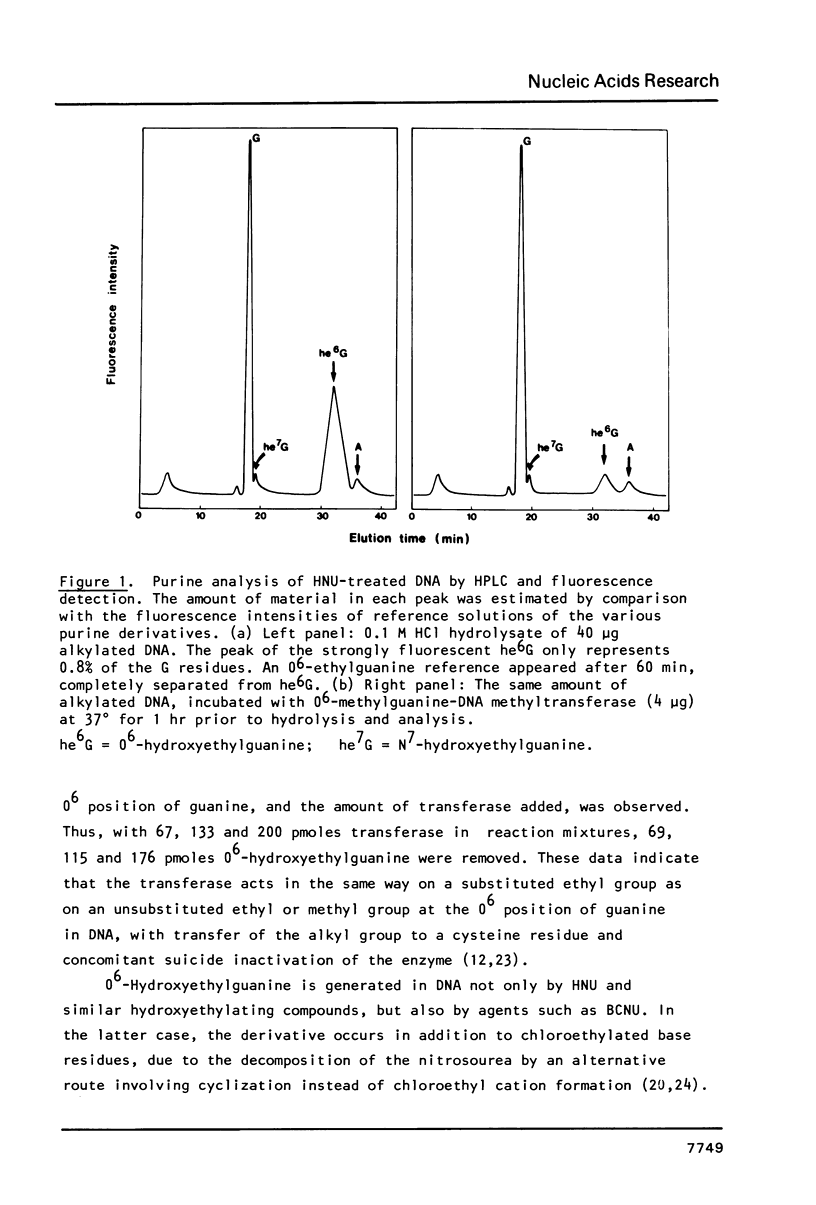
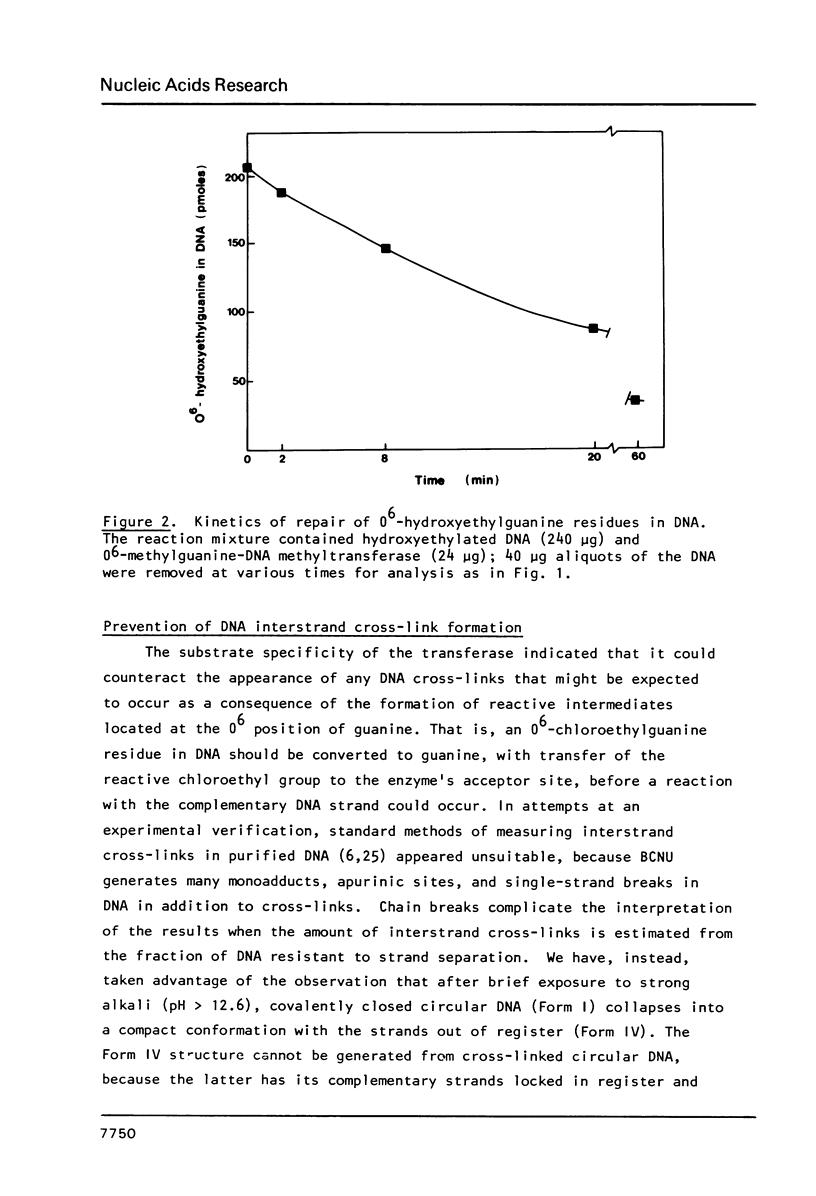
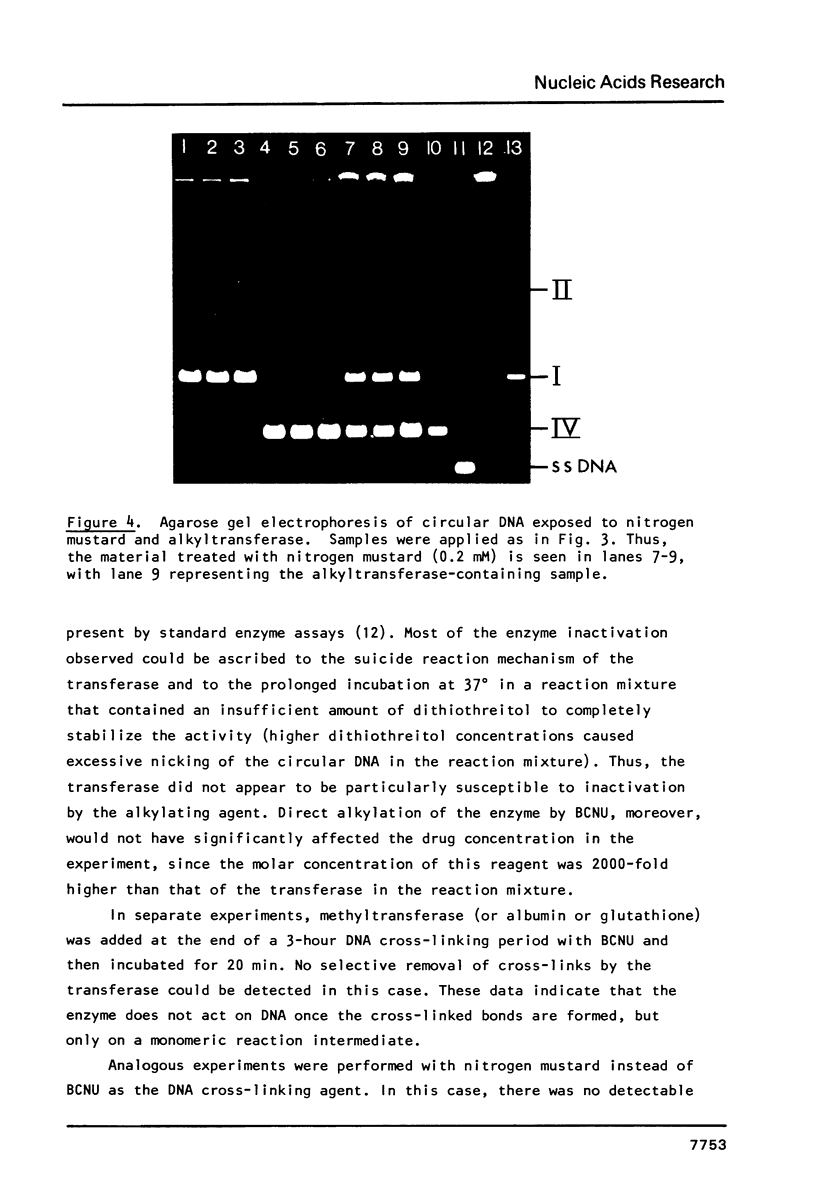
Abstract
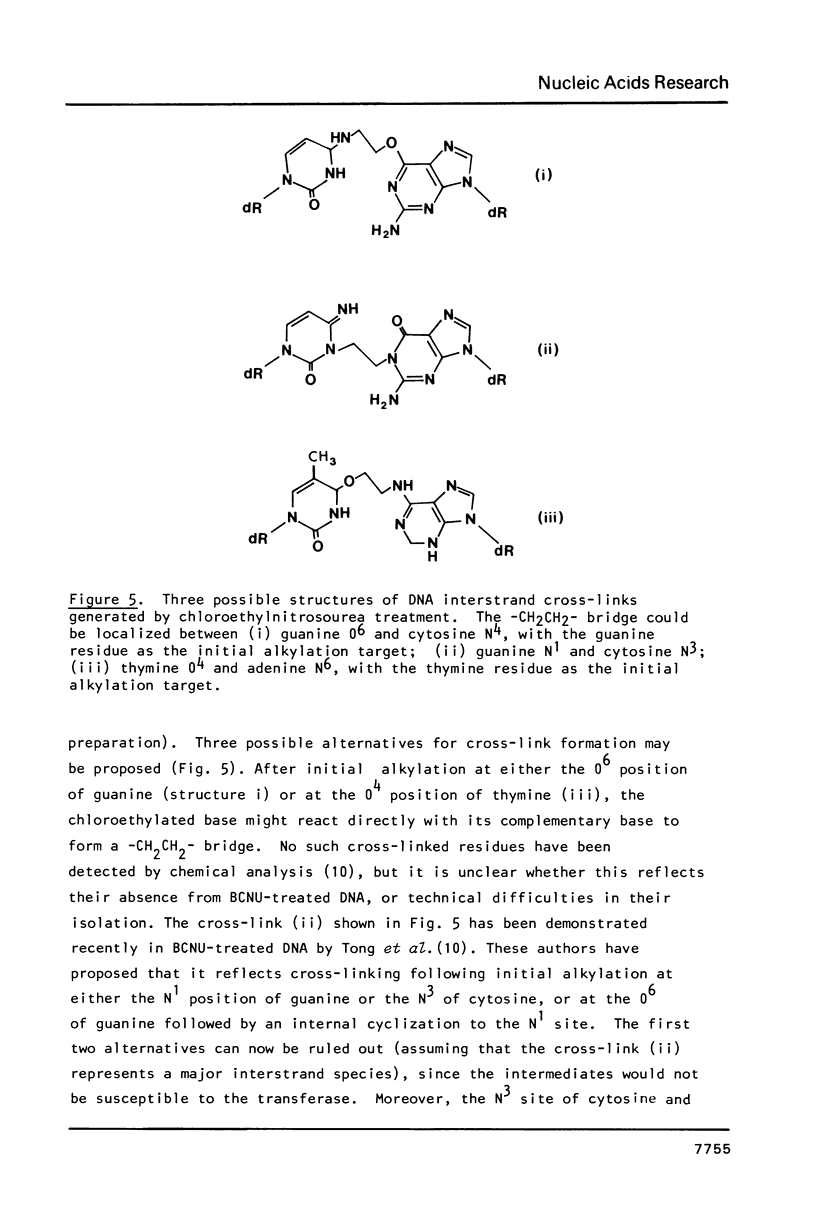
The DNA repair enzyme O6-methylguanine-DNA methyltransferase has been used as a reagent to analyse the initial reaction sites of alkylating agents such as chloroethylnitrosourea that cross-link DNA. The transferase can be employed for this purpose because it removes substituted ethyl groups from DNA, as shown by its ability to act on O6-hydroxyethylguanine residues in DNA. The enzyme counteracts the formation of interstrand cross-links induced by bis-chloroethylnitrosourea, but not those induced by nitrogen mustard. Once formed, chloroethylnitrosourea-induced cross-links are not broken by the enzyme. In agreement with deductions from experiments with living cells, it is concluded that chloroethylnitrosourea act by forming reactive monoadducts at the O6 position of guanine and/or the O4 position of thymine, which subsequently generate -CH2CH2- bridges to the complementary DNA strand. A new method for quantitating interstrand cross-links in DNA has been employed.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby J., Paton D., Styles J. A., Greatbanks D., Wright B. Synthesis of N7-hydroxyethylguanine and O6-hydroxyethylguanine. Markers for the reaction of ethylene oxide (EO) with DNA. Mutat Res. 1982 Mar;103(3-6):257–261. doi: 10.1016/0165-7992(82)90051-3. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner M., Burke R. L., Alberts B. M. Purification of the T4 gene 32 protein free from detectable deoxyribonuclease activities. J Biol Chem. 1979 Oct 10;254(19):9565–9572. [PubMed] [Google Scholar]
