Abstract
Megakaryocytes are large, polyploid cells that produce platelets. We have previously reported that calcium- and integrin-binding protein 1 (CIB1) regulates endomitosis in Dami cells. To further characterize the role of CIB1 in megakaryopoiesis, we used a Cib1−/− mouse model. Cib1−/− mice have more platelets and BM megakaryocytes than wild-type (WT) controls (P < .05). Furthermore, subsequent analysis of megakaryocyte-CFU production revealed an increase with Cib1 deletion compared with WT (P < .05). In addition, BM from Cib1−/− mice, cultured with thrombopoietin (TPO) for 24 hours, produced more highly polyploid megakaryocytes than WT BM (P < .05). Subsequent analysis of TPO signaling revealed enhanced Akt and ERK1/2 phosphorylation, whereas FAKY925 phosphorylation was reduced in Cib1−/− megakaryocytes treated with TPO. Conversely, platelet recovery in Cib1−/− mice after platelet depletion was attenuated compared with WT (P < .05). This could be the result of impaired adhesion and migration, as adhesion to fibrinogen and fibronectin and migration toward an SDF-1α gradient were reduced in Cib1−/− megakaryocytes compared with WT (P < .05). In addition, Cib1−/− megakaryocytes formed fewer proplatelets compared with WT (P < .05), when plated on fibrinogen. These data suggest that CIB1 plays a dual role in megakaryopoiesis, initially by negatively regulating TPO signaling and later by augmenting proplatelet production.
Introduction
Mature megakaryocytes produce platelets by extending proplatelet projections into sinusoidal vessels in BM.1 Before this process, the megakaryocyte must differentiate from progenitor cells by undergoing multiple rounds of endomitosis. Megakaryocyte endomitosis occurs primarily through cell signaling initiated by the cytokine thrombopoietin (TPO), which is produced constitutively in the liver.2 TPO binding to its receptor, c-Mpl, results in the activation of Janus kinase 2 (Jak2). Jak2 phosphorylates tyrosine residues on c-Mpl, which enables the subsequent activation of other signaling pathways, such as PI3K/Akt and MAPK.3–8 In addition, focal adhesion kinase (FAK) is activated and acts as a negative regulator of TPO-induced signaling by activating Lyn, an Src-family kinase.9 Specifically, Western blot analysis revealed that impaired TPO-induced FAK phosphorylation is concomitant with heightened Akt and ERK1/2 phosphorylation.9
Once mature, megakaryocytes must migrate from an “osteoblastic niche” to a “vascular niche” where they interact with BM endothelial cells.10 Integrins play a key role in cell migration, and the most abundantly expressed integrin on the megakaryocyte is αIIbβ3, which is the major receptor for the highly expressed BM extracellular matrix (ECM) protein fibronectin (Fn).11,12 The exact mechanism underlying megakaryocyte migration toward the vasculature is unknown, but stromal cell-derived factor-1α (SDF-1α) appears to provide the homing signal.13
Calcium- and integrin-binding protein 1 (CIB1) was first identified as a binding partner of the megakaryocyte-lineage specific integrin subunit αIIb cytoplasmic domain.14 Recently, we reported that the expression of CIB1 increases in endomitotic cells and that overexpression of CIB1 augmented phorbol 12-myristate 13-acetate (PMA)–induced ploidy in Dami cells, whereas RNAi-mediated knockdown of CIB1 reduced PMA-induced ploidy.15 Furthermore, using Chinese hamster ovary (CHO) cells, we revealed that CIB1 associates with and up-regulates the activity of FAK.16
FAK is also an important mediator of focal adhesion formation and cell migration.17 We have recently shown that CIB1 overexpression in CHO cells causes increased cell migration, enhanced focal adhesion formation, and heightened FAK activity.16 In addition, Zayed et al have demonstrated that cell migration is inhibited in CIB1-depleted endothelial cells and primary endothelial cells isolated from Cib1−/− mice.18
The role of CIB1 in megakaryocyte differentiation and migration is not yet understood. Therefore, we used a Cib1−/− mouse model to determine the function of CIB1 in megakaryocytes. We found that Cib1−/− mice have more platelets and megakaryocytes than wild-type (WT) controls. In addition, TPO treatment of BM revealed that Cib1−/− megakaryocytes achieves higher ploidy than WT, perhaps because of increased Akt and ERK1/2 activity that was concomitant with decreased FAK activity. Likewise, Cib1−/− BM also produced more megakaryocyte progenitors than WT. In contrast, we observed decreased recovery of platelet counts in Cib1−/− mice after immune-induced thrombocytopenia. Furthermore, we observed decreased attachment of Cib1−/− megakaryocytes to Fn and fibrinogen (Fg), as well as decreased migration toward an SDF-1α gradient compared with WT.
Methods
Antibodies and reagents
All reagents were purchased from Sigma-Aldrich unless otherwise stated. Polyclonal antibodies against phospho-Akt (Thr 308), Akt, phospho-ERK1/2, ERK1/2, phospho-FAK (Tyr 925), and FAK were purchased from Cell Signaling Technology. A polyclonal antibody against phospho-FAK (Tyr 397) was purchased from Biosource (Invitrogen). FITC-labeled anti-CD41 was purchased from BD Biosciences. Polyclonal antibodies against c-Mpl were purchased from Santa Cruz Biotechnology and Abcam. Recombinant mouse TPO, IL-3, and SDF-1α were purchased from PeproTech. Cell culture reagents, such as IMDM, penicillin/streptomycin, and heat-inactivated FBS were purchased from Invitrogen.
Animals
Cib1−/− mice were generously provided by Leslie Parise (University of North Carolina, Chapel Hill, NC) and further characterized in our laboratory.19 All procedures were approved by the University of Delaware Institutional Animal Care and Use Committee.
Measurement of hematologic parameters
Platelets and leukocytes in the peripheral blood from 8- to 12-week-old Cib1−/− and WT mice were counted using the Unopette system (BD Biosciences). Submandibular bleeds were used to collect small (20-50 μL) amounts of blood, which was used for either quantification of hematocrit or prepared for platelet and leukocyte enumeration.20 To quantify megakaryocyte number, BM was isolated (described in “Megakaryocyte CFU assay”), stained with FITC-CD41 antibody and 10 μg/mL propidium iodide, and then analyzed by flow cytometry (FACSAria, BD Biosciences). Megakaryocytes were defined as CD41+, nucleated cells, and expressed as a ratio of total nucleated cells.
