Abstract
Anxiety is a relevant problem in dental practice. The Visual Analogue Scale for Anxiety (VAS‐A), introduced in dentistry in 1988, has not yet been validated in large series. The aim of this study is to check VAS‐A effectiveness in more than 1000 patients submitted to implantology. The VAS‐A and the Dental Anxiety Scale (DAS) were administered preoperatively to 1114 patients (459 males and 655 females, age 54.7 ± 13.1 years). Statistical analysis was conducted with Pearson correlation coefficient, the receiver operating characteristic (ROC) curve, and McNemar tests. A close correlation between DAS and VAS‐A was found (r = 0.57, P < .0001); the VAS‐A thresholds of dental anxiety and phobia were 5.1 and 7.0 cm, respectively. Despite a significant concordance of tests in 800 cases (72%), disagreement was found in the remaining 314 cases (28%), and low DAS was associated with high VAS‐A (230 cases) or vice versa (84 cases). Our study confirms that VAS‐A is a simple, sensitive, fast, and reliable tool in dental anxiety assessment. The rate of disagreement between VAS‐A and DAS is probably due to different test sensitivities to different components of dental anxiety. VAS‐A can be used effectively in the assessment of dental patients, using the values of 5.1 cm and 7.0 cm as cutoff values for anxiety and phobia, respectively.
Key Words: Dental anxiety, Dental Anxiety Scale, Visual Analogue Scale, Dentistry, Psychological tests
The relevance of psychology and behavioral sciences is ever increasing both in dental education and in clinical practice.1 A large number of patients are so fearful of dental care as to delay or avoid attendance; dental anxiety involves a wide‐ranging and dynamic impact on patients' lives, in addition to avoidance behavior.2–9 The incidence of dental anxiety and phobia ranges from 10 to 30% of the population and depends on several factors, such as nationality, sociocultural background, previous experience, and type of intervention.3,10–14 Oral surgery is a stressful condition that causes a relevant increase in anxiety, expected suffering, and pain perception immediately before the operation15,16; dental anxiety also impairs the patient's capability of understanding provided information.17 Therefore, careful anxiety assessment and management are essential steps toward appropriate patient management and overall high‐quality care.
Dental anxiety can be evaluated through a wide range of approaches, including several psychological tests that can be used to explore general aspects of anxiety and/or dental anxiety; a comprehensive review of main tests for anxiety and pain evaluation in dentistry has been published by Newton and Buck in 2000.18 Of 15 tests mentioned in this review, Corah's Dental Anxiety Scale (DAS) is the most widely used.19–21 Despite its wide use and effectiveness, DAS has been criticized as exhibiting a narrow range of scores22 and showing low resolution for intermediate levels of anxiety23; furthermore, DAS cannot check all aspects of anxiety, such as personality‐related components (e.g., the role of pessimism and negative thoughts, proneness to anxiety) and behavioral and cognitive responses. As a result, it might underestimate or overestimate the level of fear, especially in patients with intermediate levels of anxiety.
Nonverbal tests are not affected by interpretation of words and phrases, may be more easily understood, and do not restrain patients' responses within the administered scenario, unlike DAS. The concept of visual analogue scale (VAS) was introduced in the 60s by Aitken24 to measure psychological states, then pain25; nowadays, it is universally accepted as a measure of pain intensity, but it is also used to assess other subjective experiences. Because it is simple and rapid, the VAS has gained a wide range of applications in clinical studies. When the key word “visual analogue scale” is inserted into PUBMED, more than 7500 papers are retrieved, most of which deal with pain. The Visual Analogue Scale for Anxiety (VAS‐A) was introduced in 197626 and was first used in dental patients in 198827; in recent years, it has been seldom used for the assessment of both dental anxiety15,16,28–34 and other medical conditions.35–39
VAS‐A was validated in a sample of 45 dental patients,27 showing a significant correlation with DAS and the A‐State portion of the Spielberger's State‐Trait Anxiety Inventory (STAI) in both preoperative and postoperative anxiety assessment. However, VAS‐A has not been checked so far in large series. The aim of this study is to assess its reliability and define the thresholds of anxiety and phobia that best correlate with DAS in a large sample of patients undergoing oral implantation surgery.
