Abstract
Rationale: Although airway inflammation can persist for years after smoking cessation in patients with chronic obstructive pulmonary disease (COPD), the mechanisms of persistent inflammation are largely unknown.
Objectives: We investigated relationships between bronchial epithelial remodeling, polymeric immunoglobulin receptor (pIgR) expression, secretory IgA (SIgA), airway inflammation, and mural remodeling in COPD.
Methods: Lung tissue specimens and bronchoalveolar lavage were obtained from lifetime nonsmokers and former smokers with or without COPD. Epithelial structural changes were quantified by morphometric analysis. Expression of pIgR was determined by immunostaining and real-time polymerase chain reaction. Immunohistochemistry was performed for IgA, CD4 and CD8 lymphocytes, and cytomegalovirus and Epstein-Barr virus antigens. Total IgA and SIgA were measured by ELISA and IgA transcytosis was studied using cultured human bronchial epithelial cells.
Measurements and Main Results: Areas of bronchial mucosa covered by normal pseudostratified ciliated epithelium were characterized by pIgR expression with SIgA present on the mucosal surface. In contrast, areas of bronchial epithelial remodeling had reduced pIgR expression, localized SIgA deficiency, and increased CD4+ and CD8+ lymphocyte infiltration. In small airways (<2 mm), these changes were associated with presence of herpesvirus antigens, airway wall remodeling, and airflow limitation in patients with COPD. Patients with COPD had reduced SIgA in bronchoalveolar lavage. Air–liquid interface epithelial cell cultures revealed that complete epithelial differentiation was required for normal pIgR expression and IgA transcytosis.
Conclusions: Our findings indicate that epithelial structural abnormalities lead to localized SIgA deficiency in COPD airways. Impaired mucosal immunity may contribute to persistent airway inflammation and progressive airway remodeling in COPD.
Keywords: polymeric immunoglobulin receptor, bronchial epithelium, cell differentiation, epithelial remodeling, mucosal host defense
At a Glance Commentary
Scientific Knowledge on the Subject
Airway inflammation often persists for a prolonged period of time after smoking cessation in patients with chronic obstructive pulmonary disease (COPD), but the reason for this finding is obscure. Secretory immunoglobulin A (SIgA), together with mucociliary clearance, prevents adherence to or invasion of bronchial epithelium by pathogens and other foreign antigens, acting as a scavenger through so-called “immune exclusion.” Therefore, impaired mucosal immunity could contribute to chronic or recurrent airway inflammation in patients with COPD.
What This Study Adds to the Field
In patients with COPD, bronchial epithelial remodeling is associated with down-regulation of polymeric Ig receptor expression and SIgA deficiency on the epithelial surface of large and small airways. In small airways, SIgA deficiency colocalizes with herpesvirus antigens and correlates with CD8+ lymphocyte accumulation, airway remodeling, and airflow limitation. These data support the concept that reduced polymeric Ig receptor expression secondary to altered epithelial cell differentiation leads to defective mucosal immunity, potentially contributing to a pathologic cycle of chronic airway inflammation and remodeling in COPD.
In chronic obstructive pulmonary disease (COPD), airway inflammation can persist for years, even after cessation of tobacco smoke exposure (1, 2). Recent studies have shown that structural and functional disorders of bronchial epithelium also persist in former smokers with COPD (3–5). Therefore, it is plausible that defective physical and immunobarrier functions of structurally abnormal bronchial epithelium may be associated with perpetuation (or progression) of airway inflammation, which is thought to be a key factor in the pathogenesis of COPD.
Together with the mucociliary escalator, secretory immunoglobulin A (SIgA) prevents adherence to or invasion of bronchial epithelium by viral or bacterial pathogens and other foreign antigens, acting as a scavenger through so-called “immune exclusion” (6, 7). Structurally, SIgA consists of a secretory component (SC) and two or more IgA monomers joined with J chain. IgA monomers and J chains are synthesized and assembled to polymeric IgA (pIgA) by subepithelial or glandular interstitial plasma cells, whereas SC is derived from the pIgA receptor (pIgR) expressed in ciliated cells of bronchial epithelium and the serous cells of submucosal glands. Selective binding of J chain with pIgR and subsequent transcytosis of pIgR–IgA complexes across bronchial and glandular epithelium is the basic mechanism of SIgA secretion (7–9). SIgA then accumulates in the periciliary fluid that forms a thin continuous film on epithelial surface. In large airways, surface SIgA may be derived from local transcytosis across bronchial epithelium and secretion from submucosal glands. Because small airways lack submucosal glands, only the former mechanism can account for surface SIgA delivery in distal bronchi (6, 7).
In COPD, reduced SC expression in bronchial epithelium has been associated with neutrophil infiltration and airflow limitation (6, 10). Whether a generalized SIgA deficiency exists in the lungs of patients with COPD is unknown, because pulmonary SIgA levels have not been investigated in this clinical setting. However, a previous study reported reduced IgA levels in bronchoalveolar lavage (BAL) fluid from patients with chronic bronchitis (11). Consistent with the notion of impaired host defense in COPD, bacteria and viruses, including Epstein-Barr virus (EBV), are often found in airways of patients with COPD (12–14). We postulated that bronchial epithelial remodeling, which is common in COPD, leads to abnormalities in SIgA trafficking to the airway that impair local host defenses, increase exposure to inhaled antigens, and perpetuate airway inflammation. Together, our results shed light on the cause and consequences of chronic airway inflammation in former smokers with COPD. Some of the findings of the present study have been published in abstract form (15, 16).
Methods
Clinical Material
Lung tissue specimens containing large (segmental and subsegmental) or small (diameter <2 mm) airways were collected from lifetime nonsmokers without known lung or cardiovascular disease (never smokers) and former smokers with or without COPD. Tissue specimens from 30 lifetime nonsmokers and 6 former smokers without COPD were obtained from donor lungs that were not used for lung transplantation. Tissue specimens from 10 former smokers without COPD and 22 patients with mild-to-moderate COPD (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage I–II) (17) were obtained from lungs resected for solitary tumors, whereas tissue specimens from 32 patients with severe-to-very-severe COPD (GOLD stage III–IV) (17) were obtained from the explanted lungs of transplant recipients. All individuals included in this study had abstained from smoking for more than 1 year (Table 1). The study was approved by the Institutional Review Board of Vanderbilt University, Nashville, Tennessee.
TABLE 1.
