Abstract
Sepsis is the most common cause of acute lung injury (ALI), leading to organ dysfunction and death in critically ill patients. Previous studies associated variants of interleukin-1 receptor–associated kinase genes (IRAKs) with differential immune responses to pathogens and with outcomes during sepsis, and revealed that increased expression levels of the IRAK3 gene were correlated with poor outcomes during sepsis. Here we explored whether common variants of the IRAK3 gene were associated with susceptibility to, and outcomes of, severe sepsis. After our discovery of polymorphism, we genotyped a subset of seven single-nucleotide polymorphisms (SNPs) in 336 population-based control subjects and 214 patients with severe sepsis, collected as part of a prospective study of adults from a Spanish network of intensive care units. Whereas IRAK3 SNPs were not associated with susceptibility to severe sepsis, rs10506481 showed a significant association with the development of ALI among patients with sepsis (P = 0.007). The association remained significant after adjusting for multiple comparisons, population stratification, and clinical variables (odds ratio, 2.50; 95% confidence interval, 1.15–5.47; P = 0.021). By imputation, we revealed three additional SNPs independently associated with ALI (P < 0.01). One of these (rs1732887) predicted the disruption of a putative human–mouse conserved transcription factor binding site, and demonstrated functional effects in vitro (P = 0.017). Despite the need for replication in independent studies, our data suggest that common SNPs in the IRAK3 gene may be determinants of sepsis-induced ALI.
Keywords: SNP, polymorphism, case-control, Toll-like receptor, severe sepsis
Clinical Relevance
Genes encoding key molecules of the Toll-like receptor (TLR) signaling pathway are known to abolish or alter the immune response, and were associated with disease susceptibility and outcomes. Genetic variants in interleukin-1 receptor–associated kinase 1 (IRAK1) and IRAK4 were shown to modify the immune response to pathogens, and to increase the risk for severe complications during sepsis. We show that a functional variant of the IRAK3 gene, another key element of this signaling pathway, is associated with the development of acute lung injury in patients with severe sepsis.
Sepsis is a devastating clinical syndrome characterized by systemic inflammation, and occurring in the setting of a severe infection (1). It constitutes the most common cause of acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Severe forms manifest an estimated incidence of 50–100 cases per 100,000 people (2), with an associated mortality in intensive care units (ICUs) at above 40% (3). Clinical and experimental reports indicate that the host response to pathogens is affected by genetic factors (4). In particular, twin studies (5) and inbred-strain murine models (6) support a considerable genetic influence on immune responses to bacterial LPS and a susceptibility to infections. Thus, genetic variations disrupting or modifying innate immune responses may also underlie the diversity of clinical presentations in sepsis, the development of organ dysfunction, and the response to current medical treatments.
Genes encoding key molecules of the Toll-like receptor (TLR) signaling pathway, which is involved in innate immune recognition and cellular activation in response to microbial agents (7), constitute potential determinants of the development and outcomes of sepsis. Both rare and common variants of genes, including LBP (8), CD14 (9), TLR4 (10), TLR2 (11–13), TIRAP (14), and others (15, 16), are known to abolish or alter the immune response, and are associated with disease susceptibility and outcomes. Genetic variants in interleukin-1 receptor–associated kinase 1 (IRAK1) and 4 (IRAK4) were shown to modify the immune response to pathogens, and to increase the risk for severe complications during sepsis (17, 18).
The human gene IRAK3, located in chromosome 12q14.3, encodes the IRAK-M (also known as IRAK-3) protein, as first described in cells of the monocytic lineage (19), and negatively regulates the Toll/NF-κβ signaling pathway. IRAK-3–deficient mice demonstrated a disproportionate response to LPS stimulation, suggesting that IRAK-3 may be implicated in TLR ligand tolerance (20). Congruently, the expression of IRAK3 in LPS-treated human leukocytes increased early during response (21) and peaked higher and more rapidly when tolerant cells were restimulated with LPS, a situation demonstrated in cells from patients with sepsis (22). Similarly, the marked up-regulation of IRAK-3 positively correlated with lung injury in septic animals, particularly in Type II epithelial cells and in the interstitial macrophages (23). These findings support a pivotal role of IRAK-3 in the suppression of downstream signaling pathways required for the inflammatory process and its important role in regulating the generation of protective innate immune responses in the lung in vivo (24). In fact, a reduced capacity of peripheral leukocytes from patients with sepsis to produce cytokines, compared with that capacity in healthy control subjects (usually referred to as endotoxin tolerance), is a well-known phenomenon (25), and it may instigate subsequent damage during the course of sepsis. In line with this concept, increased expression levels of the IRAK3 gene are associated with a decreased capacity to release proinflammatory cytokines from mononuclear cells in patients with sepsis after ex vivo stimulation with LPS, and are also associated with poor outcomes (26). Based on this evidence, we explored whether common variants of the IRAK3 gene were associated with susceptibility to severe sepsis. We also evaluated whether IRAK3 gene variants were associated with outcomes among patients with severe sepsis, including ALI and mortality.
