Abstract
Objectives. This study aims to report our experience in the management of HNBCC in ethnic Chinese over a 10-year period. Methods. A retrospective review of all ethnic Chinese patients with HNBCC treated in a tertiary centre from 1999 to 2009. Results. From 1999 to 2009, 225 patients underwent surgical excision for HNBCC. Majority were elderly female patients. Commonest presentation was a pigmented (76.2%) ulcer (64.8%) over the nose (31.6%). Median skin margin taken on tumour excision was 2.0 mm; primary skin closure was achieved in 51.8%. Postresection skin margin was clear in 75.4%. Of those with inadequate skin margins, 56.7% opted for further treatment, 43.4% for observation. Recurrence rates were 2.6% and 13.8%, respectively (P = 0.106). Overall recurrence rate was 5.5%. Conclusions. HNBCC commonly presented as pigmented ulcers over the nose of elderly female patients in our locality. Adequate tumour excision ± reconstruction offered the best chance of cure. Reexcision of those with inadequate skin margins improved local tumour control.
1. Introduction
The incidence of skin cancer is increasing worldwide, possibly arising from an ageing population and increasing sunlight exposure. Basal cell carcinoma (BCC) is the most prevalent. The incidence of which is increasing at 3% per annum [1–7].
BCC most often arise in areas of long-term sun exposure with a high predilection for the head and neck area. Classical clinical features include raised and rolled edges, pearly central area with telangiectasia. Lesions may be pigmented or nonpigmented; nodular, ulcerative, erythematous patches or even mimic benign lesions. Most BCCs are indolent with a low incidence of metastasis [8–11].
The mainstay of treatment for BCC of the head and neck region (HNBCC) is surgical excision with adequate margins. Radiotherapy may be advocated as definitive treatment for a selected group of patients, for those unfit for surgery or as an adjuvant treatment for those with inadequate margins. Other treatment modalities such as curettage and electrodessication, cryosurgery have been reported with variable treatment outcomes; similarly, topical and intralesional agents, photodynamic therapy have also been described, again with variable outcomes, warranting careful patient selection [11–14].
Much has been published regarding BCCs in Caucasian populations, but data on ethnic Chinese are less readily available. We herein report the patient demographics, tumour characteristics, surgical management, and outcome of HNBCCs in ethnic Chinese patients in Hong Kong.
2. Materials and Methods
A retrospective review of all ethnic Chinese patients with previously untreated HNBCC managed in the Queen Mary Hospital, The University of Hong Kong, Hong Kong, between 1999 and 2009. Queen Mary Hospital was one of the largest regional hospitals in Hong Kong. It was a tertiary and quaternary referral centre for the entire territory. Outcome measures included patient demographics, tumour characteristics, surgical management, management of patients with inadequate margins, and recurrence rates. Statistical analysis was performed using SPSS 18.0.
3. Results
3.1. Patient Demographics
A total of 226 HNBCC patients of Chinese ethnicity were treated in our centre from 1999 to 2009. Mean age was 73.1 (22–100) years. There were 132 female and 94 male patients with a male to female ratio of 0.7. 25 patients had multiple BCC lesions. There were a total of 273 HNBCC lesions.
3.2. Tumour Characteristics
There were 65 (23.8%) nonpigmented and 208 (76.2%) pigmented lesions. The most common presentation was in the form of an ulcer (64.8%, n = 177), followed by nodule (19.3%, n = 53), erythema (1.1%, n = 3), and lesions that mimic a benign lesion, for example, keratosis (14.7%, n = 40) (Figure 1). Common sites of involvement included the nose (31.6%, n = 86) and cheek (16.5%, n = 45) (Figure 2).
Figure 1.