SUBJECTS AND METHODS
This study was approved by our local ethical committee, and all patients gave informed consent; 1114 consecutive patients (459 male and 655 female, age 54.7 ± 13.1 years) submitted to implantology were included. Patients who were unwilling or unable to fill out DAS because of their clinical condition (e.g., neurologic or psychiatric disorders) or foreign nationality were discarded from the study.
At the beginning of the preoperative examination, all patients filled out the Italian version of DAS14; then VAS‐A was administered, before any other evaluation of patients' physical condition or information about sedation was provided. Examination included an assessment of clinical conditions and their rating according to the American Society of Anesthesiologists (ASA) Physical Status Classification40; pharmacologic treatment for coexisting diseases was also recorded. All information regarding conscious sedation, the postoperative period, and preemptive analgesia was then delivered to patients. Conscious sedation, which included presedation with oral delorazepam and sedation with intravenous diazepam according to the Manani protocol,41,42 was proposed to all patients and was planned at the end of the examination.
Statistical analysis was conducted with the Pearson correlation coefficient, the receiver operating characteristic (ROC) curve, and McNemar tests, using the Statistical Package for the Social Sciences (SPSS), version 13.0 for Windows (SPSS Inc, Chicago, Ill), for a significance level of P = .05.
RESULTS
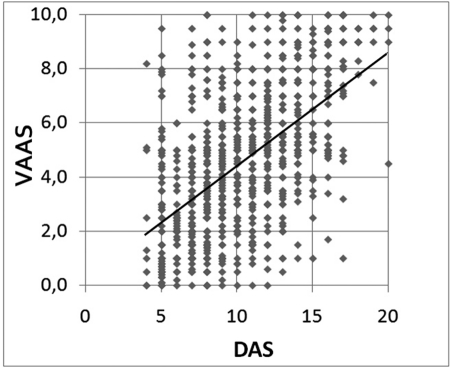
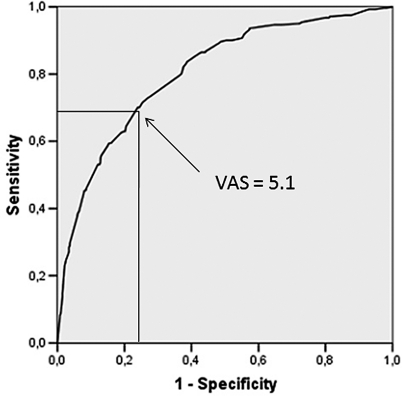
Close correlation was found between DAS and VAS‐A (Figure 1; r = 0.57; P < .001), but a large dispersion of data was present; each value of DAS corresponded to a wide range of VAS‐A data, which, apart from high DAS scores, included almost all VAS‐A values. ROC curve analysis was allowed to estimate the cutoff value for anxiety in VAS‐A that best fitted DAS data using a dichotomic grading of the DAS whereby anxiety was defined by a score >12. The area under the curve (AUC) in the ROC curve analysis was equal to 0.805 (Figure 2; P < .001), and the VAS‐A cutoff value, corresponding to the best product of sensitivity (69.5%) and specificity (72.6%), was equal to 5.1 cm; therefore, the DAS score of 12, which is the midpoint of the scale (defining the ranges 4 to 12 and 13 to 20), was paralleled by an equivalent division of VAS‐A into 2 symmetrical parts, that is, 0 to 5 and 5.1 to 10 cm.
Figure 1.
Distribution and linear regression of preoperative Visual Analogue Scale for Anxiety (VAS‐A) and Dental Anxiety Scale (DAS) scores in 1114 patients submitted to implantology. A significant correlation between the tests is observed, but a large dispersion of data is present with a wide range of VAS‐A values for each DAS score.
Figure 2.
Receiver operating characteristic (ROC) curve calculated for Visual Analogue Scale for Anxiety (VAS‐A) using the Dental Anxiety Scale (DAS) as the stated variable, where a DAS > 12 was selected as an indicator of dental anxiety (area under the curve [AUC] = 0.805; P < .001). A VAS‐A cutoff value = 5.1, corresponding to the best product of sensitivity (69.5%) and specificity (72.6%), has been chosen as a threshold for anxiety.