Materials And Methods
Study Population
This study was approved by the Ethics Committees of the Hospital Universitario Nuestra Señora de Candelaria and the Hospital Universitario Río Hortega. Written, informed consent was obtained from each subject or from appropriate surrogates. The case group of 214 patients, from a Spanish network of ICUs, fulfilled the international criteria for severe sepsis (27, 28), and were followed prospectively for the development of ALI as defined by the American–European Consensus Conference (29). For the purpose of this study, patients with ALI and ARDS were analyzed as a single group of ALI patients. The control group comprised 336 population-based subjects. Table 1. and the online supplement contain a detailed description of samples.
TABLE 1.
DEMOGRAPHIC AND CLINICAL FEATURES OF POPULATION-BASED CONTROL SUBJECTS AND PATIENTS WITH SEVERE SEPSIS
| Variable | Patients (n = 214) | Control Subjects (n = 336) | P Value |
| Gender (male %) | 60.0 | 48.5 | 0.012* |
| Median age (years) (P25–P75) | 67 (54–75) | 44 (34–56) | < 0.001† |
| Previous surgery (%) | 72.9 | 38.8 | < 0.001* |
| Hospitalized > 24 hours (%) | 79.2 | 48.2 | < 0.001* |
| Diabetes (%) | 18.4 | 11.4 | 0.124* |
| Hypertension (%) | 39.2 | 22.0 | 0.004* |
| Ischemic cardiac disease (%) | 9.7 | 1.6 | 0.002* |
| Smoker (%) | 30.3 | 26.1 | 0.679* |
| Source of infection (%) | |||
| Pulmonary | 35.3 | ||
| Extrapulmonary | 64.7 | ||
| Pathogen (%) | |||
| Gram-negative | 23.0 | ||
| Gram-positive | 15.7 | ||
| Fungi | 2.1 | ||
| Mixed Gram-negative and Gram-positive | 8.4 | ||
| Negative blood cultures | 50.8 | ||
| Median APACHE II score (P25–P75) | 24 (18–27) | ||
| ALI/ARDS (%) | 86.0 | ||
| ICU mortality (%) | 45.5 |
Definition of abbreviations: ALI, acute lung injury; APACHE II, Acute Physiology and Chronic Health Evaluation II; ARDS, acute respiratory distress syndrome; ICU, intensive care unit; P25, Percentile 25; P75, Percentile 75.
χ2 test.
Mann-Whitney U test.
Discovery of IRAK3 Gene Polymorphism, Selection of Polymorphisms, and Genotyping
DNA samples from 32 unrelated, healthy Spanish individuals were sequenced (the primers are listed in Table E1) to search for common variations in the IRAK3 gene. Seven tagging single-nucleotide polymorphisms (tSNPs) from the IRAK3 gene were selected and genotyped, using the iPLEX Gold assay in the MassARRAY system (Sequenom, San Diego, CA), by the Santiago de Compostela Node of the Spanish National Genotyping Center (CeGen, http://www.cegen.org/). SNaPshot Multiplex Kit reactions (Applied Biosystems, Foster City, CA) were used to verify the association of imputed SNPs. See the online supplement for further details.
Luciferase Activity Assay
PupaSuite (http://pupasuite.bioinfo.cipf.es) was used to predict the putative functional effects of all associated SNPs (30). The putative IRAK3 promoter region (spanning −1 to −1698 from the transcription start site), containing the SNP rs1732887, was identified and synthesized with alleles rs1732887_A (−1464A) and rs1732887_G (−1464G) by GenScript (Piscataway, NJ), according to the sequence with National Center for Biotechnology Information accession number NC_000012.10, and was subsequently cloned to the pGL3 basic vector. Human pulmonary artery endothelial cells (Cambrex, Walkersville, MD) were transfected with pGL3 fused constructs and with a plasmid with the renilla luciferase gene as transfection efficacy control. The activity of luciferase was measured 48 hours after transfection with Dual-Luciferase Assay Kits (Promega, Madison, WI), following the manufacturer's recommendations. Values were normalized to the activity of the −1464A reference allele construct. A Student t test was used for comparisons, with two-tailed statistical significance set at P < 0.05. See the online supplement for details.
Statistical Analysis
Clinical and demographic data were analyzed using SPSS, version 15.0 (SPSS, Inc., Chicago, IL). Genotype associations were tested by means of the Cochran–Armitage test, assuming an additive model. These associations were adjusted for population stratification (31), using previously reported data from 20 independent polymorphisms (32). To control the Type I error rate in multiple comparisons, the false-discovery rate (FDR) was set at 5% (33). For comparison, this rate was also assessed by a permutation procedure (34). Logistic regression with backward elimination was used to adjust for covariates and to calculate effects as odds ratios (ORs) with 95% confidence intervals (CIs). Linear regression was used to test associations with quantitative measures of clinical severity. TUNA (http://www.stat.uchicago.edu/∼wen/tuna) (35) was used to test the associations of imputed SNPs. EPIDAT 3.0 software (http://dxsp.sergas.es) was used to estimate the additive effects of risk alleles from independent SNPs. See the online supplement for details.