Platelet production assay
To assess platelet production in vivo, a small sample of blood was collected from the submandibular region and placed in anticoagulant. The blood was then diluted 20× in 2mM EDTA in PBS before the addition of 10 μg/mL thiazole orange for 30 minutes at room temperature (RT) to label reticulated platelets. The samples were then fixed in 1% formalin for 15 minutes and analyzed by flow cytometry. Thiazole orange-positive platelets were considered reticulated.21
Analysis of platelet clearance
To determine platelet clearance in both Cib1−/− and WT mice, an in vivo biotinylation approach was used.21 Briefly, 8- to 12-week-old Cib1−/− and WT mice were injected via tail vein with 35 μg/g body weight sulfo-NHS-biotin (Pierce Chemical). Submandibular bleeds were used to collect blood at the indicated time points. After collection, the blood was diluted 20× in 2mM EDTA in PBS and incubated with streptavidin-PE (BD Biosciences) to label biotinylated platelets for 30 minutes at 4°C. Thiazole orange (10 μg/mL) was then added, and the samples were incubated for 15 minutes at RT. After fixation in 1% formalin, the samples were analyzed via flow cytometry with appropriate color compensation.
Megakaryocyte CFU assay
Megakaryocyte CFUs were assayed as previously described with minor modifications.22 Briefly, BM was flushed from femurs and tibiae of Cib1+/+ and Cib1−/− mice using IMDM. A single-cell suspension was created using a 22-gauge needle, and red cells were lysed using the following buffer: 0.15M NH4Cl, 10mM KHCO3, 0.1mM Na2EDTA, pH 7.4. BM cells were washed in PBS, and 105 cells per well were added to a 48-well dish containing IMDM supplemented with 10 ng/mL IL-3 and 50 ng/mL TPO. The cells were allowed to grow for 7 to 12 days and stained for acetylcholinesterase activity as described by MegaCult-C (StemCell Technologies). In brief, the colonies were fixed in ice-cold acetone for 5 minutes and incubated for 6 hours with a staining solution containing 0.05% acetylcholiniodide, 5mM sodium citrate, 3mM copper sulfate, and 0.5mM potassium ferricyanide in 0.1M phosphate buffer, pH 6.0. The number of colonies (at least 3 cells per colony) in each well was recorded. The colonies were imaged with a 20× objective using a Nikon light microscope equipped with a Coolpix camera (Melvin).
Megakaryocyte ploidy analysis
BM cell preparation was performed as described in “Megakaryocyte CFU assay.” For baseline ploidy analysis, cells were analyzed as described previously with minor modifications.23 After BM isolation, 2 × 106 cells were labeled with an FITC-conjugated CD41 antibody for 30 minutes at 4°C. The cells were then washed twice in 2mM EDTA in PBS and fixed in 0.5% formalin for 10 minutes at RT. After fixing, the washed cells were permeabilized with 70% methanol for 1 hour at 4°C. The cells were washed again, and RNA was digested by 10 minutes of RNAse treatment. Propidium iodide (10 μg/mL) was then added, and cells were analyzed via flow cytometry.
Confocal microscopy
Spleens from WT and Cib1−/− mice were embedded in an optimal cutting temperature compound (Tissue Tek), sectioned at 7 μm on a cryostat, and fixed in acetone/methanol for 20 minutes at 4°C. The sections were then blocked in 3% BSA for 1 hour at RT before staining with FITC-conjugated anti-CD41 antibody (1:200) for 30 minutes at RT. The sections were then washed with PBS containing Draq5 (1:5000) for 10 minutes at RT, followed by 3 successive 10-minute washes with PBS at RT. The sections were imaged using a Zeiss (Carl Zeiss) 5-live confocal microscope.
Platelet recovery experiments
Platelet recovery was assessed after experimental immune-induced thrombocytopenia.9 Briefly, baseline platelet counts were measured in WT and Cib1−/− mice before intraperitoneal injection with 0.5 μg/g anti–mouse CD41 antibody. Platelet counts were monitored every 24 hours for 7 days via submandibular bleed. In a separate set of experiments, WT and Cib1−/− mice were killed 72 hours after injection, and their BM was collected for ploidy analysis.
Western blotting
The megakaryocyte population was enriched by culturing BM in IMDM with 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50 ng/mL recombinant mouse TPO for 5 days. Megakaryocytes were purified using a discontinuous BSA gradient and serum-starved overnight. Megakaryocytes were then washed in HBSS, resuspended in HBSS, and treated with 50 ng/mL TPO for 10 minutes. The cells were then centrifuged at 340g for 3 minutes and lysed using ice-cold lysis buffer (1% NP-40, 150mM NaCl, 50mM Tris-HCl, 10 μg/mL each aprotinin and leupeptin, and 1mM each NaF, sodium orthovanadate, and PMSF). The lysate was boiled with 2× Laemmeli sample buffer before loading. The proteins were then resolved using SDS-PAGE and transferred to polyvinylidene membrane. Membranes were probed for phospho-AktT308 or phospho-ERK1/2. Equal loading was assessed after stripping the membrane in Western Restore (Thermo Scientific) and reprobing with anti-Akt, anti-ERK1/2, or anti–β-actin. To evaluate FAK phosphorylation in Cib1−/− and WT megakaryocytes after TPO treatment, megakaryocytes were lysed in sample buffer consisting of equal parts 2× Laemmeli buffer and lysis buffer before resolution via SDS-PAGE.
Transwell migration assay
To assess megakaryocyte migration, we used a Neuro Probe, 96-well chemotaxis chamber with a 12-μm pore membrane coated with 20 μg/mL Fn. The bottom chamber contained serum-free IMDM with 0.2% BSA and 50 ng/mL recombinant SDF-1α. Megakaryocytes were serum starved overnight, and 2 × 105 cells were loaded in the upper chamber containing serum-free IMDM with 0.2% BSA. The cells were allowed to migrate for 5 hours at 37°C, the unmigrated cells were removed, and the membrane was stained with Diff-Quik (Dade Behring) to visualize the megakaryocytes. The total number of migrated cells was recorded for each well.
Adhesion assay
Enriched megakaryocytes were loaded with 5μM calcein-AM according to the manufacturer's instructions (Invitrogen). Calcein-labeled WT or Cib1−/− megakaryocytes (105) were allowed to attach to 96-well plates precoated with 10 μg/mL Fg, 20 μg/mL Fn, or 3% BSA for 3 hours at 37°C. The cells were then washed 4 times in PBS before determining the fluorescence of the attached cells using a Victor2 96-well plate reader (PerkinElmer Life and Analytical Sciences). The fluorescence of the total plated cells was determined before washing.
Proplatelet production
Proplatelet production was assessed as previously described, with minor modifications.24 Briefly, BM megakaryocytes were expanded and purified using a discontinuous BSA gradient before plating on 200 μg/mL immobilized Fg for 5 hours at 37°C. The megakaryocytes were then imaged using a Zeiss Axiovert microscope, and the number of megakaryocytes producing proplatelets was enumerated.