CLINICAL CHARACTERISTICS OF STUDY PARTICIPANTS*
| Patients with COPD Ranged by GOLD Criteria (17) |
||||||
| Never Smokers | Former Smokers without COPD† | COPD I | COPD II | COPD III | COPD IV | |
| Number of study participants | ||||||
| VUMC | 3 | 10 | 8 | 14 | 10 | 22 |
| UCSF | 27 | 6 | 0 | 0 | 0 | 0 |
| FEV1,% of predicted | N/A | 100.2 ± 11.3 | 86.8 ± 5‡ | 63.9 ± 7.6‡ | 37.1 ± 6.5‡ | 21.1 ± 4.4‡ |
| FEV1 / FVC, % of FVC | N/A | 0.89 ± 0.33 | 0.65 ± 0.04‡ | 0.54 ± 0.08‡ | 0.41 ± 0.07‡ | 0.32 ± 0.09‡ |
| Age, yr | 49.3 ± 16.6 | 56.4 ± 15.1 | 64.1 ± 5.5§ | 68.7 ± 8‡§ | 62.2 ± 7.4§ | 55.2 ± 5.9 |
| Sex | ||||||
| Male | 13 | 9 | 4 | 8 | 6 | 10 |
| Female | 17 | 7 | 4 | 6 | 4 | 12 |
| Smoking history | ||||||
| Pack-year | N/A | 35.6 ± 20.6 | 48.6 ± 13.6 | 61.3 ± 25.3‡ | 54.7 ± 19.3‡ | 45.3 ± 16.2 |
| Smoke free, if quit, yr | N/A | 10.1 ± 7.6 | 5.3 ± 2.8‡ | 9.6 ± 5.7 | 7.7 ± 6.2 | 5.3 ± 4.5‡ |
Definition of abbreviations: GOLD = Global Initiative for Chronic Obstructive Lung Disease; UCSF = University of California, San Francisco; VUMC = Vanderbilt University Medical Center.
SD is indicated for each parameter.
Spirometry data were available for 10 study participants who underwent lung resection for solitary tumors.
P < 0.05 compared with former smokers without COPD.
P < 0.05 compared with never smokers.
Airway Histology and Immunohistochemistry
Paraffin sections (5 μm) were stained with hematoxylin and eosin for routine histologic evaluation, periodic acid–Schiff reaction for detection of mucin, or Masson trichrome for analysis of fibrous remodeling. Double immunofluorescence microscopy was performed using five different pairs of primary antibodies: (1) IgA and SC/pIgR, (2) IgA and CD138, (3) IgA and CD8, (4) IgA and cytomegalovirus (CMV) late antigen, (5) or IgA and EBV-latent membrane protein. The murine monoclonal anti-SC/pIgR antibody used (clone SC-05; Abcam Inc., Cambridge, MA) demonstrated consistent staining on the basolateral surfaces, but not the apical surfaces of ciliated epithelial cells, suggesting that the antibody binds only pIgR but not the SC of SIgA molecules. Primary antibodies used were a rabbit polyclonal for IgA; CD138 (DakoCytomation, Carpinteria, CA); CMV (Millipore Corporation, Billerica, MA); EBV; and CD4 or CD8 (Abcam, Cambridge, MA). The specificity of immunohistochemistry (IHC) was verified using an antibody isotype control replacing the primary antiserum with an identical concentration of nonimmunized mouse or rabbit serum (Invitrogen Corporation, Camarillo, CA).
Morphometry
In large airway tissue samples, the percentage of bronchial mucosa covered by normal-appearing pseudostratified ciliated epithelium and its various pathologic states (Table 2) (18) was determined for each tissue sample by recording the length of basement membrane subjacent to each morphologic category. For each histologically defined region of bronchial epithelium, the numbers of intraepithelial and subepithelial CD4+ and CD8+ lymphocytes were calculated per 1 mm2 of epithelium or lamina propria (the space between the reticular basement membrane and smooth muscle layer).
TABLE 2.
HISTOLOGIC CHARACTERISTICS OF BRONCHIAL EPITHELIAL REMODELING
| Structural Pattern | Histologic Characteristics |
| I. Normal-appearing pseudostratified ciliated | 1. Pseudostratified structure |
| 2. Ratio of goblet cells to ciliated cells ∼1:8–20 | |
| 3. Complete differentiation of goblet and ciliated cells | |
| II. Deviated cell differentiation (goblet cell hyperplasia) | 1. Pseudostratified structure |
| 2. Predomination of goblet cells among differentiated cells | |
| 3. Mild-to-moderate damage to ciliated cells | |
| 4. Variable basal cell hyperplasia | |
| IIIa. Incomplete cell differentiation (pseudostratified variant) | 1. Pseudostratified structure |
| 2. Surface columnar cells do not show any specific features of ciliated or goblet cells | |
| 3. Severe damage to surface columnar cells | |
| 4. Cilia absent | |
| 5. Variable basal cell hyperplasia | |
| IIIb. Incomplete cell differentiation (stratified variant or immature squamous metaplasia) | 1. Stratified structure |
| 2. Surface cells cuboidal-shaped, do not contact basement membrane, and do not show any features of either ciliated or goblet cells | |
| 3. Severe damage to surface cells | |
| 4. Cilia absent | |
| 5. Excessive basal cell hyperplasia | |
| IV. Altered cell differentiation (complete squamous metaplasia) | 1. Stratified structure |
| 2. Surface cells are flattened | |
| 3. Cilia absent | |
| 4. Predomination of polygonal cells with multiple cell-to-cell bridges among intermediate cells |
In small airways, the amount of IgA on the epithelial surface was quantified by measurement of IgA-specific fluorescent signal. Data are presented as actual pixel value. Airways with actual pixel value less than 30 were considered IgA-deficient.
On tissue sections immunostained with anti-IgA antibodies, small airway remodeling was analyzed by morphometry. Airway wall remodeling was evaluated by measurement of subepithelial connective tissue volume density (VVsub) as the difference in the area, delimited by the basement membrane and the outer edge of the airway adventitia, divided by the length of subepithelial basement membrane according to the recommendations of Hogg and colleagues (19).
On tissue sections double immunostained for IgA and CD8, the number of CD8+ lymphocytes within the epithelium (intraepithelial) or localized between the basement membrane and the outer edge of adventitia (subepithelial) were enumerated and normalized to the length of basement membrane. Average VVsub and CD8+ cell counts were calculated for each study participant. These parameters were also calculated separately for IgA-positive and IgA-deficient airways in each study subject. For further technical details regarding morphometric analyses, refer to the online supplement. All morphometric measurements were made using Image-Pro Express software (Media Cybernetics, Bethesda, MD).
Laser Capture Microdissection
Lung parenchymal tissue specimens from five lifelong nonsmokers without COPD and five former smokers with very severe COPD (GOLD stage IV) were immediately snap frozen with liquid nitrogen and then kept in −80°C. Three serial sections were made from each tissue block and bronchial epithelial cells from small airways from all three sections were harvested by laser capture microdissection (Veritac LCM; Arcturus Bioscience, San Francisco, CA).
Determination of IgA and SIgA in BAL Fluid
BAL fluid was obtained from 9 lifelong nonsmokers, 8 former smokers without COPD, and 10 former smokers with COPD (see Table E1). Bronchoscopy with collection of BAL was approved by the Institutional Review Board of National Jewish Health, Denver, Colorado. BAL fluid was recovered using 60-ml saline lavage with the bronchoscope wedged in the anterior segment of the right upper lobe. Collected fluids were centrifuged at 1,500 × g for 10 minutes at 4°C and the supernatant was immediately frozen at −80°C. Total IgA and SIgA concentrations were measured using specific ELISAs.
