both controls (Fig. 1B). The siRNA and erlotinib treatment was repeated in additional well-characterized pancreatic cancer cell lines, Hs 700T, MIA PaCa-2, and PANC-1, to determine if the sensitizing effects of MAPK1 siRNA was cell-line specific. Of the three cell lines, Hs 700T had an IC50 fold difference of 2.3 and 1.4 compared to the Neg siRNA control (Fig. S2A), while MIA PaCa-2 and PANC-1 exhibited no sensitizing effects in either MAPK1 siRNA (Fig. S2B-C).
Figure 1.
Effect of MAPK1 knock-down by siRNA oligonucleotides on the anti-proliferation activity of erlotinib in BxPC-3 cells. A) Two siRNA sequences (MAPK1_1 and MAPK1_2) effectively knocked down the expression of MAPK1 protein in BxPC-3 cells detected Western blotting. A non-targeting siRNA (Neg siRNA) was used as a negative control. B) Treatment of BxPC-3 cells with MAPK1 siRNA caused a left shift of the erlotinib dose response curve. The % Cell Viability of the siRNA treated cells was normalized to that of the siRNA only treatment. Data are means ± SD from three independent experiments and each experiment was carried out with four replicates per drug concentration
It is interesting to note that BxPC-3 and Hs 700T have previously been reported to have wildtype KRAS whereas MIA PaCa-2 and PANC-1 harbor a mutation at codon 12 (21). Sequencing of the KRAS open reading frame (ORF) of the 4 cell lines used in this study confirms the mutational status (data not shown). We therefore hypothesized that the sensitizing effect of MAPK1 siRNA might be specific to cells harboring wildtype KRAS.
MEK inhibitors synergize with erlotinib to inhibit the proliferation of KRAS wildtype pancreatic cells
Since MAP kinase kinase (MEK) proteins phosphorylate and activate MAPK1, we postulated that small molecular weight inhibitors of MEK1/2 might be able to sensitize pancreatic cancer cells to the treatment of erlotinib. We obtained two MEK inhibitors, RDEA119 and AZD6244 and evaluated their synergism with erlotinib in multiple pancreatic cancer cell lines. The synergistic effect of the combination of RDEA119 and erlotinib was evaluated in the same four pancreatic cancer cell lines used in siRNA studies. It is noted that BxPC-3 and Hs 700T are moderately sensitive to erlotinib, while MIA PaCa-2 and PANC-1 are relatively less sensitive, which is consistent to other studies (Fig. S4) (22). As to RDEA119, PANC-1 is the least sensitive with an IC50 of 1.5 μM, BxPC-3 and Hs 700T are moderately sensitive at with IC50s ranging between 300-700 nM, and MIA PaCa-2 is exquisitely sensitive with an IC50 of 50 nM (15). Similar sensitivity profiles were observed for AZD6244 in these pancreatic cancer cell lines (data not shown).
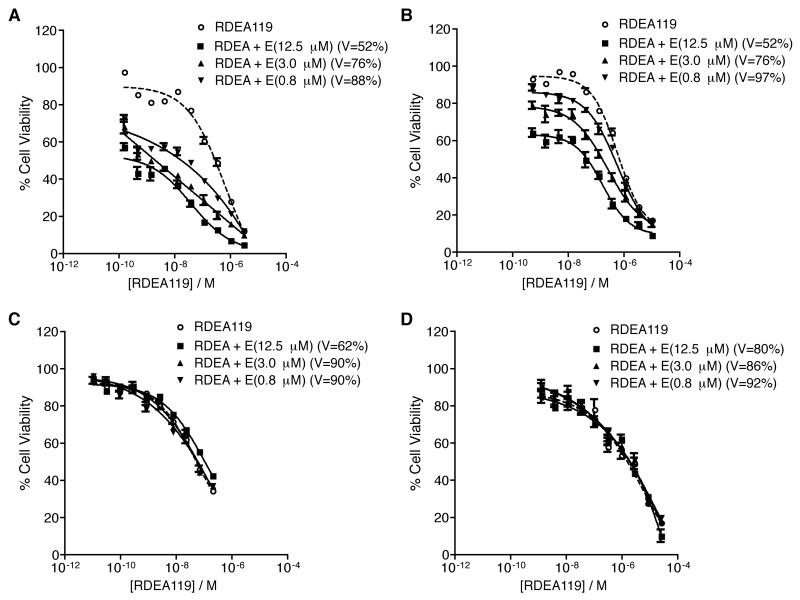
When fixed concentrations of erlotinib (12.5, 3.0, or 0.8 μM) were added to a dose response curve of RDEA119, a significant synergistic effect was observed in BxPC-3 and Hs 700T cells (Fig. 2A-B). As the concentration of erlotinib decreased from 12.5 μM to 0.8 μM, the level of synergy (left shift of the dose response curves) decreased as well, indicating that the level of synergy was dose dependent between erlotinib and RDEA119. The synergistic effect is much more dramatic in BxPC-3 cell line (764-fold at 12.5 μM of erlotinib) than in Hs 700T (14-fold at 12.5 μM of erlotinib) (Table 2). No significant synergy was observed in MIA PaCa-2 and PANC-1 cells at the same drug concentrations (Fig. 2C-D). Higher concentrations of erlotinib (up to 50 μM) did not show any significant synergy with RDEA119 (data not shown) in these two cell lines. Table 2 lists the IC50 values of RDEA119 alone and RDEA119 plus erlotinib as well as the fold reduction when compared to RDEA119 alone in the four pancreatic cancer cell lines. Other pancreatic cancer cell lines, such as AsPC-1, CFPAC-1, and L3.6pl, are similar in sensitivity to EGFR inhibitors as BxPC-3 and Hs 700T, yet contain mutant KRAS (23, 24). No significant synergy was observed in these KRAS mutant cell lines at the same drug combination concentrations (Fig. S5A-C).
Figure 2.
Combination treatment of RDEA119 and erlotinib in pancreatic cancer cells. Three erlotinib concentrations (12.5, 3, and 0.8 μM), each of which was concurrently added on to a serial dilution of RDEA119 in 4 pancreatic cancer cell lines, BxPC-3 (A), Hs 700T (B), MIA PaCa-2 (C), and PANC-1 (D). The drug dose response curves for the combinations have been normalized to the % cell viability of corresponding erlotinib alone treatment (denoted as V= % in the legends). Data are means ± SD from three independent experiments, each of which had four replicates per drug concentration.
Table 2.