Expression of c-Mpl
Expression of c-Mpl protein in platelets and BM cells was first determined by Western blot analysis using anti–c-Mpl (Abcam), which recognizes a 95-kDa mature c-Mpl protein.25 Surface expression of c-Mpl protein in platelets and BM cells was also determined by flow cytometry (Accuri6, BD Biosciences) using anti–c-Mpl (Santa Cruz Biotechnology).
Statistical analysis
The data presented in this report are the results of at least 3 independent experiments. Statistical analysis was performed using a 2-tailed Student t test, and a P value < .05 was considered statistically significant.
Results
Deletion of Cib1 results in an increased number of circulating platelets
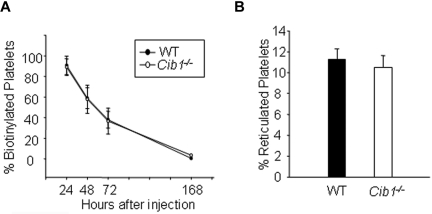
When platelets and leukocytes were enumerated using the unopette system, we observed that deletion of Cib1 significantly heightened the counts of each cell type compared with WT mice (Table 1). Increased leukocyte number was also observed in Cib1−/− mouse blood than the WT when the blood smears were stained with Wright-Giemsa, further supporting this observation (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The increased platelet counts in Cib1−/− mice could arise from increased production or decreased destruction. To test whether platelet production is enhanced in Cib1−/− mice, we chose to analyze platelet production using thiazole orange, which specifically labels newly formed reticulated platelets. There was no difference in the percentage of platelets that were reticulated in WT and Cib1−/− mice (Figure 1A; supplemental Figure 2). Therefore, we assessed platelet clearance in both WT and Cib1−/− mice using an in vivo biotinylation technique.21 As with platelet production, the percentage of platelets cleared was unaltered on Cib1 deletion (Figure 1B; supplemental Figure 2). Increased platelet count, combined with normal platelet production and clearance, suggests that, in terms of platelet regulation, Cib1−/− mice simply operate at a higher baseline than the WT. These data strongly suggest that megakaryopoiesis may be altered in Cib1−/− mice.
Table 1.
Cib1−/− mice have more platelets and leukocytes than WT mice
| Parameter | WT | Cib1−/− |
|---|---|---|
| Mice analyzed | 9 | 9 |
| WBC, × 103/μL | 7.02 ± 0.78 | 10.71 ± 1.19* |
| Hematocrit, % | 53.24 ± 0.82 | 50.44 ± 2.19 |
| Platelets, × 103/μL | 1169 ± 48.6 | 1547 ± 135.6* |
P < .05.
Figure 1.
Platelet production and platelet clearance are unchanged in Cib1−/− mice. (A) Quantification of in vivo biotinylated platelets 24, 48, 72, and 168 hours after thiazole orange injection expressed as a percentage of baseline (1 hour after injection; n = 5). (B) Quantification of the percentage of reticulated platelets from WT and Cib1−/− mouse blood (n = 5).
Ablation of Cib1 results in an increased number of BM megakaryocytes
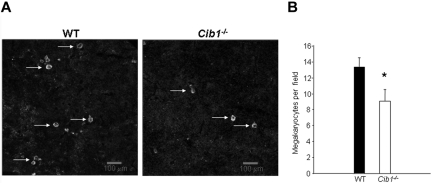
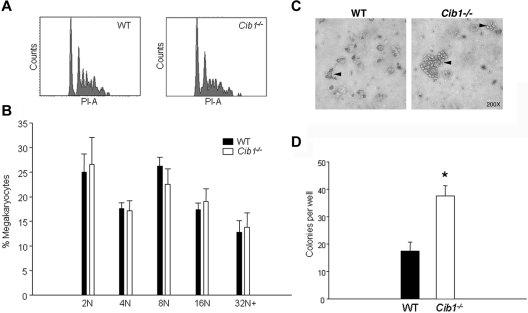
Using flow cytometric analysis, we determined that Cib1−/− mice had more megakaryocytes per nucleated BM cell than WT controls (Table 2). One possibility is that heightened megakaryocyte numbers are the result of increased circulating TPO in Cib1−/− mice compared with WT. To test this, we analyzed serum TPO concentrations in WT and Cib1−/− mice. However, we found no difference in serum TPO concentrations between WT and Cib1−/− mice, ruling out the possibility that TPO is the cause for the observed increase in MK number in Cib1−/− mice. In addition to the BM, the spleen is also a primary site of hematopoiesis in the mouse. Therefore, we analyzed megakaryocyte number within the spleens of WT and Cib1−/− mice. Surprisingly, we found that there were actually fewer megakaryocytes in the spleen of Cib1−/− compared with WT mice (Figure 2). In addition, c-Mpl expression was not altered on Cib1 deletion in both BM cells as well as platelets (supplemental Figure 3), precluding the possibility that altered expression of the TPO receptor is responsible for heightened platelet counts in Cib1−/− mice. Platelet production correlates positively with megakaryocyte ploidy. Therefore, we performed flow cytometric analysis to determine whether BM megakaryocyte DNA content was altered as a result of Cib1 deletion. BM megakaryocytes were purified using discontinuous BSA gradient, and the purity was assessed microscopically and by flow cytometry (supplemental Figure 4). However, no difference in megakaryocyte polyploidy was noted in Cib1−/− megakaryocytes compared with WT megakaryocytes (Figure 3A-B). In addition, Wright-Giemsa staining of BM touch preps shows no difference in BM megakaryocyte morphology between Cib1−/− and WT (supplemental Figure 1).
Table 2.
Cib1−/− mice have more BM megakaryocytes than WT mice
| Parameter | WT | Cib1−/− |
|---|---|---|
| Megakaryocytes, % BM cells | 0.235 ± 0.02 (n = 6) | 0.305 ± 0.04* (n = 5) |
| Serum TPO, pg/mL | 3404 ± 316 (n = 5) | 3547 ± 407 (n = 4) |
P < .05.
Figure 2.
Spleens of Cib1−/− mice contain fewer megakaryocytes than WT mice. (A) Fluorescent microscopic images of mouse spleen sections stained with anti-CD41 to label the megakaryocytes and Draq-5 to label nuclei. White arrows indicate megakaryocytes. Images were captured using a Zeiss 5-live confocal microscope (original magnification ×100). (B) Quantification of panel A expressed as megakaryocytes per field. *P < .05 vs WT (n = 7).
Figure 3.
