Abstract
Several arenaviruses cause hemorrhagic fever disease in humans and pose a significant public health concern in their endemic regions. On the other hand, the prototypic arenavirus LCMV is a superb workhorse for the investigation of virus-host interactions and associated disease. The arenavirus small RING finger protein called Z has been shown to be the main driving force of virus budding. The budding activity of Z is mediated by late (L) domain motifs, PT/SAP, and PPXY, located at the C-terminus of Z. This paper will present the current knowledge on arenavirus budding including the diversity of L domain motifs used by different arenaviruses. We will also discuss how improved knowledge of arenavirus budding may facilitate the development of novel antiviral strategies to combat human pathogenic arenaviruses.
1. Introduction
Arenaviruses are enveloped viruses with a bisegmented negative strand (NS) RNA genome with coding capability for four known genes: nucleoprotein (NP), surface glycoprotein precursor (GPC), polymerase (L), and matrix-like (Z) proteins. Despite their limited genome and proteomic complexity, arenaviruses are able to exhibit very different phenotypic infection outcomes ranging from long-term subclinical chronic infections on their natural rodent hosts [1] to hemorrhagic fever (HF) disease in humans, infected through mucosal exposure to aerosols or by direct contact of abrade skin with infectious material. Thus, Lassa virus (LASV), the causative agent of Lassa fever (LF) is estimated to infect several hundred thousand individuals yearly in its endemic regions of West Africa, resulting in a high number of LF cases associated with high morbidity and significant mortality. Likewise, Junin virus (JUNV) causes Argentine HF, a severe illness with hemorrhagic and neurological manifestations and a case fatality of 15–30%, whereas the Machupo (MACV) and Guanarito (GTOV) arenaviruses emerged as causative agents of HF in Bolivia and Venezuela, respectively. On the other hand, the prototypic arenavirus, lymphocytic choriomeningitis virus (LCMV), is a superb workhorse for the investigation of virus-host interactions including mechanisms of virus control and clearance by the host immune defenses, as well as viral counteracting measures leading to chronic infection and associated disease [2, 3]. Moreover, evidence indicates that the globally distributed prototypic arenavirus LCMV is a neglected human pathogen of clinical significance, especially in cases of congenital infection. In addition, LCMV poses a special threat to immunocompromised individuals, as illustrated by cases of transplant-associated infections by LCMV with a fatal outcome in the USA and Australia. Public health concerns about arenavirus infections are aggravated by the lack of licensed vaccines and current therapy being limited to the use of the nucleoside analog ribavirin, which is only partially effective, requires early and intravenous administration for optimal activity, and can cause significant side effects. Therefore, it is important to develop novel and effective antiarenaviral strategies, a task that should be facilitated by a better understanding of the arenavirus molecular and cell biology.
The arenavirus small RING finger Z protein has been shown to be the main driving force of budding. This paper will examine our current understanding of arenavirus budding and discuss potential implications for the development of novel targeting strategies to combat human pathogenic arenaviruses.
2. Arenavirus Genome Organization and Life Cycle
Arenaviruses are enveloped viruses with a bisegmented negative strand (NS) RNA genome and a life cycle restricted to the cell cytoplasm. Virions are pleomorphic but often spherical and covered with surface glycoprotein spikes. Both the large, L (ca 7.3 kb) and small, S (ca 3.5 kb) genome RNA species use an ambisense-coding strategy to direct the synthesis of two polypeptides in opposite orientation, separated by a noncoding intergenic region (IGR) with a predicted folding of a stable hairpin structure [1] (Figure 1). The S RNA encodes the viral glycoprotein precursor, GPC, (ca 75 kDa) and the nucleoprotein, NP, (ca 63 kDa), whereas the L RNA encodes the viral RNA-dependent RNA polymerase (RdRp, or L polymerase) (ca 200 kDa) and a small (ca 11 kDa) RING finger protein Z that is functionally the counterpart of the matrix (M) protein found in many enveloped NS RNA viruses.
Figure 1.
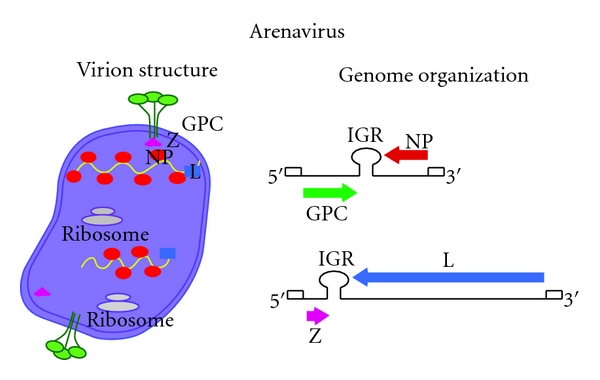
Arenavirus virion structure and genome organization. Arenaviruses are enveloped viruses with a bisegmented negative strand RNA genome. Each genome segment uses an ambisense-coding strategy to direct the synthesis of two viral polypeptides. The S (ca 3.5 kb) segment encodes for the viral nucleoprotein (NP) and glycoprotein precursor (GPC). GPC is posttranslational processed by the cellular protease S1P into the mature virion surface GP1 and GP2. The L (ca 7.3 kb) segment encodes for the virus RNA-dependent RNA polymerase (L) and a small RING finger protein (Z) that is functionally the arenavirus counterpart of the matrix (M) protein found in many enveloped negative strand RNA viruses. IGR, noncoding intergenic region.