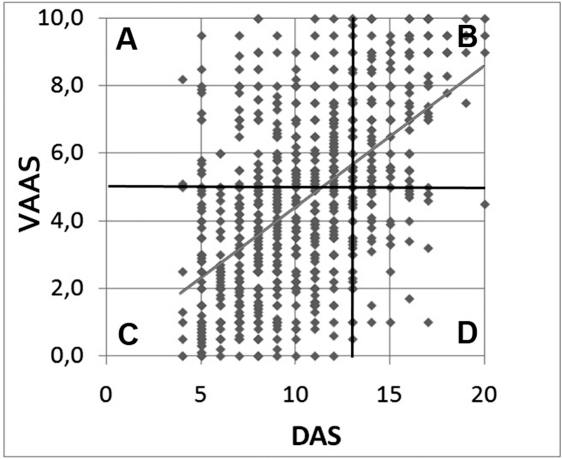
VAS‐A grading obtained by ROC curve was allowed to divide Figure 1 into 4 quadrants (Figure 3), showing the areas where both tests provided concurrent results (B and C) and those where the results were discordant (A through D). Both tests provided the same information in most cases (Table; concurrence index = 0.72); in the remaining 28% of patients with discordant results, VAS‐A mainly overestimated the level of anxiety in comparison with DAS (230 with high VAS‐A and low DAS vs 84 with low VAS‐A and high DAS; P < .001).
Figure 3.
Distribution of preoperative Visual Analogue Scale for Anxiety (VAS‐A) and Dental Anxiety Scale (DAS) scores in 1114 patients submitted to implantology. Using DAS > 12 and VAS‐A > 5.0 as indicators of dental anxiety, the figure can be divided into the following four quadrants: (A) area of test discordance, where a low DAS is associated with a high VAS‐A; (B) area of anxiety, where both tests show a high score; (C) area of no anxiety, where both tests show a low score; and (D) area of inverse test discordance, where high DAS scores are associated with low VAS‐A values.
Relationship Between Visual Analogue Scale for Anxiety (VAS‐A) and Dental Anxiety Scale (DAS)
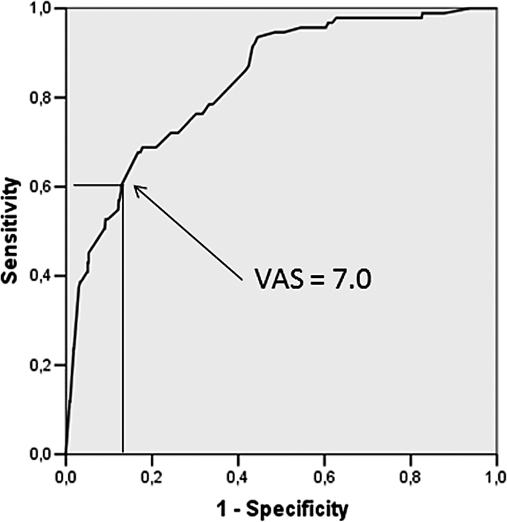
Of 1114 patients, 93 (8.3%) had a phobic level of anxiety. The ROC curve using a DAS score of 16 as a threshold for dental phobia had an AUC = 0.833 (P < .001), with a VAS‐A cutoff equal to 7.0 cm, corresponding to a sensitivity of 60.2% and a specificity of 87.2% (Figure 4).
Figure 4.
Receiver operating characteristic (ROC) curve calculated for Visual Analogue Scale for Anxiety (VAS‐A) using the Dental Anxiety Scale (DAS) as the stated variable, where a DAS > 15 was selected as an indicator of dental phobia (area under the curve [AUC] = 0.833; P < .001). A VAS‐A cutoff value = 7.0, corresponding to the best product of sensitivity (60.2%) and specificity (87.2%), has been chosen as a threshold for dental phobia.
DISCUSSION
Dental fear can be considered a universal phenomenon with different cultural features.3 The origin of dental anxiety is multidimensional and includes both endogenous and exogenous causes.43 Several psychological disorders (e.g., low self‐esteem, general fearfulness, conduct disorder, agoraphobia, simple phobia, alcohol dependence, multiple Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition [DMS IV] diagnoses) are more frequent in patients with high dental anxiety as defined by DAS.44–46 Exogenous factors include conditioned fear (yielded by previous bad experiences or information), fear of somatic intraoperative reactions, and distrust of dental professionals43; the latter, in turn, is usually caused by dentists' inappropriate behavior and traumatic dental treatments, leading to patients' helplessness and threats of loss of autonomy and violation.47 Finally, patients with severe systemic disease show a higher level of dental anxiety, related to previous experience with their diseases and interventions.14 As a result, dental anxiety is far from being a simple entity that is easily detectable with a single test.