Results
Sequencing 22.6 kb of the IRAK3 gene per sample revealed a total of 59 polymorphisms, 58 of which were biallelic (Table E2). Three polymorphisms (one insertion/deletion, one SNP, and the multiallelic locus) did not have an assigned reference in the database for SNPs (dbSNPs), and were considered novel. Most of the polymorphisms revealed by sequencing (81.4%) showed minor allele frequencies (MAFs) of at least 5%. Two coding SNPs were detected, and one of them (rs1152888) predicted a common, nonsynonymous amino-acid change (Val147Ile). A subset of seven tSNPs (Table E2) allowed for the accurate inference of more than 80% of the common variants of the gene (see the online supplement for further details), and was used to genotype case–control samples.
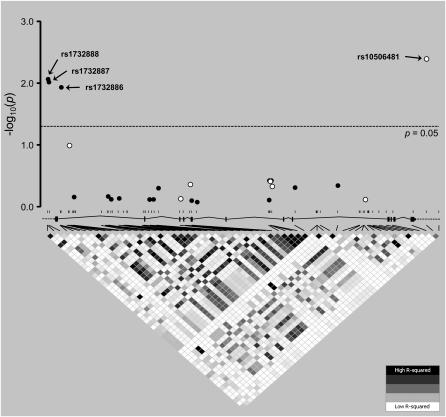
Quality-control measures for the tSNP genotype data are described in Table E3. We did not observe significant deviations from Hardy–Weinberg equilibrium in cases or control subjects. None of the tSNPs (Table 2) or imputed SNPs (not shown) showed a significant association with susceptibility when comparing patients with severe sepsis and control subjects. Similarly, no significant association was evident for any SNP with ICU mortality among patients with severe sepsis (data not shown). However, the tSNP rs10506481 from the 3′ flanking region showed a significant association with the development of ALI among patients with severe sepsis (OR for the T allele, 2.51; 95% CI, 1.29–4.86; P = 0.007; Figure 1; genotype counts are listed in Table E4), and the FDR for this result was 4.5%. An alternative method, based on permutations (34), supported the significance of this result in the context of multiple comparisons (permuted P = 0.021). Moreover, the association remained significant after applying a conservative adjustment for population stratification (P = 0.025). Significance was further maintained in a regression model adjusting for covariates, with significant differences between patients manifesting severe sepsis with and without ALI (Table 3), including gender, source of infection, and ICU mortality (OR for the T allele, 2.50; 95% CI, 1.15–5.47; P = 0.021; Table E5). An in silico exploration using PupaSuite (30), which in addition to predicting nonsynonymous changes and splicing effects for polymorphisms also scans for allelic differences in binding sites for transcription factors and microRNAs, did not predict any functional effects for rs10506481.
TABLE 2.
ASSOCIATION OF IRAK3 GENE TAGGING SINGLE-NUCLEOTIDE PPLYMORPHISMS WITH SEVERE SEPSIS AND ALI
| rs/ss Number | Location (Effect) | Position* | MAF, Control Subjects | MAF, Patients with Severe Sepsis | P Value† | MAF, Non-ALI | MAF, ALI | P Value† |
| ss289570834 | Intron 1 | 64871319 | 0.30 | 0.28 | 0.551 | 0.37 | 0.26 | 0.084 |
| rs11465955 | Intron 3 | 64889666 | 0.33 | 0.31 | 0.489 | 0.33 | 0.31 | 0.763 |
| rs1152888 | Exon 5 (Val147Ile) | 64891495 | 0.10 | 0.08 | 0.346 | 0.10 | 0.07 | 0.400 |
| rs1624395 | Intron 6 | 64904483 | 0.40 | 0.40 | 0.870 | 0.45 | 0.39 | 0.370 |
| rs1370128 | Intron 6 | 64904905 | 0.42 | 0.41 | 0.652 | 0.45 | 0.40 | 0.440 |
| rs1152912 | Intron 8 | 64920175 | 0.49 | 0.50 | 0.645 | 0.48 | 0.50 | 0.795 |
| rs10506481 | 3′ flanking region | 64930378 | 0.13 | 0.12 | 0.543 | 0.23 | 0.10 | 0.007 |
Definition of abbreviations: ALI, acute lung injury; IRAK3 gene, interleukin-1 receptor–associated kinase 3 gene; MAF, minor allele frequency.
According to the National Center for Biotechnology Information build 36.
Unadjusted P value.
Figure 1.
