Summary
Background and objectives
Non-renal transplant recipients who subsequently develop ESRD and undergo kidney transplantation are medically and immunologically complex due to comorbidities, high cumulative exposure to immunosuppressants, and sensitization to alloantigen from the prior transplant. Although prior non-renal transplant recipients are one of the fastest growing segments of the kidney wait list, minimal data exist to guide the use of antibody induction therapy (IT+) at the time of kidney after lung (KALu), heart (KAH), and liver (KALi) transplant.
Design, setting, participants, & measurements
This retrospective cohort study used national registry data to examine IT use and survival after kidney transplantation. Separate multivariate Cox regression models were constructed to assess patient survival for IT+ and IT− KALu (n=232), KAH (n=588), and KALi (n=736) recipients.
Results
Use of IT increased during the study period. The percentage of patients considered highly sensitized (panel reactive antibody ≥20%) was not statistically significant between IT+ and IT− groups. IT+ was not associated with improvement in 1- and 10-year patient survival for KALu (P=0.20 and P=0.22, respectively) or for KAH (P=0.90 and P=0.14, respectively). However, IT+ among KALi was associated with inferior patient survival at 1 and 10 years (P=0.04 and P=0.02, respectively).
Conclusions
Use of IT for kidney transplantation among prior non-renal transplant recipients may not offer a survival advantage in KALu or KAH. However, due to limited power, these findings should be interpreted cautiously. IT+ was associated with inferior outcomes for KALi. Use of IT should be judicially reconsidered in this complex group of recipients.
Introduction
The success of lung, liver, and heart transplantation is reflected by the increasing number of surviving recipients. These patients are at greater risk of developing CKD and ESRD, likely as a result of episodes of AKI, prolonged exposure to calcineurin inhibitors, and in association with contributing comorbidities such as diabetes, hepatitis C, and hypertension (1–4). A retrospective analysis of national registry data indicated that 15.8% of lung transplant recipients, 18.1% of liver recipients, and 10.9% of heart recipients will develop CKD within 5 years of transplant (5). The development of CKD and ESRD after non-renal transplant is associated with an increased risk of mortality (5–7). Although the number of patients is small, non-renal transplant recipients represent a growing segment of the kidney transplant wait list. Evidence indicates that these patients receive a survival benefit from kidney transplantation compared with remaining on the kidney wait list (8–12). Taken together, these findings indicate that non-renal transplant recipients with subsequent chronic renal disease are at increased risk of mortality and those who are candidates for kidney transplantation receive a substantial survival benefit.
Clinical trials have revealed that induction immunosuppression using antibody therapy decreases the risk of acute rejection for isolated kidney transplants (13,14). Induction therapy may also reduce exposure to calcineurin inhibitors in the peri-transplant period (15,16). Although kidney transplant after prior heart, liver, or lung transplant has become more common, little evidence exists to guide transplant centers in their decisions regarding the use of induction therapy for this group. Decisions regarding immunosuppression at the time of renal transplant can be challenging in patients with a prior non-renal transplant. The judgment to use induction therapy in patients with a prior heart, lung, or liver transplant requires careful consideration of the potential adverse consequences as well as benefits of this therapy. In general, non-renal transplant recipients considered for renal transplantation are medically complex, often with residual comorbidities related to the primary disease. From an immunologic perspective, these patients may be at increased risk for rejection because of exposure to alloantigens from the prior transplant. Consideration for induction therapy may reduce the risk of acute rejection. However, non-renal transplant recipients have accumulated significant exposure to chronic immunosuppression, placing them at increased risk of infection and immunosuppression-related malignancies.
Evidence to help make informed decisions regarding induction therapy in patients presenting for kidney after liver (KALi), kidney after lung (KALu), and kidney after heart (KAH) transplants is sparse (17). The aims of this study were to examine national trends with regard to induction therapy and to determine whether there is evidence of benefit in the use of antibody induction therapy in a medically and immunologically challenged group of transplant recipients.
Materials and Methods
Study Design
This was a retrospective cohort study of kidney transplant recipients between January 1995 and February 2009 using registry data from the United Network of Organ Sharing (UNOS). This study was approved by the Hospital of the University of Pennsylvania Institutional Review Board.
UNOS kidney, thoracic, and liver data sets were used to identify patients who received a kidney transplant in one of the following clinical scenarios: kidney after lung transplant (KALu), kidney after heart transplant (KAH), and kidney after liver transplant (KALi). UNOS immunosuppressant data were used to identify patients who had received induction therapy (IT+) at the time of kidney transplantation. The induction therapy group included those patients who received antilymphocyte globulin, antithymocyte globulin, alemtuzumab, muromonab-CD3, basiliximab, or daclizumab.
