Summary
Background and objectives
Hemodialysis treatment induces markers of inflammation and oxidative stress, which could affect hemoglobin levels and the response to erythropoietin use. This study sought to determine whether high-flux dialysis would help improve markers of renal anemia, inflammation, and oxidative stress compared with low-flux dialysis.
Design, settings, participants, & measurements
In a prospective, controlled study, 221 patients undergoing maintenance hemodialysis and receiving darbepoetin-alfa treatment (mean age, 66 years; 55% male) from 19 centers were screened in a 20-week run-in period of low-flux hemodialysis with a synthetic dialysis membrane. Thereafter, 166 patients were enrolled and randomly assigned to receive a synthetic high-flux membrane or to continue on low-flux dialysis for 52 weeks. Data on myeloperoxidase, oxidized LDL, high-sensitivity C-reactive protein, and the Malnutrition Inflammation Score were collected at baseline and after 52 weeks; routine laboratory data, such as hemoglobin, ferritin, and albumin, and the use of darbepoetin-alfa, were also measured in the run-in period.
Results
After 52 weeks, the low-flux and the high-flux groups did not differ with respect to hemoglobin (mean ± SD, 11.7±0.9 g/dl versus 11.7±1.1 g/dl; P=0.62) or use of darbepoetin-alfa (mean dosage ± SD, 29.8±24.8 μg/wk versus 26.0±31.1 μg/wk; P=0.85). Markers of inflammation, oxidative stress, or nutritional status also did not differ between groups.
Conclusion
Over 1 year, high-flux dialysis had no superior effects on hemoglobin levels or markers of inflammation, oxidative stress, and nutritional status. These data do not support the hypothesis that enhanced convective toxin removal would improve patient outcome.
Introduction
Patients with CKD are prone to develop renal anemia at an early stage of the disease (1). The reduction in hemoglobin concentrations is associated with impaired quality of life and may be accompanied by reduced survival (2–5). On the other hand, large-scale studies found no mortality benefits by targeting higher hematocrit and hemoglobin levels in patients undergoing hemodialysis (6,7). The main cause of renal anemia is the reduced renal production of the hormone erythropoietin, which promotes the maturation of red blood cells in the bone marrow (8). The effects of uremic toxins on bone marrow and the blood loss associated with venipuncture and dialysis also contribute to anemia.
One important aspect of the optimization of hemodialysis treatment has been the development of dialysis membranes that eliminate larger molecules. These “high-flux” membranes also eliminate molecules of medium molecular weight that accumulate in renal failure and that have been suggested as causing adverse effects on erythropoiesis (9). However, various clinical studies aiming to demonstrate the superiority of high-flux over low-flux membranes for renal anemia have delivered conflicting results (9–13). Consequently, it remains unclear whether and how the removal of higher-molecular-weight molecules would benefit hemoglobin levels.
Inflammation is an important factor associated with renal anemia because it leads to hyporesponsiveness of erythropoiesis-stimulating agent (ESA), as indicated by significantly increased ESA dose requirements in patients with elevated C-reactive protein (CRP) levels (14,15). One trigger of inflammation is the contact between the blood and dialysis membranes during hemodialysis, which may lead to the release of cytokines, such as TNF-α, IL-6, and IL-1 (16).
Furthermore, the increased cytokine levels in dialysis patients are positively correlated with markers of oxidative stress and symptoms of malnutrition (17). Experimental and clinical studies have also indicated that oxidative stress and malnutrition are implicated in the pathogenesis of anemia (18). This finding seems to be true in clinical practice; in hemodialysis patients, a lower serum albumin level is associated with ESA hyporesponsiveness (19).
The purpose of the MINOXIS (Modulation of INflammation and OXidative stress by high-flux hemodialysIS) study was to determine in a prospective, controlled clinical trial whether high-flux dialysis helps improve renal anemia and anemia-related markers of inflammation, oxidative stress, and nutritional status.
Materials and Methods
The study was performed in accordance with the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from each participant before study entry. The study was approved by the University Ethics Committee of the University of Würzburg and was conducted according to “good clinical practice” guidelines. The trial registry number is DE-2003-5054.
Study Design and Participants
This prospective, open-label, multicenter clinical study used a centralized randomization procedure in two parallel groups. In total, 221 patients from 19 German centers were eligible to attend the screening visit. Patients with CKD who were treated by hemodialysis had to be ≥18 years of age and to have been receiving treatment for anemia with the ESA darbepoetin-alfa for at least 6 months. Incident patients undergoing hemodialysis for ≥1 or ≤3 months (i.e., those recently diagnosed) had to be treated by low-flux dialyzers. Prevalent patients undergoing hemodialysis for more than 3 months had to be treated with low-flux dialyzers for ≥3 months before inclusion in the study. Exclusion criteria were serious comorbid conditions with a life expectancy of <2 years, single-needle hemodialysis, use of a temporary or permanent catheter, planned kidney transplantation, and pregnancy.