Consistent with a broad host range and cell-type tropism, a highly conserved and widely expressed cell surface protein α-Dystroglycan (α-DG) has been identified as a main receptor for LCMV, LASV, and several other arenaviruses [4, 5], whereas the human transferrin receptor (TfR) was identified as the primary receptor used by several New World (NW) arenavirus [6]. Upon receptor binding, virions are internalized using an endocytotic pathway that is either clathrin-independent or clathrin-dependent for Old World (OW) and NW arenavirus, respectively [5]. Interestingly, cell entry of OW LCMV and LASV are independent of caveolin, dynamin, actin, or small GTPases Rab5 and Rab7 but cholesterol-dependent [7–9]. Following the release of the viral ribonucleoprotein into the cytoplasm of the infected cells, the associated polymerase directs the biosynthetic processes involved in RNA replication and gene transcription. Assembly and cell release of infectious progeny involve the association of the viral ribonucleoprotein core with the surface GP complex, a process that is required for the production of infectious virions, which bud from the plasma membrane (PM).
3. Arenavirus Z Structure and Function
Results derived from minigenome- (MG-) based assays identified NP and L as the minimal viral transacting factors required for efficient RNA synthesis mediated by the virus polymerase [10–12]. Z was not required for RNA replication or transcription, but rather Z has been shown to exhibit a dose-dependent inhibitory effect on both transcription and replication of LCMV, Tacaribe virus (TACV), and LASV MGs [10, 12–14]. The inhibitory activity of Z on RNA synthesis by the LCMV polymerase did not require the N-terminus or C-terminus of Z, whereas the RING domain was strictly required but not sufficient [13, 14]. RING domains are known to mediate protein-protein interactions, and Z protein has been documented to interact with a variety of host cellular proteins including PML [15, 16] and translation initiation factor eIF4E [15–18]. The Z-PML interaction was reported to result in disruption of PML nuclear bodies and redistribution of PML to the cytoplasm, but the biological implications of this remain to be determined. On the other hand the Z-eIF4E interaction was found to impair eIF4E-dependent translation through its RING domain [16]. Interestingly, expression of Interferon regulatory factor 7 (IRF7), a key factor in the regulation of type I interferon (IFN) production by pDCs, is highly dependent on 4E [19]. Therefore, it is plausible that Z might mediate inhibition of IRF7 expression in arenavirus-infected pDCs and, thus, contributing to the mechanisms by which arenaviruses overcome the innate immune response by the host.
The possible contribution of RING-mediated Z-host cellular protein interactions to arenavirus budding is currently unknown. Notably, arenavirus Z proteins have a strictly conserved W residue in proximity to the second conserved C residue within the RING, a feature characteristic of RING proteins with E3 ligase activity involved in ubiquitin-dependent protein degradation. However, preliminary evidence indicated that LASV Z protein lacked ubiquitin-ligating activity in the presence of a variety of E2 enzymes including Ubc4 and Cdc34/Ubc3 [16]. Whether arenavirus Z proteins may exhibit E3 ligase activity in the presence of other E2 ubiquitin-conjugating enzymes and their biological implications remain to be determined. Z has also been implicated in antagonizing the host innate immune response. NW, but not OW, arenavirus Z was shown to bind RIG-I and inhibits IFN-β activation [20]. The recently reported NMR structure of LASV Z [17] should facilitate future structure-function studies aimed at the elucidation of the likely several roles played by Z in the arenavirus life cycle.
4. The Z Protein Is the Driving Force of Arenavirus Budding
The arenavirus Z protein has been shown to have bona fide budding activity [21–23]. Many enveloped viruses possess a matrix (M) protein that is often the main driving force of viral budding. Accordingly, the sole expression of this M protein can produce virus-like particles (VLPs). Frequently M proteins contain short amino acid motifs, called L (late) domains that play a critical role in virus budding. To date, the sequences PT/SAP, PPXY, and YPXnL (YPXL) have been well established as L domain motifs [24–26]. In addition to these L domains, the FPDL motif and several other short amino acid motifs have also been reported to function as L domains [24–26]. These L domain motifs exert their activity in virus budding by mediating the interaction with specific host cellular factors. Thus, the PT/SAP motif binds to Tsg101, a component of ESCRT-I (endosomal sorting complex required for transport-I) and initiates the budding process [27, 28]. Vps4A/B is AAA-type ATPases involved in catalyzing the disassembly and recycling of the membrane-bound ESCRT complexes [29–31]. Evidence indicates that the M protein of many, but not all, enveloped viruses have the ability to recruit ESCRT complex to their budding sites [24–26]. In addition to M, several other viral proteins, including Sendai virus (SeV) C protein and Marburg virus (MARV) NP, have been found to bind directly to ESCRT proteins, Alix/AIP1, or both and contribute to the budding process [24, 32–35]. Interestingly, some enveloped viruses, including influenza, are able to execute very efficiently the budding process without the ESCRT machinery [24, 36]. It is worth noting that although the M protein of VSV contains both PTAP and PPPY L domain motifs that interact with Tsg101 and Nedd4; respectively, Tsg101 and Vps4A were not required for efficient budding of VSV [37]. Rous sarcoma virus (RSV) Gag has a PPPY motif whose activity has been shown to be regulated by late-domain-interacting protein (LDI-1, Nedd4 chicken homolog) [38, 39]. Recently LYPSL motif in RSV Gag was shown to serve as a second L domain motif [40]. Table 1 summaries the variety of interactions observed between L domains present in M proteins of enveloped viruses and their host cellular interacting partners.
Table 1.