CFU-MK production is heightened with Cib1 deletion. (A) Representative histograms of DNA content of CD41+ BM cells derived from WT and Cib1−/− mice. (B) Quantification of the ploidy distribution of WT and Cib1−/− BM megakaryocytes expressed as a percentage of total megakaryocytes (n = 6). (C) Representative light-microscopic images of CFU-MK stained with acetylcholinesterase after 7 days in culture. Arrows indicate CFU-MK colonies. (D) Quantification of the number of CFU-MK in each well after 12 days of incubation. Colonies were composed of at least 3 cells. *P < .05 vs WT (n = 4).
Cib1 deletion results in enhanced CFU-megakaryocyte production
It is possible that the increase in megakaryocyte number observed in Cib1−/− mice is the result of enhanced production of progenitor cells. To test this possibility, we performed a CFU-MK assay using total BM from WT and Cib1−/− mice. After 5 to 7 days in culture, more and larger CFU-MK colonies were visible in Cib1−/− BM cultures compared with WT as determined by acetylcholinesterase staining (Figure 3C). By 10 to 12 days in culture, these differences became more prominent. Furthermore, quantification of the number of colonies showed that Cib1 deletion results in a significantly greater number of CFU-MK colonies compared with WT (Figure 3D). This supports our observation that Cib1−/− mice have more megakaryocytes than WT, and suggests that TPO signaling may be altered with Cib1 deletion.
CIB1 is an endogenous negative regulator of TPO signaling
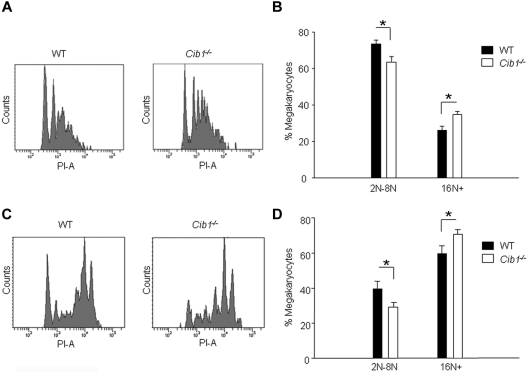
Enhanced CFU-MK production suggests that TPO signaling may be altered with Cib1 deletion. Therefore, we chose to investigate whether or not CIB1 had any effect on TPO-dependent signaling. First, we treated BM from both Cib1−/− and WT mice with 50 ng/mL recombinant mouse TPO for either 1 day or 5 days and analyzed the cultures via flow cytometry for DNA content of CD41+ cells. Interestingly, Cib1 deletion caused a statistically significant increase in ploidy after only 1 day of TPO treatment, which was characterized by a decreased 2N population, and enhanced 16N and 32N populations (Figure 4A-B). Similarly, 5 days of TPO treatment also caused increased ploidy in Cib1−/− BM as 2N to 8N populations were depressed, whereas the 16N+ population was greater compared with WT (Figure 4C-D). Furthermore, the 2N population was greatly reduced in Cib1−/− megakaryocytes compared with WT megakaryocytes after 5 days of TPO treatment, but the number of megakaryocytes remained unchanged (1.022% ± 0.212% vs 1.382% ± 0.235%, respectively). This suggests that Cib1−/− BM cells (2N population) undergo endomitosis more efficiently compared with WT BM cells on treatment with TPO.
Figure 4.
Cib1 deletion increases megakaryocyte ploidy in response to exogenous TPO. (A) Representative histograms of CD41+ BM cells from WT and Cib1−/− mice after addition of 50 ng/mL TPO for 24 hours. (B) Quantification of megakaryocyte ploidy from panel A. *P < .05 vs WT (n = 7). (C) Representative histograms of CD41+ BM cells from WT and Cib1−/− mice after 50 ng/mL TPO treatment for 5 days. (D) Quantification of megakaryocyte ploidy from panel C. *P < .05 vs WT (n = 6).
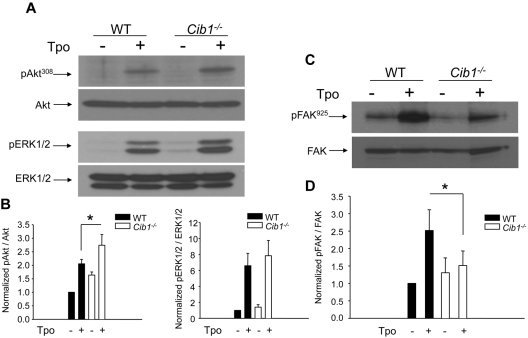
Our observation that Cib1−/− BM had increased TPO-dependent ploidy compared with WT suggests that CIB1 may regulate TPO signaling. Therefore, we determined whether key regulators of TPO signaling were altered with Cib1 deletion by Western blot analysis. Two important positive regulators of TPO-dependent signaling are ERK1/2 and Akt.3,4,26 Therefore, we analyzed the phosphorylation state of each, after 10 minutes of exogenous TPO treatment, and found enhanced phosphorylation of both AktT308 and ERK1/2 in Cib1−/− megakaryocytes compared with WT megakaryocytes (Figure 5A). Although the increase in Akt phosphorylation was significant, the increase in ERK1/2 phosphorylation was not (Figure 5B). Recently, Hitchcock et al revealed that FAK is a negative regulator of TPO-dependent signaling.9 We have previously shown that CIB1 interacts with and activates FAK.16 Therefore, we examined the phosphorylation of FAKY925 in Cib1−/− and WT megakaryocytes after TPO treatment and found that phosphorylation of FAKY925 was attenuated in Cib1−/− compared with WT (Figure 5C-D). These data strongly suggest that CIB1 is an endogenous negative regulator of TPO-dependent signaling, perhaps by enhancing FAK activity.
Figure 5.
TPO-induced signaling is enhanced in Cib1−/− megakaryocytes. (A) Western blots of lysates from untreated or TPO-treated WT and Cib1−/− BM-derived megakaryocytes purified using a discontinuous BSA gradient. Membranes were initially probed for phosphorylated AktT308 and phosphorylated ERK1/2, then stripped and reprobed for total Akt or total ERK1/2, respectively. (B) Quantification of band intensity from panel A normalized to untreated WT sample. *P < .05 vs WT + TPO (n = 5). (C) Western blots of lysates from WT and Cib1−/− megakaryocytes treated with or without TPO. Membranes were probed for phosphorylated FAKY925, then stripped and reprobed for total FAK. (D) Quantification of band intensity from panel B, normalized to untreated WT sample. *P < .05 vs WT + TPO (n = 5).