The DAS is the most widely used and well‐validated test in the assessment of dental anxiety and phobia. It shapes 4 dentally related situations, that is, the day before dental care plus 3 scenarios (the attending room, the dentist preparing a drill, and the dental hygiene session). Each of these includes 5 responses of increasing anxiety (scores 1 to 5), with the sum of responses ranging between 4 and 20. Scores higher than 12 indicate anxious patients,48–53 and scores higher than 15 indicate phobic levels of anxiety.18 DAS has shown high internal consistency14,19 and test‐retest reliability19 and is available in 5 European languages (Dutch, Hungarian, German, Italian, and Norwegian).14,54–57 Nevertheless, it has some limitations: (a) narrow range of total scores22; (b) low resolution in intermediate level of anxiety23; and (c) dyshomogeneous answers, including descriptions of both anxiety and physical reactions.23 Furthermore, the DAS, describing a dental scenario only, may skip relevant aspects of the multidimensional nature of dental anxiety; if so, anxiety assessment in dental patients might be improved by adding other anxiety tests unrelated to the dental scenario.
VAS provides a useful measure of experience in clinical settings with the advantage of being nonverbal and thus independent from any specific descriptor of the investigated phenomenon. Apart from pain, the VAS has been used to test anxiety, well‐being, satisfaction, and physical concerns in other medical conditions, such as irritable bowel syndrome,58 rheumatoid arthritis,38 pelvic disorders,35 oocyte retrieval,36 cesarean section,59 and mechanical ventilation.60
As far as anxiety in dentistry is concerned, the VAS‐A has been checked in small series, correlating it with cortisol level28 or other psychological tests.16,34,39 The most recent study deals with implantology in 98 cases and reports a significant correlation between VAS‐A, DAS, and the expectation of experiencing pain,34 but no further information is available on the relationship between VAS‐A and DAS. Therefore, the paper by Luyk et al27 on 45 patients remains the only study providing detailed data on the relationship between VAS‐A and DAS, but it reports only the correlation between the 2 (r = 0.58; P < .01) and with stated anxiety (r = 0.76; P < .001). These data show that VAS‐A may be a useful indicator of anxiety in dentistry but do not allow us to pinpoint a VAS‐A threshold for anxiety and phobia; furthermore, use of the VAS‐A in clinical practice calls for checking the rate of discordance between the 2 tests in a large series.
Our study confirms the significant correlation between VAS‐A and DAS but shows the large dispersion of data, whereby almost all VAS‐A values are displayed for each DAS score, especially in the intermediate DAS values (Figure 1). The best relationship between VAS‐A and DAS can be obtained by classifying the VAS‐A as follows: (a) 0 to 5 cm = nonanxious patients; (b) 5.1 to 6.9 cm = anxious patients; and (c) 7.0 to 10 cm = phobic patients. However, even with this VAS‐A grading, the concurrence index is 0.72, showing that in 28% of cases, results of the tests are discordant, raising the question of which, if any, may fail in assessing anxiety in this subgroup.
Analysis of discordant data shows that about 50% of patients with VAS‐A > 5.0 have a DAS of 12 or less, while only 12.1% of those with VAS ≤ 5.0 have a high DAS value. This indicates that VAS‐A has a higher risk of overestimating preoperative anxiety, or, conversely, that DAS has a higher risk of underestimating it. Such a discrepancy calls for further study using other psychological tests (such as the Spielberger's State‐Trait Anxiety Inventory) to check which test, if any, fails to assess the patient. Another, more plausible explanation is that VAS‐A and DAS may detect different components of dental anxiety, whereby the former, because it is unrelated to the dental setting, may disclose factors undetected by DAS; for example, a patient may not be fearful of the dentist and the operation (taken by themselves) but may be concerned about possible complications of that specific intervention (e.g., alveolar nerve damage). If so, the apparent discordance does not necessarily imply that the result of one test should be considered as “false.” In case of error, it is better to overestimate than underestimate the level of anxiety; therefore, our results suggest greater effectiveness of VAS‐A, which was able to recognize as anxious 191 patients who went undetected by DAS (vs 84 detected by DAS only) or, better, to indicate the need for using both tests in clinical practice, thus classifying the patient as anxious when at least 1 test is positive. The latter approach can maximize dental anxiety detection.