Patients aged <17 years at the time of transplant and patients with prior or subsequent transplants other than the initial lung, heart, or liver were excluded. A total of 153 KALu, 356 KAH, and 416 KALi IT+ recipients, and 79 KALu, 232 KAH, and 320 KALi recipients who did not receive induction (IT−) were identified for this study.
Survival Analysis
The primary endpoints of the study were graft and patient survival. Unadjusted patient survival was determined with Kaplan–Meier analysis. Independent multivariable Cox hazard models were constructed to compare IT+ and IT− recipients within the three transplant populations (KALu, KAH, and KALi). Variables nominally associated with the outcome (P<0.10) in univariate analyses were entered into the multivariate models. Covariates not meeting the entry threshold were included if considered clinically significant. Graft and patient survival models included the following covariates: recipient and donor age, cold ischemic time, peak panel reactive antibody (PRA) greater or less than 20%, donor type (standard criteria deceased, extended criteria deceased, living donor), and antibody immunosuppression group (IT+ or IT−). Recipient hepatitis C status was included in the model for KALi but not the other groups due to the low number of KALu and KAH recipients who had positive hepatitis C results. Additional KALi models were created specific to recipients with and without hepatitis C virus (HCV+ and HCV−, respectively) so as to better evaluate the use of induction therapy within this cohort.
Missing Data
Our primary approach was to perform multivariable regression analyses using data from recipients with compete data sets regarding the covariates of interest. PRA and cold ischemic time were missing in 24.0% and 23.4% of recipients, respectively. We performed a series of secondary analyses to demonstrate that these missing data did not significantly affect the primary relationships between induction therapy and outcomes. In these secondary analyses, missing values for PRA and cold ischemic time were imputed using the 10th and 90th percentiles, as well as mean values. In these secondary analyses, imputation did not significantly change the results of the primary analysis and are not shown.
Statistical Analyses
Means of continuous variables were compared between groups using ANOVA. Categorical variables were compared between groups using the chi-squared test. All analyses were performed using SPSS software (version 17.0; SPSS Inc, Chicago, IL). Microsoft Excel 2003 was used for construction of some of the figures.
Results
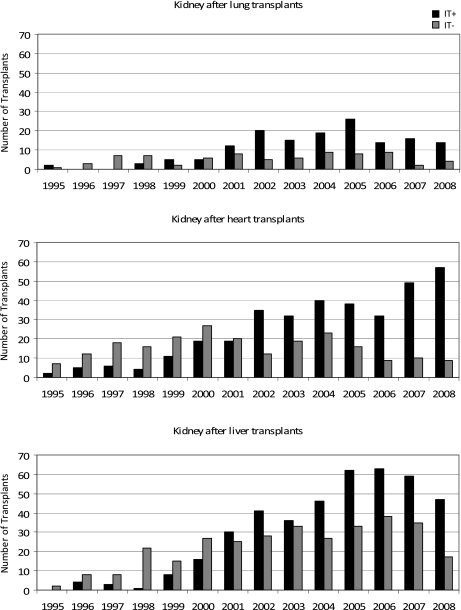
Our results showed that 65.9% of KALu, 60.5% of KAH, and 56.5% of KALi received induction at the time of kidney transplant. The number of patients undergoing kidney after previous lung, heart, or liver transplant, as well as the percentage receiving induction immunosuppression at the time of kidney transplant increased during the study period (Figure 1). In the last 5 years of this study, the percentage of renal recipients given induction therapy as a component of their immunosuppression regimen increased from 70% to 78% for KALu, 64% to 86% for KAH, and 63% to 73% for KALi. The types and distribution of induction therapy are listed in Table 1. Patients receiving induction therapy were more likely to receive steroids at the time of transplant and were less likely to receive maintenance steroids at the time of discharge (Table 1).
Figure 1.
Annual number of kidney transplants following previous lung, heart, and liver transplants from 1995 to 2008. IT+, received induction therapy; IT−, did not receive induction therapy.
Table 1.