Platelet recovery is delayed in Cib1−/− mice after experimental immune-induced thrombocytopenia
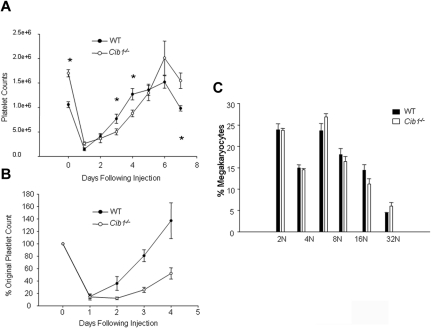
Because we observed increased TPO-dependent signaling, enhanced ploidy, and enhanced CFU-MK formation with Cib1 deletion, we hypothesized that Cib1−/− mice would recover from experimental immune-induced thrombocytopenia faster than WT controls. Therefore, we depleted circulating platelet counts by injecting anti–mouse CD41 antibody into WT and Cib1−/− mice. We were routinely able to achieve reductions in platelet counts of 85% to 90% in both WT and Cib1−/−. Increases in platelet counts in WT mice after immune-induced thrombocytopenia appeared nearly linear, and reactive thrombocytopenia occurred 96 hours after injection. In Cib1−/− mice, however, the initial rate of recovery was reduced and reactive thrombocytopenia was not observed until 144 hours after injection. Surprisingly, we observed a “lag” in recovery in Cib1−/− mice compared with WT (Figure 6A-B).
Figure 6.
Platelet recovery is attenuated in Cib1−/− mice after immune-induced thrombocytopenia. (A) Time course of platelet recovery from WT and Cib1−/− mice, every 24 hours for 7 days after injection of an anti–mouse CD41 antibody. *P < .05 vs WT (n = 8). (B) Time course of platelet recovery after experimentally induced thrombocytopenia expressed as a percentage of original platelet count. (C) Quantification of DNA content from WT and Cib1−/− megakaryocytes 72 hours after injection of a mouse anti-CD41 antibody.
Megakaryocyte ploidy was enhanced with TPO treatment in Cib1−/− BM compared with WT. It is possible that Cib1−/− megakaryocytes are protected from apoptosis and subsequent platelet production. Therefore, we examined the ploidy of BM megakaryocytes from Cib1−/− and WT mice 72 hours after the onset of immune-induced thrombocytopenia. No differences in megakaryocyte ploidy were observed (Figure 6C). These data suggest that megakaryocyte differentiation in vivo is normal in Cib1−/− mice after platelet depletion and that the reduction in platelet recovery observed in Cib1−/− mice after platelet depletion must be caused by another mechanism.
Impaired adhesion to ECM in megakaryocytes derived from Cib1−/− mice
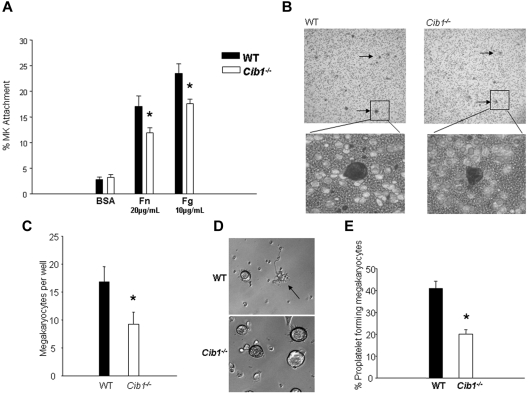
The initial lag in platelet recovery after platelet depletion could be the result of impaired interaction between integrins and the ECM in Cib1−/− mouse megakaryocytes. Therefore, we allowed megakaryocytes from WT and Cib1−/− mice to attach to either 10 μg/mL Fg or 20 μg/mL Fn for 3 hours, and the percentage of attached cells was determined. Indeed, attachment to both Fg and Fn was significantly reduced with Cib1 deletion (Figure 7A). These data are consistent with our previous data, which demonstrate that overexpression of CIB1 enhances attachment to ECM proteins.16
Figure 7.
Cib1 deletion causes inhibition of megakaryocyte migration and attachment to ECM proteins. (A) Percentage of WT and Cib1−/− megakaryocytes attached to BSA, Fg, or Fn. *P < .05 vs WT (n = 6). (B) Diff-Quik stained membranes from transwell migration assays using WT and Cib1−/− megakaryocytes. Arrows point to megakaryocytes. Images were captured using a Nikon Eclipse TS100 microscope: top (original magnification ×40) and bottom (original magnification ×200). (C) Quantification of megakaryocytes per well after transwell migration assays. *P < .05 vs WT (n = 7). (D) Phase-contrast images of BM-derived megakaryocytes purified using discontinuous BSA gradient from WT and Cib1−/− mice attached for 5 hours to immobilized Fg. The arrow indicates a proplatelet-producing megakaryocyte. Images were captured using a Zeiss Axiovert microscope (original magnification ×200). (E) Quantification of the percentage of proplatelet-producing megakaryocytes derived from WT and Cib1−/− mice. *P < .05 vs WT (n = 6).
Deletion of Cib1 inhibits megakaryocyte migration toward an SDF-1α gradient
We, and others, have previously shown that CIB1 regulates focal adhesion formation and cell migration.16,18 Therefore, it is possible that Cib1 deletion reduces megakaryocyte migration by limiting the ability of integrins to interact with the ECM. To test this hypothesis, we used a transwell migration assay. After 5 hours of incubation, we observed decreased migration in Cib1−/− megakaryocytes compared with WT megakaryocytes toward an SDF-1α gradient (Figure 7B-C). Because CIB1 is known to regulate integrin attachment to ECM, it is possible that some megakaryocytes detached from the Fn-coated membrane after migration. However, this was not the case as there were no unattached, transmigrated megakaryocytes found in the lower chamber of any Cib1−/− or WT samples. These data suggest that CIB1 is a key regulator of megakaryocyte migration and that the delay observed in platelet recovery after depletion in Cib1−/− mice could be the result of reduced megakaryocyte migration toward the vascular niche.
Cib1−/− mouse megakaryocytes form fewer proplatelets when plated on immobilized Fg
Because Cib1−/− megakaryocytes have an impaired ability to adhere to immobilized Fg, we analyzed proplatelet formation in WT and Cib1−/− megakaryocytes after attachment to immobilized Fg. We found that a significantly lower percentage of Cib1−/− megakaryocytes produced proplatelets compared with WT megakaryocytes (Figure 7D-E). This suggests that CIB1 regulates proplatelet production and could contribute to the delay observed in platelet recovery after immune-induced thrombocytopenia observed in Cib1−/− mice. Further analysis is warranted to determine exactly how CIB1 regulates this process.