Finally, the fixed sequence of test administration (DAS first, then VAS‐A) is a methodologic limitation that may have caused a bias in this study; in fact, patients' anxiety might have increased while filling out the DAS, thus artifactually increasing the sensitivity of VAS‐A. This limitation calls for further study by adding other anxiety tests and randomizing their sequence of administration (Facco et al., study in progress) to properly check the correlation between tests. However, the results of this study keep all their value for routine clinical practice, where randomization is meaningless. The used fixed sequence of tests is most suitable in that the DAS can be self‐completed before the preoperative examination is performed, while the VAS can be easily administered at its beginning. In this setting, the VAS‐A grading suggested here keeps its effectiveness when used after the DAS.
In conclusion, the VAS‐A is a valid test for preoperative anxiety, with greater sensitivity than the DAS in this series. It can be used alone or in combination with DAS to improve assessment of dental anxiety. Patients with VAS‐A > 5.0 cm should be regarded as anxious, and those with VAS‐A ≥ 7.0 as phobic; further study is required, which can be conducted by adding other psychological tests (such as STAI and Beck Depression Inventory) and randomizing the order of test administration, to check the concurrence of DAS and VAS‐A and provide further insight on discordant data.
REFERENCES
- 1.Cowpe J, Plasschaert A, Harzer W, Vinka‐Puhakka A, Walmsley AD. Profile and competences for the European dentist, update 2008. Available at: www.adee.org. Accessed January 23, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Berggreb U, Linde A. Dental fear and avoidance: a comparison of two modes of treatment. J Dent Res. 1984;63:1223–1227. doi: 10.1177/00220345840630101201. [DOI] [PubMed] [Google Scholar]
- 3.Berggren U, Pierce CJ, Eli I. Characteristics of adult dentally fearful individuals: a cross‐cultural study. Eur J Oral Sci. 2000;108:268–274. doi: 10.1034/j.1600-0722.2000.108004268.x. [DOI] [PubMed] [Google Scholar]
- 4.Cohen SM, Fiske J, Newton JT. The impact of dental anxiety on daily living. Br Dent J. 2000;189:385–390. doi: 10.1038/sj.bdj.4800777. [DOI] [PubMed] [Google Scholar]
- 5.Hakeberg M, Berggren U, Carlsson SG, Grondahl HG. Long‐term effects on dental care behavior and dental health after treatments for dental fear. Anesth Prog. 1993;40:72–77. [PMC free article] [PubMed] [Google Scholar]
- 6.Moore R, Brodsgaard I, Mao TK, Kwan HW, Shiau YY, Knudsen R. Fear of injections and report of negative dentist behavior among Caucasian American and Taiwanese adults from dental school clinics. Community Dent Oral Epidemiol. 1996;24:292–295. doi: 10.1111/j.1600-0528.1996.tb00862.x. [DOI] [PubMed] [Google Scholar]
- 7.McGrath C, Bedi R. The association between dental anxiety and oral health‐related quality of life in Britain. Community Dent Oral Epidemiol. 2004;32:67–72. doi: 10.1111/j.1600-0528.2004.00119.x. [DOI] [PubMed] [Google Scholar]
- 8.Haugejorden O, Klock KS. Avoidance of dental visits: the predictive validity of three dental anxiety scales. Acta Odontol Scand. 2000;58:255–259. doi: 10.1080/00016350050217091. [DOI] [PubMed] [Google Scholar]
- 9.Mellor AC. Dental anxiety and attendance in the north‐west of England. J Dent. 1992;20:207–210. doi: 10.1016/0300-5712(92)90077-p. [DOI] [PubMed] [Google Scholar]
- 10.Erten H, Akarslan ZZ, Bodrumlu E. Dental fear and anxiety levels of patients attending a dental clinic. Quintessence Int. 2006;37:304–310. [PubMed] [Google Scholar]