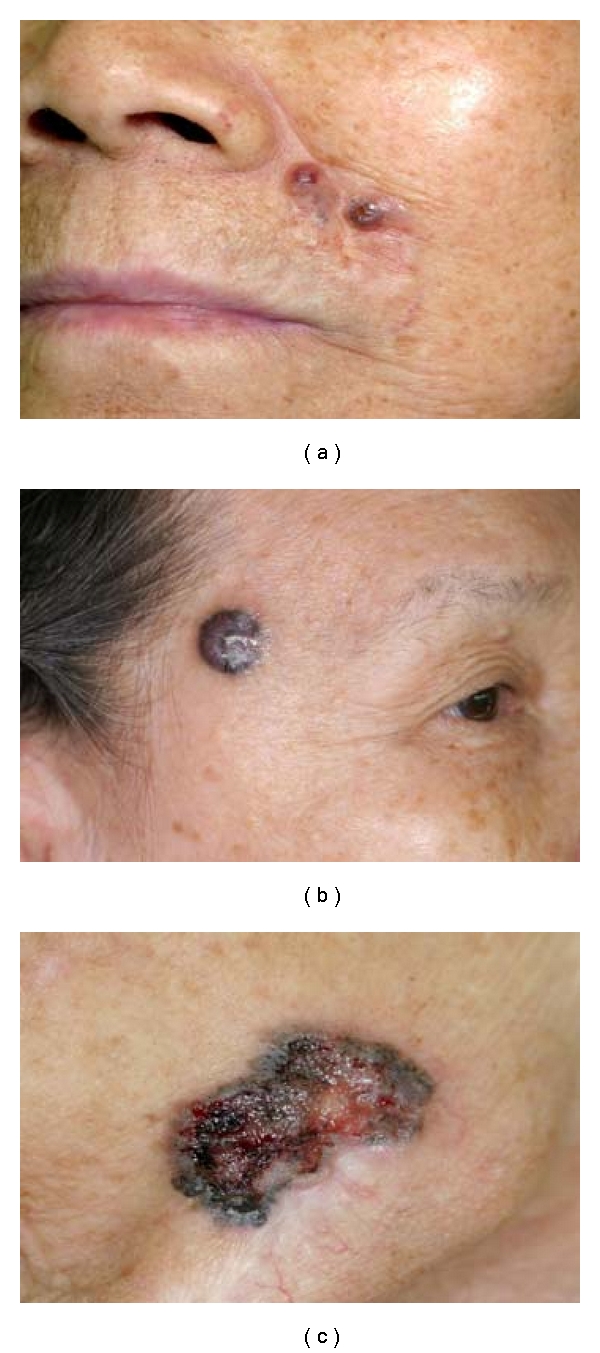
Common presentation of HNBCC in ethnic Chinese: pigmented lesion with well-defined borders, rolled ulcer edges, central pearly area, and overlying telangiectasia.
Figure 2.
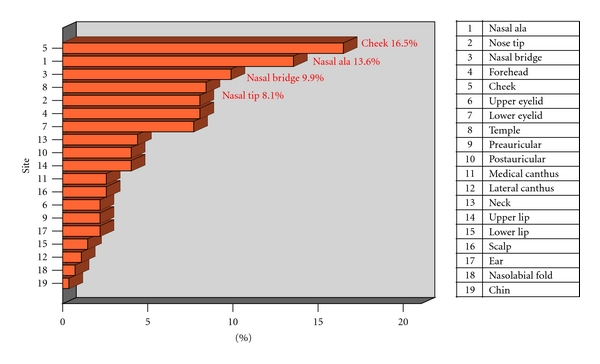
HNBCC presentation—anatomical sites. The commonest site was on the nose.
3.3. Management
One patient with solitary HNBCC refused treatment. All others underwent surgical excision. Median skin margin taken on tumour excision was 2.0 (0–20) mm. Primary skin closure was achieved in 51.8% (n = 141). Other patients required reconstruction in the form of skin graft (11.7%, n = 32), local flap (35.3%, n = 96), and free flap (1.1%, n = 3) (Figure 3).
Figure 3.
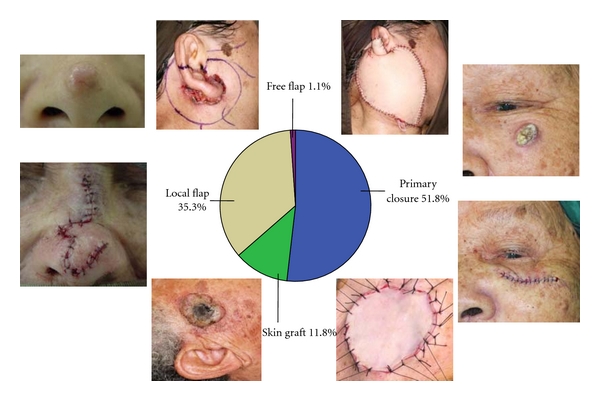
Methods of wound closure. From right to left in a clockwise direction: an 80-year-old lady with a pigmented ulcerative BCC over her left cheek, pathology excised with a 2 mm margins, wound closed primarily; a 60-year-old lady with a pigmented ulcerative BCC over her right preauricular region, the wound was too extensive for primary closure after tumour resection, and yet there was insufficient tissue for local flap reconstruction, hence a full thickness skin graft was harvested from the postauricular region for wound coverage; a 70-year-old lady with a nonpigmented nodular BCC over her nose tip. Excision was performed with a 2 mm margin followed by reconstruction with a bilobed flap; a 50-year-old lady who presented with a pigmented ulcerative BCC over her right auricle which invaded into the superficial and deep lobes of the parotid gland; facial nerve was intact. Wide local excision of tumour with total conservative parotidectomy was performed. The defect was reconstructed with a free anterolateral thigh myocutaneous flap.