Air–Liquid Interface Cultures
Primary human bronchial epithelial cells (HBECs) were obtained from three lifelong nonsmokers and were cultured as previously described (20). Culture medium with or without retinoic acid (RA) was used to promote pseudostratified mucociliary or stratified squamous differentiation, respectively (21). The day of confluence was designated as Day 0 and at Day 28 human plasma IgA enriched with the dimeric form (1:1 ratio of monomeric and dimeric; Athens Research and Technology, Athens, GA) was added to the basolateral media. Twenty-four hours later, apical washings were collected and replicate wells were harvested for histology and pIgR gene and protein analyses. Goat polyclonal anti-SC/pIgR antibody (R&D Systems, Minneapolis, MN) was used for IHC and Western blotting. Total IgA and SIgA concentrations in apical washings were measured using specific ELISAs.
Real-time Polymerase Chain Reaction
Total RNA from microdissected bronchial epithelial cells was extracted using the RNAqueous-Micro Kit (Applied Biosystems/Ambion, Austin, TX), according to the manufacturer's protocol. Total RNA from air-liquid interface (ALI) cultured cells was isolated using the RNeasy Mini kit (Qiagen, Valencia, CA), according to the manufacturer's specifications. Primer sequences were as follows: PIGR (Forward 5′-CTCTCTGGAGGACCACCGT-3′, Reverse 5′-CAGCCGTGACATTCCCTG-3′), HPRT (Forward 5′-TGCTCGAGATGTGATGAAGGAG- 3′, Reverse 5′-TGATGTAATCCAGCAGGTCAGC-3′).
Statistical Analyses
Differences among groups were assessed using Kruskal-Wallis rank analysis of variance with post hoc Dunn multiple comparisons tests. Differences between pairs were assessed using a Student t test. Correlations were assessed using a Spearman test and frequencies of viral infections were compared using a chi-square test. Results are presented as means ± standard error of the mean (SEM). P values less than 0.05 were considered significant.
Results
SIgA Deficiency in Large Airways
To analyze bronchial epithelium remodeling in this study, we used a previously published classification scheme based on characteristics of epithelial cell differentiation that was devised to capture the entire spectrum of pathologic changes of the bronchial epithelium found in individuals with COPD (Table 2) (18). In lifelong nonsmokers and former smokers without COPD, epithelium with normal pseudostratified ciliated appearance predominated (Figures 1A and 1F). In early stage COPD (GOLD stage I–II), 35.3 ± 6.4% of the mucosal surface was covered by epithelium with goblet cell hyperplasia and 10.5 ± 3.7% by epithelium with structural patterns of incomplete or altered cell differentiation. In advanced COPD (GOLD stage III–IV), most epithelium exhibited abnormal structure. Goblet cell hyperplasia covered 49.4 ± 7.1% of the mucosal surface and epithelium with incomplete or altered cell differentiation covered 32.6 ± 6.6% of the mucosal surface (Figures 1B and 1F).
Figure 1.
Structural remodeling of large airway epithelium in chronic obstructive pulmonary disease (COPD) (18). Rows I and II: (A) Pseudostratified ciliated bronchial epithelium with normal ratio of basal, ciliated, and goblet cells. (B) Pseudostratified bronchial epithelium with deviated cell differentiation (goblet cell hyperplasia) showing predomination of goblet cells among scattered ciliated cells. (C) Pseudostratified bronchial epithelium with incomplete cell differentiation; surface cells have columnar shape but do not show morphologic features of ciliated or goblet cells. (D) Stratified bronchial epithelium with incomplete cell differentiation (immature squamous metaplasia); surface cells have cuboid shape and do not contact basement membrane. (E) Bronchial epithelium with altered cell differentiation (complete squamous metaplasia). Row III: Abundant polymeric immunoglobulin receptor (pIgR) (red) is present within normal-appearing pseudostratified epithelium (A), but is reduced in goblet cell hyperplasia (B) and absent in epithelia with incomplete and altered cell differentiation (C–E). Reduced pIgR expression in bronchial epithelium correlates with significant reduction in the amount of IgA (green) on the epithelial surface (A–E). Row I, hematoxylin and eosin–stained paraffin sections (original magnification ×300); row II, electron micrographs (original magnification ×3,000); row III, double immunofluorescence with anti-pIgR (red) and anti-IgA (green) antibodies, confocal microscope images (original magnification ×400). (F) Distribution of epithelial types shown in A–E lining bronchial mucosal surfaces from lifelong nonsmokers (never smokers), former smokers without COPD, and patients with mild-moderate (Global Initiative for Chronic Obstructive Lung Disease [GOLD] stage I–II) or severe-to-very-severe (GOLD stage III–IV) COPD. Mean ± SEM is indicated for each epithelial subtype. * P < 0.01 compared with never smokers and former smokers without COPD; ** Epithelial disorders not detected in never smokers and former smokers without COPD. Increase in intraepithelial and subepithelial CD8+ lymphocytes (G) or CD4+ lymphocytes (H) with progression of bronchial epithelial structural disorders. Deviated COPD = cell count in areas with deviated epithelial cell differentiation in patients with COPD; Inc-Alt COPD = cell count in areas with incomplete or altered epithelial cell differentiation in patients with COPD; Norm-app COPD = cell count in areas with normal-appearing epithelium in patients with COPD; Norm-app NS = cell count for nonsmokers (both lifelong nonsmokers and former smokers without COPD). Mean ± SEM is indicated for each structural variant. * P < 0.01 compared with nonsmoker group.
Double immunofluorescence stains demonstrated basolateral localization of pIgR and apical surface staining for IgA only in bronchial mucosa covered by normal-appearing pseudostratified ciliated epithelium. In contrast, areas of the bronchial mucosa covered by structurally altered epithelium had reduced pIgR expression in epithelial cells and decreased surface IgA (Figure 1, row III; see Figure E1). Although pIgR-positive ciliated cells were present in areas of goblet cell hyperplasia, surface IgA levels were markedly reduced, likely related to the predominance of goblet cells in these areas where pIgR expression was minimal or absent. More advanced structural changes of the bronchial epithelium (incomplete and altered cell differentiation) were characterized by the absence of both pIgR expression and surface IgA.
Next, we measured the numbers of CD8+ and CD4+ lymphocytes in intraepithelial and subepithelial compartments in relation to the structure of the overlying epithelium. No differences in lymphocyte numbers were observed in control subjects (both lifelong nonsmokers and former smokers without COPD) and patients with COPD in areas of bronchial mucosa covered by normal-appearing pseudostratified epithelium (Figures 1G and 1H). In contrast, both CD8+ and CD4+ cell numbers were modestly increased in areas of bronchial mucosa with goblet cell hyperplasia and markedly increased in areas covered by epithelium with incomplete or altered differentiation (Figures 1G and 1H). These findings show that epithelial remodeling is associated with SIgA deficiency and lymphocyte accumulation in large airways.
Robust pIgR expression within the serous cells of bronchial submucosal glands and numerous interstitial IgA-producing plasma cells (IgA+/CD138+) were observed in normal-appearing submucosal glands of lifelong nonsmokers or former smokers without COPD and hyperplastic submucosal glands seen in patients with COPD (see Figure E2). Because submucosal glands are a major source of SIgA (6, 7), this finding suggests that deficiency of IgA on the airway surface develops despite SIgA secretion in adjacent submucosal glands. Therefore, production of SIgA by submucosal glands seems unable to compensate for decreased IgA transcytosis through abnormal surface epithelia.