- 11.Gatchel RJ. The prevalence of dental fear and avoidance: expanded adult and recent adolescent surveys. J Am Dent Assoc. 1989;118:591–593. doi: 10.14219/jada.archive.1989.0068. [DOI] [PubMed] [Google Scholar]
- 12.Kaakko T, Milgrom P, Coldwell SE, Getz T, Weinstein P, Ramsay DS. Dental fear among university students: implications for pharmacological research. Anesth Prog. 1998;45:62–67. [PMC free article] [PubMed] [Google Scholar]
- 13.Moore R, Birn H, Kirkegaard E, Brodsgaard I, Scheutz F. Prevalence and characteristics of dental anxiety in Danish adults. Community Dent Oral Epidemiol. 1993;21:292–296. doi: 10.1111/j.1600-0528.1993.tb00777.x. [DOI] [PubMed] [Google Scholar]
- 14.Facco E, Zanette G, Manani G. Italian version of Corah's Dental Anxiety Scale: normative data in patients undergoing oral surgery and relationship with the ASA physical status classification. Anesth Prog. 2008;55:109–115. doi: 10.2344/0003-3006-55.4.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eli I, Baht R, Kozlovsky A, Simon H. Effect of gender on acute pain prediction and memory in periodontal surgery. Eur J Oral Sci. 2000;108:99–103. doi: 10.1034/j.1600-0722.2000.00777.x. [DOI] [PubMed] [Google Scholar]
- 16.Eli I, Schwartz‐Arad D, Baht R, Ben‐Tuvim H. Effect of anxiety on the experience of pain in implant insertion. Clin Oral Implants Res. 2003;14:115–118. doi: 10.1034/j.1600-0501.2003.140115.x. [DOI] [PubMed] [Google Scholar]
- 17.Eli I, Schwartz‐Arad D, Bartal Y. Anxiety and ability to recognize clinical information in dentistry. J Dent Res. 2008;87:65–68. doi: 10.1177/154405910808700111. [DOI] [PubMed] [Google Scholar]
- 18.Newton JT, Buck DJ. Anxiety and pain measures in dentistry: a guide to their quality and application. J Am Dent Assoc. 2000;131:1449–1457. doi: 10.14219/jada.archive.2000.0056. [DOI] [PubMed] [Google Scholar]
- 19.Corah NL, Gale EN, Illig SJ. Assessment of a dental anxiety scale. J Am Dent Assoc. 1978;97:816–819. doi: 10.14219/jada.archive.1978.0394. [DOI] [PubMed] [Google Scholar]
- 20.Corah NL. Dental anxiety: assessment, reduction and increasing patient satisfaction. Dent Clin North Am. 1988;32:779–790. [PubMed] [Google Scholar]
- 21.Corah NL. Development of a dental anxiety scale. J Dent Res. 1969;48:596. doi: 10.1177/00220345690480041801. [DOI] [PubMed] [Google Scholar]
- 22.Schuurs AH, Hoogstraten J. Appraisal of dental anxiety and fear questionnaires: a review. Community Dent Oral Epidemiol. 1993;21:329–339. doi: 10.1111/j.1600-0528.1993.tb01095.x. [DOI] [PubMed] [Google Scholar]
- 23.Humphris GM, Morrison T, Lindsay SJ. The Modified Dental Anxiety Scale: validation and United Kingdom norms. Community Dent Health. 1995;12:143–150. [PubMed] [Google Scholar]
- 24.Aitken RC. Measurement of feelings using visual analogue scales. Proc R Soc Med. 1969;62:989–993. doi: 10.1177/003591576906201005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohnhaus EE, Adler R. Methodological problems in the measurement of pain: a comparison between the verbal rating scale and the visual analogue scale. Pain. 1975;1:379–384. doi: 10.1016/0304-3959(75)90075-5. [DOI] [PubMed] [Google Scholar]
- 26.Hornblow AR, Kidson MA. The visual analogue scale for anxiety: a validation study. Aust N Z J Psychiatry. 1976;10:339–341. doi: 10.3109/00048677609159523. [DOI] [PubMed] [Google Scholar]
- 27.Luyk NH, Beck FM, Weaver JM. A visual analogue scale in the assessment of dental anxiety. Anesth Prog. 1988;35:121–123. [PMC free article] [PubMed] [Google Scholar]