Recipient and donor demographics
| Kidney after Lung Transplant | Kidney after Heart Transplant | Kidney after Liver Transplant | ||||
|---|---|---|---|---|---|---|
| IT+ | IT− | IT+ | IT− | IT+ | IT− | |
| Recipient data | ||||||
| n | 153 | 79 | 356 | 232 | 416 | 320 |
| age at transplant, yr (SD) | 49.1 (13.3) | 46.1 (13.7) | 58.0 (11.7) | 57.0 (11.0) | 54.5 (10.8) | 53.4 (10.5) |
| sex, % | ||||||
| male | 41.8 | 50.6 | 81.0 | 81.3 | 68.8 | 65.1 |
| female | 58.2 | 49.4 | 19.0 | 18.7 | 31.3 | 34.9 |
| race, % | ||||||
| white | 96.7 | 93.7 | 85.8 | 81.3 | 78.6 | 71.4 |
| black | 1.3 | 2.5 | 8.8 | 12.3 | 7.2 | 7.9 |
| other | 2.0 | 3.8 | 5.4 | 6.4 | 14.2 | 20.8 |
| diabetes, % | 28.8 | 25.3 | 27.2 | 28.8 | 38.7 | 36.2 |
| dialysis at time of transplant, % | 52.9 | 48.1 | 63.2 | 66.2 | 65.6 | 69.2 |
| hepatitis C positive, % | 7.1 | 1.6 | 4.7 | 3.8 | 37.7 | 38.5 |
| peak panel reactive antibody, %a | ||||||
| <20% | 91.8 | 86.5 | 88.5 | 91.2 | 77.5 | 81.4 |
| ≥20% | 8.2 | 13.5 | 11.5 | 8.8 | 22.5 | 18.6 |
| missing | 35.8 | 32.5 | 26.5 | 22.4 | 16.8 | 25.8 |
| induction therapy agents, %b | ||||||
| ALG | 1.3 | — | 0.3 | — | 0.2 | — |
| ATG | 41.8 | — | 46.3 | — | 49.8 | — |
| alemtuzumab | 3.3 | — | 5.9 | — | 2.9 | — |
| muromonab-CD3 | 0.7 | — | 4.2 | — | 1.2 | — |
| basiliximab | 34.0 | — | 30.3 | — | 33.2 | — |
| daclizumab | 29.0 | — | 19.1 | — | 20.2 | — |
| steroids at transplant, % | 76.5c | 55.7c | 74.2c | 50.9c | 74c | 54.7c |
| maintenance steroids, % | 88.9c | 96.2c | 85.1c | 96.1c | 81.3c | 90.9c |
| days on wait list | 366 (405)c | 250 (237)c | 432 (503) | 372 (417) | 382 (471) | 352 (389) |
| years between previous and kidney transplant (SD) | 7.7 (3.1)c | 6.6 (2.9)c | 7.3 (4.8) | 7.1 (4.1) | 4.8 (3.7)c | 3.4 (3.4)c |
| median follow-up, yr (range) | 2.9 (10.1) | 2.9 (12.0) | 2.0 (13.7) | 3.9 (13.1) | 2.0 (11.9) | 3.0 (13.1) |
| Donor data | ||||||
| age, yr (SD) | 41.4 (13.8) | 42.7 (14.3) | 40.4 (14.6)c | 37.4 (15.3)c | 39.3 (14.9) | 38.7 (14.1) |
| sex, % | ||||||
| male | 40.5 | 44.3 | 52.7 | 50.7 | 55.5 | 52.2 |
| female | 59.5 | 55.7 | 47.3 | 49.3 | 44.5 | 47.8 |
| race, % | c | c | ||||
| white | 93.5 | 91.1 | 86.1 | 79.0 | 76.7 | 72.0 |
| black | 2.6 | 5.1 | 7.1 | 12.3 | 10.3 | 7.2 |
| other | 3.9 | 3.8 | 6.8 | 8.7 | 13.0 | 20.8 |
| diabetes, % | 0c | 8.9c | 4.8 | 4.5 | 6.0 | 4.6 |
| hepatitis C status, % | c | c | ||||
| negative | 94.8 | 86.1 | 94.3 | 92.2 | 86.5 | 88.1 |
| positive | 0.7 | 0.0 | 1.4 | 1.8 | 10.1 | 7.5 |
| unknown/missing | 4.6 | 13.9 | 4.2 | 5.9 | 3.4 | 4.4 |
| donor type, % | ||||||
| living | 68.0 | 72.2 | 38.8 | 37.9 | 24.5 | 28.9 |
| standard criteria (deceased) | 27.5 | 24.1 | 50.1 | 53.9 | 64.2 | 62.3 |
| expanded criteria (deceased) | 4.6 | 3.8 | 11.0 | 8.2 | 11.3 | 8.8 |
| cold ischemic time, h (SD) | 8.6 (10.3) | 8.0 (10.2) | 13.2 (11.4) | 12.3 (11.1) | 14.8 (10.2) | 14.9 (11.1) |
| missing, % | 33.8 | 20.8 | 20.1 | 15.5 | 23.1 | 26.7 |
IT, induction therapy; ALG, antilymphocyte globulin; ATG, antithymocyte globulin.