Summary of different matrix (M) protein L domain motifs and cellular-interacting partners. Characterized viral M proteins, accessory proteins, and their L domains are shown. *1: Alix/AIP1 has been shown to connect Z and NP [45, 46]. *2: there is a discrepancy between the groups for Alix/AIP1 and Vps4 necessity for the budding [34, 35, 47].
| Virus | M protein | Accessary protein | L domain | Nedd4-like Ubiquitin ligase | Tsg101 | Alix/AIP1 | Vps4 |
|---|---|---|---|---|---|---|---|
| HIV-1 | Gag | PTAP | ○ | ○ | ○ | ||
| YPXnL | ○ | ||||||
|
| |||||||
| RSV | Gag | PPPY | ○ | ○ | |||
| YPXnL | |||||||
|
| |||||||
| VSV | M | PTAP | x | x | |||
| PPPY | ○ | ||||||
|
| |||||||
| Ebola virus | VP40 | PTAP | ○ | ○ | |||
| PPPY | ○ | ||||||
|
| |||||||
| Marburg virus | VP40 | PPPY | ○ | ○ | ○ | ||
| NP | PSAP | ○ | |||||
|
| |||||||
| Lassa virus | Z | PTAP | ○ | ○ | |||
| PPPY | |||||||
|
| |||||||
| LCMV | Z | PPPY | ○ | ○ | |||
|
| |||||||
| Tacaribe virus | Z | ASAP | x | ○ | |||
|
| |||||||
| Mopeia virus | Z | PTAP | ○*1 | ||||
| PPPY | |||||||
| NP | |||||||
|
| |||||||
| Sendai virus | M | YLDL | ○*2 | ○*2 | |||
| C | ○*2 | ||||||
|
| |||||||
| Influenza virus | x | ||||||
The family Arenaviridae currently includes 23 antigenically related viruses classified into two groups: OW and NW. This classification was originally established based on serological cross-reactivity but is well supported by recent sequence-based phylogenetic studies. OW arenaviruses constitute a single lineage, while NW arenaviruses segregate into clades A, B, and C. Recently, Lujo (LUJV) and Merino Walk (MWAV) viruses were identified as newly identified members of the OW group [41, 42]. Interestingly, among different arenaviruses, there are significant differences in the type of L domain motifs present within their Z proteins (Figure 2). LCMV Z contains a canonical PPPY L domain and the PT/SAP-like domain STAP, whereas the Z of LASV and Mopeia virus (MOPV) which as LCMV are also members of the OW arenavirus group, contain, however, both PTAP and PPPY canonical L domains, but the Z of the also OW member Lujo virus contains only the PTAP L domain. On the other hand, the Z of many of the NW arenaviruses including JUNV, MACV, GATV, and Sabia (SABV) viruses contain the PT/SAP L domain. In addition Z proteins of Pichinde (PICV) and Whitewater Arroyo- (WWAV) viruses contain PTAP and APPY- (PPPY-like-) overlapping L domains, similar to those of Ebola virus (EBOV) VP40 L domain (PTAPPEY). Some arenavirus Z proteins do not contain canonical L domains but rather closely related motifs as in the case of the NW TACV and OW MWAV whose Z proteins contain ASAP and PTCP, respectively, L-like domains. In addition, all known arenavirus Z proteins contain an YxxL motif within the RING domain, but at least for TACV Z protein, it did not influence the Z budding activity [43, 44] (Figure 2). The relative contribution of the different types of L domains to Z-mediated budding appears to be influenced by different factors including the virus species. Thus, for LASV the PPPY L domain appears to have a stronger contribution to budding than the PTAP motif [22, 23]. PPPY motif seems to have critical function compared to PTAP motif. In the case of TACV, the L-like domain ASAP was found to lack budding activity, and TACV Z-mediated budding was also Tsg101-independent but Vps4A/B-dependent [43, 44].
Figure 2.
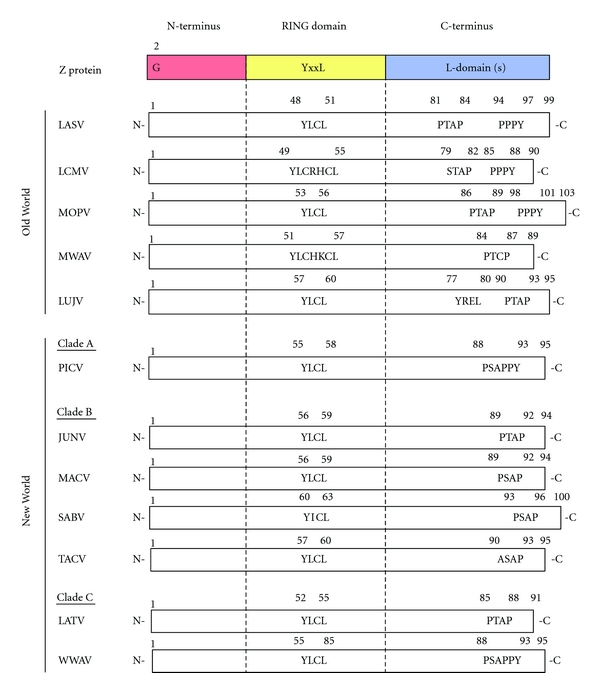
Organization of arenavirus Z protein. All arenavirus Z proteins have G at position 2 (G2). Within the centrally located RING domain, all known arenavirus Z proteins possess an YxxL (or YxxL-like) motif. At their C-terminus, arenavirus Z proteins have different types of L domains. Lassa virus (LASV), lymphocytic choriomeningitis virus (LCMV), Mopeia virus (MOPV), Merino Walk virus (MWAV), Lujo virus (LUJV), Pichinde virus (PICV), Junin virus (JUNV), Machupo virus (MACV), Sabia virus (SABV), Tacaribe virus (TACV), Latino virus (LATV), Whitewater Arroyo virus (WWAV).
In addition to the critical role played by the L domain motifs in Z-mediated budding, glycine at position two (G2) was strictly required for Z-mediated budding. G2 is conserved among all known arenavirus Z proteins (Figure 2) and is required for Z myristoylation and its subsequent targeting of membranes [43, 48, 49]. Accordingly, mutation G2A abrogated Z-mediated budding. Consistent with these findings, treatment with 2-OHM (DL-2-hydroxymyristic acid), an inhibitor of protein myristoylation, caused a dramatic reduction on Z-mediated budding and production of infectious virus progeny [43, 48].