3.4. Treatment Outcomes
Skin margins were uninvolved, involved, and close in 75.4% (n = 205), 15.4% (n = 42), and 9.2% (n = 25), respectively, with close skin margin being defined as a pathological margin of less than one millimeter. Involved and close skin margins were classified as inadequate skin margins. The rate of tumour clearance was increased with an increase in skin margin taken on tumour excision—25.3% (n = 40) inadequate margins for 2 mm margins versus 16.9% (n = 13) for 3 mm margins (P = 0.089) (Figure 4).
Figure 4.
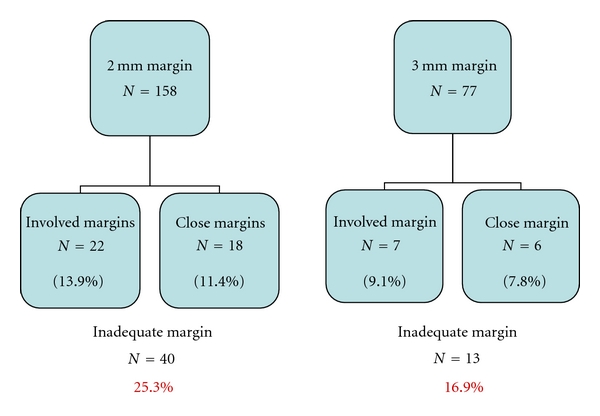
Treatment outcome—the greater the skin margin taken on tumour excision, the better the tumour clearance rate.
Most patients with involved margins (76.2%, n = 32) underwent reexcision; 4 (9.5%) underwent radiation therapy. One developed local recurrence 2 years after reexcision. The majority of those with close margins (92.0%, n = 23) opted for observation. Four developed local recurrence thereafter. Two patients opted for reexcision, and none recurred to date (Figure 5).
Figure 5.
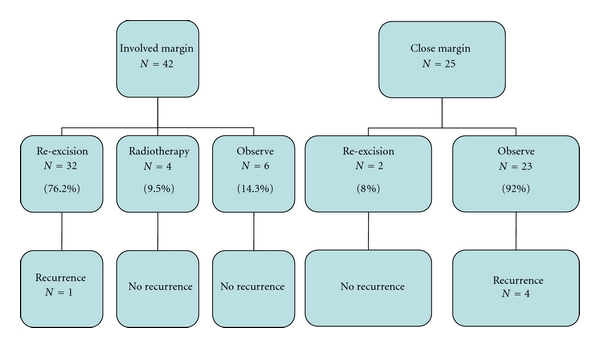
Management of patients with involved and close margins and associated recurrence rates.
Further treatment in the form of reexcision or radiation therapy in those with inadequate skin margins led to a lower recurrence rate than those who opted for observation (2.6% versus 13.8%, P = 0.106) (Figure 6). Follow-up period was indefinite for all patients. Overall recurrence rate was 5.5% (n = 15) over a mean follow-up period of 73.0 (16–195) months. Mean interval to recurrence was 36.6 (9–78) months (Figure 7). The commonest site of recurrence was over the nose 20% (n = 3).
Figure 6.
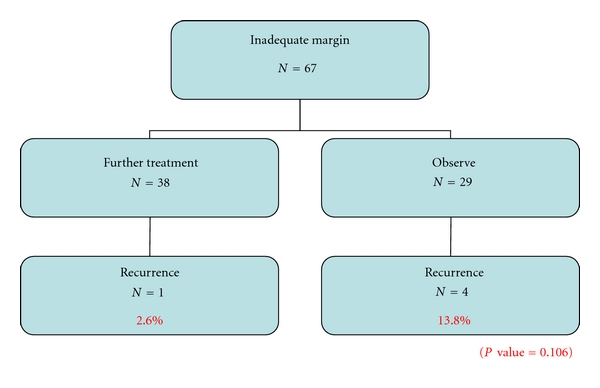
Treatment outcomes of patients who chose to undergo further treatment versus observation in those with inadequate margins.