During an initial 20-week run-in period, patients underwent hemodialysis with low-flux dialyzers to stabilize hemoglobin levels. On completion of week 20, patients were randomly assigned to one of two treatment groups: low-flux or high-flux dialysis (1:1 ratio). To be eligible for randomization, patients must have had a high-sensitivity CRP (hsCRP) level <50 mg/L and a hemoglobin concentration within the target range of 10.0–13.0 g/dl. After randomization, patients were followed up for 1 year (main study period). The total duration of the study was 2 years and 5 months (Figure 1).
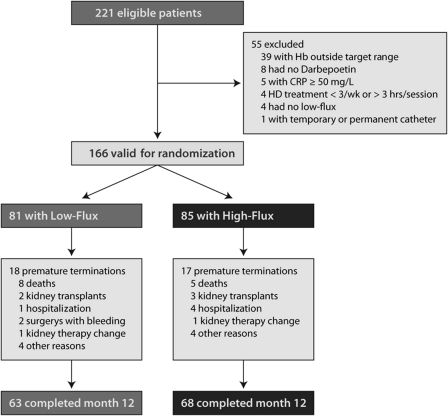
Figure 1.
Numbers of patients who entered the study, were assigned to a study group, and completed the protocol.
Anemia Therapy and Erythropoietin Resistance Index
Darbepoetin alfa administered intravenously or subcutaneously was used to treat anemia. During the study run-in period, the hemoglobin concentration had to be maintained within a range of 10.0–13.0 g/dl, with appropriate dose adjustment of darbepoetin alfa if required. If hemoglobin concentration at two successive measurements (within a 7-day period) was outside the target range of 10.0–13.0 g/dl, the darbepoetin alfa dose was adjusted in accordance with the following criteria: (1) hemoglobin concentration <10 g/dl: increase ESA dose by approximately 25%, (2) hemoglobin concentration >13.0 g/dl: reduce ESA dose by approximately 25%, (3) hemoglobin concentration >14 g/dl: immediately discontinue use of darbepoetin alfa, wait until hemoglobin concentration is again within the target range, and reinstitute darbepoetin alfa at the next lower dose level.
Blood iron status (including ferritin and transferrin saturation) was analyzed every 3 months. If patients exhibited signs of iron deficiency (serum ferritin <100 µg/L or transferrin saturation <20%), centers provided iron replacement therapy in accordance with the standard practice at the center.
As specified in the European Best Practice Guidelines, the required target range for serum ferritin was 200–500 µg/L. A value within an extended range of 200–800 µg/L was considered acceptable. If the value was outside this range, iron replacement therapy had to be immediately adjusted.
The erythropoietin resistance index (ERI) was calculated as the weekly weight-adjusted dose of ESA divided by the hemoglobin value. This index is used to identify patients not responding to ESA (those who require doses 25%–100% higher than doses for the average patient).
Study Dialyzers and Hemodialysis Treatment
In this study, synthetic polysulfone dialyzers (Fresenius Medical Care Deutschland GmbH, Bad Homburg, Germany) were used exclusively. For patients undergoing low-flux dialysis, surface areas between 1.3 and 2.4 m2 were used (Fresenius Hemoflow F6HPS to F10HPS, steam sterile). High-flux dialysis was administered with surface areas between 1.0 and 2.4 m2 (Fresenius FX50 to FX100, steam sterile). All randomly assigned patients had undergone hemodialysis before inclusion in the study, most via an arterio-venous fistula (90.1% of low-flux group and 91.8% of high-flux group). Hemodialysis treatments were performed three times per week. Most patients had undergone low-flux dialysis for >180 days before the run-in period (65.4% of low-flux group and 57.6% of high-flux group).
Data Collection
Within the first 10 weeks of the 20-week run-in period, hemoglobin, ferritin, transferrin, transferrin saturation, and iron status of patients were measured twice. During the main study period, values for hemoglobin, platelets, leukocytes, BP, any periods of hospitalization, blood transfusions, and data on ESA and iron substitution were documented every 4 weeks. Blood samples for routine analyses (e.g., albumin, hemoglobin) were measured in the local laboratories.