- Chun E. H., Gonzales L., Lewis F. S., Jones J., Rutman R. J. Differences in the in vivo alkylation and cross-linking of nitrogen mustard-sensitive and -resistant lines of Lettré-Ehrlich asites tumors. Cancer Res. 1969 Jun;29(6):1184–1194. [PubMed] [Google Scholar]
- Day R. S., 3rd, Ziolkowski C. H., Scudiero D. A., Meyer S. A., Lubiniecki A. S., Girardi A. J., Galloway S. M., Bynum G. D. Defective repair of alkylated DNA by human tumour and SV40-transformed human cell strains. Nature. 1980 Dec 25;288(5792):724–727. doi: 10.1038/288724a0. [DOI] [PubMed] [Google Scholar]
- Demple B., Jacobsson A., Olsson M., Robins P., Lindahl T. Repair of alkylated DNA in Escherichia coli. Physical properties of O6-methylguanine-DNA methyltransferase. J Biol Chem. 1982 Nov 25;257(22):13776–13780. [PubMed] [Google Scholar]
- Erickson L. C., Laurent G., Sharkey N. A., Kohn K. W. DNA cross-linking and monoadduct repair in nitrosourea-treated human tumour cells. Nature. 1980 Dec 25;288(5792):727–729. doi: 10.1038/288727a0. [DOI] [PubMed] [Google Scholar]
- Evensen G., Seeberg E. Adaptation to alkylation resistance involves the induction of a DNA glycosylase. Nature. 1982 Apr 22;296(5859):773–775. doi: 10.1038/296773a0. [DOI] [PubMed] [Google Scholar]
- Harris A. L., Karran P., Lindahl T. O6-Methylguanine-DNA methyltransferase of human lymphoid cells: structural and kinetic properties and absence in repair-deficient cells. Cancer Res. 1983 Jul;43(7):3247–3252. [PubMed] [Google Scholar]