Figure 7.
A graph depicting tumour recurrence over time.
4. Discussion
According to the Hong Kong Hospital Authority statistical report 2007-2008, there was an increasing trend of skin cancer in Hong Kong [15]. The rate of increase in skin cancer and BCC incidence in ethnic Chinese and other Asian countries was less than that of the fair-skinned Caucasian population [16].
BCC had a high predilection for the head and neck region. HNBCC predominantly affected the elderly population with a slight female preponderance in our locality. Common presentation was in the form of a pigmented ulcer over the nose.
Our results were similar to those reported by Sng et al., Kikuchi et al., and Cho et al. [6, 17, 18]. This study, corroborated by reviews in other Asian countries, showed that HNBCC in ethnic Chinese and other Asian populations presented differently compared to the Caucasian population, whereby HNBCC commonly presented as nonpigmented nodules in male patients (Table 1) [6, 11, 17–19].
Table 1.
A table comparing our results with other Asian and Caucasian populations.
| Hong Kong (QMH) N = 273 |
Singapore N = 292 |
Japan N = 243 |
Korea N = 78 |
Australia N = 6252 |
|
|---|---|---|---|---|---|
| Mean age (yrs) | 73.1 | 70.9 | 59.0 | 58.2 | 62.0 |
| M:F | 0.70 | 0.95 | 0.97 | 0.90 | 1.13 |
| Clinical features | Pigmented (76.2%) | Pigmented (63%) | Pigmented (75%) | Pigmented (55%) | Nonpigmented (93%) |
| Ulcer (64.8%) | Ulcer (NA) | Nodule (NA) | Ulcer (NA) | Nodule (50%) | |
| Site | Nose (32.3%) | Nose (37.0%) | Nose (NA) | Nose (26.9%) | Nose (40.6%) |
The difference in trends, rates, and presentation in the two ethnic groups could be accounted for the difference in skin types (Fitzpatrick types III and IV in Chinese versus I and II in Caucasians), geographical latitude, sociocultural differences, varying occupational and sun exposure, skin protection, and differences in disease awareness and surveillance.
There is no consensus as to the amount of skin margin taken on tumour excision. However, as one would expect, the greater the skin margin taken on tumour excision, the better the tumour control. In excising tumour over the head and neck region, there is always a balance between adequate tumour control and conservation of normal tissue in an attempt to achieve acceptable functional and cosmetic outcome. Excision of HNBCC with a 2 mm skin margin for well-defined lesions was adequate in most cases, with an overall recurrence rate of 5.5%, which was comparable to that of large-scale studies conducted worldwide [20–23].
As cited in other reviews, our data also showed that the nose was the site with the highest incidence of recurrence and inadequate margins. This could represent embryonic fusion planes where tumour can spread aggressively or because of a scarcity of surplus skin tissue which may present technical difficulty on skin closure, resulting in a more conservative excision margin [24–27].
For such difficult mid-face lesions, excision under frozen section guidance or even Mohs micrographic surgery could be advocated to enhance complete tumour removal whilst preserving the maximal amount of normal tissue [11, 19, 23, 28–30]. Various reconstructive techniques such as skin graft, local flaps, or even free flaps could be used for skin coverage if the defect is too extensive for primary closure.
In cases of inadequate skin margins, reexcision should be advocated to prevent recurrence and to decrease the chance of more radical surgery in the future; incompletely excised tumour and recurrent tumours are contributing factors to more aggressive tumour behaviour. The presence of scar tissue obscures monitoring and delays clinical detection. Fibrotic scar tissue entraps malignant cells and favours deep extension by preventing upward migration [27, 31, 32].
5. Conclusions
HNBCC commonly presented as pigmented ulcers over the cheek and nose of elderly female patients of Chinese ethnicity in our locality. This corroborated with studies conducted in other Asian countries but contrasted with those of Caucasian populations in that HNBCC commonly presented as nonpigmented nodules in the male population. Tumour excision with a 2 mm skin margin for HNBCC with well-defined margins yielded a tumour control rate comparable with other large-scale studies. Various reconstructive techniques could be adapted in cases where primary skin closure could not be achieved after adequate tumour resection. Reexcision of lesions with inadequate margins improved local tumour control.
Conflict of Interests
The authors declare that there is no conflict of interests.