- 28.Brand HS. Anxiety and cortisol excretion correlate prior to dental treatment. Int Dent J. 1999;49:330–336. doi: 10.1111/j.1875-595x.1999.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 29.Campbell C, Hosey MT, McHugh S. Facilitating coping behavior in children prior to dental general anesthesia: a randomized controlled trial. Paediatr Anaesth. 2005;15:831–838. doi: 10.1111/j.1460-9592.2004.01565.x. [DOI] [PubMed] [Google Scholar]
- 30.Hosey MT, Blinkhorn AS. An evaluation of four methods of assessing the behaviour of anxious child dental patients. Int J Paediatr Dent. 1995;5:87–95. doi: 10.1111/j.1365-263x.1995.tb00170.x. [DOI] [PubMed] [Google Scholar]
- 31.Palmer‐Bouva C, Oosting J, Devries R, Abraham‐Inpijn L. Stress in elective dental treatment: epinephrine, norepinephrine, the VAS, and CDAS in four different procedures. Gen Dent. 1998;46:356–360. [PubMed] [Google Scholar]
- 32.Peretz B, Nazarian Y, Bimstein E. Dental anxiety in a students' paediatric dental clinic: children, parents and students. Int J Paediatr Dent. 2004;14:192–198. doi: 10.1111/j.1365-263X.2004.00545.x. [DOI] [PubMed] [Google Scholar]
- 33.Stopperich PS, Moore PA, Finder RL, McGirl BE, Weyant RJ. Oral triazolam pretreatment for intravenous sedation. Anesth Prog. 1993;40:117–121. [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz‐Arad D, Bar‐Tal Y, Eli I. Effect of stress on information processing in the dental implant surgery setting. Clin Oral Implants Res. 2007;18:9–12. doi: 10.1111/j.1600-0501.2006.01290.x. [DOI] [PubMed] [Google Scholar]
- 35.Coolen JC, Florisson JM, Bissett IP, Parry BR. Evaluation of knowledge and anxiety level of patients visiting the colorectal pelvic floor clinic. Colorectal Dis. 2006;8:208–211. doi: 10.1111/j.1463-1318.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- 36.Hong JY, Kang IS, Koong MK. Preoperative anxiety and propofol requirement in conscious sedation for ovum retrieval. J Korean Med Sci. 2003;18:863–868. doi: 10.3346/jkms.2003.18.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kidson M, Hornblow A. Examination anxiety in medical students: experiences with the visual analogue scale for anxiety. Med Educ. 1982;16:247–250. doi: 10.1111/j.1365-2923.1982.tb01259.x. [DOI] [PubMed] [Google Scholar]
- 38.Tamiya N, Araki S, Ohi G. Assessment of pain, depression, and anxiety by visual analogue scale in Japanese women with rheumatoid arthritis. Scand J Caring Sci. 2002;16:137–141. doi: 10.1046/j.1471-6712.2002.00067.x. [DOI] [PubMed] [Google Scholar]
- 39.Videbech M, Carlsson PS, Jensen NC, Videbech P. Measuring of preoperative anxiety by three self‐reporting scales: State Trait Anxiety Inventory, Symptoms CheckList 92 and visual analogue scale. Ugeskr Laeger. 2003;165:569–574. [PubMed] [Google Scholar]
- 40.ASA physical status classification system, 2008. Available at: http://www.asahq.org/clinical/physicalstatus.htm. Accessed December 1, 2009. [Google Scholar]
- 41.Manani G, Baldinelli L, Cordioloi G, Consolati E, Luisetto F, Galzigna L. Premedication with chlordemethyldiazepam and anxiolytic effect of diazepeam in implantology. Anesth Prog. 1995;42:107–112. [PMC free article] [PubMed] [Google Scholar]
- 42.Manani G, Alberton L, Bazzato MF. Analysis of an anxiolytic technique applied in 1179 patients undergoing oral surgery. Minerva Stomatol. 2005;54:551–568. [PubMed] [Google Scholar]
- 43.Liddell A, Locker D. Changes in levels of dental anxiety as a function of dental experience. Behav Modif. 2000;24:57–68. doi: 10.1177/0145445500241003. [DOI] [PubMed] [Google Scholar]