SIgA Deficiency in Small Airways
In small airways, varying degrees of goblet cell metaplasia were demonstrated by periodic acid–Schiff staining (Figures 2A–2C). In addition, some small airways in patients with COPD showed a stratified appearance reminiscent of immature squamous metaplasia seen in large airways (Figure 2D). A progressive increase in the proportion of airways with abnormal epithelium was observed among lifelong nonsmokers, former smokers without COPD, patients with mild-to-moderate COPD (GOLD stage I–II), and patients with severe-to-very-severe COPD (GOLD stage III–IV) (Figure 2E). Immunofluorescence microscopy demonstrated that only normal-appearing epithelium or epithelium with focal goblet cell metaplasia (involving <25% of mucosal surface) was positive for pIgR and surface IgA, whereas both extensive goblet cell metaplasia (involving ≥25% of mucosal surface) and stratified transformation were characterized by absence of pIgR expression and surface IgA (Figures 2A–2D, see Figure E1). Quantitative analysis demonstrated an association between decreased surface IgA and the clinical severity of COPD (Figure 2F). The mean intensity of the fluorescent signal for surface IgA was slightly reduced in former smokers without COPD compared with lifelong nonsmokers; however, patients with COPD had a marked reduction in this parameter compared with either group without COPD, and patients with the most severe COPD (GOLD stages III–IV) had the lowest fluorescent signal. In total, there was a striking direct correlation between surface SIgA and severity of airflow obstruction as measured by FEV1 (Figure 2G).
Figure 2.
Small airway mucosal secretory immunoglobulin A (SIgA) deficiency. (A) Airway with normal-appearing respiratory epithelium showing polymeric Ig receptor (pIgR) expression (red) in epithelial cells and surface IgA (green). (B) Focal goblet cell metaplasia showing pIgR expression (red) in epithelial cells and surface SIgA expression (green). (C) Extensive goblet cell metaplasia with profound reduction in pIgR in remaining ciliated cells (red) and absence of surface SIgA (green). (D) Stratified epithelium negative for pIgR and surface SIgA. Row I, hematoxylin and eosin (HE)–stained sections (original magnification ×100); row II, periodic acid–Schiff (PAS)–stained tissue sections (original magnification ×400); row III, immunofluorescence with anti-IgA antibody (green), confocal microscope images (original magnification ×100); row IV, double immunofluorescence with anti-pIgR (red) and anti-IgA (green) antibodies, confocal microscope images (original magnification ×400). (E) Distribution of epithelial types shown in 2A–2D lining small airway mucosa according to clinical status. Mean ± SEM is indicated for each epithelial subtype. * P < 0.01 compared with lifelong non-smokers (never smokers); ** Epithelial disorders not detected in never smokers and former smokers without chronic obstructive pulmonary disease (COPD). (F) Progressive reduction of IgA-specific fluorescent signal on epithelial surfaces of small airways in lifelong non-smokers (never smokers), former smokers without COPD, and patients with COPD. Mean ± SEM is indicated for each clinical group. * P < 0.01 compared with never smokers. (G) Correlation between small airway surface SIgA (estimated by IgA-specific fluorescent signal on epithelial surface) and airflow estimated by FEV1 parameter in former smokers and patients with Grade I–II or Grade III–IV COPD. (H) PIGR mRNA expression in airway epithelial cells. Mean ± SEM is indicated for each clinical group. * P < 0.01 compared with never smokers. Apv = actual pixel value.
To corroborate the reduction of pIgR expression in structurally abnormal small airways, we microdissected epithelial cells from small airways of lifelong nonsmokers and patients with severe COPD. Quantitative real-time polymerase chain reaction (RT-PCR) analysis from these samples revealed a significant down-regulation of PIGR mRNA expression in small airway epithelium from patients with very severe COPD (Figure 2H).
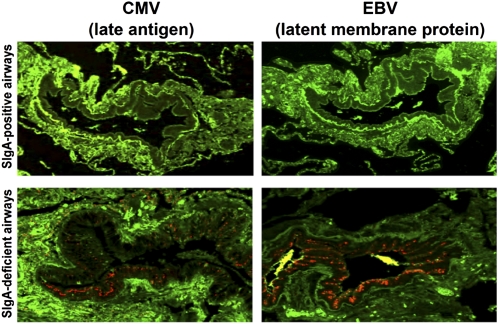
We investigated for the presence of viral antigens in small airway samples to evaluate the consequences of localized mucosal SIgA deficiency. Based on a recent report of increased herpesvirus prevalence in COPD (14), we chose to perform double immunofluorescence studies with primary antibodies against IgA and EBV or CMV antigens (Figure 3). The proportion of patients with COPD harboring latent viral antigens in the epithelium was significantly greater than in lifelong nonsmokers and former smokers without COPD. Interestingly, we found that more than 90% of virally infected airways were surface IgA-deficient in patients with COPD (Table 3). Both parameters (percentage of infected patients and percentage of small airways infected) were higher in patients with severe-to-very-severe COPD (Table 3). These findings suggest that an impaired immunobarrier resulting from localized SIgA deficiency increases the risk of viral infection, including herpesviruses, in abnormal airways.
Figure 3.
Association of secretory immunoglobulin A (SIgA) deficiency and herpesvirus infection in small airways. Images of infected and uninfected airways are from the same patient with chronic obstructive pulmonary disease. Double immunofluorescence with primary anti-IgA (green) and anti–Epstein-Barr virus (EBV) or anti-cytomegalovirus (CMV) antibodies (red), confocal microscope images (original magnification ×100).
TABLE 3.
ANALYSIS OF EBV AND CMV ANTIGENS IN BRONCHIAL EPITHELIUM
| COPD |
||||
| Never Smokers | Former Smokers without COPD | GOLD Stage I–II | GOLD Stage III–IV | |
| Number of study participants | 30 | 16 | 22 | 32 |
| Number of subjects with EBV+ airways (%) | 2 (6.7) | 2 (12.5) | 15 (68.2)* | 27 (84.4)*† |
| EBV+ airways / total airways, % | 2.6 | 3.3 | 24.4* | 40.6*† |
| IgA+ and EBV+, % | 2.6 | 2.2 | 2.2 | 0.6 |
| IgA− and EBV+, % | 0 | 1.1 | 22.2*‡ | 40*†‡ |
| Number of subjects with CMV+ airways (%) | 1 (3.3) | 1 (6.3) | 8 (36.4)* | 18 (56.3)*† |
| CMV+ airways / total airways, % | 1.5 | 1.1 | 12.9* | 34.4*† |
| IgA+ and CMV+, % | 1 | 1.1 | 1.1 | 0.6 |
| IgA− and CMV+, % | 0.5 | 0 | 11.8*‡ | 33.8*†‡ |
Definition of abbreviations: CMV = cytomegalovirus; EBV = Epstein-Barr virus; GOLD = Global Initiative for Chronic Obstructive Lung Disease; IgA = immunoglobulin A.