The degree of sensitization before transplant: First kidney transplant, 10.3 (22.9); repeat kidney transplant, 38.1 (38.5); kidney after lung, 7.0 (17.8); kidney after heart, 7.2 (17.2); and kidney after liver, 14.0 (26.3).11
Some patients received more than one induction therapy agent.
Indicates P<0.05 when comparing induction therapy (IT+) and non-induction therapy (IT−) populations within a specific transplant setting, such as kidney after lung transplant.
The degree of allosensitization was examined in the three clinical groups. The mean PRA of those patients receiving induction was similar to patients who did not receive induction (KALu IT+ = 6.8, KALu IT− = 8.0, P=0.69; KAH IT+ = 7.6, KAH IT− = 6.3, P=0.44; KALi IT+ = 15.3, KALi IT− = 12.1, P=0.15). Despite similar levels of immunologic sensitization between IT+ and IT− groups as measured by PRA, we examined waiting times and the interval between the non-renal transplant and kidney transplant as surrogate markers that might represent immunologically complex patients for which compatible donors were less likely to become available (Table 1). Wait list time was longer for patients who received induction therapy; however, this only reached statistical significance for KALu (IT+ = 366±405, IT− = 250±237 days, P<0.05). The length of time between transplants was longer among IT+ KALu (7.7±3.1 versus 6.6±2.9 years, P=0.01) and IT+ KALi (4.8±3.7 versus 3.4±3.4 years, P<0.001) compared with IT− recipients. The difference among IT+ and IT− recipients with regard to wait list time or time interval between non-renal transplant and renal transplant was not associated with an increased number of living donors in the IT+ groups (Table 1).
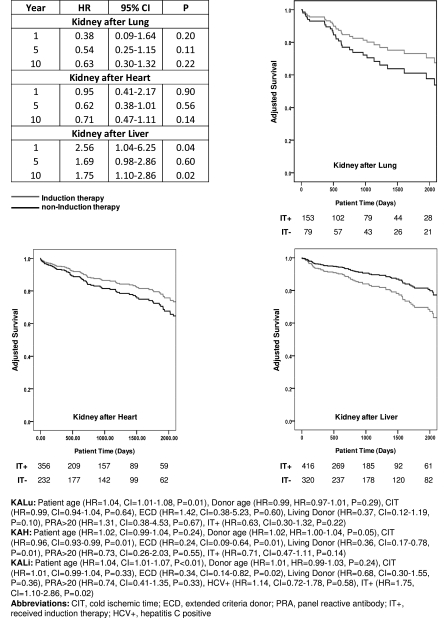
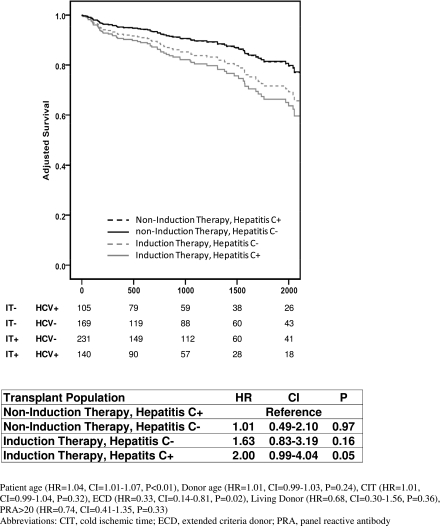
Multivariable regression modeling of patient survival did not demonstrate a significant difference at 1, 5, or 10 years between IT+ and IT− kidney recipients of a prior lung or heart transplant. However, induction therapy was associated with an increased risk of 1-year (hazard ratio [HR], 2.56; confidence interval [CI], 1.04–6.25; P=0.04) and 10-year patient mortality (HR, 1.75; CI, 1.10–2.86; P=0.02) among KALi recipients (Figure 2). To determine if hepatitis C might account for the increased risk of mortality among IT+ liver recipients, we created adjusted models in which KALi recipients were stratified by hepatitis C status and use of induction therapy. HCV+ IT+ recipients had inferior survival compared with HCV+ IT− recipients (HR, 2.0; CI, 0.99–4.04; P=0.05) and the outcome of HCV−IT+ recipients also was inferior compared with HCV−IT− recipients, although this did not quite reach statistical significance (HR, 1.75; CI, 0.94–3.25; P=0.08). These results suggest that HCV status did not account for differences in survival of the IT+ and IT− populations (Figure 3).