Discussion
In this report, we demonstrate that CIB1 is an important endogenous mediator of both TPO-induced signaling and megakaryocyte migration. Cib1−/− mice have enhanced CFU-MK development and increased megakaryocyte ploidy in culture after exogenous TPO treatment. In addition, TPO-induced signaling is altered with Cib1 deletion. Furthermore, Cib1−/− mice experience a lag in platelet recovery after platelet depletion perhaps because of impaired migration and reduced proplatelet formation by Cib1−/− megakaryocytes compared with WT megakaryocytes.
TPO-induced phosphorylation of 2 positive regulators of TPO signaling, ERK1/2 and Akt, is augmented, and phosphorylation of a negative regulator of TPO signaling, FAK, is attenuated after TPO treatment of Cib1−/− megakaryocytes compared with WT megakaryocytes. The increased Akt and ERK1/2 phosphorylation shown in Cib1−/− megakaryocytes could explain our observation that Cib1−/− mice have more platelets and BM megakaryocytes than WT controls. These data suggest that CIB1 is part of a negative feedback loop in which TPO-dependent FAK activation leads to suppression of Akt and ERK1/2.9 These data are in agreement with Hitchcock et al, who demonstrated that FAK−/− mice have increased megakaryopoiesis.9 Like Cib1−/− megakaryocytes, the phosphorylation of both Akt and ERK1/2 was enhanced in FAK−/− megakaryocytes treated with TPO. In addition, the activity of Lyn kinase was significantly depressed. Furthermore, Lyn−/− mice have also been produced and are reported to have increased megakaryopoiesis.22 Specifically, the phosphorylation of both Akt and ERK1/2 is enhanced in Lyn−/− megakaryocytes treated with TPO. These data suggest that FAK and Lyn are part of a negative feedback loop initiated by TPO. The fact that Cib1−/− mouse megakaryocytes also have increased Akt and ERK1/2 phosphorylation in response to TPO, as well as reduced TPO-dependent FAKY925 phosphorylation, suggests that CIB1 is also involved in the FAK-Lyn negative feedback loop, perhaps by regulating FAK activity. It is curious that, although an increase in TPO-dependent ERK1/2 phosphorylation was seen consistently, we were not able to observe a statistically significant increase as seen in both the FAK−/− and Lyn−/− megakaryocytes. Because CIB1 was previously shown to positively influence the activity of ERK1/2, it is likely that the absence of Cib1 may partially mask TPO-dependent ERK1/2 phosphorylation.27
We have recently demonstrated that manipulation of CIB1 expression alters endomitosis in Dami cells treated with PMA.15 We showed that overexpression of CIB1 causes increased PMA-dependent ploidy, whereas RNAi-mediated reduction of CIB1 has the reverse effect. The in vitro data presented in that report are not consistent with the in vivo data presented here. This could be for a variety of reasons. (1) PMA is a nonphysiologic agonist, and signaling initiated by PMA is only partially understood. (2) Bypassing the TPO receptor, c-Mpl, using PMA may nullify the aforementioned FAK-dependent negative feedback loop. (3) PMA, which is known to induce cell spreading and FAK activation, may alter FAK localization.28 Cell adhesion to ECM can result in of FAK translocation and sequestration to the focal adhesions. Thus, the addition of PMA could prevent FAK from acting as a negative regulator of endomitosis in Dami cells. Therefore, it is not entirely surprising that the PMA-induced effect on Dami cells is different from that seen when primary megakaryocytes were treated with a specific physiologic agonist, such as TPO. Another possibility is that CIB1 is known to bind and inhibit Plk3, a mitotic kinase important for cell-cycle regulation.29 Forced overexpression of CIB1 could inhibit Plk3 and thus affect proper regulation of cell cycle leading to polyploidy in Dami cells. Indeed, such an effect of CIB1 has been reported in a variety of cell lines.30
Cib1 deletion inhibited the ability of megakaryocytes to attach to both Fn and Fg, and reduced megakaryocyte migration toward an SDF-1α gradient. These data are supported by our previous results, which demonstrate that CIB1 binds and activates FAK when αIIbβ3 is bound to Fg.16 Megakaryocyte migration is dependent on several factors, including, but not limited to, integrin expression, cytoskeletal rearrangement, and polarization of the SDF-1α receptor CXCR4.11,31,32 In this report, we demonstrate that Cib1−/− mouse megakaryocytes have a defect in migration toward an SDF-1α gradient. Our results suggest that this is the result of impaired attachment to Fn. However, it is also possible that CIB1 is partially responsible for regulating polarity of CXCR4; thus, deletion of Cib1 may prevent proper polarization. However, we have previously shown that CIB1 localizes to focal adhesions during cell spreading and migration.16 In addition, CIB1 interacts with αIIbβ3 and FAK, and overexpression of CIB1 induces focal adhesion formation and cell migration.14,16,33 Therefore, it is likely that CIB1 regulates megakaryocyte migration by enhancing focal adhesion formation through interactions with αIIbβ3 and FAK.
Our observation of the impaired ability of Cib1−/− megakaryocytes to form proplatelets suggests a role for CIB1 in platelet production. CIB1 was first identified as an interacting partner of the integrin αIIb subunit and subsequently established that it regulates Fg-dependent signaling through integrin αIIbβ3 in platelets.14,19 It has recently been shown that Fg binding to integrin αIIbβ3 is vital to the production of proplatelets by megakaryocytes.24 Therefore, it is possible that the absence of CIB1 in megakaryocytes impairs proper signaling through αIIbβ3 on Fg binding. Alternatively, we and others have recently demonstrated that CIB1 regulates Polo-like kinase 3 (Plk3), a known regulator of microtubule dynamics.29,30,34–36 It is therefore possible that CIB1 is integral to the regulation of microtubule dynamics during proplatelet formation, perhaps through an interaction with Plk3.
We report here that platelet recovery in Cib1−/− mice after platelet depletion is inhibited because of decreased attachment of BM megakaryocytes to ECM, reduced megakaryocyte migration, and reduced proplatelet production compared with WT. We have previously shown that CIB1 overexpression enhances cell migration by regulating FAK activity.16 However, Hitchcock et al reported that platelet recovery in FAK−/− mice after platelet depletion is enhanced compared with WT.9 Although the Cib1−/− mice indeed produce more platelets than WT controls, 5 days after the onset of thrombocytopenia, similar to FAK−/− mice, FAK−/− mice do not experience the initial delay in platelet recovery that Cib1−/− mice do. One possible explanation is that Cib1−/− mouse megakaryocytes do not produce proplatelets as efficiently as WT mouse megakaryocytes (Figure 6D-E). Another possible explanation for this discrepancy is compensation by the FAK homologue, proline-rich tyrosine kinase 2 (Pyk2) in FAK−/− mice.37 Pyk2 is a tyrosine kinase that regulates cell migration, and its expression and function have been reported in megakaryocytic cell lines, including phosphorylation when cells are plated on Fn.38,39 Indeed, others observed increased Pyk2 phosphorylation in FAK−/− megakaryocytes spread on Fg. Therefore, it is possible that migration and adhesion defects in FAK−/− megakaryocytes were masked because of compensation by Pyk2.