5. Novel Strategies to Identify Cellular Factors Contributing to Z-Mediated Budding and Small Molecule Inhibitors of Z-Mediated Budding
As with many other bona fide viral budding proteins, Z-mediated budding requires its interaction with specific cellular factors within the endosomal/multivesicular body pathway as we discuss below. The identification and characterization of LASV-Z-host protein interactions involved in virus budding may uncover novel anti-arenavirus targets, and facilitate the development of screening strategies to identify drugs capable of disrupting viral budding and thereby preventing virus propagation. The ability of Z to direct self-budding in the absence of other viral proteins should facilitate the development of assays amenable to both genetics and chemical High Throughput Screening (HTS) to identify host cellular proteins required for Z-mediated budding, as well as small molecule inhibitors of this process. To this end, the emergence of RNA interference as a pathway that allows the modulation of gene expression has enabled functional genetic screens in mammalian cell types. Likewise, combinatorial chemical libraries have emerged as a leading source of compounds for biological screens, and; therefore, it should be feasible to identify small molecule inhibitors of Z-mediated budding by screening chemical libraries using appropriately designed cell-based assays of Z-mediated budding. In this regard, recent findings have shown that the fusion of the smaller (185 amino acids) luciferase from Gaussia princeps (GLuc) to Z resulted in a chimeric protein (Z-GLuc) that retain wild-type Z budding activity that could be monitored by direct measuring of GLuc activity in tissue culture supernatant of Z-GLuc transfected cells. Initial studies have shown that this Z-GLuc-based budding assay consistently exhibits high signal-to-noise ratio (S/N) values (average 10-fold) [50], suggesting that it should be amenable for the development of both genetic and chemical HTS to identify host cellular genes contributing to Z-mediated budding and small molecule inhibitors of Z-mediated budding, respectively.
6. Contribution of the ESCRT Machinery to Arenavirus Budding
The ESCRT machinery was originally identified as the Class E subset of vacuolar protein sorting (VPS) genes required for the correct sorting of soluble hydrolases from the yeast Golgi to the vacuole [51–53]. The essential VPS-mediated sorting step occurs during MVB (multi vesicular body) formation, when ubiquitinated proteins and lipids present on the limiting endosomal membrane are recognized and sorted into endosomal membrane microdomains, which ultimately invaginate and form vesicles that bud into the lumen to create the MVB [54–56]. Vesicles of the MVB subsequently fuse with lysosome, thereby exposing the internal vesicles to the degrading lipases and proteases in this organelle. ESCRT pathway is also involved in the membrane abscission event at the conclusion of cell division [57, 58]. ESCRT-I contains Tsg101, Vps37, Vps23, and Mvb12A/B and has been shown to be recruited from the cytoplasm to the surface of maturing endosomes [29, 54–56, 59, 60]. Components of ESCRT-I, especially Tsg101, recognize ubiquitinated protein cargos and interact with ESCRT-II that participates in protein sorting and vesicle formation. It should be noted that Alix/AIP1 was found to bridge ESCRT-I and ESCRT-III using a different way to that used by ESCRT-II [61, 62]. ESCRT-III components also recruit another class E proteins, Vps4A/B, which are AAA-type ATPases involved in catalyzing the disassembly and recycling of the membrane-bound ESCRT complexes [29–31]. Notably, the abscission event during cellular cytokinesis, a process topologically similar to endosomal vesicle formation, utilizes the same ESCRT machinery [29, 58, 63].
Myristoylation of Z facilitates its attachment to the PM where Z is likely recognized by Tsg101 through the PT/SAP L domain motif present in Z (Figure 3). However, there is not direct biochemical evidence that Z binds to Tsg101 directly. Nevertheless, depletion of Tsg101 by siRNA resulted in decreased levels of both LASV and LCMV Z-mediated budding [21, 23]. Intriguingly, when the ASAP motif present on TACV-Z protein was mutated to AAAA, VLP production levels were not affected, suggesting in contrast to LCMV and LASV that TACV Z-mediated budding does not utilize Tsg101 [43, 44]. However, results of siRNA-mediated depletion and the use of dominant negative mutants indicated that Vps4A/B was necessary for both LASV and TACV Z-mediated budding activity [21, 43]. These results would suggest that the last step within the ESCRT pathway is necessary for both LASV and TACV Z-mediated budding.
Figure 3.
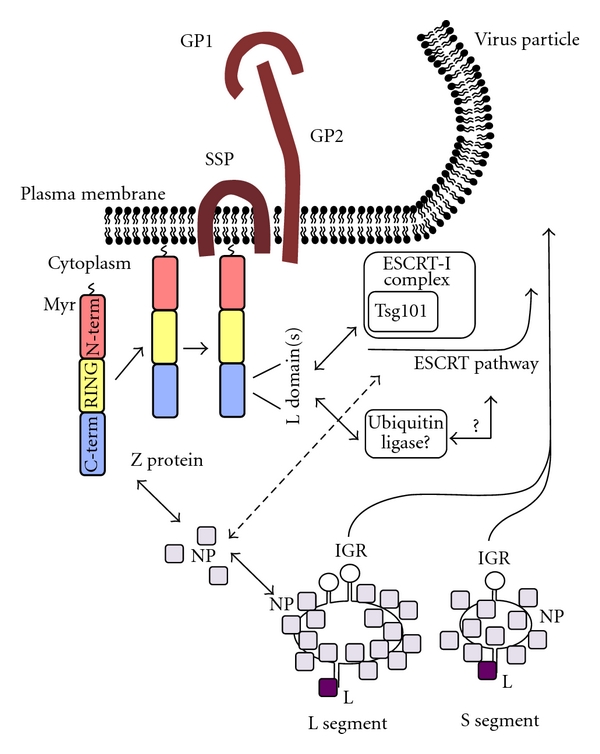
Model of arenavirus budding. Myristoylation of Z at G2 facilitates its interaction with the plasma membrane (PM), where Z likely forms higher-order complexes. L domains located within the C-terminus of Z facilitate its interactions with host cellular factors to allow Z to utilize the ESCRT machinery of the cell for cell egress (budding). SSP, stable signal peptide.