At the time of randomization (month 0) and at completion of the study (month 12), additional blood samples were drawn for hsCRP, albumin, and the markers of oxidative stress (oxidized LDL and myeloperoxidase). These blood samples were frozen at −20°C. HsCRP was measured by immunoturbidimetry on a Modular analyzer (Roche Diagnostics, Mannheim, Germany) using reagents and standards from Roche Diagnostics. Albumin was measured using a photometric test (Roche Diagnostics). The oxidative stress variables were measured by ELISA (Immunodiagnostic, Benshein, Germany). The coefficients of variation (CVs) were as follows: (1) Albumin—intra-assay CV, 1.1%; interassay CV, 1.7%; (2) CRP—intra-assay CV, 2.7%; interassay CV, 3.6%; (3) oxidative LDL—intra-assay CV, 4.8%; interassay CV, 8.7%; (4) myeloperoxidase—intra-assay CV, 4.4%; interassay CV, 9.3%.
A central laboratory was involved in the analysis of these samples. At months 0 and 12, the nutritional and inflammatory status of each patient was documented on the basis of the Malnutrition Inflammation Score (20). This test was developed to allow comparative assessments of individual patients. It evaluates various components, such as patient history, results of physical examination, and laboratory measures (20).
Statistical Analyses
Parametric continuous data were expressed as means ± SDs and were compared by t test. Nonparametric continuous data were expressed as median with interquartile range (IQR) and compared using the Wilcoxon rank-sum test. Categorical variables were expressed as frequency counts and percentages. The primary endpoint of the study was the effect of high-flux versus low-flux hemodialysis on hemoglobin concentration. Therefore, the intraindividual change in hemoglobin concentration between beginning (month 0) and end (month 12) of the study period was calculated and compared between the two treatment groups. Secondary endpoints were the effects of high-flux versus low-flux hemodialysis on inflammatory status, oxidative stress, and nutritional status. As was done for calculations of the primary endpoint, the intraindividual changes in albumin, hsCRP, oxidative LDL, and myeloperoxidase concentrations, as well as changes in the Malnutrition Inflammation Score, between months 0 and 12 were determined and compared between the two treatment groups.
For the analyses, a multivariate analysis for repeated measures was conducted using a general linear random-effects model (Proc Mixed in SAS). This model takes into account within-subject effects and was adjusted for age, sex, diabetes status, time trend, ESF (expressed as µg/wk), and the number of center as random effects. P≤0.05 (two-sided) was considered to represent a statistically significant difference. All statistical procedures were carried out using SAS software for Windows, version 9.1 (SAS Institute, Cary, NC).
Results
Patient Characteristics
A total of 221 patients from 19 sites were eligible to attend the screening visit. Of these patients, 166 were eligible for randomization; 131 completed the trial according to the protocol. Premature study termination was reported for 18 of the 81 patients (22.2%) undergoing low-flux dialysis and for 17 of the 85 patients (20.0%) undergoing high-flux dialysis. Eight patients in the low-flux group and five patients in the high-flux group died.
Baseline characteristics for all randomly assigned patients are given in Table 1. All characteristics were similar between the low-flux and the high-flux groups, except for a slightly higher proportion of men in the high-flux group (P=0.02). Cause of CKD was diabetic nephropathy in most cases (28.4% in the low-flux group and 37.6% in the high-flux group), followed by GN (17.3% and 16.5%, respectively).
Table 1.
Baseline characteristics of the study population
| Characteristic | Low-Flux Hemodialysis Group (n=81) | High-Flux Hemodialysis Group (n=85) | P Value |
|---|---|---|---|
| Demographic variables | |||
| mean age (yr) | 66.1±10.9 | 66.0±12.7 | 0.96 |
| age ≥ 70 yr (%) | 42.0 | 47.0 | 0.54 |
| mean height (cm) | 167±9 | 170±10 | 0.07 |
| mean weight (kg) | 76.7±16.8 | 77.4±17.5 | 0.79 |
| men (%) | 44.4 | 63.5 | 0.02 |