Association P values for interleukin-1 receptor–associated kinase 3 gene (IRAK3) single-nucleotide polymorphisms (SNPs) with acute lung injury among patients with severe sepsis. Open and solid circles correspond to P values (in −log10 scale) resulting from tagging SNPs and imputed SNPs tested for association, respectively. Significantly associated SNPs (i.e., with values above the dotted line corresponding to an unadjusted significance at P = 0.05) are indicated. For reference, a linkage disequilibrium (LD) plot of r2 values across the region, with the approximate location of all 58 biallelic SNPs discovered and a schematic representation of the IRAK3 gene structure (vertical lines, exons; horizontal lines, introns), is shown below. This plot is based on resequencing data from 32 unrelated, healthy Spanish individuals. Each diamond in the LD plot represents a pairwise SNP comparison with its r2 value, schematically symbolized by a color gradient ranging from black (r2 = 1, corresponding to complete LD) to gray (1 < r2 < 0, corresponding to moderate LD) and white (r2 = 0, corresponding to an absence of LD).
TABLE 3.
RELEVANT DEMOGRAPHIC AND CLINICAL FEATURES OF PATIENTS WITH AND WITHOUT ALI
| Variable | ALI (n = 184) | Non-ALI (n = 30) | P Value |
| Gender, male % (95% CI) | 63.8 (56.2–70.5) | 33.3 (17.9–52.9) | 0.003* |
| Age, median years (P25–P75) | 67 (54–76) | 66.5 (48–74) | 0.496† |
| Clinical features: | |||
| APACHE II, median (P25–P75) | 24 (18–28) | 22 (16–24) | 0.035† |
| Source of infection, % (95% CI) | |||
| Pulmonary | 40.5 (33.7–48.3) | 37.0 (20.6–56.1) | |
| Extrapulmonary | 59.5 (51.8–66.3) | 63.0 (43.9–79.5) | <0.001* |
| ICU mortality, % (95% CI) | 51.1 (43.6–58.5) | 11.1 (2.6–27.7) | <0.001* |
Definition of abbreviations: ALI, acute lung injury; APACHE II, Acute Physiology and Chronic Health Evaluation score II; CI, confidence interval; ICU, intensive care unit; P25, Percentile 25; P75, Percentile 75.
χ2 test.
Mann-Whitney U test.
We next used TUNA software to impute and test the associations of 17 SNPs of the gene with ALI. This procedure also predicted three largely correlated SNPs (r2 ≥ 0.8, MAFs ≥ 0.25), located on the 5′ region of the gene, that were significantly associated with ALI, all three with P < 0.01: rs1732888 (−1494 T/C), rs1732887 (−1464 A/G), and rs1732886 (c.133 + 550 A/G) (Figure 1 and Table E6). To demonstrate that these results were not spurious, we validated the association by genotyping two of these SNPs (rs1732886 and rs1732887) in all patients with severe sepsis (genotype counts are listed in Table E4). Borderline significance was obtained for both SNPs, using a regression model to adjust for covariates (OR for the rs1732887 G allele, 2.37; 95% CI, 0.99–5.65; P = 0.053; OR for the rs1732886 G allele, 2.57; 95% CI, 1.02–6.10; P = 0.033; see Table E5 for further details).
In an overall analysis across all 24 SNPs tested (seven genotyped tSNPs plus 17 imputed SNPs), the FDR was estimated at 7% for all four SNPs with P < 0.05 of an association with ALI (i.e., rs1732888, rs1732887, rs1732886, and rs10506481). However, this value must be regarded with caution, because the underlying linkage disequilibrium (LD) was not taken into account when calculating the FDR.
In a post hoc analysis, we tested the association of rs10506481, rs1732887, and rs1732886 with quantitative measures of clinical severity during sepsis, including the number of organs failing in the ICU, the number of days in the ICU, and the Acute Physiology and Chronic Health Evaluation II (APACHE II) score. We did not find rs10506481 to be significantly associated with any of these measures of severity (Table E7). However, borderline associations were evident for rs1732887 and rs1732886 with the number of days in the ICU (Table E7), where the G allele for both SNPs was associated with more days in ICU (not shown). No significant association was evident for any of these SNPs with the number of organs failing in ICU or the APACHE II score.
The SNPs from the 5′ and 3′ regions of the IRAK3 gene were poorly correlated (average pairwise r2 < 0.01; Figure 1), indicating the independence of both associations with ALI. We confirmed the independence by reasoning that if risk alleles from both regions were not correlated, the combination of their genotypes would have to show an increased risk for ALI in a dose-dependent manner. Under this assumption, a linear tendency of risk increasement resulting from the addition of risk alleles from the two regions would have to be evident. Because of the strong LD among the three SNPs from the 5′ flanking region, this trend was investigated only between rs1732887 and rs10506481, assuming a dominant model because of the small sample size (for this reason, we did not test for interaction effects). As expected, a significant trend for the accumulation of risks from alleles at rs1732887 and rs10506481 was evident (χ2 = 5.24; P for trend = 0.022).