The budding activity of some M proteins has been shown to be increased by the contribution of other viral proteins as illustrated in the case of the nucleoprotein (NP) and glycoprotein (GP) of the EBOV and MARV viruses [64, 65]. However, studies on LCMV and LASV Z-mediated budding failed to uncover a contribution to budding by other viral protein. In contrast, TACV NP was shown to enhance Z-mediated VLP production [44].
Nevertheless, recently published work has shown that Mopeia virus (MOPV) NP incorporation into VLP is mediated by AIP1/Alix via interaction with the YLCL motif present on Z [45, 46].
7. Tetherin/BST-2/CD317 as an Antagonist of Arenavirus Budding
Tetherin was identified as an IFN-inducible antiviral cellular factor that tether HIV virions at the PM [66, 67]. Subsequently studies have extended these findings to other viruses [68]. As with other host innate immune defense factors, several viruses have evolved mechanisms to counteract tetherin-mediated antiviral activity [69]. Tetherin has been shown to inhibit LASV Z-mediated budding [70]. Accordingly, 293T cells constitutively expressing tetherin resulted in decreased production levels of LASV and MACV virion particle production, whereas siRNA-mediated knockdown of endogenous tetherin in HeLa cells resulted in increased production levels of LASV and MACV virion particle production. These results would suggest that LASV does not possess any tetherin-antagonizing function as described for other viral proteins including HIV-1 Vpu, EBOV GP, and KSHV K5 [66, 71–73].
An issue that remains to be investigated relates to the contribution of tetherin to host protection and viral pathogenesis. An attractive hypothesis, but still without experimental support, would be that tetherin could do to some degree slow the process of virus propagation in vivo and thereby facilitating both the action of the host innate immune defense mechanisms and antigen presentation leading to a more robust host adaptive immune response that could control and eliminate the virus.
8. Perspectives on Arenavirus Z-Mediated Budding
Current evidence indicates that many enveloped viruses use the ESCRT machinery to exit from the cell. In the case of arenavirus budding, Z-Tsg101 interaction appears to facilitate access of Z to the ESCRT machinery. Despite significant recent progress in defining the basic aspects of arenavirus budding, there are still a large number of issues that have not been investigated including: (1) Identification and functional characterization of host cellular factors that interact with the PPXY L domain motif present in LASV, LCMV, and some other arenavirus Z to facilitate virus budding. PPXY L domain motifs present in EBOV and MARV VP40 have shown to mediate interaction with Nedd4.1 [74–76]. Likewise, for several retroviruses, the interaction of Gag PPXY L domains with ubiquitin ligases has been shown to contribute to the regulation of viral budding [24, 25, 38, 39]. How ubiquitin ligases may regulate budding remains unknown, but recent published data have shown arrestin-related proteins to connect ubiquitin ligases and the ESCRT machinery [77]. It is important to know whether specific ubiquitin ligase may regulate Z-mediated budding, and if so, what are the mechanism underlying this regulation. (2) Ubiquitin or ubiquitin-like molecules (UBLs) have been shown to modify the properties and budding activity of HIV-1 Gag and EBOV VP40 proteins [78]. It is currently unknown whether these protein modifiers may also have a role in the regulation of arenavirus budding. (3) The mechanisms by which arenavirus RNP interacts with Z and GP to form budding mature infectious progeny are largely unknown. (4) Whether the species-specific and type of cell influence arenavirus budding and the biological implications regarding the outcome of infection are issues that have not been investigated.
Detailed understanding of the virus-host cell protein interactions that direct arenavirus budding may uncover novel targets for the development of antiviral drugs to combat human pathogenic arenaviruses.
Acknowledgments
This is Publication no. 21130 from the Department of Immunology and Microbial Science, The Scripps Research Institute (TSRI), La Jolla, Calif, USA. Because space limitations, we have relied extensively in recent reviews where more extensive detailed information, including all the original citations, can be found regarding the different topics discussed in this paper. Recognition and apologies are given in advance to the many colleagues whose original contributions have not been possible to cite. The research contributed by the authors of this paper was supported by NIH grants RO1 AI047140, RO1 AI077719 and RO1 AI079665 to J. C. de la Torre and T32-AI0735419 to S. Urata.