IC50 values of the RDEA119 and Erlotinib combination treatments in pancreatic cancer cell lines
| Cell Line | Treatments | RDEA119 IC50 (nM)* | Fold Reduction of IC50 |
|---|---|---|---|
| BxPC-3 | RDEA119 | 268.6 | – |
| RDEA + Erlotinib(12.5μM) | 0.4 | 764.6 | |
| RDEA + Erlotinib(3.0μM) | 2.5 | 108.6 | |
| RDEA + Erlotinib(0.8μM) | 24.5 | 11 | |
| Hs 700T | RDEA119 | 684.1 | – |
| RDEA + Erlotinib(12.5μM) | 48.3 | 14.2 | |
| RDEA + Erlotinib(3.0μM) | 166.8 | 4.1 | |
| RDEA + Erlotinib(0.8μM) | 459.1 | 1.5 | |
| MIA PaCa-2 | RDEA119 | 53.3 | – |
| RDEA + Erlotinib(12.5μM) | 107 | 0.5 | |
| RDEA + Erlotinib(3.0μM) | 60.3 | 0.9 | |
| RDEA + Erlotinib(0.8μM) | 58.5 | 0.9 | |
| PANC-1 | RDEA119 | 1411.1 | – |
| RDEA + Erlotinib(12.5μM) | 1620.6 | 0.9 | |
| RDEA + Erlotinib(3.0μM) | 1608.1 | 0.9 | |
| RDEA + Erlotinib(0.8μM) | 1597.2 | 0 |
IC50 was calculated after the RDEA119 dose response curve was normalized to the effect of the erlotinib only control.
Similar to RDEA119, AZD6244 showed a significant synergistic effect with erlotinib in BxPC-3 and Hs 700T (Fig. S6A-B). The synergism between AZD6244 and erlotinib is also dose dependent, although it is more apparent in Hs 700T than in BxPC-3. Interestingly, the synergistic effect between AZD6244 and erlotinib is more dramatic in Hs 700T cells than in BxCP-3 cells (64 fold vs. 15 fold at 12.5 μM of erlotinib) (Table S1). There was no significant synergy between the two drugs in MIA PaCa-2 and PANC-1 cells (Fig. S6C-D).
Based upon our current observations, we hypothesized that the efficacy of the EGFR and MEK inhibitor combination was limited to cells with wildtype KRAS. To determine if mutant KRAS was the determining factor for the lack of synergy, siRNA targeting the specific KRAS mutation in MIA PaCa-2 and PANC-1 (25) was transfected in combination with the treatment of erlotinib and RDEA119. In MIA PaCa-2, the inhibition of mutant KRAS yielded a synergistic effect between RDEA119 and 50 μM of erlotinib (2.4 fold compared to erlotinib plus RDEA119) (Fig. S7A). As the dose of erlotinib was reducted to 25 μM (Fig. S7B), the synergistic effect was lost. In PANC-1, sensitivity with the KRAS mutatnt specific siRNA plus drug combination was restored to by 1.5 fold compared RDEA119 in combination with erlotinib at 50 μM (Fig S7C), but no difference at 25 μM (Fig. S7D).
Together, these results indicate that the synergism between erlotinib and the MEK inhibitors is restricted to pancreatic cancer cell lines with wildtype KRAS, further supporting the hypothesis that inhibition of MAPK1 function specifically sensitizes KRAS wildtype pancreatic cancer cells to the treatment of erlotinib.
The erlotinib plus MEK inhibitor combination disrupts a negative feedback loop of the EGFR-MAPK signaling pathways
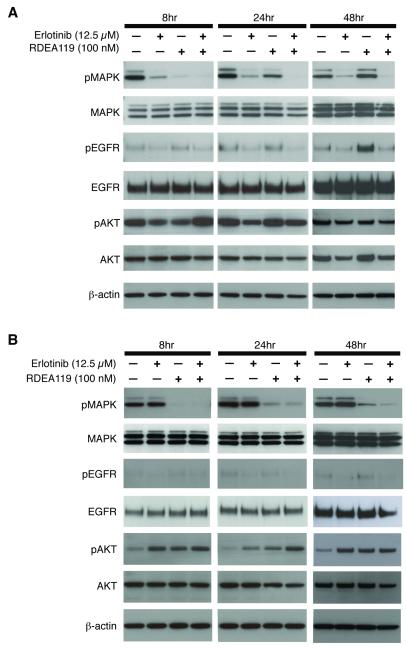
Based on the observed synergistic effect of the erlotinib-RDEA119 combination in inhibiting cell proliferation, we assessed the phosphorylation activities within the EGFR-MAPK signaling pathway. The MAPK signaling pathway regulates cell proliferation and survival, and we hypothesized that the synergy observed in the KRAS wildtype cells were, at least in part, due to the increased inhibition of the MAPK signaling. To examine the effect of drug treatment on MAPK signaling, both BxPC-3 and MIA PaCa-2 cells were treated with 12.5 μM of erlotinib, 100 nM of RDEA119, or the combination for a time course of 8, 24, and 48 hr. The erlotinib concentration used in our experiments (12.5 μM) is comparable to the pharmacokinetic variables documented in patients, which showed the trough, peak plasma, and average steady-state plasma concentrations of 9.6, 15.1, and 11.0 μM, respectively (26-28). The 100 nM concentration of the MEK inhibitor is below pharmacokinetic plasma concentrations obtained in recent clinical studies (29, 30).
In BxPC-3, erlotinib significantly decreased the pMAPK level over the time course while in MIA PaCa-2 there was little effect (Fig. 3), which is expected based on their KRAS mutational status. With the treatment of RDEA119 alone, the pMAPK level was effectively suppressed at 8 hr in both BxPC-3 and MIA PaCa-2. As time progressed the phosphorylation of MAPK was recovered in both cell lines but at a higher level in BxPC-3 to near basal levels by 48 hr. In the combination treatment, BxPC-3 showed sustained ablation of pMAPK activity throughout the time course, while MIA PaCa-2 recovered activity similar to RDEA119 alone by 48 hr.
Figure 3.
The effect of erlotinib and RDEA119 treatment on the phosphorylation of MAPK, EGFR, and AKT in BxPC-3 (A) and MIA PaCa-2 (B) cell lines. Cells were treated with erlotinib (12.5 μM), RDEA119 (100 nM), or a combination of erlotinib and RDEA119 and harvested at indicated hours.
In addition, MEK inhibition led to a marked increase in pEGFR in BxPC-3 cells indicating a feedback between MEK inhibition and EGFR activation (Fig. 3A). This was most apparent at 48 hrs of RDEA119 treatment in BxPC-3, when the level of pEGFR dramatically increased compared to basal levels whereas the total EGFR levels remain the same. This observation was previously also reported in breast and lung cancer cell lines when treated with another MEK inhibitor, AZD6244 (31). The level of pEGFR was not changed in MIA PaCa-2 in any of the treatments. With the increased activation of EGFR by MEK inhibitors, the rationale of the combination of erlotinib and RDEA119 is evident in BxPC-3 as it inhibits both the activity from the receptor tyrosine kinase and its signaling cascade to MAPK.