The fact that Cib1−/− mice contain fewer megakaryocytes in the spleen presents another potential explanation for their initial lag in platelet recovery after immune-induced thrombocytopenia. It was recently reported that adult mice injected with an anti-CD41 antibody for a 7-day period displayed an increase in both size and number of megakaryocytes in the spleen, whereas no change was noted in megakaryocytes in the BM.40 It is therefore possible that the pool of megakaryocytes housed in the spleen is responsible for initial platelet recovery after thrombocytopenia. Given that Cib1−/− mice have fewer megakaryocytes in the spleen than WT controls, it is possible that Cib1−/− mice do not have a sufficient number of megakaryocytes in the spleen to respond to the thrombocytopenia initially. This may explain the observed initial delay in recovery after thrombocytopenia.
The data presented in this report suggest that CIB1 regulates megakaryocyte progenitor production and megakaryocyte differentiation by negatively regulating TPO-dependent signaling while also promoting megakaryocyte migration and proplatelet production. These data shed new light on TPO-dependent signaling by further characterizing the negative feedback loop arising from TPO engagement of c-Mpl. Furthermore, our data suggest that CIB1 may be an important regulator of megakaryocyte migration and attachment to important BM-ECM proteins.
Supplementary Material
Acknowledgments
The authors thank Pani Apostolidis and Stephan Lindsey for their expert assistance with flow cytometry, Sharmila Chatterjee for her assistance with confocal microscopy, and Arjit Nigam for genotyping.
This work was supported by the National Institutes of Health (grants HL57630 and HL 69360) and the National Center for Research Resources (grants 015588-10 RR and 2P20RR016472-11).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.C.K. designed, collected, interpreted, and analyzed data and wrote the manuscript; M.U.N. designed, collected, interpreted, and analyzed data; and U.P.N designed and interpreted data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.C.K. is Department of Physiology, Temple University School of Medicine, Philadelphia, PA.
Correspondence: Ulhas P. Naik, Department of Biological Sciences, University of Delaware, 329 Wolf Hall, Newark, DE 19716; e-mail: unaik@udel.edu.
References
- 1.Junt T, Schulze H, Chen Z, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317(5845):1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 2.de Sauvage FJ, Hass PE, Spencer SD, et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994;369(6481):533–538. doi: 10.1038/369533a0. [DOI] [PubMed] [Google Scholar]
- 3.Mazharian A, Watson SP, Severin S. Critical role for ERK1/2 in bone marrow and fetal liver-derived primary megakaryocyte differentiation, motility, and proplatelet formation. Exp Hematol. 2009;37(10):1238–1249. doi: 10.1016/j.exphem.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakao T, Geddis AE, Fox NE, Kaushansky K. PI3K/Akt/FOXO3a pathway contributes to thrombopoietin-induced proliferation of primary megakaryocytes in vitro and in vivo via modulation of p27(Kip1). Cell Cycle. 2008;7(2):257–266. doi: 10.4161/cc.7.2.5148. [DOI] [PubMed] [Google Scholar]
- 5.Rojnuckarin P, Drachman JG, Kaushansky K. Thrombopoietin-induced activation of the mitogen-activated protein kinase (MAPK) pathway in normal megakaryocytes: role in endomitosis. Blood. 1999;94(4):1273–1282. [PubMed] [Google Scholar]
- 6.Chanprasert S, Geddis AE, Barroga C, Fox NE, Kaushansky K. Thrombopoietin (TPO) induces c-myc expression through a PI3K- and MAPK-dependent pathway that is not mediated by Akt, PKCzeta or mTOR in TPO-dependent cell lines and primary megakaryocytes. Cell Signal. 2006;18(8):1212–1218. doi: 10.1016/j.cellsig.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Tortolani PJ, Johnston JA, Bacon CM, et al. Thrombopoietin induces tyrosine phosphorylation and activation of the Janus kinase, JAK2. Blood. 1995;85(12):3444–3451. [PubMed] [Google Scholar]
- 8.Bacon CM, Tortolani PJ, Shimosaka A, Rees RC, Longo DL, O'Shea JJ. Thrombopoietin (TPO) induces tyrosine phosphorylation and activation of STAT5 and STAT3. FEBS Lett. 1995;370(1):63–68. doi: 10.1016/0014-5793(95)00796-c. [DOI] [PubMed] [Google Scholar]
- 9.Hitchcock IS, Fox NE, Prevost N, Sear K, Shattil SJ, Kaushansky K. Roles of focal adhesion kinase (FAK) in megakaryopoiesis and platelet function: studies using a megakaryocyte lineage specific FAK knockout. Blood. 2008;111(2):596–604. doi: 10.1182/blood-2007-05-089680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel SR, Hartwig JH, Italiano JE., Jr The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest. 2005;115(12):3348–3354. doi: 10.1172/JCI26891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mossuz P, Schweitzer A, Molla A, Berthier R. Expression and function of receptors for extracellular matrix molecules in the differentiation of human megakaryocytes in vitro. Br J Haematol. 1997;98(4):819–827. doi: 10.1046/j.1365-2141.1997.3013118.x. [DOI] [PubMed] [Google Scholar]
- 12.Bentley SA, Tralka TS. Fibronectin-mediated attachment of hematopoietic cells to stromal elements in continuous bone marrow culture. Exp Hematol. 1983;11(2):129–138. [PubMed] [Google Scholar]
- 13.Hamada T, Mohle R, Hesselgesser J, et al. Transendothelial migration of megakaryocytes in response to stromal cell-derived factor 1 (SDF-1) enhances platelet formation. J Exp Med. 1998;188(3):539–548. doi: 10.1084/jem.188.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naik UP, Patel PM, Parise LV. Identification of a novel calcium-binding protein that interacts with the integrin alphaIIb cytoplasmic domain. J Biol Chem. 1997;272(8):4651–4654. doi: 10.1074/jbc.272.8.4651. [DOI] [PubMed] [Google Scholar]
- 15.Kostyak JC, Naik UP. Calcium- and integrin-binding protein 1 regulates endomitosis and its interaction with polo-like kinase 3 is enhanced in endomitotic dami cells. PLoS One. 2011;6(1):e14513. doi: 10.1371/journal.pone.0014513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naik MU, Naik UP. Calcium-and integrin-binding protein regulates focal adhesion kinase activity during platelet spreading on immobilized fibrinogen. Blood. 2003;102(10):3629–3636. doi: 10.1182/blood-2003-05-1703. [DOI] [PubMed] [Google Scholar]