References
- 1.Buchmeier MJ, Peters CJ, de la Torre JC. Arenaviridae: the virus and their replication. Fields Virology. 2007;2:1792–1827. [Google Scholar]
- 2.Oldstone MB. Biology and pathogenesis of lymphocytic choriomeningitis virus infection. Current Topics in Microbiology and Immunology. 2002;263:83–117. doi: 10.1007/978-3-642-56055-2_6. [DOI] [PubMed] [Google Scholar]
- 3.Zinkernagel RM. Lymphocytic choriomeningitis virus and immunology. Current Topics in Microbiology and Immunology. 2002;263:1–5. doi: 10.1007/978-3-642-56055-2_1. [DOI] [PubMed] [Google Scholar]
- 4.Cao W, Henry MD, Borrow P, et al. Identification of α-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282(5396):2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 5.Kunz S, Borrow P, Oldstone MB. Receptor structure, binding, and cell entry of arenaviruses. Current Topics in Microbiology and Immunology. 2002;262:111–137. doi: 10.1007/978-3-642-56029-3_5. [DOI] [PubMed] [Google Scholar]
- 6.Radoshitzky SR, Abraham J, Spiropoulou CF, et al. Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature. 2007;446(7131):92–96. doi: 10.1038/nature05539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunz S. Receptor binding and cell entry of Old World arenaviruses reveal novel aspects of virus-host interaction. Virology. 2009;387(2):245–249. doi: 10.1016/j.virol.2009.02.042. [DOI] [PubMed] [Google Scholar]
- 8.Rojek JM, Perez M, Kunz S. Cellular entry of lymphocytic choriomeningitis virus. Journal of Virology. 2008;82(3):1505–1517. doi: 10.1128/JVI.01331-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rojek JM, Sanchez AB, Nguyen NT, de La Torre JC, Kunz S. Different mechanisms of cell entry by human-pathogenic Old World and New World arenaviruses. Journal of Virology. 2008;82(15):7677–7687. doi: 10.1128/JVI.00560-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hass M, Gölnitz U, Müller S, Becker-Ziaja B, Günther S. Replicon system for Lassa virus. Journal of Virology. 2004;78(24):13793–13803. doi: 10.1128/JVI.78.24.13793-13803.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee KJ, Novella IS, Teng MN, Oldstone MB, de La Torre JC. NP and L proteins of lymphocytic choriomeningitis virus (LCMV) are sufficient for efficient transcription and replication of LCMV genomic RNA analogs. Journal of Virology. 2000;74(8):3470–3477. doi: 10.1128/jvi.74.8.3470-3477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.López N, Jácamo R, Franze-fernández MT. Transcription and RNA replication of tacaribe virus genome and antigenome analogs require n and l proteins: Z protein is an inhibitor of these processes. Journal of Virology. 2001;75(24):12241–12251. doi: 10.1128/JVI.75.24.12241-12251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornu TI, de La Torre JC. Ring finger z protein of lymphocytic choriomeningitis virus (lcmv) inhibits transcription and rna replication of an lcmv s-segment minigenome. Journal of Virology. 2001;75(19):9415–9426. doi: 10.1128/JVI.75.19.9415-9426.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornu TI, de la Torre JC. Characterization of the arenavirus RING finger Z protein regions required for Z-mediated inhibition of viral RNA synthesist. Journal of Virology. 2002;76(13):6678–6688. doi: 10.1128/JVI.76.13.6678-6688.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borden KL, Campbell Dwyer EJ, Salvato MS. An arenavirus RING (zinc-binding) protein binds the oncoprotein promyelocyte leukemia protein (PML) and relocates PML nuclear bodies to the cytoplasm. Journal of Virology. 1998;72(1):758–766. doi: 10.1128/jvi.72.1.758-766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kentsis A, Dwyer EC, Perez JM, et al. The RING domains of the promyelocytic leukemia protein PML and the arenaviral protein Z repress translation by directly inhibiting translation initiation factor eIF4E. Journal of Molecular Biology. 2001;312(4):609–623. doi: 10.1006/jmbi.2001.5003. [DOI] [PubMed] [Google Scholar]
- 17.Volpon L, Osborne MJ, Capul AA, de La Torre JC, Borden KL. Structural characterization of the Z RING-eIF4E complex reveals a distinct mode of control for eIF4E. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(12):5441–5446. doi: 10.1073/pnas.0909877107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell Dwyer EJ, Lai H, MacDonald RC, Salvato MS, Borden KL. The lymphocytic choriomeningitis virus RING protein Z associates with eukaryotic initiation factor 4E and selectively represses translation in a RING-dependent manner. Journal of Virology. 2000;74(7):3293–3300. doi: 10.1128/jvi.74.7.3293-3300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Colina R, Costa-Mattioli M, Dowling RJ, et al. Translational control of the innate immune response through IRF-7. Nature. 2008;452(7185):323–328. doi: 10.1038/nature06730. [DOI] [PubMed] [Google Scholar]
- 20.Fan L, Briese T, Lipkin WI. Z proteins of new world arenaviruses bind RIG-I and interfere with type i interferon induction. Journal of Virology. 2010;84(4):1785–1791. doi: 10.1128/JVI.01362-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urata S, Noda T, Kawaoka Y, Yokosawa H, Yasuda J. Cellular factors required for Lassa virus budding. Journal of Virology. 2006;80(8):4191–4195. doi: 10.1128/JVI.80.8.4191-4195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strecker T, Eichler R, Meulen J, et al. Lassa virus Z protein is a matrix protein sufficient for the release of virus-like particles. Journal of Virology. 2003;77(19):10700–10705. doi: 10.1128/JVI.77.19.10700-10705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez M, Craven RC, de la Torre JC. The small RING finger protein Z drives arenavirus budding: implications for antiviral strategies. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(22):12978–12983. doi: 10.1073/pnas.2133782100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen BJ, Lamb RA. Mechanisms for enveloped virus budding: can some viruses do without an ESCRT? Virology. 2008;372(2):221–232. doi: 10.1016/j.virol.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bieniasz PD. Late budding domains and host proteins in enveloped virus release. Virology. 2006;344(1):55–63. doi: 10.1016/j.virol.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 26.Morita E, Sundquist WI. Retrovirus budding. Annual Review of Cell and Developmental Biology. 2004;20:395–425. doi: 10.1146/annurev.cellbio.20.010403.102350. [DOI] [PubMed] [Google Scholar]