It has been noted in the literature that disruption of the EGFR-MAPK signaling pathway can shift signaling to AKT as a mode to maintain cell survival (32-34). In either of the drug treatments in MIA PaCa-2, pAKT levels did increase when compared to basal levels of the cells only control, yet the levels were not differentially affected in each respective group as time progressed (Fig. 3B). For BxPC-3, the combination increased pAKT levels at 8 hrs of treatment by 20% above the basal control, but as time progressed to 48 hrs, the combination reduced pAKT levels by 20% compared to basal control levels. RDEA119 alone increased pAKT in BxPC-3, while the erlotinib treatment slightly reduced it (Fig. 3A).
The exquisite sensitivity of MIA PaCa-2 to the MEK inhibitors in our study is comparable to previous reports (35, 36). Results from a recent study reported that sensitivity to MEK inhibition was inversely correlated with the basal level of pAKT (37). In MIA PaCa-2, the basal level of pAKT was comparably lower than BxPC-3 (Fig. 3). Despite its sensitivity, the combination of MEK inhibition and erlotinib was not synergistic in MIA PaCa-2.
Overall, these results showed that the synergy of the drug combination observed in BxPC-3 is likely due to the complete repression of the EGFR-MAPK signaling pathway and reduction of pAKT activity, whereas the lack of synergy in MIA PaCa-2 may be attributed to the residual activity of pMAPK and the increased levels of pAKT.
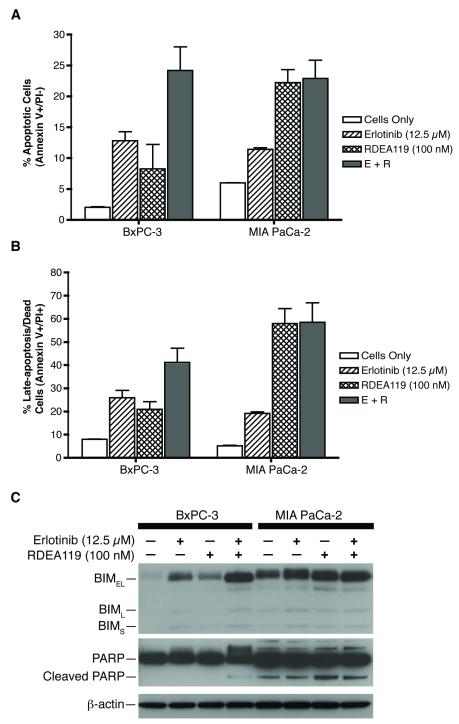
Dual EGFR and MEK inhibition cooperatively induces apoptotic cell death
To further understand the mechanism of the synergism between EGFR and MEK inhibitions, we evaluated the degree of apoptosis with FITC Annexin V flow cytometric analysis and the expression of BIM and PARP in cells treated with erlotinib, RDEA119, or the combination. As shown in Figure 4A, the percentage of cells that were undergoing apoptosis (Annexin V positive/ PI negative) in BxPC-3 were significantly higher in the combination (28%) than the respective single drug treatments (erlotinib, 14% and RDEA119, 12%). The combination of erlotinib and RDEA119 cooperatively induces and enhances apoptotic cell death more than the single agents. The percentage of BxPC-3 cells in late-apoptosis or already dead (Annexin V positive/PI positive) was consistent with 26% with erlotinib, 21% with RDEA119, and 41% with the combination treatment (Fig. 4B). In contrast, in MIA PaCa-2, the erlotinib treatment elicited 12% of the cell population to undergo apoptosis, while RDEA119 was 24% and the combination was 26%. Similarly, the percentage of late-apoptosis was 19% with erlotinib, 58% with RDEA119, and 58% with combination treatment. Comparing RDEA119 and the combination, the level of apoptosis observed in the combination treatment in MIA PaCa-2 is due to mostly the effect of RDEA119 alone. Erlotinib does not synergistically nor additively enhance apoptosis in the combination in MIA PaCa-2.
Figure 4.
Induction of apoptosis by erlotinib and RDEA119 in pancreatic cancer cells. A and B) Apoptosis levels upon erlotinib and RDEA119 treatment detected by Annexin V assay in BxPC-3 and MIA PaCa-2 cells. E+R: erlotinib (12.5 μM) + RDEA119 (100 nM). C) Effect of erlotinib and RDEA119 treatment on BIM expression and PARP cleavage in BxPC-3 and MIA PaCa-2 cells. Data are means ± SD from three independent experiments.
It has been reported that MAPK can inhibit the activity of several pro-apoptotic proteins by promoting its ubiquitination for proteasome-dependent degradation, more notably the BIM protein (38-40). Several studies have reported that BIM is a major target of MAPK-dependent survival signaling, and the up-regulation of BIM was required for committed apoptosis (41, 42). As single agents, erlotinib and RDEA119 had different effects on the pro-apoptotic BIM protein levels (Fig. 4C). In the cell lines treated with the inhibitors, BIMEL was the predominant isoform expressed. BIMEL expression moderately increased in BxPC-3 and MIA PaCa-2 cells treated with either erlotinib or RDEA119, yet the drug combination induced the dephosporylated, stabilized, active form at 48 hrs more so in BxPC-3 than in MIA PaCa-2. Erlotinib or RDEA119 induced comparable levels of BIM expression and moderate apoptosis, but the combination is necessary to potently inhibit the EGFR-MAPK signaling pathway in BxPC-3 and dramatically increase apoptotic cell death. The magnitude and duration of pMAPK inhibition committed BxPC-3 to pro-apoptotic signaling as seen by the up-regulation of BIM expression.
Next, the analysis of cells undergoing apoptosis was evaluated by PARP cleavage. In MIA PaCa-2, there exists a basal level of PARP cleavage in the cells only control, but there was not a significant differential change in PARP cleavage with either drug treatments (Fig. 4C). The basal level of PARP cleavage in MIA PaCa-2 was similar in a previous study examining the in vitro activity of AZD6244 plus rapamycin (43). It is of note that the study used a high dose of AZD6244 at 1 μM to induce PARP cleavage in both BxPC-3 and MIA PaCa-2. In this study, we used 100 nM of RDEA119, which increased PARP cleavage slightly in MIA PaCa-2 compared to those in the cells only control, erlotinib, and the combination. In contrast, PARP cleavage was observed only in the drug combination in BxPC-3, which is consistent with the results from the FITC Annexin V analysis.
Finally, the effects of erlotinib and RDEA119 on the cell cycle distribution were examined. Consistent with the apoptotic immunoblot profiles, a substantial increase in the sub-G1 population was only observed in BxPC-3 in the combination treatment (Table S2). As the concentration of erlotinib was reduced from 12.5 μM to 3 μM, the differential affect on cell cycle was not significant. In MIA PaCa-2, the sub-G1 population was minimal in all of the drug treatments, but did exhibit some cell cycle arrest with an increase of G0/G1 population (Table S3). Accordingly, cyclin D1 levels were down-regulated with an increase of p27 (Fig. S8).