Figure 2.
Adjusted hazard model comparing patient survival between induction therapy and non-induction (reference) therapy populations within the setting of kidney after lung, heart, or liver transplantation. Graphs represent adjusted patient survival with the number of remaining patients displayed below. The 10-year model is presented as a footnote.
Figure 3.
Adjusted patient survival of kidney after liver transplantation stratified by hepatitis C status and induction therapy. Graph represents adjusted patient survival with the number of remaining patients displayed below. The model is presented as a footnote.
Although induction therapy did not seem to influence the survival of KALu and KAH recipients, we investigated whether induction therapy was related to acute rejection within the first year after kidney transplant. Our results showed that 8.7% of KALu IT+ recipients were treated for acute rejection within 1 year, compared with 17.9% in IT− recipients (P=0.09). KAH recipients showed a similar trend toward less rejection in the IT+ recipients, with 9.6% IT+ and 16.3% IT− being treated for acute rejection within 1 year after kidney transplant (P=0.06). Despite a trend toward decreased rejection among IT+ KALu and KAH recipients, 5-year renal allograft survival for IT+ and IT− KALu was 64.6% and 58.1% (P=0.46) and 68.1% and 57.8% for KAH (P=0.19), respectively. Among KALi recipients, 14.5% KALi IT+ and 11.9% IT− recipients were treated for acute rejection within 1 year of the kidney transplant (P=0.41) with similar 5-year allograft survival (62.9% and 70.6%; P=0.09), respectively.
Discussion
As kidney transplantation after non-renal solid organ transplantation becomes more common, clinicians are increasingly confronting decisions about the optimal medical management of this complex group of patients. Recent publications emphasize the growing number of kidney wait list patients with a prior non-renal transplant and the survival benefit of a kidney transplant (10–12). The identification of key issues in the clinical management of this complex group will help to improve patient and graft survival. Little guidance is available concerning the use of induction therapy in this population. We conducted this study to determine if induction immunosuppression therapy imparts a substantial benefit to kidney and patient survival in patients with a prior non-renal transplant.
Previous studies of kidney transplant recipients without a prior non-renal transplant reveal the risk–benefit tradeoffs of antibody induction therapy, although the generalizability of these findings to individuals with a prior non-renal transplant is unclear. Substantial evidence demonstrates that induction therapy decreases the incidence of acute rejection, may improve short term allograft survival, and reduce the incidence of delayed graft function (13,14,16,18). However, there is little evidence that induction therapy improves long-term kidney survival (19,20). A body of data suggests that the decreased incidence of acute rejection and improvement in graft survival associated with induction therapy may preferentially benefit kidney transplant candidates with a high PRA level and other populations at greater risk of rejection (15,21,22). Induction therapy at the time of renal transplantation has been recommended by Kidney Disease Improving Global Outcomes guidelines, although this is not without controversy (23). Unfortunately, most of these studies did not include a substantial number of patients with a prior non-renal organ transplant.
Despite a lack of data, the use of induction therapy at the time of kidney transplantation in recipients of a prior non-renal transplant is increasing. Our results showed that 78% of KALu, 86% of KAH, and 73% of KALi received induction therapy at the time of kidney transplant. This finding reflects trends among kidney transplant recipients without a prior non-renal transplant, in which induction therapy increased to 72% of all recipients in 2003 compared with 46% of recipients in 1995 (18). Our survival analyses demonstrated that induction therapy was not associated with better patient or graft survival in KALu and KAH, even though the incidence of rejection within the first year of transplant was lower among those who received induction. However, these results should be interpreted with caution given the limited power and wide confidence intervals. In the setting of kidney after liver transplantation, however, induction was associated with inferior short- and long-term patient survival but no difference in the risk of rejection within the first year after transplant. It is concerning that these potent immunosuppressives are finding greater acceptance without evidence of benefit, and perhaps increased risk for recipients with a prior liver transplant.