- Hora J. F., Eastman A., Bresnick E. O6-methylguanine methyltransferase in rat liver. Biochemistry. 1983 Aug 2;22(16):3759–3763. doi: 10.1021/bi00285a007. [DOI] [PubMed] [Google Scholar]
- Karran P., Hjelmgren T., Lindahl T. Induction of a DNA glycosylase for N-methylated purines is part of the adaptive response to alkylating agents. Nature. 1982 Apr 22;296(5859):770–773. doi: 10.1038/296770a0. [DOI] [PubMed] [Google Scholar]
- Kohn K. W. Interstrand cross-linking of DNA by 1,3-bis(2-chloroethyl)-1-nitrosourea and other 1-(2-haloethyl)-1-nitrosoureas. Cancer Res. 1977 May;37(5):1450–1454. [PubMed] [Google Scholar]
- Lawley P. D. Effects of some chemical mutagens and carcinogens on nucleic acids. Prog Nucleic Acid Res Mol Biol. 1966;5:89–131. doi: 10.1016/s0079-6603(08)60232-9. [DOI] [PubMed] [Google Scholar]
- Lijinsky W., Reuber M. D. Carcinogenicity of hydroxylated alkylnitrosoureas and of nitrosooxazolidones by mouse skin painting and by gavage in rats. Cancer Res. 1983 Jan;43(1):214–221. [PubMed] [Google Scholar]
- Lindahl T., Andersson A. Rate of chain breakage at apurinic sites in double-stranded deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3618–3623. doi: 10.1021/bi00769a019. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Demple B., Robins P. Suicide inactivation of the E. coli O6-methylguanine-DNA methyltransferase. EMBO J. 1982;1(11):1359–1363. doi: 10.1002/j.1460-2075.1982.tb01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lown J. W., Chauhan S. M. Mechanism of action of (2-haloethyl)nitrosoureas on DNA. Isolation and reactions of postulated 