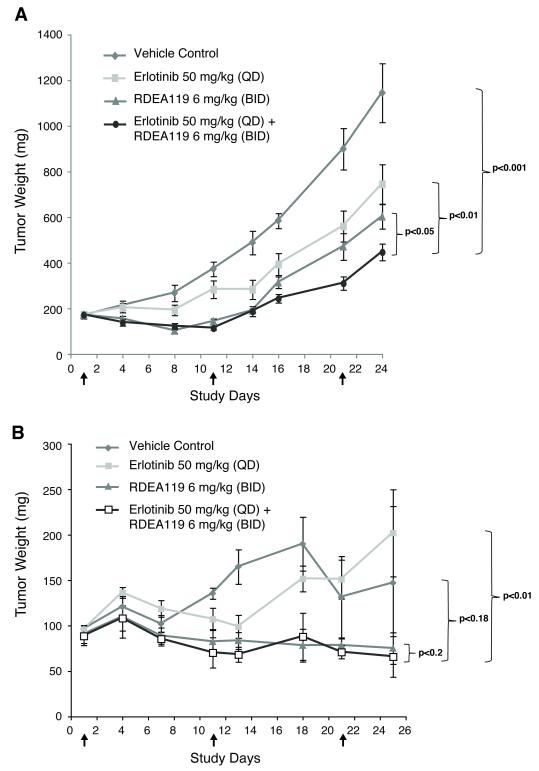
The combination of erlotinib and RDEA119 demonstrates antitumor efficacy in vivo
The antitumor efficacy of the erlotinib plus RDEA119 combination was evaluated in BxPC-3 and MIA PaCa-2 mouse xenografts. Consistent with in vitro observations, a significant effect of tumor growth suppression was seen in BxPC-3 in the combination treatment of erlotinib at 50 mg/kg and RDEA119 at 6 mg/kg with a TGI of 67% when compared to vehicle (p<0.001), and single drug dose controls of erlotinib had a TGI of 42% (p<0.05) and RDEA119 of 52% (p<0.01) by Day 24 (Fig. 5A). Similar antitumor effects were observed for a second dosing regimen (erlotinib at 50 mg/kg and RDEA119 at 12 mg/kg) (Data not shown). In MIA PaCa-2, no significant effect of tumor growth suppression was observed in the drug combination compared to vehicle (p=0.18) and RDEA119 (p=0.2) (Fig. 5B).
Figure 5.
Inhibition of tumor growth by erlotinib and RDEA119 as single agents or in combination in the BxPC-3 and MIA PaCa-2 xenograft model. Tumor growth curves for the different treatment groups in BxPC-3 (A) and MIA PaCa-2 (B). Mouse body weight curves for different treatment groups in BxPC-3 (C) and MIA PaCa-2 (D). Arrows indicate the treatment start and end dates.
Since the combination of erlotinib plus gemcitabine has shown small yet statistically significant survival advantage over patients treated with gemcitabine alone, we evaluated if the addition of gemcitabine to the erlotinib and RDEA119 combination treatment would enhance tumor growth suppression. The addition of gemcitabine significantly reduced tumor growth in BxPC-3 when compared to the combination treatment (p<0.01) (Fig. S9A). In MIA PaCa-2, gemcitabine did not enhance tumor suppression in addition to the erlotinib and RDEA119 combination treatment (p=0.13) (Fig. S9B).
The effects of drug treatment did not notably affect the body weight of the mice during the treatment course. All treatment regiments were well tolerated with minimal weight losses (<10%) that are similar to that of vehicle control (Fig. 5C-D and S9C-D).
Discussion
There have been multiple investigations of chemotherapeutics that target EGFR, and thereby inhibiting the EGFR-MAPK signaling pathway in solid tumors. However, response rates for single agent remain relatively modest unless the EGFR-targeted therapy is combined with other chemotherapeutics or radiation (44). The use of high-throughput siRNA (HT-siRNA) screening is a powerful method in the development of therapeutic compounds, from target discovery to validation and elucidating mechanisms of action (45). In this study, we presented evidence of how efficacious the HT-siRNA screen was in discovering a rational drug combination to erlotinib in pancreatic cancer cells. Results from the screen identified several gene targets, that when inhibited, would enhance the cytotoxic activity of erlotinib. The targets identified in the screen may also present potential use molecular targets and provide interesting insight into molecular interactions of EGFR signaling inhibition in pancreatic cancer. Bone morphogenetic protein 2 (BMP-2) and its receptors BMPR1 and BMPR2 are overexpressed in pancreatic cancer cells. The aberrant activation of BMP-2 and its receptors have been linked to significantly activate MAPK1 in pancreatic cancer cell lines, and treatment of a MAPK inhibitor blocks the BMP-2 mitogenic effects (46). Recently, in BRAF wildtype cells, RAF inhibitors can increase CRAF activity resulting in an increase of MEK/ERK phosphorylation and enhanced growth (47). The identification of the inhibition of BMPR2 and BRAF as enhancers to erlotinib further highlights how the activation of alternative protein kinases in the EGFR-MAPK pathway enables cancer cells to maintain the oncogenic potential when EGFR is inhibited.
Sunitinib, a multi-targeted receptor tyrosine kinase inhibitor, FLT3 among them (48), has been reported to sensitize pancreatic cancer cells to ionizing radiation by attenuating pAKT and pERK levels (49). GCK phosphorylates glucose to provide glucose-6-phosphate for the synthesis of glycogen and preferentially expresses in hepatocytes and pancreatic beta cells. PRKAB2 is a regulatory subunit of AMP-activated protein kinase, which regulates the intercellular metabolism of fatty acids and glycogen. Although both GCK and PRKAB2 have not been studied in the context of pancreatic cancer, both genes have been associated to diabetes (50-52).
The use of MEK inhibitors to perturb the activity of MAPK has been reported to have efficacy in pancreatic models (36, 53). Other groups have reported on the synergistic effect of EGFR and MEK inhibition. Jimeno et al reported on the efficacy of the combined EGFR and MAPK inhibitors in biliary and pancreatic cancer. Their discovery was based upon global analysis of gene expression profiles on biliary cancer cell lines that were resistant to EGFR-targeted therapeutics, gefitinib and erlotinib, and then confirmed the activity in vivo in human pancreatic cancer xenografts with minimal mechanistic studies to explain the synergy in their pancreatic cancer model (54). In EGFR-dependent lung cancer cell lines, Balko et al observed synergistic cytotoxicity with the combination of EGFR and MEK inhibitors only in cells with wildtype KRAS (55). Yoon et al recently reported the synergistic effect between gefitinib and AZD6244 in gastric and lung cancer cells (56, 57). The authors found that the combination treatment repressed AKT and ERK activation and consequently enhanced apoptosis.