The use of induction therapy at the time of liver or kidney transplantation among patients with hepatitis C is controversial. The effect of these intense immunosuppressive medications on viral activity and progression of disease in the transplanted or native liver remains uncertain. We conducted a stratified analysis to determine if hepatitis C might account for decreased survival among liver recipients who subsequently undergo kidney transplantation. Our findings indicate that hepatitis C status was not associated with a significantly increased risk of mortality. Both HCV+ and HCV− recipients tended to have inferior survival with the use of induction (Figure 3). Among liver transplant recipients with hepatitis C, some studies have shown that induction therapy has no effect on disease progression, whereas others have found a harmful effect (24–28). A more recent analysis of the UNOS database provides evidence of a small survival benefit with induction therapy regardless of recipient HCV status (29). Among kidney transplant recipients who have not undergone prior non-renal transplant, immunosuppression increased HCV replication, although recent studies have shown relatively slow progression of liver fibrosis (30–33). Despite conflicting data, most studies show worse graft and patient survival in HCV+ compared with HCV− kidney recipients with higher rates of infections and death among the HCV+ kidney recipients (34–37). In contrast to our findings among KALi, a recent analysis of UNOS data found that the use of antibody induction in kidney recipients with HCV+ serology did not negatively affect survival (38).
One of the primary justifications for the use of induction therapy in kidney transplant patients, who have previously received a solid organ, is the increased risk of rejection associated with allosensitization. Our analysis suggests that, in general, sensitization among previous non-renal transplant recipients as measured by the PRA level is limited. A majority of patients had panel reactive antibody levels <20%. Levels of allosensitization seem to be similar to the entire kidney wait list, but lower than patients with isolated renal failure awaiting a repeat kidney transplant (11). Given that most non-renal transplant patients who undergo subsequent renal transplant are not highly sensitized, the benefit of induction therapy may be overestimated. The potential for infections and malignancies (39,40) and increased cost should be considered in the decision to use induction therapy for renal transplantation after a non-renal transplant.
Our study has several limitations. Because these data are from a national registry, unmeasured clinical attributes may produce residual confounding. For example, patient attributes such as opportunistic infections or malignancies identified before the kidney transplant—but that would influence the decision to use induction therapy at the time of kidney transplant—are not well captured by the UNOS registry. An additional limitation was our analysis of lymphocyte-depleting and nondepleting agents as a single exposure due to small cohort size. Induction agents are recognized to have different mechanisms, efficacy, and safety profiles. Lastly, because of the relatively small sample size, in particular KALu and KAH, the study had limited statistical power to detect a significant (P<0.05) or clinically meaningful difference between induction therapy groups. As such, a type II error was possible.
In conclusion, induction therapy in KAH and KALu may not affect graft or patient survival. In contrast, induction therapy in KALi was associated with decreased short- and long-term patient survival. Taking into account the increasing number of kidney transplants after the transplantation of a non-renal organ, the potential complications, and expense associated with induction therapy, its use in this patient subset deserves further scrutiny.
Disclosures
None.
Acknowledgments
J.C. was supported by an American Society of Transplantation–Roche Presidential Student Mentor Award. M.H.L. was supported by NIH Career Development Award K08-1K08DK092282-01. P.P.R. was supported by NIH Career Development Award K23-DK078688-01. This work was supported in part by Health Resources and Services Administration Contract 231-00-0115.