2-(alkylimino)-3-nitrosooxazolidine intermediates in the decomposition of 1,3-bis(2-chloroethyl)-, 1-(2-chloroethyl)-3-cyclohexyl-, and 1-(2-chloroethyl)-3-(4'-trans-methylcyclohexyl)-1-nitrosourea. J Med Chem. 1981 Mar;24(3):270–279. doi: 10.1021/jm00135a007. [DOI] [PubMed] [Google Scholar]
- Ludlum D. B., Kramer B. S., Wang J., Fenselau C. Reaction of 1,3-bis(2-chloroethyl)-1-nitrosourea with synthetic polynucleotides. Biochemistry. 1975 Dec 16;14(25):5480–5485. doi: 10.1021/bi00696a016. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Wiest L., Foote R. S., Mitra S., Perry W. Purification and properties of O6-methylguanine-DNA transmethylase from rat liver. J Biol Chem. 1983 Feb 25;258(4):2327–2333. [PubMed] [Google Scholar]
- Pouwels P. H., van Rotterdam J., Cohen J. A. Structure of the replicative form of bacteriophage phi-X-174. VII. Renaturation of denatured double-stranded phi-X DNA. J Mol Biol. 1969 Mar 28;40(3):379–390. doi: 10.1016/0022-2836(69)90160-0. [DOI] [PubMed] [Google Scholar]
- Samson L., Cairns J. A new pathway for DNA repair in Escherichia coli. Nature. 1977 May 19;267(5608):281–283. doi: 10.1038/267281a0. [DOI] [PubMed] [Google Scholar]
- Sedgwick B., Lindahl T. A common mechanism for repair of O6-methylguanine and O6-ethylguanine in DNA. J Mol Biol. 1982 Jan 5;154(1):169–175. doi: 10.1016/0022-2836(82)90424-7. [DOI] [PubMed] [Google Scholar]
- Singer B., Kuśmierek J. T. Chemical mutagenesis. Annu Rev Biochem. 1982;51:655–693. doi: 10.1146/annurev.bi.51.070182.003255. [DOI] [PubMed] [Google Scholar]