P < 0.001 compared with lifelong nonsmokers or former smokers without COPD.
P < 0.01 compared with GOLD Stage I–II patients with COPD.
P < 0.001 compared with IgA-positive airways.
Chronic accumulation of CD8+ T lymphocytes in small airways has been considered a major pathogenetic factor in COPD progression, because these cells correlate with disease severity and persist for years after smoking cessation (22–24). To analyze the association between CD8+ infiltrates and surface SIgA deficiency, double immunofluorescence studies were performed with anti-IgA and anti-CD8 antibodies (Figures 4A–4C). Increased intraepithelial and submucosal CD8+ infiltrates were observed in surface IgA-deficient airways compared with IgA-positive airways in all patient groups (Figure 4D). The number of CD8+ cells was also inversely correlated with the amount of surface IgA (r = −0.842; P < 0.001) (Figure 4E). The increased weighted averages of CD8+ cell infiltrates in patients with COPD compared with lifelong nonsmokers and former smokers without COPD was caused by the increased proportion of IgA-deficient airways in the patients with COPD (Figure 4D).
Figure 4.
Surface secretory immunoglobulin A (SIgA) deficiency is associated with increased CD8+ T lymphocyte accumulation in small airways. (A) Normal-appearing surface IgA-positive small airway in lifelong nonsmoker with few adjacent CD8+ cells. (B) Normal-appearing surface IgA-positive small airway in a patient with chronic obstructive pulmonary disease (COPD) with slight increase in intraepithelial and submucosal CD8+ cells. (C) Surface IgA-deficient small airway in a patient with COPD with a significant increase in intraepithelial and submucosal CD8+ cells. (A–C) Double immunofluorescence with primary anti-IgA (green) and anti-CD8 (red) antibodies, confocal microscope images (original magnification ×100). (D) CD8+ cell accumulation predominates in IgA-deficient airways, leading to increased weighted averages. Mean ± SEM is indicated for IgA-positive and IgA-deficient airways and weighted average in each clinical group. * P < 0.01 compared with lifelong nonsmokers (never smokers). ** P < 0.01 compared with surface IgA-positive airways from the respective clinical group. (E) Inverse correlation between small airway surface IgA (estimated by IgA-specific fluorescent signal on epithelial surface) and airway-associated CD8+ cells. Apv = actual pixel value.
To determine how SIgA deficiency and chronic airway inflammation could impact airflow obstruction, we evaluated airway wall remodeling by morphometric analysis in airways with intact and deficient SIgA (Figures 5A–5C). Increased VVsub (a parameter of airway wall thickness) was observed in patients with COPD compared with lifelong nonsmokers and former smokers without COPD (Figure 5D). VVsub was increased only in small airways deficient in surface IgA; the wall thickness of surface IgA-positive airways was not significantly different among any of the study groups. As with CD8+ T lymphocyte infiltration, there was a significant inverse correlation between the amount of surface IgA present and the VVsub of each individual airway (Figure 5E).
Figure 5.
Surface secretory immunoglobulin A (SIgA) deficiency is associated with mural remodeling of small airways. (A) Normal-appearing surface SIgA-positive small airway in lifelong nonsmoker showing a thin, nonfibrotic submucosa. (B) Normal-appearing surface SIgA-positive small airway in a patient with chronic obstructive pulmonary disease (COPD) showing moderate submucosal fibrosis, but without significantly increased wall thickness. (C) Surface SIgA-deficient small airway in COPD patient showing marked submucosal fibrosis and significant thickening of the airway wall. Row I, immunofluorescence with anti-IgA antibody (green) (original magnification ×100); red lines delineate airway wall thickness. Row II, trichrome-stained tissue sections (original magnification ×100). (D) Wall thickness (VVsub) predominates in SIgA-deficient airways, leading to increased weighted averages. Mean ± SEM is indicated for SIgA-positive and SIgA-deficient airways and weighted average in each clinical group. * P < 0.01 compared with lifelong nonsmokers (never smokers). ** P < 0.01 compared with surface SIgA-positive airways from the same clinical group. (E) Inverse correlation between small airway surface SIgA (estimated by IgA-specific fluorescent signal on epithelial surface) and airway wall thickness (VVsub). Apv = actual pixel value.
Reduced SIgA in BAL Fluid from Patients with COPD
To investigate whether the reduction in pIgR expression in COPD airways results in a general deficiency of SIgA, we obtained BAL samples from a separate group of individuals, including 9 lifelong nonsmokers, 8 former smokers without COPD, and 10 former smokers with COPD. Compared with lifelong nonsmokers and former smokers without COPD, former smokers with COPD showed significantly reduced SIgA concentration in BAL fluid without significant differences in total IgA concentrations (Figure 6A). These data indicate a generalized SIgA deficiency in patients with COPD that correlates with airflow limitation (Figure 6B).
Figure 6.
Determination of immunoglobulin A (IgA) and secretory IgA (SIgA) in bronchoalveolar lavage (BAL) fluid. (A) Concentration of total IgA and SIgA in BAL fluid from lifelong nonsmokers and former smokers with or without chronic obstructive pulmonary disease. Mean ± SEM is indicated for each clinical group.* P < 0.05 compared with never smokers. (B) Correlation between SIgA concentration in BAL fluid and FEV1. COPD = chronic obstructive pulmonary disease.
Complete Bronchial Epithelial Cell Differentiation Is Required for pIgR Expression and IgA Transcytosis In Vitro
Given the association between epithelial remodeling, reduced pIgR expression, and absence of IgA on mucosal surfaces in airways of patients with COPD, we wanted to test whether complete epithelial differentiation is required for IgA transcytosis across the bronchial epithelium. Therefore, we used ALI cultures of primary HBECs to model complete and incomplete epithelial cell differentiation in vitro. In the presence of RA in basal medium, HBECs fully differentiate to a pseudostratified ciliated structure. In the absence of RA, HBECs assume a stratified epithelial structure (Figure 7A). Using IHC techniques with antibodies against SC/pIgR, we detected SC/pIgR expression in ciliated epithelial cells in RA+ cultures, but no SC/pIgR expression in RA− ALI cultures (Figure 7A). In addition, Western blot showed pIgR expression exclusively in RA+ ALI cultures. As demonstrated in Figure 7B, pIgR was detected as a double band at approximately 100 kD in the cell lysates obtained from cells grown in presence of RA. These two fractions are nonglycosylated immature (92 kD) and glycosylated mature (107 kD) pIgR, whereas an additional band at 85 kD is considered as SC (25). We also performed quantitative RT-PCR, which showed a significant down-regulation of PIGR mRNA expression in incompletely differentiated (RA−) HBECs (Figure 7C).
Figure 7.