- 17.Pelletier AJ, Kunicki T, Ruggeri ZM, Quaranta V. The activation state of the integrin alpha IIb beta 3 affects outside-in signals leading to cell spreading and focal adhesion kinase phosphorylation. J Biol Chem. 1995;270(30):18133–18140. doi: 10.1074/jbc.270.30.18133. [DOI] [PubMed] [Google Scholar]
- 18.Zayed MA, Yuan W, Leisner TM, et al. CIB1 regulates endothelial cells and ischemia-induced pathological and adaptive angiogenesis. Circ Res. 2007;101(11):1185–1193. doi: 10.1161/CIRCRESAHA.107.157586. [DOI] [PubMed] [Google Scholar]
- 19.Naik MU, Nigam A, Manrai P, et al. CIB1 deficiency results in impaired thrombosis: the potential role of CIB1 in outside-in signaling through integrin alpha IIb beta 3. J Thromb Haemost. 2009;7(11):1906–1914. doi: 10.1111/j.1538-7836.2009.03581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY) 2005;34(9):39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- 21.Prislovsky A, Marathe B, Hosni A, et al. Rapid platelet turnover in WASP(−) mice correlates with increased ex vivo phagocytosis of opsonized WASP(−) platelets. Exp Hematol. 2008;36(5):609–623. doi: 10.1016/j.exphem.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lannutti BJ, Minear J, Blake N, Drachman JG. Increased megakaryocytopoiesis in Lyn-deficient mice. Oncogene. 2006;25(23):3316–3324. doi: 10.1038/sj.onc.1209351. [DOI] [PubMed] [Google Scholar]
- 23.Fuhrken PG, Apostolidis PA, Lindsey S, Miller WM, Papoutsakis ET. Tumor suppressor protein p53 regulates megakaryocytic polyploidization and apoptosis. J Biol Chem. 2008;283(23):15589–15600. doi: 10.1074/jbc.M801923200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larson MK, Watson SP. Regulation of proplatelet formation and platelet release by integrin alpha IIb beta3. Blood. 2006;108(5):1509–1514. doi: 10.1182/blood-2005-11-011957. [DOI] [PubMed] [Google Scholar]
- 25.van den Oudenrijn S. Thrombopoietin receptor (CD110). Protein Review On The Web. 2000;1:22–25. [Google Scholar]
- 26.Majka M, Ratajczak J, Villaire G, et al. Thrombopoietin, but not cytokines binding to gp130 protein-coupled receptors, activates MAPKp42/44, AKT, and STAT proteins in normal human CD34+ cells, megakaryocytes, and platelets. Exp Hematol. 2002;30(7):751–760. doi: 10.1016/s0301-472x(02)00810-x. [DOI] [PubMed] [Google Scholar]
- 27.Naik MU, Naik UP. Contra-regulation of calcium- and integrin-binding protein 1-induced cell migration on fibronectin by PAK1 and MAP kinase signaling. J Cell Biochem. 2011;112(11):3289–3299. doi: 10.1002/jcb.23255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee MS, Kim YB, Lee SY, et al. Integrin signaling and cell spreading mediated by phorbol 12-myristate 13-acetate treatment. J Cell Biochem. 2006;99(1):88–95. doi: 10.1002/jcb.20830. [DOI] [PubMed] [Google Scholar]
- 29.Naik MU, Pham NT, Beebe K, Dai W, Naik UP. Calcium-dependent inhibition of polo-like kinase 3 activity by CIB1 in breast cancer cells. Int J Cancer. 2011;128(3):587–596. doi: 10.1002/ijc.25388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naik MU, Naik UP. Calcium- and integrin-binding protein 1 regulates microtubule organization and centrosome segregation through polo like kinase 3 during cell cycle progression. Int J Biochem Cell Biol. 2011;43(1):120–129. doi: 10.1016/j.biocel.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lane WJ, Dias S, Hattori K, et al. Stromal-derived factor 1-induced megakaryocyte migration and platelet production is dependent on matrix metalloproteinases. Blood. 2000;96(13):4152–4159. [PubMed] [Google Scholar]
- 32.Mazharian A, Thomas SG, Dhanjal TS, Buckley CD, Watson SP. Critical role of Src-Syk-PLCγ2 signaling in megakaryocyte migration and thrombopoiesis. Blood. 2010;116(5):793–800. doi: 10.1182/blood-2010-03-275990. [DOI] [PubMed] [Google Scholar]
- 33.Naik UP, Naik MU. Association of CIB with GPIIb/IIIa during outside-in signaling is required for platelet spreading on fibrinogen. Blood. 2003;102(4):1355–1362. doi: 10.1182/blood-2003-02-0591. [DOI] [PubMed] [Google Scholar]
- 34.Kauselmann G, Weiler M, Wulff P, et al. The polo-like protein kinases Fnk and Snk associate with a Ca(2+)- and integrin-binding protein and are regulated dynamically with synaptic plasticity. EMBO J. 1999;18(20):5528–5539. doi: 10.1093/emboj/18.20.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kostyak JC, Naik UP. Calcium- and integrin-binding protein 1 regulates endomitosis and its interaction with polo-like kinase 3 is enhanced in endomitotic dami cells. PLoS One. 2011;6(1):e14513. doi: 10.1371/journal.pone.0014513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Q, Xie S, Chen J, et al. Cell cycle arrest and apoptosis induced by human Polo-like kinase 3 is mediated through perturbation of microtubule integrity. Mol Cell Biol. 2002;22(10):3450–3459. doi: 10.1128/MCB.22.10.3450-3459.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sieg DJ, Ilic D, Jones KC, Damsky CH, Hunter T, Schlaepfer DD. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK- cell migration. EMBO J. 1998;17(20):5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiregowdara D, Avraham H, Fu Y, London R, Avraham S. Tyrosine phosphorylation of the related adhesion focal tyrosine kinase in megakaryocytes upon stem cell factor and phorbol myristate acetate stimulation and its association with paxillin. J Biol Chem. 1997;272(16):10804–10810. doi: 10.1074/jbc.272.16.10804. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Avraham H, Rogers RA, Raja S, Avraham S. Characterization of RAFTK, a novel focal adhesion kinase, and its integrin-dependent phosphorylation and activation in megakaryocytes. Blood. 1996;88(2):417–428. [PubMed] [Google Scholar]
- 40.Hu Z, Slayton WB, Rimsza LM, Bailey M, Sallmon H, Sola-Visner MC. Differences between newborn and adult mice in their response to immune thrombocytopenia. Neonatology. 2010;98(1):100–108. doi: 10.1159/000280413. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.