The occurrence of somatic KRAS mutations is a highly predictive marker of resistance to anti-EGFR chemotherapies. In colorectal cancer, KRAS mutations were associated with resistance to anti-EGFR therapies (cetuximab and panitumumab) in patients with metastatic disease, while KRAS wildtype predicted efficacy in terms of tumor response and patient survival (58-60). In non-small cell lung cancer, KRAS mutations are attributed to the poor response to erlotinib and gefitinib (61). In pancreatic cancer, the combination of gemcitabine and erlotinib exhibited modest (6.24 vs. 5.91 months) but statistically significant improvement in overall survival (7). As KRAS mutations are reported so prevalently in pancreatic cancer, our study does raise the issue of the status of KRAS for patients that may benefit from the treatment of erlotinib plus a MEK inhibitor.
In summary, we have shown a synergistic effect between erlotinib and two clinically relevant MEK inhibitors, RDEA119 and AZD6244, in a subset of KRAS wildtype pancreatic cancer cells in vitro and in vivo. Our results provide a clear biological rationale for the investigation of erlotinib in combination with a MEK inhibitor in KRAS wildtype pancreatic cancer.
Supplementary Material
Figure S1. Drug dose response curves of the treatment of BxPC-3 cells by combination of the respective siRNA oligonucleotides and erlotinib from the siRNA library screen. siRNA and erlotinib combination curves shown have been normalized to the percent cell viability of the respective siRNA sequence. (A) GCK, (B) MAPK1, (C) FLT3, (D) BRAF, (E) BMPR2, and (F) PRKAB2.
Figure S2. Effect of MAPK1 knock-down by siRNA oligonucleotides on the anti-proliferation activity of erlotinib in pancreatic cells. Treatment of KRAS wildtype Hs 700T cells with MAPK1 siRNA caused a left shift of erlotinib dose response curves (A), but none in KRAS mutant cells, MIA PaCa-2 (B), and PANC-1 (C). The % Cell Viability of the siRNA treated cells was normalized to that of the siRNA treatment. All experiments were repeated three times independently and data are means ± SD. Dose response curves had four replicates per drug treatment group.
Figure S3. Two siRNA sequences (MAPK1_1 and MAPK1_2) effectively knocked down the expression of MAPK1 in Hs700T, MIA PaCa-2, and PANC-1 cells at both mRNA (A) and protein level (B) detected by real-time RT-PCR and Western blotting, respectively. A scrambled, non-targeting siRNA (Neg siRNA) was used as a mock-transfected control. All experiments were repeated three times independently and data are means ± SD. Immunoblots shown are the best representatives of all experiments repeated two times independently with two separate blots analyzed.
Figure S4. Drug dose response curves of the pancreatic cancer cells to the treatment of erlotinib. The concentrations of erlotinib were made in 1:2 serial dilutions in serum-free medium and then add to the cells for a 96-hr incubation period. The IC50 value of BxPC-3 is 12 μM and Hs 700T is 5 μM. The IC50s for MIA PaCa-2 and PANC-1 were not reached at the high concentration of erlotinib. All experiments were repeated three times independently and data are means ± SD. Dose response curves had four replicates per drug treatment group.
Figure S5. Combination treatment of AZD6244 and erlotinib in pancreatic cancer cells. Three erlotinib concentrations (12.5, 3, and 0.8 μM), each of which was concurrently added on to a serial dilution of AZD6244 in 4 pancreatic cancer cell lines, BxPC-3 (A), Hs 700T (B), MIA PaCa-2 (C), and PANC-1 (D). The drug dose response curves for the combinations have been normalized to % cell viability of corresponding erlotinib alone treatment (denoted as V= % in the legends). All experiments were repeated three times independently and data are means ± SD. Dose response curves had four replicates per drug treatment group.
Figure S6. Combination treatment of RDEA119 and erlotinib in pancreatic cancer cells harboring KRAS mutations. Four erlotinib concentrations (12.5, 3, 0.8, and 0.2 μM), each of which was concurrently added on to a serial dilution of RDEA119 in 3 pancreatic cancer cell lines, AsPC-1 (A), CFPAC-1 (B), and L3.6pl (C), The drug dose response curves for the combinations have been normalized to % cell viability of corresponding erlotinib alone treatment (denoted as V= % in the legends). All experiments were repeated three times independently and data are means ± SD. Dose response curves had four replicates per drug treatment group.
Figure S7. Effect of mutant KRAS knock-down by siRNA oligonucleotides in the combination treatment of the RDEA119 and erlotinib in pancreatic cells. Treatment of KRAS mutant specific siRNA in MIA PaCa-2 caused a left shift of the RDEA119 plus 50 μM erlotinib dose response curve (A), but not in the RDEA119 plus 25 μM erlotinib (B). In PANC-1, the left shift of the KRAS siRNA treatment in the drug combination of RDEA119 and erlotinib was also observed at 50 μM of erlotinib (C), but not at 25 μM erlotinib (D). The % Cell Viability of the siRNA treated cells was normalized to that of the siRNA treatment. Dose response curves had four replicates per drug treatment group.
Figure S8. The effect of erlotinib and RDEA119 treatment on cyclin D1 and p27 protein levels in pancreatic cancer cell lines.
Figure S9. Inhibition of tumor growth by gemcitabine as a single agent or in combination with erlotinib and RDEA119 in the BxPC-3 and MIA PaCa-2 xenograft models. Tumor growth curves for the different treatment groups in BxPC-3 (A) and MIA PaCa-2 (B). Mouse body weight curves for different treatment groups in BxPC-3 (C) and MIA PaCa-2 (D).
Statement of Translational Relevance.
With a 5-year survival rate of less than 5%, pancreatic cancer is among the most lethal types of human cancers. Current therapies are mostly ineffective and new therapies are desperately needed. This manuscript describes the synergistic effect between erlotinib and MEK inhibitors in pancreatic cancer cells and animal models. We demonstrate that the combination of erlotinib, an EGFR inhibitor, and several MEK inhibitors have enhanced efficacy in pancreatic cancer cells with wildtype KRAS. This work is highly translational since further evaluation of the combination treatment may result in new and improved treatment for patients with pancreatic cancer.
Acknowledgements
We would like to thank Jill Muehling and Kristen Bisanz for assistance with the STR profiling of the cell lines, and Carole Viso and Tammy Brehm-Gibson for cell cycle analysis. This work was supported by funding from the National Foundation for Cancer Research (NFCR) and a NCI grant CA109552.