- Sklar R., Strauss B. Removal of O6-methylguanine from DNA of normal and xeroderma pigmentosum-derived lymphoblastoid lines. Nature. 1981 Jan 29;289(5796):417–420. doi: 10.1038/289417a0. [DOI] [PubMed] [Google Scholar]
- Swenson D. H., Frei J. V., Lawley P. D. Synthesis of 1-(2-hydroxyethyl)-1-nitrosourea and comparison of its carcinogenicity with that of 1-ethyl-1-nitrosourea. J Natl Cancer Inst. 1979 Dec;63(6):1469–1473. [PubMed] [Google Scholar]
- Tong W. P., Kirk M. C., Ludlum D. B. Formation of the cross-link 1-[N3-deoxycytidyl),2-[N1-deoxyguanosinyl]ethane in DNA treated with N,N'-bis(2-chloroethyl)-N-nitrosourea. Cancer Res. 1982 Aug;42(8):3102–3105. [PubMed] [Google Scholar]
- Tong W. P., Kohn K. W., Ludlum D. B. Modifications of DNA by different haloethylnitrosoureas. Cancer Res. 1982 Nov;42(11):4460–4464. [PubMed] [Google Scholar]
- Tong W. P., Ludlum D. B. Formation of the cross-linked base, diguanylethane, in DNA treated with N,N'-bis(2-chloroethyl)-N-nitrosourea. Cancer Res. 1981 Feb;41(2):380–382. [PubMed] [Google Scholar]
- Yarosh D. B., Foote R. S., Mitra S., Day R. S., 3rd Repair of O6-methylguanine in DNA by demethylation is lacking in Mer- human tumor cell strains. Carcinogenesis. 1983;4(2):199–205. doi: 10.1093/carcin/4.2.199. [DOI] [PubMed] [Google Scholar]
- Zlotogorski C., Erickson L. C. Pretreatment of normal human fibroblasts and human colon carcinoma cells with MNNG allows chloroethylnitrosourea to produce DNA interstrand crosslinks not observed in cells treated with chloroethylnitrosourea alone. Carcinogenesis. 1983;4(6):759–763. doi: 10.1093/carcin/4.6.759. [DOI] [PubMed] [Google Scholar]