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Chandrakantan A, de Mattos AM, Naftel D, Crosswy A, Kirklin J, Curtis JJ: Increasing referral for renal transplant evaluation in recipients of nonrenal solid-organ transplants: A single-center experience. Clin J Am Soc Nephrol 1: 832–836, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bennett WM, DeMattos A, Meyer MM, Andoh T, Barry JM: Chronic cyclosporine nephropathy: The Achilles’ heel of immunosuppressive therapy. Kidney Int 50: 1089–1100, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Lindelöw B, Bergh CH, Herlitz H, Waagstein F: Predictors and evolution of renal function during 9 years following heart transplantation. J Am Soc Nephrol 11: 951–957, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Hamour IM, Omar F, Lyster HS, Palmer A, Banner NR: Chronic kidney disease after heart transplantation. Nephrol Dial Transplant 24: 1655–1662, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM: Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 349: 931–940, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Al Riyami D, Alam A, Badovinac K, Ivis F, Trpeski L, Cantarovich M: Decreased survival in liver transplant patients requiring chronic dialysis: A Canadian experience. Transplantation 85: 1277–1280, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Alam A, Badovinac K, Ivis F, Trpeski L, Cantarovich M: The outcome of heart transplant recipients following the development of end-stage renal disease: Analysis of the Canadian Organ Replacement Register (CORR). Am J Transplant 7: 461–465, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Gupta J, Amaral S, Mahle WT: Renal transplantation after previous pediatric heart transplantation. J Heart Lung Transplant 27: 217–221, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Coopersmith CM, Brennan DC, Miller B, Wang C, Hmiel P, Shenoy S, Ramachandran V, Jendrisak MD, Ceriotti CS, Mohanakumar T, Lowell JA: Renal transplantation following previous heart, liver, and lung transplantation: An 8-year single-center experience. Surgery 130: 457–462, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Lonze BE, Warren DS, Stewart ZA, Dagher NN, Singer AL, Shah AS, Montgomery RA, Segev DL: Kidney transplantation in previous heart or lung recipients. Am J Transplant 9: 578–585, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Cassuto JR, Reese PP, Sonnad S, Bloom RD, Levine MH, Olthoff KM, Shaked A, Naji A, Abt P: Wait list death and survival benefit of kidney transplantation among nonrenal transplant recipients. Am J Transplant 10: 2502–2511, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Srinivas TR, Stephany BR, Budev M, Mason DP, Starling RC, Miller C, Goldfarb DA, Flechner SM, Poggio ED, Schold JD: An emerging population: Kidney transplant candidates who are placed on the waiting list after liver, heart, and lung transplantation. Clin J Am Soc Nephrol 5: 1881–1886, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szczech LA, Berlin JA, Feldman HI. Anti-Lymphocyte Antibody Induction Therapy Study Group: The effect of antilymphocyte induction therapy on renal allograft survival. A meta-analysis of individual patient-level data. Ann Intern Med 128: 817–826, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Thibaudin D, Alamartine E, de Filippis JP, Diab N, Laurent B, Berthoux F: Advantage of antithymocyte globulin induction in sensitized kidney recipients: A randomized prospective study comparing induction with and without antithymocyte globulin. Nephrol Dial Transplant 13: 711–715, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Charpentier B, Rostaing L, Berthoux F, Lang P, Civati G, Touraine JL, Squifflet JP, Vialtel P, Abramowicz D, Mourad G, Wolf P, Cassuto E, Moulin B, Rifle G, Pruna A, Merville P, Mignon F, Legendre C, Le Pogamp P, Lebranchu Y, Toupance O, Hurault De Ligny B, Touchard G, Olmer M, Purgus R, Pouteil-Noble C, Glotz D, Bourbigot B, Leski M, Wauters JP, Kessler M: A three-arm study comparing immediate tacrolimus therapy with antithymocyte globulin induction therapy followed by tacrolimus or cyclosporine A in adult renal transplant recipients. Transplantation 75: 844–851, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Goggins WC, Pascual MA, Powelson JA, Magee C, Tolkoff-Rubin N, Farrell ML, Ko DS, Williams WW, Chandraker A, Delmonico FL, Auchincloss H, Cosimi AB: A prospective, randomized, clinical trial of intraoperative versus postoperative thymoglobulin in adult cadaveric renal transplant recipients. Transplantation 76: 798–802, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Ranney DN, Englesbe MJ, Muhammad W, Al-Holou SN, Park JM, Pelletier SJ, Punch JD, Lynch RJ: Should heart, lung, and liver transplant recipients receive immunosuppression induction for kidney transplantation? Clin Transplant 24: 67–72, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Meier-Kriesche HU, Li S, Gruessner RW, Fung JJ, Bustami RT, Barr ML, Leichtman AB: Immunosuppression: Evolution in practice and trends, 1994-2004. Am J Transplant 6: 1111–1131, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Cantarovich M, Durrbach A, Hiesse C, Ladouceur M, Benoit G, Charpentier B: 20-year follow-up results of a randomized controlled trial comparing antilymphocyte globulin induction to no induction in renal transplant patients. Transplantation 86: 1732–1737, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Gill JS, Johnston O, Rose CL, Landsberg D: Are there any benefits to using depleting antibodies in low risk kidney transplant recipients [Abstract]? Am J Transplant 8[suppl 2]: 215, 2008 [Google Scholar]