References
- 1.Cancer Facts & Figures 2010. American Cancer Society; Atlanta: 2010. [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 3.Scheithauer W, Schull B, Ulrich-Pur H, Schmid K, Raderer M, Haider K, et al. Biweekly high-dose gemcitabine alone or in combination with capecitabine in patients with metastatic pancreatic adenocarcinoma: a randomized phase II trial. Ann Oncol. 2003;14:97–104. doi: 10.1093/annonc/mdg029. [DOI] [PubMed] [Google Scholar]
- 4.Colucci G, Labianca R, Di Costanzo F, Gebbia V, Carteni G, Massidda B, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol. 28:1645–51. doi: 10.1200/JCO.2009.25.4433. [DOI] [PubMed] [Google Scholar]
- 5.Lima CM Rocha, Green MR, Rotche R, Miller WH, Jr., Jeffrey GM, Cisar LA, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22:3776–83. doi: 10.1200/JCO.2004.12.082. [DOI] [PubMed] [Google Scholar]
- 6.Dancer J, Takei H, Ro JY, Lowery-Nordberg M. Coexpression of EGFR and HER-2 in pancreatic ductal adenocarcinoma: a comparative study using immunohistochemistry correlated with gene amplification by fluorescencent in situ hybridization. Oncol Rep. 2007;18:151–5. [PubMed] [Google Scholar]
- 7.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 8.Bamford S, Dawson E, Forbes S, Clements J, Pettett R, Dogan A, et al. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br J Cancer. 2004;91:355–8. doi: 10.1038/sj.bjc.6601894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deramaudt T, Rustgi AK. Mutant KRAS in the initiation of pancreatic cancer. Biochim Biophys Acta. 2005;1756:97–101. doi: 10.1016/j.bbcan.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Hruban RH, van Mansfeld AD, Offerhaus GJ, van Weering DH, Allison DC, Goodman SN, et al. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993;143:545–54. [PMC free article] [PubMed] [Google Scholar]
- 11.van Heek NT, Meeker AK, Kern SE, Yeo CJ, Lillemoe KD, Cameron JL, et al. Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am J Pathol. 2002;161:1541–7. doi: 10.1016/S0002-9440(10)64432-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurent-Puig P, Lievre A, Blons H. Mutations and response to epidermal growth factor receptor inhibitors. Clin Cancer Res. 2009;15:1133–9. doi: 10.1158/1078-0432.CCR-08-0905. [DOI] [PubMed] [Google Scholar]
- 13.Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature. 2002;418:934. doi: 10.1038/418934a. [DOI] [PubMed] [Google Scholar]
- 14.Yeh TC, Marsh V, Bernat BA, Ballard J, Colwell H, Evans RJ, et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin Cancer Res. 2007;13:1576–83. doi: 10.1158/1078-0432.CCR-06-1150. [DOI] [PubMed] [Google Scholar]
- 15.Iverson C, Larson G, Lai C, Yeh LT, Dadson C, Weingarten P, et al. RDEA119/BAY 869766: a potent, selective, allosteric inhibitor of MEK1/2 for the treatment of cancer. Cancer Res. 2009;69:6839–47. doi: 10.1158/0008-5472.CAN-09-0679. [DOI] [PubMed] [Google Scholar]
- 16.Collins PJ, Hennessy LK, Leibelt CS, Roby RK, Reeder DJ, Foxall PA. Developmental validation of a single-tube amplification of the 13 CODIS STR loci, D2S1338, D19S433, and amelogenin: the AmpFlSTR Identifiler PCR Amplification Kit. J Forensic Sci. 2004;49:1265–77. [PubMed] [Google Scholar]
- 17.Azorsa DO, Gonzales IM, Basu GD, Choudhary A, Arora S, Bisanz KM, et al. Synthetic lethal RNAi screening identifies sensitizing targets for gemcitabine therapy in pancreatic cancer. J Transl Med. 2009;7:43. doi: 10.1186/1479-5876-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975;66:188–93. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Britten CD, Hilsenbeck SG, Eckhardt SG, Marty J, Mangold G, MacDonald JR, et al. Enhanced antitumor activity of 6-hydroxymethylacylfulvene in combination with irinotecan and 5-fluorouracil in the HT29 human colon tumor xenograft model. Cancer Res. 1999;59:1049–53. [PubMed] [Google Scholar]
- 21.Ohnami S, Matsumoto N, Nakano M, Aoki K, Nagasaki K, Sugimura T, et al. Identification of genes showing differential expression in antisense K-ras-transduced pancreatic cancer cells with suppressed tumorigenicity. Cancer Res. 1999;59:5565–71. [PubMed] [Google Scholar]
- 22.Buck E, Eyzaguirre A, Haley JD, Gibson NW, Cagnoni P, Iwata KK. Inactivation of Akt by the epidermal growth factor receptor inhibitor erlotinib is mediated by HER-3 in pancreatic and colorectal tumor cell lines and contributes to erlotinib sensitivity. Mol Cancer Ther. 2006;5:2051–9. doi: 10.1158/1535-7163.MCT-06-0007. [DOI] [PubMed] [Google Scholar]
- 23.Pino MS, Shrader M, Baker CH, Cognetti F, Xiong HQ, Abbruzzese JL, et al. Transforming growth factor alpha expression drives constitutive epidermal growth factor receptor pathway activation and sensitivity to gefitinib (Iressa) in human pancreatic cancer cell lines. Cancer Res. 2006;66:3802–12. doi: 10.1158/0008-5472.CAN-05-3753. [DOI] [PubMed] [Google Scholar]
- 24.Buck E, Eyzaguirre A, Barr S, Thompson S, Sennello R, Young D, et al. Loss of homotypic cell adhesion by epithelial-mesenchymal transition or mutation limits sensitivity to epidermal growth factor receptor inhibition. Mol Cancer Ther. 2007;6:532–41. doi: 10.1158/1535-7163.MCT-06-0462. [DOI] [PubMed] [Google Scholar]
- 25.Fleming JB, Shen GL, Holloway SE, Davis M, Brekken RA. Molecular consequences of silencing mutant K-ras in pancreatic cancer cells: justification for K-ras-directed therapy. Mol Cancer Res. 2005;3:413–23. doi: 10.1158/1541-7786.MCR-04-0206. [DOI] [PubMed] [Google Scholar]
- 26.Petty WJ, Dragnev KH, Memoli VA, Ma Y, Desai NB, Biddle A, et al. Epidermal growth factor receptor tyrosine kinase inhibition represses cyclin D1 in aerodigestive tract cancers. Clin Cancer Res. 2004;10:7547–54. doi: 10.1158/1078-0432.CCR-04-1169. [DOI] [PubMed] [Google Scholar]
- 27.Tan AR, Yang X, Hewitt SM, Berman A, Lepper ER, Sparreboom A, et al. Evaluation of biologic end points and pharmacokinetics in patients with metastatic breast cancer after treatment with erlotinib, an epidermal growth factor receptor tyrosine kinase inhibitor. J Clin Oncol. 2004;22:3080–90. doi: 10.1200/JCO.2004.08.189. [DOI] [PubMed] [Google Scholar]