Polymeric immunoglobulin receptor (pIgR) expression and IgA transcytosis in air-liquid interface (ALI) cultured human bronchial epithelial cells (HBECs) grown with or without retinoic acid (RA). (A) Pseudostratified ciliated structure of bronchial epithelium and marked secretory component (SC)/pIgR expression in culture with RA added; squamous stratified structure of bronchial epithelium and absent SC/pIgR expression in culture grown without RA. Top row: hematoxylin and eosin (HE)–stained paraffin sections (original magnification ×400). Bottom row: immunohistochemistry of paraffin tissue sections with goat polyclonal anti–SC-pIgR antibody (original magnification ×400). (B) Western blot showing expression of SC/pIgR in cell cultures with RA added and no pIgR expression in cells grown without RA. (C) PIGR mRNA expression in RA+ and RA− cultures (normalized to HPRT). (D) IgA and secretory IgA (SIgA) concentrations in apical washings of ALI cultured HBECs grown with or without RA. * P < 0.001 (compared with RA+ cultures). Mean ± SEM is indicated for each concentration.
To assess transcytosis of IgA across bronchial epithelial cells, IgA was added to the basal media and apical washings were obtained 24 hours later. Analysis of apical washings demonstrated successful transepithelial delivery of IgA in RA+ cultures versus minimal delivery in RA− cultures (Figure 7D). Separate measurements of total IgA and SIgA using specific ELISAs showed similar concentrations, suggesting that all IgA detected in apical washings was SIgA. Together, these studies show a direct relationship between epithelial cell differentiation, pIgR expression, and SIgA delivery to the bronchial epithelial surface, and are consistent with the idea that epithelial remodeling in COPD is a primary factor in producing localized SIgA deficiency.
Discussion
Our data indicate that bronchial epithelium remains structurally and functionally abnormal in patients with COPD for years after smoking cessation and that the extent of structural changes to the epithelium in both large and small airways correlates with disease severity. We found a strong relationship between epithelial abnormalities, localized deficiency of SIgA on the bronchial mucosal surface, and the presence of CD4+ and CD8+ lymphocytes. In addition, we found that mucosal SIgA deficiency is associated with latent or persistent herpesvirus infection in small conducting airways, submucosal thickening, fibrotic remodeling of the airway walls, and severity of airway obstruction. These results extend previous reports demonstrating associations of airflow limitation with immune and inflammatory cell infiltration (24, 26, 27) and mural fibrotic remodeling of small airways in COPD (19, 28) by linking these changes with epithelial remodeling and deficiency of SIgA on the epithelial surface. In cultured airway epithelial cells, we found that complete differentiation to a pseudostratified structure was necessary for IgA transcytosis. Together, our findings point to a possible role for epithelial structural abnormalities and impaired mucosal immunity in persistent airway inflammation and functional decline in former smokers with COPD.
We showed that SIgA-mediated bronchial mucosal host defense is closely associated with bronchial epithelial cell differentiation and that only normal-appearing pseudostratified ciliated epithelium is able to express pIgR and transport pIgA to the bronchial mucosal surface. In areas of goblet cell hyperplasia, we found limited SIgA delivery to the epithelial surface despite pIgR expression in ciliated cells, suggesting that reduced SIgA delivery in these areas results from a reduction in the number of epithelial cells responsible for IgA transport rather than alterations in molecular mechanisms of delivery. More severe epithelial structural disorders with incomplete or altered epithelial cell differentiation were characterized by a lack of pIgR expression in epithelial cells and striking SIgA deficiency on the luminal surface. Because only a small portion of the bronchial mucosa in tissue specimens from patients with severe COPD was covered by normal differentiated bronchial epithelium, our data suggest widespread bronchial mucosal SIgA deficiency in these patients. This concept was further supported by identification of reduced SIgA in BAL from patients with COPD.
Because the pIgR makes only one passage across epithelial cells before being cleaved (8), one molecule of pIgR must be produced by the epithelial cell for every pIgA transported across the epithelial layer. Therefore, diminution of pIgR expression results in a proportional reduction in pIgA delivery to the epithelial surface. In airway and intestinal mucosa, pIgR expression can be transcriptionally up-regulated by proinflammatory cytokines (29–32). Thus, our finding that pIgR is reduced in the inflammatory environment of the COPD airway is paradoxical. Whereas the molecular mechanisms responsible for down-regulation of pIgR in COPD airways requires further investigation, we speculate that the reduction in pIgR expression results from altered transcriptional profiles in epithelial cells that are not completely differentiated. This idea is supported by our finding of reduced PIGR mRNA expression in airway epithelium from patients with COPD and incompletely differentiated epithelium in vitro. Alternatively, it is possible that reduction in pIgR results from increased protein degradation, given the prior report that neutrophil serine proteases can cleave pIgR (33); however, if this were the case it would be hard to explain the precise correlation between pIgR expression and epithelial structure in the same airway.
Although surface SIgA deficiency occurs in both large and small airways, there are differences in the pathophysiologic mechanisms at these different anatomic sites. In small airways, SIgA secretion is possible only by local transcytosis via a pIgR-mediated mechanism (6). Progressive epithelial remodeling and the associated reduction in pIgR expression subsequently result in profound surface SIgA deficiency. However, in large airways, SIgA may be secreted from serous cells of submucosal glands as an alternative mechanism to local transcytosis (6). Although hyperplasia of submucosal glands in COPD might suggest increased SIgA secretion and abundance in large airways, we demonstrate that SIgA deficiency develops on the mucosal surface in large airways despite the presence of submucosal IgA-producing plasma cells and pIgR expression in submucosal glandular cells. Although the cause is not clear from our data, it is possible that damage to the bronchial cilia apparatus and decreased mucociliary clearance limit mucosal distribution of SIgA secreted by submucosal glands. Furthermore, dehydration of periciliary liquid on the epithelial surface, frequently seen in patients with COPD (34), may preclude adequate storage and maintenance of surface SIgA. Interestingly, we found reduced SIgA, but not total IgA, in BAL fluid from patients with COPD. Although the reason for this discrepancy is not entirely clear, this imbalance could be caused by increased passive leakage of serum IgA into airways in patients with COPD instead of secretion via pIgR-dependent mechanisms.
Recent studies have demonstrated an important role for viral and bacterial pathogens in COPD exacerbations (12, 35). The most common viral pathogens implicated are rhinovirus, influenza, parainfluenza, and respiratory syncytial virus (12, 36, 37). Respiratory syncytial virus and adenovirus have been detected by RT-PCR in a high percentage of patients with stable COPD, raising the possibility that latent or low-grade viral infection may have implications in the pathogenesis of COPD (12, 13). In this study, we detected EBV and CMV antigens in bronchial epithelial cells almost exclusively in SIgA-deficient small airways. These herpesviruses were chosen for evaluation because they can establish long-standing latency in host tissues (38, 39). In addition, EBV and CMV infections have been previously demonstrated in patients with COPD (14) and idiopathic pulmonary fibrosis (40, 41). Because one of the host defense functions of surface SIgA is direct intraepithelial viral clearance (42–46), reduced pIgR expression and impaired pIgA transcytosis may be critical factors for persistent herpesvirus infection of bronchial epithelium in COPD.
CD8+ cytotoxic T lymphocytes predominate in the inflammatory cell infiltrates of small airways in patients with COPD (22–24). The mechanisms by which these cells are recruited to airways in COPD are not fully understood, but one possibility is the immune response to respiratory viral infection (22). Because CD8+ cells play a substantial role in clearance of respiratory virus via contact-dependent effector functions (47), chronic colonization or recurrent or persistent viral infections may be responsible for T cell–mediated airway cell and tissue injury in patients with COPD. Chronic inflammatory responses also seem to induce subepithelial fibrosis that characterizes small airways in COPD and that contributes to airflow limitation (19, 28, 48).