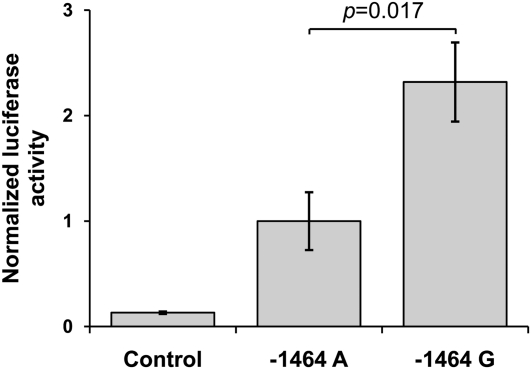
To examine the potential functionality of any of this 5′ region's SNPs, we performed in silico analysis using a transcription factor database (TRANSFAC) exploration (30). This predicted that rs1732887 (−1464 A/G) would potentially interrupt a highly conserved putative transcription factor binding site for the human–murine forkhead box P3 (FOXP3), a protein recently detected not only in regulatory T cells but also in activated T lymphocytes (36). We next used an in vitro luciferase activity assay to assess the functional effects of the −1464 A/G alleles of this SNP in gene expression, because it was the only associated SNP with predicted functionality. Constructs included positions −1 to −1698 from the transcription start site of the gene, and varied only at position −1464 with respect to the sequence NC_000012.10. Luciferase activity, as a proxy for gene expression, was significantly higher for the −1464G construct compared with −1464A construct (P = 0.017). The activity of both constructs was significantly higher than the activity of a promoter-less control (P ≤ 0.01; Figure 2). Because the risk alleles of the other two 5′ region SNPs that were associated with ALI were not included in the construct (i.e., the DNA fragment did not contain the rs1732886 locus, and contained only the nonrisk T allele at rs1732888 locus), these results suggest that the −1464 A/G SNP is sufficient to alter the transcriptional control of IRAK3 gene in vitro.
Figure 2.
Normalized luciferase activity of the putative IRAK3 promoter constructs carrying −1464A and −1464G alleles. Control sample is represented by a promoter-less construct, whereas −1464A and −1464G stand for the A and G allele constructs for rs1732887, respectively. Both show higher luciferase activity than the control sample (P ≤ 0.01). The data represent mean values ± SEMs from four independent experiments, normalized to the −1464A construct.
Discussion
To our knowledge, this is the first report examining the role of IRAK3 gene variants in susceptibility to severe sepsis and sepsis-associated outcomes. We largely characterized common variations across the nonrepetitive sequence of the gene in the population under study, and exhaustively evaluated the tSNP subset selected in the association study for their capacity to impute untyped SNPs. Our findings suggest that common variants of the IRAK3 gene are associated with the development of ALI during severe sepsis. These findings were confirmed by ruling out effects attributable to the presence of major systematic errors during genotyping, the existence of population stratification, differences in clinical and demographic factors, and chance in the performance of multiple comparisons. Reinforcing these results, the SNPs rs1732887 and rs1732886 also showed borderline association with number of days in the ICU. Finally, we demonstrated that one of the associated variants from the 5′ flanking region (rs1732887 and −1464 A/G) modified luciferase activity in vitro, suggesting that it may modify the expression of the IRAK3 gene in vivo.
Critical illness in adults is often followed by ALI. Epidemiologic studies indicate that the incidence and outcomes of ALI partly depend on the nature of the precipitating disease and individual susceptibility. Current evidence suggests that variations in genes involved in pulmonary or systemic inflammatory processes are associated with a high risk of developing ALI (16, 37). Therefore, genes/pathways involved in the development of other complex diseases are likely to be relevant in the onset, course, or severity of ALI. The IRAK3 gene is critical for the early recognition of bacterial products by the Toll-like family of receptors (7, 20), and although it has been poorly studied for associations with susceptibility to other complex diseases and no published reports have associated IRAK3 polymorphisms with sepsis or ALI (http://geneticassociationdb.nih.gov; accessed May 2010), the IRAK3 gene was recently studied in association with susceptibility to chronic inflammatory conditions such as asthma (38, 39) and inflammatory bowel disease (40). In fact, strong evidence for the role of IRAK3 variants in susceptibility to asthma was found by Balaci and colleagues (39), who positionally cloned the gene in asthma after using successive linkage and association analyses of samples from families and unrelated case-control subjects from Southern European populations. Notably, rs11465955 and rs1370128, which tagged rs1732887 in our sample, were associated with asthma in samples from Sardinia and Italy (39). This finding may be indicative of a gene region harboring variants with pleiotropic effects in both asthma and ALI, and deserving of further study.
The region where −1464 A/G is located was predicted to be a highly conserved putative human–murine FOXP3 transcription factor binding site that would be disrupted in the presence of the G allele. Because FOXP3 was demonstrated to be a transcriptional repressor (41), patients with severe sepsis and carrying the −1464G allele may manifest increased IRAK3 gene transcription and higher levels of mRNA expression. This would imply a greater negative regulatory capacity, favoring an imbalance of the inflammatory and anti-inflammatory processes that would enhance the immunosuppression occurring as part of the septic process (25, 42). This hypothesis is not only congruent with our in vitro observations of significantly higher luciferase activity for constructs with −1464G (which is the allele at-risk for ALI), but also with the increased levels of the IRAK3 gene in monocytes from patients with sepsis (22), with the decreased capacity of monocytes to release proinflammatory cytokines after ex vivo stimulation with LPS or Burkholderia species, and with the positive correlation of IRAK3 gene expression with poor outcomes in sepsis (26). This evidence testifies to the pivotal role of IRAK-3 in the generation of protective, innate immune responses in the lung (23, 24). However, experimental validation of the binding of FOXP3 to the −1464 A/G region and a demonstration of the functionality of −1464 A/G in its native genomic context (e.g., by gene expression analysis in relevant cell lines or in cells isolated from patients) are necessary.