- 28.Hidalgo M, Siu LL, Nemunaitis J, Rizzo J, Hammond LA, Takimoto C, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol. 2001;19:3267–79. doi: 10.1200/JCO.2001.19.13.3267. [DOI] [PubMed] [Google Scholar]
- 29.Adjei AA, Cohen RB, Franklin W, Morris C, Wilson D, Molina JR, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol. 2008;26:2139–46. doi: 10.1200/JCO.2007.14.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banerji U, Camidge DR, Verheul HM, Agarwal R, Sarker D, Kaye SB, et al. The first-in-human study of the hydrogen sulfate (Hyd-sulfate) capsule of the MEK1/2 inhibitor AZD6244 (ARRY-142886): a phase I open-label multicenter trial in patients with advanced cancer. Clin Cancer Res. 2010;16:1613–23. doi: 10.1158/1078-0432.CCR-09-2483. [DOI] [PubMed] [Google Scholar]
- 31.Faber AC, Li D, Song Y, Liang MC, Yeap BY, Bronson RT, et al. Differential induction of apoptosis in HER2 and EGFR addicted cancers following PI3K inhibition. Proc Natl Acad Sci U S A. 2009;106:19503–8. doi: 10.1073/pnas.0905056106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Normanno N, De Luca A, Maiello MR, Campiglio M, Napolitano M, Mancino M, et al. The MEK/MAPK pathway is involved in the resistance of breast cancer cells to the EGFR tyrosine kinase inhibitor gefitinib. J Cell Physiol. 2006;207:420–7. doi: 10.1002/jcp.20588. [DOI] [PubMed] [Google Scholar]
- 33.Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–41. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buck E, Eyzaguirre A, Rosenfeld-Franklin M, Thomson S, Mulvihill M, Barr S, et al. Feedback mechanisms promote cooperativity for small molecule inhibitors of epidermal and insulin-like growth factor receptors. Cancer Res. 2008;68:8322–32. doi: 10.1158/0008-5472.CAN-07-6720. [DOI] [PubMed] [Google Scholar]
- 35.Allen LF, Sebolt-Leopold J, Meyer MB. CI-1040 (PD184352), a targeted signal transduction inhibitor of MEK (MAPKK) Semin Oncol. 2003;30:105–16. doi: 10.1053/j.seminoncol.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Davies BR, Logie A, McKay JS, Martin P, Steele S, Jenkins R, et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther. 2007;6:2209–19. doi: 10.1158/1535-7163.MCT-07-0231. [DOI] [PubMed] [Google Scholar]
- 37.Pratilas CA, Hanrahan AJ, Halilovic E, Persaud Y, Soh J, Chitale D, et al. Genetic predictors of MEK dependence in non-small cell lung cancer. Cancer Res. 2008;68:9375–83. doi: 10.1158/0008-5472.CAN-08-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ewings KE, Hadfield-Moorhouse K, Wiggins CM, Wickenden JA, Balmanno K, Gilley R, et al. ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL. EMBO J. 2007;26:2856–67. doi: 10.1038/sj.emboj.7601723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hubner A, Barrett T, Flavell RA, Davis RJ. Multisite phosphorylation regulates Bim stability and apoptotic activity. Mol Cell. 2008;30:415–25. doi: 10.1016/j.molcel.2008.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J Biol Chem. 2003;278:18811–6. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 41.Deng J, Shimamura T, Perera S, Carlson NE, Cai D, Shapiro GI, et al. Proapoptotic BH3-only BCL-2 family protein BIM connects death signaling from epidermal growth factor receptor inhibition to the mitochondrion. Cancer Res. 2007;67:11867–75. doi: 10.1158/0008-5472.CAN-07-1961. [DOI] [PubMed] [Google Scholar]
- 42.Gong Y, Somwar R, Politi K, Balak M, Chmielecki J, Jiang X, et al. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med. 2007;4:e294. doi: 10.1371/journal.pmed.0040294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang Q, Chen E, Hedley DW. Effects of combined inhibition of MEK and mTOR on downstream signaling and tumor growth in pancreatic cancer xenograft models. Cancer Biol Ther. 2009;8:1893–901. doi: 10.4161/cbt.8.20.9430. [DOI] [PubMed] [Google Scholar]
- 44.Baselga J, Arteaga CL. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol. 2005;23:2445–59. doi: 10.1200/JCO.2005.11.890. [DOI] [PubMed] [Google Scholar]
- 45.Kramer R, Cohen D. Functional genomics to new drug targets. Nat Rev Drug Discov. 2004;3:965–72. doi: 10.1038/nrd1552. [DOI] [PubMed] [Google Scholar]
- 46.Kleeff J, Maruyama H, Ishiwata T, Sawhney H, Friess H, Buchler MW, et al. Bone morphogenetic protein 2 exerts diverse effects on cell growth in vitro and is expressed in human pancreatic cancer in vivo. Gastroenterology. 1999;116:1202–16. doi: 10.1016/s0016-5085(99)70024-7. [DOI] [PubMed] [Google Scholar]
- 47.Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 464:431–5. doi: 10.1038/nature08833. [DOI] [PubMed] [Google Scholar]
- 48.O’Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101:3597–605. doi: 10.1182/blood-2002-07-2307. [DOI] [PubMed] [Google Scholar]
- 49.Cuneo KC, Geng L, Fu A, Orton D, Hallahan DE, Chakravarthy AB. SU11248 (sunitinib) sensitizes pancreatic cancer to the cytotoxic effects of ionizing radiation. Int J Radiat Oncol Biol Phys. 2008;71:873–9. doi: 10.1016/j.ijrobp.2008.02.062. [DOI] [PubMed] [Google Scholar]
- 50.Hanson RL, Ehm MG, Pettitt DJ, Prochazka M, Thompson DB, Timberlake D, et al. An autosomal genomic scan for loci linked to type II diabetes mellitus and body-mass index in Pima Indians. Am J Hum Genet. 1998;63:1130–8. doi: 10.1086/302061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iynedjian PB. Molecular physiology of mammalian glucokinase. Cell Mol Life Sci. 2009;66:27–42. doi: 10.1007/s00018-008-8322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iynedjian PB. Mammalian glucokinase and its gene. Biochem J. 1993;293(Pt 1):1–13. doi: 10.1042/bj2930001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rinehart J, Adjei AA, Lorusso PM, Waterhouse D, Hecht JR, Natale RB, et al. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol. 2004;22:4456–62. doi: 10.1200/JCO.2004.01.185. [DOI] [PubMed] [Google Scholar]
- 54.Jimeno A, Rubio-Viqueira B, Amador ML, Grunwald V, Maitra A, Iacobuzio-Donahue C, et al. Dual mitogen-activated protein kinase and epidermal growth factor receptor inhibition in biliary and pancreatic cancer. Mol Cancer Ther. 2007;6:1079–88. doi: 10.1158/1535-7163.MCT-06-0448. [DOI] [PubMed] [Google Scholar]