References
- 1.American Cancer Society. Detailed guide: skin cancer–basal and squamous cell
- 2.Cancer Research UK. CancerStats Key facts on skin cancer. How common is skin cancer? http://info.cancerresearchuk.org/cancerstats/types/skin/#incidence.
- 3.Katalinic A, Kunze U, Schäfer T. Epidemiology of cutaneous melanoma and non-melanoma skin cancer in Schleswig-Holstein, Germany: incidence, clinical subtypes, tumour stages and localization (epidemiology of skin cancer) British Journal of Dermatology. 2003;149(6):1200–1206. doi: 10.1111/j.1365-2133.2003.05554.x. [DOI] [PubMed] [Google Scholar]
- 4.Staples MP, Elwood M, Burton RC, Williams JL, Marks R, Giles GG. Non-melanoma skin cancer in Australia: the 2002 national survey and trends since 1985. Medical Journal of Australia. 2006;184(1):6–10. doi: 10.5694/j.1326-5377.2006.tb00086.x. [DOI] [PubMed] [Google Scholar]
- 5.Hayes RC, Leonfellner S, Pilgrim W, Liu J, Keeling DN. Incidence of nonmelanoma skin cancer in New Brunswick, Canada, 1992 to 2001. Journal of Cutaneous Medicine and Surgery. 2007;11(2):45–52. doi: 10.2310/7750.2007.00010. [DOI] [PubMed] [Google Scholar]
- 6.Sng J, Koh D, Siong WC, Choo TB. Skin cancer trends among Asians living in Singapore from 1968 to 2006. Journal of the American Academy of Dermatology. 2009;61(3):426–432. doi: 10.1016/j.jaad.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 7.Diffey BL, Langtry JAA. Skin cancer incidence and the ageing population. British Journal of Dermatology. 2005;153(3):679–680. doi: 10.1111/j.1365-2133.2005.06799.x. [DOI] [PubMed] [Google Scholar]
- 8.Rünger TM. How different wavelengths of the ultraviolet spectrum contribute to skin carcinogenesis: the role of cellular damage responses. Journal of Investigative Dermatology. 2007;127(9):2103–2105. doi: 10.1038/sj.jid.5700988. [DOI] [PubMed] [Google Scholar]
- 9.Ridley AJ, Whiteside JR, McMillan TJ, Allinson SL. Cellular and sub-cellular responses to UVA in relation to carcinogenesis. International Journal of Radiation Biology. 2009;85(3):177–185. doi: 10.1080/09553000902740150. [DOI] [PubMed] [Google Scholar]
- 10.Rosso S, Zanetti R, Martinez C, et al. The multicentre south European study ’Helios’ II: different sun exposure patterns in the aetiology of basal cell and squamous cell carcinomas of the skin. British Journal of Cancer. 1996;73(11):1447–1454. doi: 10.1038/bjc.1996.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madan V, Lear JT, Szeimies RM. Non-melanoma skin cancer. The Lancet. 2010;375(9715):673–685. doi: 10.1016/S0140-6736(09)61196-X. [DOI] [PubMed] [Google Scholar]
- 12.Good LM, Miller MD, High WA. Intralesional agents in the management of cutaneous malignancy: a review. Journal of the American Academy of Dermatology. 2011;64(2):413–422. doi: 10.1016/j.jaad.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Love WE, Bernhard JD, Bordeaux JS. Topical imiquimod or fluorouracil therapy for basal and squamous cell carcinoma: a systematic review. Archives of Dermatology. 2009;145(12):1431–1438. doi: 10.1001/archdermatol.2009.291. [DOI] [PubMed] [Google Scholar]
- 14.Marmur ES, Schmults CD, Goldberg DJ. A review of laser and photodynamic therapy for the treatment of nonmelanoma skin cancer. Dermatologic Surgery. 2004;30(2):264–271. doi: 10.1111/j.1524-4725.2004.30083.x. [DOI] [PubMed] [Google Scholar]
- 15.Hospital Authority Statistical Report (2007–2008)
- 16.Koh D, Wang H, Lee J, Chia KS, Lee HP, Goh CL. Basal cell carcinoma, squamous cell carcinoma and melanoma of the skin: analysis of the Singapore Cancer Registry data 1968–97. British Journal of Dermatology. 2003;148(6):1161–1166. doi: 10.1046/j.1365-2133.2003.05223.x. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi A, Shimizu H, Nishikawa T. Clinical and histopathological characteristics of basal cell carcinoma in Japanese patients. Archives of Dermatology. 1996;132(3):320–324. [PubMed] [Google Scholar]