- 44.Locker D, Poulton R, Thomson WM. Psychological disorders and dental anxiety in a young adult population. Community Dent Oral Epidemiol. 2001;29:456–463. doi: 10.1034/j.1600-0528.2001.290607.x. [DOI] [PubMed] [Google Scholar]
- 45.Locker D. Psychosocial consequences of dental fear and anxiety. Community Dent Oral Epidemiol. 2003;31:144–151. doi: 10.1034/j.1600-0528.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- 46.Kvale G, Raadal M, Vika M. Treatment of dental anxiety disorders: outcome related to DSM‐IV diagnoses. Eur J Oral Sci. 2002;110:69–74. doi: 10.1034/j.1600-0722.2002.11204.x. [DOI] [PubMed] [Google Scholar]
- 47.Abrahamsson KH, Berggren U, Hallberg L, Carlsson SG. Dental phobic patients' view of dental anxiety and experiences in dental care: a qualitative study. Scand J Caring Sci. 2002;16:188–196. doi: 10.1046/j.1471-6712.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- 48.Sohn W, Ismail AI. Regular dental visits and dental anxiety in an adult dentate population. J Am Dent Assoc. 2005;136:58–66. doi: 10.14219/jada.archive.2005.0027. [DOI] [PubMed] [Google Scholar]
- 49.Ekanayake L, Dharmawardena D. Dental anxiety in patients seeking care at the University Dental Hospital in Sri Lanka. Community Dent Health. 2003;20:112–116. [PubMed] [Google Scholar]
- 50.Bedi R, McGrath C. Factors associated with dental anxiety among older people in Britain. Gerodontology. 2000;17:97–103. doi: 10.1111/j.1741-2358.2000.00097.x. [DOI] [PubMed] [Google Scholar]
- 51.Kruger E, Thomson WM, Poulton R, Davies S, Brown RH, Silva PA. Dental caries and changes in dental anxiety in late adolescence. Community Dent Oral Epidemiol. 1998;26:355–359. doi: 10.1111/j.1600-0528.1998.tb01973.x. [DOI] [PubMed] [Google Scholar]
- 52.Thomson WM, Poulton RG, Kruger E, Davies S, Brown RH, Silva PA. Changes in self‐reported dental anxiety in New Zealand adolescents from ages 15 to 18 years. J Dent Res. 1997;76:1287–1291. doi: 10.1177/00220345970760060801. [DOI] [PubMed] [Google Scholar]
- 53.Thomson WM, Stewart JF, Carter KD, Spencer AJ. Dental anxiety among Australians. Int Dent J. 1996;46:320–324. [PubMed] [Google Scholar]
- 54.Eijkman MA, Orlebeke JF. De factor ‘angst’ in de tandheelkundige situatie. Nederlandsche Tijdschrift voor Taandheekunde. 1975;82:114–123. [Google Scholar]
- 55.Kunzelmann KH, Dunninger P. Dental fear and pain: effect on patient's perception of the dentist. Community Dent Oral Epidemiol. 1990;18:264–266. doi: 10.1111/j.1600-0528.1990.tb00073.x. [DOI] [PubMed] [Google Scholar]
- 56.Neverlien PO. Normative data for Corah's Dental Anxiety Scale (DAS) for the Norwegian adult population. Community Dent Oral Epidemiol. 1990;18:162. doi: 10.1111/j.1600-0528.1990.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 57.Fabian TK, Kelemen P, Fabian G. Introduction of the concept of Dental Anxiety Scale in Hungary: epidemiologic studies on the Hungarian population. Fogorv Sz. 1998;91:43–52. [PubMed] [Google Scholar]
- 58.Bengtsson M, Ohlsson B, Ulander K. Development and psychometric testing of the Visual Analogue Scale for Irritable Bowel Syndrome (VAS‐IBS) BMC Gastroenterol. 2007;7:16. doi: 10.1186/1471-230X-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morgan PJ, Halpern S, Lam‐McCulloch J. Comparison of maternal satisfaction between epidural and spinal anesthesia for elective Cesarean section. Can J Anaesth. 2000;47:956–961. doi: 10.1007/BF03024865. [DOI] [PubMed] [Google Scholar]
- 60.Chlan LL. Relationship between two anxiety instruments in patients receiving mechanical ventilatory support. J Adv Nurs. 2004;48:493–499. doi: 10.1111/j.1365-2648.2004.03231.x. [DOI] [PubMed] [Google Scholar]