Although we followed current guidelines for genetic association studies (43) and used a number of approaches to reduce the chance of reporting a false-positive association, such a possibility cannot be excluded. Two major limitations of the study include its lack of a replication sample and its reduced sample size, particularly for detecting associations with secondary outcomes, given the modest effect sizes of common variants (44). In addition, we used population-based subjects as controls to test for the association of IRAK3 variants with susceptibility to severe sepsis. Control subjects from the general population are not affected by selection bias (i.e., the possibility that health concerns may have influenced participation), and nesting association studies in cohorts of the general population has been productive, leading to many firm genetic associations (45, 46). However, the use of these cohorts entails some limitations. Most importantly in this study, they did not allow us to control for important environmental factors that might explain the absence of association of IRAK3 variants with susceptibility to severe sepsis. Moreover, this study was designed to explore the effects of common variants in disease, and not to analyze the effects of rare variants, for which the study was underpowered. Thus, other variants of the gene, neither studied for functionality nor revealed by our resequencing, may also contribute to ALI. Furthermore, although rs10506481 showed a reduced LD with nearby regions, rs1732887 showed moderate-to-strong LD, extending as far as 125 kb upstream from IRAK3, where the LLPH and TMBIM4 genes lie (not shown). Thus, whether the observed association of the IRAK3 gene with ALI is attributable to its biological function or to its LD with nearby genes requires further study.
To the best of our knowledge, we provide the first evidence suggesting that common genetic variants of IRAK3 may be novel determinants of susceptibility to sepsis-induced ALI. Although our results were adjusted for multiple comparisons, relevant covariates, and population stratification, and although we used functional assays to demonstrate in vitro effects of SNPs, we underscore the limitations of our sample size and the need for replication in independent studies. The early tailoring of treatments has been beneficial for outcomes of sepsis in animal models (47). Therefore, an early classification of patients, based on their genetics to predict immunomodulatory status, may offer benefits in more specific therapeutic interventions.
Supplementary Material
Acknowledgments
The authors thank Dan Nicolae and Xiaoquan Wen for their advice using TUNA, Santiago Basaldúa for help with the statistical analysis, Tobías Felipe for Fortran scripting for handling TUNA outputs, and two anonymous reviewers for their helpful suggestions to improve the manuscript.
Footnotes
This work was supported by grants from the Ministry of Science of Spain (SAF 2004–06833) and Fundación Canaria de InvestigaciÓn y Salud (53/04), by a specific agreement between the Instituto de Salud Carlos III and the Gobierno de Canarias (EMER07/001) under the National Strategy for Science and Technology 2015 framework, and by grants HL91899, HL58064, and GM07019 from the National Heart Lung Blood Institute of the National Institutes of Health.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2010-0292OC on February 4, 2011
Author Disclosure: L.B. received lecture fees from Hamilton, a sponsored grant from Instituto Carlos III, and a patent from the Corporació Sanitària Parc Taulí for a monitoring platform, and he owns stock in Better Care SL. J.V. received lecture fees from GlaxoSmithKline and a sponsored grant from MAQUET (Solna, Sweden). M.P.-Y., S.-F.M., X.S., P.T., A.C., J.B., L.P.-M., E.E., A.M., J.G.N.G., and C.F. do not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Cohen J. The immunopathogenesis of sepsis. Nature 2002;420:885–891 [DOI] [PubMed] [Google Scholar]
- 2.Moss M. Epidemiology of sepsis: race, sex, and chronic alcohol abuse. Clin Infect Dis 2005;41:S490–S497 [DOI] [PubMed] [Google Scholar]
- 3.Blanco J, Muriel-Bombin A, Sagredo V, Taboada F, Gandia F, Tamayo L, Collado J, Garcia-Labattut A, Carriedo D, Valledor M, et al. Grupo de Estudios y Análisis en Cuidados Intensivos: incidence, organ dysfunction and mortality in severe sepsis: a Spanish multicentre study. Crit Care 2008;12:R158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frodsham AJ, Hill AV. Genetics of infectious diseases. Hum Mol Genet 2004;13:R187–R194 [DOI] [PubMed] [Google Scholar]
- 5.de Craen AJ, Posthuma D, Remarque EJ, van den Biggelaar AH, Westendorp RG, Boomsma DI. Heritability estimates of innate immunity: an extended twin study. Genes Immun 2005;6:167–170 [DOI] [PubMed] [Google Scholar]