- 27.Garrus JE, von Schwedler UK, Pornillos OW, et al. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 2001;107(1):55–65. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- 28.VerPlank L, Bouamr F, LaGrassa TJ, et al. Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, binds the L domain in HIV type 1 Pr55Gag. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(14):7724–7729. doi: 10.1073/pnas.131059198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurley JH, Hanson PI. Membrane budding and scission by the ESCRT machinery: it’s all in the neck. Nature Reviews Molecular Cell Biology. 2010;11(8):556–566. doi: 10.1038/nrm2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Babst M, Sato TK, Banta LM, Emr SD. Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO Journal. 1997;16(8):1820–1831. doi: 10.1093/emboj/16.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Babst M, Wendland B, Estepa EJ, Emr SD. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO Journal. 1998;17(11):2982–2993. doi: 10.1093/emboj/17.11.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dolnik O, Kolesnikova L, Stevermann L, Becker S. Tsg101 is recruited by a late domain of the nucleocapsid protein to support budding of Marburg virus-like particles. Journal of Virology. 2010;84(15):7847–7856. doi: 10.1128/JVI.00476-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Irie T, Nagata N, Yoshida T, Sakaguchi T. Paramyxovirus Sendai virus C proteins are essential for maintenance of negative-sense RNA genome in virus particles. Virology. 2008;374(2):495–505. doi: 10.1016/j.virol.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Sakaguchi T, Kato A, Sugahara F, et al. AIP1/Alix is a binding partner of Sendai virus C protein and facilitates virus budding. Journal of Virology. 2005;79(14):8933–8941. doi: 10.1128/JVI.79.14.8933-8941.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irie T, Shimazu Y, Yoshida T, Sakaguchi T. The YLDL sequence within sendai virus M protein is critical for budding of virus-like particles and interacts with Alix/AIP1 independently of C protein. Journal of Virology. 2007;81(5):2263–2273. doi: 10.1128/JVI.02218-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossman JS, Lamb RA. Influenza virus assembly and budding. Virology. 2011;411(2):229–236. doi: 10.1016/j.virol.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irie T, Licata JM, McGettigan JP, Schnell MJ, Harty RN. Budding of PPxY-containing rhabdoviruses is not dependent on host proteins TGS101 and VPS4A. Journal of Virology. 2004;78(6):2657–2665. doi: 10.1128/JVI.78.6.2657-2665.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kikonyogo A, Bouamr F, Vana ML, et al. Proteins related to the Nedd4 family of ubiquitin protein ligases interact with the L domain of Rous sarcoma virus and are required for gag budding from cells. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(20):11199–11204. doi: 10.1073/pnas.201268998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vana ML, Tang Y, Chen A, Medina G, Carter C, Leis J. Role of Nedd4 and ubiquitination of rous sarcoma virus Gag in budding of virus-like particles from cells. Journal of Virology. 2004;78(24):13943–13953. doi: 10.1128/JVI.78.24.13943-13953.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dilley KA, Gregory D, Johnson MC, Vogt VM. An LYPSL late domain in the Gag protein contributes to the efficient release and replication of Rous sarcoma virus. Journal of Virology. 2010;84(13):6276–6287. doi: 10.1128/JVI.00238-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Briese T, Paweska JT, McMullan LK, et al. Genetic detection and characterization of Lujo virus, a new hemorrhagic fever-associated arenavirus from southern Africa. PLoS Pathogens. 2009;5(5) doi: 10.1371/journal.ppat.1000455. Article ID e1000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palacios G, Savji N, Hui J, et al. Genomic and phylogenetic characterization of Merino Walk virus, a novel arenavirus isolated in South Africa. Journal of General Virology. 2010;91(5):1315–1324. doi: 10.1099/vir.0.017798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Urata S, Yasuda J, de La Torre JC. The Z protein of the new world arenavirus tacaribe virus has bona fide budding activity that does not depend on known late domain motifs. Journal of Virology. 2009;83(23):12651–12655. doi: 10.1128/JVI.01012-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Groseth A, Wolff S, Strecker T, Hoenen T, Becker S. Efficient budding of the tacaribe virus matrix protein Z requires the nucleoprotein. Journal of Virology. 2010;84(7):3603–3611. doi: 10.1128/JVI.02429-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shtanko O, Imai M, Goto H, et al. A role for the C terminus of mopeia virus nucleoprotein in its incorporation into Z protein-induced virus-like particles. Journal of Virology. 2010;84(10):5415–5422. doi: 10.1128/JVI.02417-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shtanko O, Watanabe S, Jasenosky LD, Watanabe T, Kawaoka Y. ALIX/AIP1 is required for NP incorporation into Mopeia virus Z-induced virus-like particles. Journal of Virology. 2011;85(7):3631–3641. doi: 10.1128/JVI.01984-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gosselin-Grenet AS, Marq JB, Abrami L, Garcin D, Roux L. Sendai virus budding in the course of an infection does not require Alix and VPS4A host factors. Virology. 2007;365(1):101–112. doi: 10.1016/j.virol.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 48.Perez M, Greenwald DL, de La Torre JC. Myristoylation of the RING finger Z protein is essential for arenavirus budding. Journal of Virology. 2004;78(20):11443–11448. doi: 10.1128/JVI.78.20.11443-11448.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strecker T, Maisa A, Daffis S, Eichler R, Lenz O, Garten W. The role of myristoylation in the membrane association of the Lassa virus matrix protein Z. Virology Journal. 2006;3, article 93 doi: 10.1186/1743-422X-3-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Capul AA, de la Torre JC. A cell-based luciferase assay amenable to high-throughput screening of inhibitors of arenavirus budding. Virology. 2008;382(1):107–114. doi: 10.1016/j.virol.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bankaitis VA, Johnson LM, Emr SD. Isolation of yeast mutants defective in protein targeting to the vacuole. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(23):9075–9079. doi: 10.1073/pnas.83.23.9075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson JS, Klionsky DJ, Banta LM, Emr SD. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Molecular and Cellular Biology. 1988;8(11):4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raymond CK, Howald-Stevenson I, Vater CA, Stevens TH. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Molecular Biology of the Cell. 1992;3(12):1389–1402. doi: 10.1091/mbc.3.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106(2):145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- 55.Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD. ESCRT-III: an endosome-associated heterooligomeric protein complex required for MVB sorting. Developmental Cell. 2002;3(2):271–282. doi: 10.1016/s1534-5807(02)00220-4. [DOI] [PubMed] [Google Scholar]
- 56.Babst M, Katzmann DJ, Snyder WB, Wendland B, Emr SD. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Developmental Cell. 2002;3(2):283–289. doi: 10.1016/s1534-5807(02)00219-8. [DOI] [PubMed] [Google Scholar]
- 57.Morita E, Sandrin V, Chung HY, et al. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO Journal. 2007;26(19):4215–4227. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carlton JG, Martin-Serrano J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science. 2007;316(5833):1908–1912. doi: 10.1126/science.1143422. [DOI] [PubMed] [Google Scholar]
- 59.Chu T, Sun J, Saksena S, Emr SD. New component of ESCRT-I regulates endosomal sorting complex assembly. Journal of Cell Biology. 2006;175(5):815–823. doi: 10.1083/jcb.200608053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morita E, Sandrin V, Alam SL, Eckert DM, Gygi SP, Sundquist WI. Identification of human MVB12 proteins as ESCRT-I subunits that function in HIV budding. Cell Host and Microbe. 2007;2(1):41–53. doi: 10.1016/j.chom.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strack B, Calistri A, Craig S, Popova E, Göttlinger HG. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell. 2003;114(6):689–699. doi: 10.1016/s0092-8674(03)00653-6. [DOI] [PubMed] [Google Scholar]
- 62.Martin-Serrano J, Yarovoy A, Perez-Caballero D, Bieniasz PD. Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(21):12414–12419. doi: 10.1073/pnas.2133846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morita E, Colf LA, Karren MA, Sandrin V, Rodesch CK, Sundquist WI. Human ESCRT-III and VPS4 proteins are required for centrosome and spindle maintenance. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(29):12889–12894. doi: 10.1073/pnas.1005938107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Urata S, Noda T, Kawaoka Y, Morikawa S, Yokosawa H, Yasuda J. Interaction of Tsg101 with Marburg virus VP40 depends on the PPPY motif, but not the PT/SAP motif as in the case of Ebola virus, and Tsg101 plays a critical role in the budding of Marburg virus-like particles induced by VP40, NP, and GP. Journal of Virology. 2007;81(9):4895–4899. doi: 10.1128/JVI.02829-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Licata JM, Johnson RF, Han Z, Harty RN. Contribution of Ebola virus glycoprotein, nucleoprotein, and VP24 to budding of VP40 virus-like particles. Journal of Virology. 2004;78(14):7344–7351. doi: 10.1128/JVI.78.14.7344-7351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451(7177):425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 67.Van Damme N, Goff D, Katsura C, et al. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell Host and Microbe. 2008;3(4):245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Evans DT, Serra-Moreno R, Singh RK, Guatelli JC. BST-2/tetherin: a new component of the innate immune response to enveloped viruses. Trends in Microbiology. 2010;18(9):388–396. doi: 10.1016/j.tim.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Douglas JL, Gustin JK, Viswanathan K, Mansouri M, Moses AV, Früh K. The great escape: viral strategies to counter BST-2/tetherin. PLoS Pathogens. 2010;6(5) doi: 10.1371/journal.ppat.1000913. Article ID e1000913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sakuma T, Noda T, Urata S, Kawaoka Y, Yasuda J. Inhibition of lassa and marburg virus production by tetherin. Journal of Virology. 2009;83(5):2382–2385. doi: 10.1128/JVI.01607-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaletsky RL, Francica JR, Agrawal-Gamse C, Bates P. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(8):2886–2891. doi: 10.1073/pnas.0811014106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Radoshitzky SR, Dong L, Chi X, et al. Infectious Lassa virus, but not filoviruses, is restricted by BST-2/tetherin. Journal of Virology. 2010;84(20):10569–10580. doi: 10.1128/JVI.00103-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bartee E, McCormack A, Früh K. Quantitative membrane proteomics reveals new cellular targets of viral immune modulators. PLoS Pathogens. 2006;2(10, article e107) doi: 10.1371/journal.ppat.0020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Urata S, Yasuda J. Regulation of Marburg virus (MARV) budding by Nedd4.1: a different WW domain of Nedd4.1 is critical for binding to MARV and Ebola virus VP40. Journal of General Virology. 2010;91(1):228–234. doi: 10.1099/vir.0.015495-0. [DOI] [PubMed] [Google Scholar]
- 75.Yasuda J, Nakao M, Kawaoka Y, Shida H. Nedd4 regulates egress of Ebola virus-like particles from host cells. Journal of Virology. 2003;77(18):9987–9992. doi: 10.1128/JVI.77.18.9987-9992.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Timmins J, Schoehn G, Ricard-Blum S, et al. Ebola virus matrix protein VP40 interaction with human cellular factors Tsg101 and Nedd4. Journal of Molecular Biology. 2003;326(2):493–502. doi: 10.1016/s0022-2836(02)01406-7. [DOI] [PubMed] [Google Scholar]
- 77.Rauch S, Martin-Serrano J. Multiple interactions between the ESCRT machinery and arrestin-related proteins: implications for PPXY-dependent budding. Journal of Virology. 2011;85(7):3546–3556. doi: 10.1128/JVI.02045-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harty RN, Pitha PM, Okumura A. Antiviral activity of innate immune protein ISG15. Journal of Innate Immunity. 2009;1(5):397–404. doi: 10.1159/000226245. [DOI] [PMC free article] [PubMed] [Google Scholar]