The strong correlations between small airway SIgA deficiency, viral infection, increased CD8+ T lymphocyte infiltration, airway wall remodeling, and clinical status demonstrated in this study suggest that mucosal SIgA deficiency may be an important pathogenetic factor in the development of progression of COPD. In addition to malfunction of the mucociliary clearance mechanism secondary to bronchial epithelial remodeling (49), concomitant surface SIgA deficiency likely contributes to derangement of mucosal immune host defense in COPD. We speculate that acquired surface SIgA deficiency increases the risk of chronic or repeated airway infection and enhances exposure of the bronchial mucosa to inhaled antigens. The resulting antigen deluge fuels persistent activation of adaptive immune responses and local accumulation of immune and inflammatory effector cells. In turn, chronic airway inflammation drives further epithelial remodeling and progressive fibrotic changes in COPD airways. This pathologic sequence may help to explain the persistence of airway inflammation and bronchial epithelial remodeling seen in patients with COPD years after smoking cessation. Importantly, the pathologic cycle proposed previously may be susceptible to therapeutic measures designed to alter the progression of disease by restoring mucosal immunity and therefore curtailing ongoing airway inflammation and fibrotic remodeling.
Supplementary Material
Acknowledgments
V.V.P. designed the study, coordinated the investigations, analyzed the results, and participated in all aspects of the study. V.V.P., J.M.C., W.E.L., A.V.K., and T.S.B. were responsible for the writing of the manuscript. V.V.P. had full access to all data in the study and had final responsibility for the decision to submit for publication. J.M.C. assisted V.V.P. with the pathologic interpretation of all study results and participated in the writing of the manuscript. W.E.L. assisted V.V.P. in the design of the study, analysis of the data, and the writing of the manuscript. R.Z. performed PIGR gene expression analysis (real-time polymerase chain reaction). A.P.M., P.P.M., S.O., L.B.W., and J.W.L. provided clinical samples. R.P.B. provided bronchoalveolar lavage samples. A.V.K. assisted V.V.P. with the design of the study, analysis of the data, and writing of the manuscript. S.H.R. assisted V.V.P. in the design of in vitro experiments, analysis of the data, and writing of the manuscript. T.S.B. provided direction and oversight in all aspects of the study, including design of the study, analysis of results, and writing of the manuscript.
Footnotes
Supported by NIH NHLBI HL085406, NIH HL080322, NIH HL088263, NIH UL1 PR024975-01, CFF R026-CR07, DK065988, and the U.S. Department of Veterans Affairs.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201010-1629OC on July 7, 2011
Author Disclosure: V.V.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.M.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. W.E.L. received grant support from the American Thoracic Society, the American Lung Association, the Parker B. Francis Fellowship Program, and Vanderbilt University Grants. R.Z. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript A.P.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. P.P.M. received grant support from the Department of Veterans Affairs. S.O. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. L.B.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.W.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. R.P.B. received lecture fees from AstraZeneca and Pfizer. A.V.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. S.H.R. was a consultant for Gilead Sciences and BellBrook Labs. He received grant support from Parion Sciences and the Cystic Fibrosis Foundation. He receives royalties from AstraZeneca. T.S.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Rutgers SR, Postma DS, ten Hacken NH, Kauffman HF, Der Mark TW, Koeter GH, Timens W. Ongoing airway inflammation in patients with COPD who do not currently smoke. Thorax 2000;55:12–18 [DOI] [PubMed] [Google Scholar]
- 2.Lapperre TS, Postma DS, Gosman MM, Snoeck-Stroband JB, ten Hacken NH, Hiemstra PS, Timens W, Sterk PJ, Mauad T. Relation between duration of smoking cessation and bronchial inflammation in COPD. Thorax 2006;61:115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verra F, Escudier E, Lebargy F, Bernaudin JF, De CH, Bignon J. Ciliary abnormalities in bronchial epithelium of smokers, ex-smokers, and nonsmokers. Am J Respir Crit Care Med 1995;151:630–634 [DOI] [PubMed] [Google Scholar]
- 4.Wistuba II, Lam S, Behrens C, Virmani AK, Fong KM, LeRiche J, Samet JM, Srivastava S, Minna JD, Gazdar AF. Molecular damage in the bronchial epithelium of current and former smokers. J Natl Cancer Inst 1997;89:1366–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lapperre TS, Sont JK, van Schadewijk A, Gosman MM, Postma DS, Bajema IM, Timens W, Mauad T, Hiemstra PS. Smoking cessation and bronchial epithelial remodelling in COPD: a cross-sectional study. Respir Res 2007;8:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pilette C, Durham SR, Vaerman JP, Sibille Y. Mucosal immunity in asthma and chronic obstructive pulmonary disease: a role for immunoglobulin A? Proc Am Thorac Soc 2004;1:125–135 [DOI] [PubMed] [Google Scholar]
- 7.Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine 2007;25:5467–5484 [DOI] [PubMed] [Google Scholar]
- 8.Kaetzel CS. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol Rev 2005;206:83–99 [DOI] [PubMed] [Google Scholar]
- 9.Corthesy B. Roundtrip ticket for secretory IgA: role in mucosal homeostasis? J Immunol 2007;178:27–32 [DOI] [PubMed] [Google Scholar]
- 10.Pilette C, Godding V, Kiss R, Delos M, Verbeken E, Decaestecker C, De Paepe K, Vaerman JP, Decramer M, Sibille Y. Reduced epithelial expression of secretory component in small airways correlates with airflow obstruction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:185–194 [DOI] [PubMed] [Google Scholar]
- 11.Atis S, Tutluoglu B, Salepci B, Ocal Z. Serum IgA and secretory IgA levels in bronchial lavages from patients with a variety of respiratory diseases. J Investig Allergol Clin Immunol 2001;11:112–117 [PubMed] [Google Scholar]
- 12.Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, Fabbri LM, Johnston SL. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med 2006;173:1114–1121 [DOI] [PubMed] [Google Scholar]
- 13.McManus TE, Marley AM, Baxter N, Christie SN, Elborn JS, Heaney LG, Coyle PV, Kidney JC. Acute and latent adenovirus in COPD. Respir Med 2007;101:2084–2090 [DOI] [PubMed] [Google Scholar]
- 14.McManus TE, Marley AM, Baxter N, Christie SN, Elborn JS, O'Neill HJ, Coyle PV, Kidney JC. High levels of Epstein-Barr virus in COPD. Eur Respir J 2008;31:1221–1226 [DOI] [PubMed] [Google Scholar]