- 6.Aziz RK, Kansal R, Abdeltawab NF, Rowe SL, Su Y, Carrigan D, Nooh MM, Attia RR, Brannen C, Gardner LA, et al. Susceptibility to severe streptococcal sepsis: use of a large set of isogenic mouse lines to study genetic and environmental factors. Genes Immun 2007;8:404–415 [DOI] [PubMed] [Google Scholar]
- 7.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol 2007;7:179–190 [DOI] [PubMed] [Google Scholar]
- 8.Flores C, Perez-Mendez L, Maca-Meyer N, Muriel A, Espinosa E, Blanco J, Sanguesa R, Muros M, Garcia JG, Villar J. A common haplotype of the LBP gene predisposes to severe sepsis. Crit Care Med 2009;37:2759–2766 [DOI] [PubMed] [Google Scholar]
- 9.Sutherland AM, Walley KR, Russell JA. Polymorphisms in CD14, mannose-binding lectin, and Toll-like receptor–2 are associated with increased prevalence of infection in critically ill adults. Crit Care Med 2005;33:638–644 [DOI] [PubMed] [Google Scholar]
- 10.Arbour NC, Lorenz E, Schutte BC, Zabner J, Kline JN, Jones M, Frees K, Watt JL, Schwartz DA. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat Genet 2000;25:187–191 [DOI] [PubMed] [Google Scholar]
- 11.Bochud PY, Hawn TR, Aderem A. Cutting edge: a Toll-like receptor 2 polymorphism that is associated with lepromatous leprosy is unable to mediate mycobacterial signaling. J Immunol 2003;170:3451–3454 [DOI] [PubMed] [Google Scholar]
- 12.Kang TJ, Lee SB, Chae GT. A polymorphism in the Toll-like receptor 2 is associated with IL-12 production from monocyte in lepromatous leprosy. Cytokine 2002;20:56–62 [DOI] [PubMed] [Google Scholar]
- 13.Lorenz E, Mira JP, Cornish KL, Arbour NC, Schwartz DA. A novel polymorphism in the Toll-like receptor 2 gene and its potential association with staphylococcal infection. Infect Immun 2000;68:6398–6401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khor CC, Chapman SJ, Vannberg FO, Dunne A, Murphy C, Ling EY, Frodsham AJ, Walley AJ, Kyrieleis O, Khan A, et al. A Mal functional variant is associated with protection against invasive pneumococcal disease, bacteremia, malaria and tuberculosis. Nat Genet 2007;39:523–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark MF, Baudouin SV. A systematic review of the quality of genetic association studies in human sepsis. Intensive Care Med 2006;32:1706–1712 [DOI] [PubMed] [Google Scholar]
- 16.Flores C, Pino-Yanes MM, Villar J. A quality assessment of genetic association studies supporting susceptibility and outcome in acute lung injury. Crit Care 2008;12:R130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picard C, Puel A, Bonnet M, Ku CL, Bustamante J, Yang K, Soudais C, Dupuis S, Feinberg J, Fieschi C, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science 2003;299:2076–2079 [DOI] [PubMed] [Google Scholar]
- 18.Arcaroli J, Silva E, Maloney JP, He Q, Svetkauskaite D, Murphy JR. Abraham E. Variant IRAK-1 haplotype is associated with increased nuclear factor–kappaB activation and worse outcomes in sepsis. Am J Respir Crit Care Med 2006;173:1335–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wesche H, Gao X, Li X, Kirschning CJ, Stark GR, Cao Z. IRAK-M is a novel member of the pelle/interleukin-1 receptor–associated kinase (IRAK) family. J Biol Chem 1999;274:19403–19410 [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi K, Hernandez LD, Galan JE, Janeway CA., Jr Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 2002;110:191–202 [DOI] [PubMed] [Google Scholar]
- 21.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, et al. Inflammatory and host response to injury: large scale collaborative research program: a network-based analysis of systemic inflammation in humans. Nature 2005;437:1032–1037 [DOI] [PubMed] [Google Scholar]
- 22.Escoll P, del Fresno C, Garcia L, Valles G, Lendinez MJ, Arnalich F, Lopez-Collazo E. Rapid up-regulation of IRAK-M expression following a second endotoxin challenge in human monocytes and in monocytes isolated from septic patients. Biochem Biophys Res Commun 2003;311:465–472 [DOI] [PubMed] [Google Scholar]
- 23.Villar J, Cabrera N, Casula M, Flores C, Valladares F, Muros M, Slutsky AS, Kacmarek RM. Mechanical ventilation modulates Toll-like receptor signaling pathway in a sepsis-induced lung injury model. Intensive Care Med 2010;36:1409–1457 [DOI] [PubMed] [Google Scholar]