- 18.Cho S, Kim MH, Whang KK, Hahm JH. Clinical and histopathological characteristics of basal cell carcinoma in Korean patients. Journal of Dermatology. 1999;26(8):494–501. doi: 10.1111/j.1346-8138.1999.tb02034.x. [DOI] [PubMed] [Google Scholar]
- 19.Leibovitch I, Huilgol SC, Selva D, Richards S, Paver R. Basal cell carcinoma treated with Mohs surgery in Australia I. Experience over 10 years. Journal of the American Academy of Dermatology. 2005;53(3):445–451. doi: 10.1016/j.jaad.2005.04.083. [DOI] [PubMed] [Google Scholar]
- 20.Bisson MA, Dunkin C, Griffiths RW, Suvarna SK. Do plastic surgeons resect basal cell carcinomas too widely? A prospective study comparing surgical and histological margins. British Journal of Plastic Surgery. 2002;55(4):293–297. doi: 10.1054/bjps.2002.3829. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths RW, Suvarna SK, Stone J. Do basal cell carcinomas recur after complete conventional surgical excision? British Journal of Plastic Surgery. 2005;58(6):795–805. doi: 10.1016/j.bjps.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Griffiths RW, Suvarna SK, Stone J. Basal cell carcinoma histological clearance margins: an analysis of 1539 conventionally excised tumours. Wider still and deeper? Journal of Plastic, Reconstructive and Aesthetic Surgery. 2007;60(1):41–47. doi: 10.1016/j.bjps.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 23.Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. The Lancet Oncology. 2008;9(12):1149–1156. doi: 10.1016/S1470-2045(08)70260-2. [DOI] [PubMed] [Google Scholar]
- 24.Gooding CA, White G, Yatsuhashi M. Significance of marginal extension in excised basal-cell carcinoma. The New England Journal of Medicine. 1965;273(17):923–924. doi: 10.1056/NEJM196510212731708. [DOI] [PubMed] [Google Scholar]
- 25.Pascal RR, Hobby LW, Lattes R, Crikelair GF. Prognosis of 'incompletely excised' versus 'completely excised' basal cell carcinoma. Plastic and Reconstructive Surgery. 1968;41(4):328–332. doi: 10.1097/00006534-196804000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Koplin L, Zarem HA. Recurrent basal cell carcinoma. A review concerning the incidence, behaviour, and management of recurrent basal cell carcinoma, with emphasis on the incompletely excised lesion. Plastic and Reconstructive Surgery. 1980;65(5):656–664. [PubMed] [Google Scholar]
- 27.Richmond JD, Davie RM. The significance of incomplete excision in patients with basal cell carcinoma. British Journal of Plastic Surgery. 1987;40(1):63–67. doi: 10.1016/0007-1226(87)90013-0. [DOI] [PubMed] [Google Scholar]
- 28.Smeets NWJ, Kuijpers DIM, Nelemans P, et al. Mohs’ micrographic surgery for treatment of basal cell carcinoma of the face—results of a retrospective study and review of the literature. British Journal of Dermatology. 2004;151(1):141–147. doi: 10.1111/j.1365-2133.2004.06047.x. [DOI] [PubMed] [Google Scholar]
- 29.Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. Journal of Drugs in Dermatology. 2009;8(10):914–922. [PubMed] [Google Scholar]
- 30.Leibovitch I, Huilgol SC, Selva D, Richards S, Paver R. Basal cell carcinoma treated with Mohs surgery in Australia II. Outcome at 5-year follow-up. Journal of the American Academy of Dermatology. 2005;53(3):452–457. doi: 10.1016/j.jaad.2005.04.087. [DOI] [PubMed] [Google Scholar]
- 31.Walling HW, Fosko SW, Geraminejad PA, Whitaker DC, Arpey CJ. Aggressive basal cell carcinoma: presentation, pathogenesis, and management. Cancer and Metastasis Reviews. 2004;23(3-4):389–402. doi: 10.1023/B:CANC.0000031775.04618.30. [DOI] [PubMed] [Google Scholar]
- 32.Lang PG, Maize JC. Histologic evolution of recurrent basal cell carcinoma and treatment implications. Journal of the American Academy of Dermatology. 1986;14(2):186–196. doi: 10.1016/s0190-9622(86)70020-0. [DOI] [PubMed] [Google Scholar]