- 15.Polosukhin VV, Lawson WE, Milstone AP, Ocak S, Massion PP, Blackwell TS. Bronchial secretory IgA deficiency in COPD is associated with alterations of epithelial cell differentiation. Am J Respir Crit Care Med 2008;177:A68 [Google Scholar]
- 16.Polosukhin VV, Lawson WE, Milstone AP, Ocak S, Massion PP, Blackwell TS. Herpesviruses are found in small airways in association with mucosal secretory IgA deficiency in COPD. Am J Respir Crit Care Med 2009;179:A2819 [Google Scholar]
- 17.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001;163:1256–1276 [DOI] [PubMed] [Google Scholar]
- 18.Polosukhin VV. Ultrastructure of the bronchial epithelium in chronic inflammation. Ultrastruct Pathol 2001;25:119–128 [DOI] [PubMed] [Google Scholar]
- 19.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004;350:2645–2653 [DOI] [PubMed] [Google Scholar]
- 20.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med 2005;107:183–206 [DOI] [PubMed] [Google Scholar]
- 21.Gray T, Koo JS, Nettesheim P. Regulation of mucous differentiation and mucin gene expression in the tracheobronchial epithelium. Toxicology 2001;160:35–46 [DOI] [PubMed] [Google Scholar]
- 22.MacNee W. Pathogenesis of chronic obstructive pulmonary disease. Clin Chest Med 2007;28:479–513 (v.) [DOI] [PubMed] [Google Scholar]
- 23.Gamble E, Grootendorst DC, Hattotuwa K, O'Shaughnessy T, Ram FS, Qiu Y, Zhu J, Vignola AM, Kroegel C, Morell F, et al. Airway mucosal inflammation in COPD is similar in smokers and ex-smokers: a pooled analysis. Eur Respir J 2007;30:467–471 [DOI] [PubMed] [Google Scholar]
- 24.Di Stefano A, Caramori G, Ricciardolo FL, Capelli A, Adcock IM, Donner CF. Cellular and molecular mechanisms in chronic obstructive pulmonary disease: an overview. Clin Exp Allergy 2004;34:1156–1167 [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto N, Asano M, Ogura Y, Takenouchi-Ohkubo N, Chihaya H, Chung-Hsing W, Ishikawa K, Zhu L, Moro I. Release of non-glycosylated polymeric immunoglobulin receptor protein. Scand J Immunol 2003;58:471–476 [DOI] [PubMed] [Google Scholar]
- 26.Saetta M, Di Stefano A, Turato G, Facchini FM, Corbino L, Mapp CE, Maestrelli P, Ciaccia A, Fabbri LM. CD8+ T-lymphocytes in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:822–826 [DOI] [PubMed] [Google Scholar]
- 27.Di Stefano A, Capelli A, Lusuardi M, Balbo P, Vecchio C, Maestrelli P, Mapp CE, Fabbri LM, Donner CF, Saetta M. Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am J Respir Crit Care Med 1998;158:1277–1285 [DOI] [PubMed] [Google Scholar]
- 28.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet 2004;364:709–721 [DOI] [PubMed] [Google Scholar]
- 29.Bruno ME, Kaetzel CS. Long-term exposure of the HT-29 human intestinal epithelial cell line to TNF causes sustained up-regulation of the polymeric Ig receptor and proinflammatory genes through transcriptional and posttranscriptional mechanisms. J Immunol 2005;174:7278–7284 [DOI] [PubMed] [Google Scholar]
- 30.Schneeman TA, Bruno ME, Schjerven H, Johansen FE, Chady L, Kaetzel CS. Regulation of the polymeric Ig receptor by signaling through TLRs 3 and 4: linking innate and adaptive immune responses. J Immunol 2005;175:376–384 [DOI] [PubMed] [Google Scholar]
- 31.Pal K, Kaetzel CS, Brundage K, Cunningham CA, Cuff CF. Regulation of polymeric immunoglobulin receptor expression by reovirus. J Gen Virol 2005;86:2347–2357 [DOI] [PubMed] [Google Scholar]
- 32.Amin PB, Diebel LN, Liberati DM. T-cell cytokines affect mucosal immunoglobulin A transport. Am J Surg 2007;194:128–133 [DOI] [PubMed] [Google Scholar]
- 33.Pilette C, Ouadrhiri Y, Dimanche F, Vaerman JP, Sibille Y. Secretory component is cleaved by neutrophil serine proteinases but its epithelial production is increased by neutrophils through NF-kappa B- and p38 mitogen-activated protein kinase-dependent mechanisms. Am J Respir Cell Mol Biol 2003;28:485–498 [DOI] [PubMed] [Google Scholar]
- 34.Randell SH, Boucher RC. Effective mucus clearance is essential for respiratory health. Am J Respir Cell Mol Biol 2006;35:20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008;359:2355–2365 [DOI] [PubMed] [Google Scholar]
- 36.Hurst JR, Wedzicha JA. The biology of a chronic obstructive pulmonary disease exacerbation. Clin Chest Med 2007;28:525–536 (v.) [DOI] [PubMed] [Google Scholar]
- 37.Sykes A, Mallia P, Johnston SL. Diagnosis of pathogens in exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2007;4:642–646 [DOI] [PubMed] [Google Scholar]
- 38.Britt W. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr Top Microbiol Immunol 2008;325:417–470 [DOI] [PubMed] [Google Scholar]
- 39.Waller EC, Day E, Sissons JG, Wills MR. Dynamics of T cell memory in human cytomegalovirus infection. Med Microbiol Immunol (Berl) 2008;197:83–96 [DOI] [PubMed] [Google Scholar]
- 40.Tang YW, Johnson JE, Browning PJ, Cruz-Gervis RA, Davis A, Graham BS, Brigham KL, Oates JA, Jr, Loyd JE, Stecenko AA. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol 2003;41:2633–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawson WE, Crossno PF, Polosukhin VV, Roldan J, Cheng DS, Lane KB, Blackwell TR, Xu C, Markin C, Ware LB, et al. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol 2008;294:L1119–L1126 [DOI] [PubMed] [Google Scholar]
- 42.Mazanec MB, Coudret CL, Fletcher DR. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J Virol 1995;69:1339–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan H, Lamm ME, Bjorling E, Huang YT. Multiple functions of immunoglobulin A in mucosal defense against viruses: an in vitro measles virus model. J Virol 2002;76:10972–10979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Renegar KB, Small PA, Jr, Boykins LG, Wright PF. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol 2004;173:1978–1986 [DOI] [PubMed] [Google Scholar]
- 45.Huang YT, Wright A, Gao X, Kulick L, Yan H, Lamm ME. Intraepithelial cell neutralization of HIV-1 replication by IgA. J Immunol 2005;174:4828–4835 [DOI] [PubMed] [Google Scholar]
- 46.Wright A, Yan H, Lamm ME, Huang YT. Immunoglobulin A antibodies against internal HIV-1 proteins neutralize HIV-1 replication inside epithelial cells. Virology 2006;356:165–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bruder D, Srikiatkhachorn A, Enelow RI. Cellular immunity and lung injury in respiratory virus infection. Viral Immunol 2006;19:147–155 [DOI] [PubMed] [Google Scholar]
- 48.Matsuba K, Wright JL, Wiggs BR, Pare PD, Hogg JC. The changes in airways structure associated with reduced forced expiratory volume in one second. Eur Respir J 1989;2:834–839 [PubMed] [Google Scholar]
- 49.Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology 2003;8:432–446 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.