- 55.Balko JM, Jones BR, Coakley VL, Black EP. Combined MEK and EGFR inhibition demonstrates synergistic activity in EGFR-dependent NSCLC. Cancer Biol Ther. 2009;8 doi: 10.4161/cbt.8.6.7690. [DOI] [PubMed] [Google Scholar]
- 56.Yoon YK, Kim HP, Han SW, Hur HS, Oh do Y, Im SA, et al. Combination of EGFR and MEK1/2 inhibitor shows synergistic effects by suppressing EGFR/HER3-dependent AKT activation in human gastric cancer cells. Mol Cancer Ther. 2009;8:2526–36. doi: 10.1158/1535-7163.MCT-09-0300. [DOI] [PubMed] [Google Scholar]
- 57.Yoon YK, Kim HP, Han SW, Oh do Y, Im SA, Bang YJ, et al. KRAS mutant lung cancer cells are differentially responsive to MEK inhibitor due to AKT or STAT3 activation: implication for combinatorial approach. Mol Carcinog. 2010;49:353–62. doi: 10.1002/mc.20607. [DOI] [PubMed] [Google Scholar]
- 58.De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, et al. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19:508–15. doi: 10.1093/annonc/mdm496. [DOI] [PubMed] [Google Scholar]
- 59.Di Fiore F, Blanchard F, Charbonnier F, Le Pessot F, Lamy A, Galais MP, et al. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer. 2007;96:1166–9. doi: 10.1038/sj.bjc.6603685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lievre A, Bachet JB, Boige V, Cayre A, Le Corre D, Buc E, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol. 2008;26:374–9. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 61.Massarelli E, Varella-Garcia M, Tang X, Xavier AC, Ozburn NC, Liu DD, et al. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin Cancer Res. 2007;13:2890–6. doi: 10.1158/1078-0432.CCR-06-3043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Drug dose response curves of the treatment of BxPC-3 cells by combination of the respective siRNA oligonucleotides and erlotinib from the siRNA library screen. siRNA and erlotinib combination curves shown have been normalized to the percent cell viability of the respective siRNA sequence. (A) GCK, (B) MAPK1, (C) FLT3, (D) BRAF, (E) BMPR2, and (F) PRKAB2.
Figure S2. Effect of MAPK1 knock-down by siRNA oligonucleotides on the anti-proliferation activity of erlotinib in pancreatic cells. Treatment of KRAS wildtype Hs 700T cells with MAPK1 siRNA caused a left shift of erlotinib dose response curves (A), but none in KRAS mutant cells, MIA PaCa-2 (B), and PANC-1 (C). The % Cell Viability of the siRNA treated cells was normalized to that of the siRNA treatment. All experiments were repeated three times independently and data are means ± SD. Dose response curves had four replicates per drug treatment group.
Figure S3. Two siRNA sequences (MAPK1_1 and MAPK1_2) effectively knocked down the expression of MAPK1 in Hs700T, MIA PaCa-2, and PANC-1 cells at both mRNA (A) and protein level (B) detected by real-time RT-PCR and Western blotting, respectively. A scrambled, non-targeting siRNA (Neg siRNA) was used as a mock-transfected control. All experiments were repeated three times independently and data are means ± SD. Immunoblots shown are the best representatives of all experiments repeated two times independently with two separate blots analyzed.
Figure S4. Drug dose response curves of the pancreatic cancer cells to the treatment of erlotinib. The concentrations of erlotinib were made in 1:2 serial dilutions in serum-free medium and then add to the cells for a 96-hr incubation period. The IC50 value of BxPC-3 is 12 μM and Hs 700T is 5 μM. The IC50s for MIA PaCa-2 and PANC-1 were not reached at the high concentration of erlotinib. All experiments were repeated three times independently and data are means ± SD. Dose response curves had four replicates per drug treatment group.
Figure S5. Combination treatment of AZD6244 and erlotinib in pancreatic cancer cells. Three erlotinib concentrations (12.5, 3, and 0.8 μM), each of which was concurrently added on to a serial dilution of AZD6244 in 4 pancreatic cancer cell lines, BxPC-3 (A), Hs 700T (B), MIA PaCa-2 (C), and PANC-1 (D). The drug dose response curves for the combinations have been normalized to % cell viability of corresponding erlotinib alone treatment (denoted as V= % in the legends). All experiments were repeated three times independently and data are means ± SD. Dose response curves had four replicates per drug treatment group.
Figure S6. Combination treatment of RDEA119 and erlotinib in pancreatic cancer cells harboring KRAS mutations. Four erlotinib concentrations (12.5, 3, 0.8, and 0.2 μM), each of which was concurrently added on to a serial dilution of RDEA119 in 3 pancreatic cancer cell lines, AsPC-1 (A), CFPAC-1 (B), and L3.6pl (C), The drug dose response curves for the combinations have been normalized to % cell viability of corresponding erlotinib alone treatment (denoted as V= % in the legends). All experiments were repeated three times independently and data are means ± SD. Dose response curves had four replicates per drug treatment group.
Figure S7. Effect of mutant KRAS knock-down by siRNA oligonucleotides in the combination treatment of the RDEA119 and erlotinib in pancreatic cells. Treatment of KRAS mutant specific siRNA in MIA PaCa-2 caused a left shift of the RDEA119 plus 50 μM erlotinib dose response curve (A), but not in the RDEA119 plus 25 μM erlotinib (B). In PANC-1, the left shift of the KRAS siRNA treatment in the drug combination of RDEA119 and erlotinib was also observed at 50 μM of erlotinib (C), but not at 25 μM erlotinib (D). The % Cell Viability of the siRNA treated cells was normalized to that of the siRNA treatment. Dose response curves had four replicates per drug treatment group.
Figure S8. The effect of erlotinib and RDEA119 treatment on cyclin D1 and p27 protein levels in pancreatic cancer cell lines.
Figure S9. Inhibition of tumor growth by gemcitabine as a single agent or in combination with erlotinib and RDEA119 in the BxPC-3 and MIA PaCa-2 xenograft models. Tumor growth curves for the different treatment groups in BxPC-3 (A) and MIA PaCa-2 (B). Mouse body weight curves for different treatment groups in BxPC-3 (C) and MIA PaCa-2 (D).