- 24.Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, Flavell RA, Standiford TJ. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest 2006;116:2532–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCall CE, Grosso-Wilmoth LM, LaRue K, Guzman RN, Cousart SL. Tolerance to endotoxin-induced expression of the interleukin-1 beta gene in blood neutrophils of humans with the sepsis syndrome. J Clin Invest 1993;91:853–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiersinga WJ, van't Veer C, van den Pangaart PS, Dondorp AM, Day NP, Peacock SJ, van der Poll T. Immunosuppression associated with interleukin-1r–associated–kinase-M upregulation predicts mortality in Gram-negative sepsis (melioidosis). Crit Care Med 2009;37:569–576 [DOI] [PubMed] [Google Scholar]
- 27.Villar J, Perez-Mendez L, Flores C, Maca-Meyer N, Espinosa E, Muriel A, Sanguesa R, Blanco J, Muros M, Kacmarek RMA. CXCL2 polymorphism is associated with better outcomes in patients with severe sepsis. Crit Care Med 2007;35:2292–2297 [DOI] [PubMed] [Google Scholar]
- 28.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 2003;31:1250–1256 [DOI] [PubMed] [Google Scholar]
- 29.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American–European Consensus Conference on ARDS: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818–824 [DOI] [PubMed] [Google Scholar]
- 30.Conde L, Vaquerizas JM, Dopazo H, Arbiza L, Reumers J, Rousseau F, Schymkowitz J, Dopazo J. PupaSuite: finding functional single nucleotide polymorphisms for large-scale genotyping purposes. Nucleic Acids Res 2006;34:W621–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang K. Testing for genetic association in the presence of population stratification in genome-wide association studies. Genet Epidemiol 2009;33:637–645 [DOI] [PubMed] [Google Scholar]
- 32.Flores C, Maca-Meyer N, Perez-Mendez L, Sanguesa R, Espinosa E, Muriel A, Blanco J, Villar JA. CXCL2 tandem repeat promoter polymorphism is associated with susceptibility to severe sepsis in the Spanish population. Genes Immun 2006;7:141–149 [DOI] [PubMed] [Google Scholar]
- 33.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc, B 1995;57:289–300 [Google Scholar]
- 34.Westfall PH, Young SS. Resampling-based multiple testing: examples and methods for P-value adjustment. New York: John Wiley & Sons; 1993 [Google Scholar]
- 35.Wen X, Nicolae DL. Association studies for untyped markers with TUNA. Bioinformatics 2008;24:435–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cassani B, Poliani PL, Moratto D, Sobacchi C, Marrella V, Imperatori L, Vairo D, Plebani A, Giliani S, Vezzoni P, et al. Defect of regulatory T cells in patients with Omenn syndrome. J Allergy Clin Immunol 2010;125:209–216 [DOI] [PubMed] [Google Scholar]
- 37.Villar J, Maca-Meyer N, Perez-Mendez L, Flores C. Bench-to-bedside review: understanding genetic predisposition to sepsis. Crit Care 2004;8:180–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakashima K, Hirota T, Obara K, Shimizu M, Jodo A, Kameda M, Doi S, Fujita K, Shirakawa T, Enomoto T, et al. An association study of asthma and related phenotypes with polymorphisms in negative regulator molecules of the TLR signaling pathway. J Hum Genet 2006;51:284–291 [DOI] [PubMed] [Google Scholar]
- 39.Balaci L, Spada MC, Olla N, Sole G, Loddo L, Anedda F, Naitza S, Zuncheddu MA, Maschio A, Altea D, et al. IRAK-M is involved in the pathogenesis of early-onset persistent asthma. Am J Hum Genet 2007;80:1103–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weersma RK, Oostenbrug LE, Nolte IM, Van Der Steege G, Oosterom E, Van Dullemen HM, Kleibeuker JH, Dijkstra G. Association of interleukin-1 receptor–associated kinase M (IRAK-M) and inflammatory bowel diseases. Scand J Gastroenterol 2007;42:827–833 [DOI] [PubMed] [Google Scholar]
- 41.Schubert LA, Jeffery E, Zhang Y, Ramsdell F, Ziegler SF. Scurfin (FOXP3) acts as a repressor of transcription and regulates T cell activation. J Biol Chem 2001;276:37672–37679 [DOI] [PubMed] [Google Scholar]
- 42.Lyn-Kew K, Standiford TJ. Immunosuppression in sepsis. Curr Pharm Des 2008;14:1870–1881 [DOI] [PubMed] [Google Scholar]
- 43.Little J, Higgins JP, Ioannidis JP, Moher D, Gagnon F, von Elm E, Khoury MJ, Cohen B, Davey-Smith G, Grimshaw J, et al. Strengthening the reporting of genetic association studies (STREGA): an extension of the STROBE statement. Hum Genet 2009;125:131–151 [DOI] [PubMed] [Google Scholar]
- 44.Altshuler D, Daly MJ, Lander ES. Genetic mapping in human disease. Science 2008;322:881–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 2007;447:661–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moffatt MF, Kabesch M, Liang L, Dixon AL, Strachan D, Heath S, Depner M, von Berg A, Bufe A, Rietschel E, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature 2007;448:470–473 [DOI] [PubMed] [Google Scholar]
- 47.Osuchowski MF, Connett J, Welch K, Granger J, Remick DG. Stratification is the key: inflammatory biomarkers accurately direct immunomodulatory therapy in experimental sepsis. Crit Care Med 2009;37:1567–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.