- 21.Hammond EB, Taber DJ, Weimert NA, Egidi MF, Bratton CF, Lin A, McGillicuddy JW, Chavin KD, Baliga PK: Efficacy of induction therapy on acute rejection and graft outcomes in African American kidney transplantation. Clin Transplant 24: 40–47, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Augustine JJ, Poggio ED, Heeger PS, Hricik DE: Preferential benefit of antibody induction therapy in kidney recipients with high pretransplant frequencies of donor-reactive interferon-γ enzyme-linked immunosorbent spots. Transplantation 86: 529–534, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group: KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 9[Suppl 3]: S1–S155, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Klintmalm GBG, Washburn WK, Rudich SM, Heffron TG, Teperman LW, Fasola C, Eckhoff DE, Netto GJ, Katz E: Corticosteroid-free immunosuppression with daclizumab in HCV(+) liver transplant recipients: 1-year interim results of the HCV-3 study. Liver Transpl 13: 1521–1531, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Nair S, Loss GE, Cohen AJ, Eason JD: Induction with rabbit antithymocyte globulin versus induction with corticosteroids in liver transplantation: Effect on recurrent hepatitis C virus infection. Transplantation 81: 620–622, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Eghtesad B, Fung JJ, Demetris AJ, Murase N, Ness R, Bass DC, Gray EA, Shakil O, Flynn B, Marcos A, Starzl TE: Immunosuppression for liver transplantation in HCV-infected patients: Mechanism-based principles. Liver Transpl 11: 1343–1352, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen HR, Shackleton CR, Higa L, Gralnek IM, Farmer DA, McDiarmid SV, Holt C, Lewin KJ, Busuttil RW, Martin P: Use of OKT3 is associated with early and severe recurrence of hepatitis C after liver transplantation. Am J Gastroenterol 92: 1453–1457, 1997 [PubMed] [Google Scholar]
- 28.Nelson DR, Soldevila-Pico C, Reed A, Abdelmalek MF, Hemming AW, Van der Werf WJ, Howard R, Davis GL: Anti-interleukin-2 receptor therapy in combination with mycophenolate mofetil is associated with more severe hepatitis C recurrence after liver transplantation. Liver Transpl 7: 1064–1070, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Moonka DK, Kim D, Kapke A, Brown KA, Yoshida A: The influence of induction therapy on graft and patient survival in patients with and without hepatitis C after liver transplantation. Am J Transplant 10: 590–601, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Roth D, Zucker K, Cirocco R, Burke G, Ciancio G, Esquenazi V, Swanson SJ, 3rd, Miller J: A prospective study of hepatitis C virus infection in renal allograft recipients. Transplantation 61: 886–889, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Pereira BJ, Milford EL, Kirkman RL, Levey AS: Transmission of hepatitis C virus by organ transplantation. N Engl J Med 325: 454–460, 1991 [DOI] [PubMed] [Google Scholar]
- 32.Alric L, Di-Martino V, Selves J, Cacoub P, Charlotte F, Reynaud D, Piette JC, Péron JM, Vinel JP, Durand D, Izopet J, Poynard T, Duffaut M, Rostaing L: Long-term effect of renal transplantation on liver fibrosis during hepatitis C virus infection. Gastroenterology 123: 1494–1499, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Kamar N, Rostaing L, Selves J, Sandres-Saune K, Alric L, Durand D, Izopet J: Natural history of hepatitis C virus-related liver fibrosis after renal transplantation. Am J Transplant 5: 1704–1712, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Meier-Kriesche HU, Ojo AO, Hanson JA, Kaplan B: Hepatitis C antibody status and outcomes in renal transplant recipients. Transplantation 72: 241–244, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G: Hepatitis C virus antibody status and survival after renal transplantation: Meta-analysis of observational studies. Am J Transplant 5: 1452–1461, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Rao KV, Ma J: Chronic viral hepatitis enhances the risk of infection but not acute rejection in renal transplant recipients. Transplantation 62: 1765–1769, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Legendre C, Garrigue V, Le Bihan C, Mamzer-Bruneel MF, Chaix ML, Landais P, Kreis H, Pol S: Harmful long-term effect of hepatitis C virus infection in kidney transplant recipients. Transplantation 65: 667–670, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Luan FL, Schaubel DE, Zhang H, Jia X, Pelletier SJ, Port FK, Magee JC, Sung RS: Effect of immunosuppressive regimen on survival of kidney transplant recipients with hepatitis C. Transplantation 85: 1601–1606, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Meier-Kriesche HU, Arndorfer JA, Kaplan B: Association of antibody induction with short- and long-term cause-specific mortality in renal transplant recipients. J Am Soc Nephrol 13: 769–772, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Chandraker A, Tullius SG: Induction therapy: Are we picking our battles? Clin J Am Soc Nephrol 1: 356–357, 2006 [DOI] [PubMed] [Google Scholar]