| mean systolic BP (mmHg) | 137±19 | 134±22 | 0.43 |
| arterio-venous fistula (%) | 90.1 | 91.8 | 0.79 |
| renal residual function > 300 ml/d (%) | 57.4 | 62.5 | 0.70 |
| Type of primary kidney disease (%) | |||
| diabetic nephropathy | 28.4 | 37.6 | 0.25 |
| glomerulonephritis | 17.3 | 16.5 | 0.68 |
| interstitial nephritis | 6.2 | 7.1 | 0.82 |
| nephrosclerosis | 8.6 | 11.8 | 0.61 |
| polycystic kidney disease | 8.6 | 4.7 | 0.36 |
| Comorbid conditions (%) | |||
| diabetes mellitus | 39.5 | 49.4 | 0.22 |
| arterial hypertension | 80.2 | 83.5 | 0.69 |
| CHD | 33.3 | 32.9 | 0.96 |
| stroke | 11.1 | 12.9 | 0.81 |
| MI | 8.6 | 10.6 | 0.80 |
| cardiac arrhythmia | 19.8 | 12.9 | 0.29 |
| CHF | 22.2 | 23.5 | 0.86 |
| Mean laboratory values | |||
| hemoglobin (g/dl) | 11.8±1.0 | 11.7±1.1 | 0.88 |
| phosphate (mmol/L) | 1.8±0.5 | 1.9±0.5 | 0.47 |
| albumin (g/dl) | 3.9±0.5 | 3.9±0.4 | 0.92 |
| hs-CRP (mg/L)a | 5.0 (3.0–10.9) | 5.0 (4.0–12.0) | 0.51 |
| urea predialytic (mg/dl) | 128±36 | 132±36 | 0.55 |
| urea postdialytic (mg/dl) | 41±14 | 44±16 | 0.39 |
| creatinine predialytic (µmol/L) | 683±241 | 720±227 | 0.34 |
| Mean hemodialysis variables | |||
| duration of hemodialysis (hr) | 4.2±0.45 | 4.2±0.51 | 0.98 |
| rate of blood flow (ml/min) | 295±38 | 294±41 | 0.82 |
| rate of dialysate flow (ml/min) | 497±22 | 498±22 | 0.99 |
| UF volume (ml) | 2378±1144 | 2495±1161 | 0.53 |
| Concomitant medication (%) | |||
| antihypertensives | 84.0 | 85.9 | 0.83 |
| statins (HMG-CoA reductase inhibitor) | 37.0 | 24.7 | 0.10 |
| ASS | 38.3 | 35.3 | 0.75 |
| clopidogrel | 7.4 | 8.2 | 0.78 |
| warfarin | 5 | 8 | 0.36 |
Values presented with a plus/minus sign are the mean ± SD. CHD, coronary heart disease; MI, myocardial infarction; CHF, congestive heart failure; hs-CRP, high-sensitivity C-reactive protein; UF, ultrafiltration; HMG-CoA, hydroxymethyl glutaryl coenzyme A; ASS, acetylsalicylic acid.
Median (interquartile range).
Renal Anemia and ERI
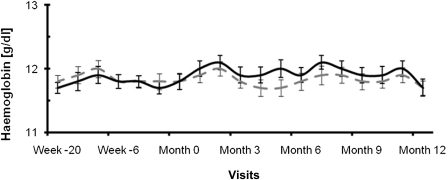
Patients randomly assigned to low-flux dialysis showed mean hemoglobin values of 11.8±1.0 g/dl at baseline and 11.7±0.9 g/dl at the end of the study. In the high-flux dialysis group, the mean hemoglobin was 11.8±1.1 g/dl at baseline and 11.7±1.1 g/dl at the end of the study. The change in hemoglobin did not significantly differ between treatment groups (P=0.62; Figure 2).
Figure 2.
Hemoglobin levels (g/dl) of the patients undergoing low-flux (dotted curve) and high-flux (solid curve) hemodialysis.
In the low-flux group, the mean ESA dosage was 29.3±22.6 μg/wk at baseline and 29.8±24.8 μg/wk at the end of the study. The corresponding values in the high-flux group were 29.6±28.9 and 26.0±31.1 μg/wk, respectively. There was no difference in the change in ESA dosage between the two groups (P=0.85).
At the time of randomization, 63.0% of the patients using low-flux dialysis required iron replacement therapy, and this proportion did not change during the study (63.0%). In the patients who underwent high-flux dialysis, the percentage of patients receiving iron preparations was 67.1% at the time of randomization and 60.3% at the end of the study. The change in the proportion of patients using iron replacement therapy did not differ between the high-flux and the low-flux groups (P=0.59).
In the low-flux dialysis group, the median iron dosages were 50 (IQR, 40–120) mg/wk at the time of randomization and 62.5 (IQR, 40–120) mg/wk at the end of the study. The corresponding values for the high-flux group were 62.5 (IQR, 40– 120) mg/wk at the time of randomization and 40 (IQR, 31–120) mg/wk at the end of the study. There was no difference in the change in iron dose between the two groups (P=0.53).
Mean ERI values were 7.1 IU/kg per week per g/dl at the time of randomization in both study groups. At the end of the study, the mean ERI levels were 7.1 and 6.3 IU/kg per week per g/dl in the low- and the high-flux groups, respectively. The change in ERI did not differ between the two groups (P=0.60).
Secondary Endpoints: Oxidative Stress, Inflammation, and Nutritional Status
In patients undergoing low-flux dialysis, the median oxidative LDL values were 647 (IQR, 488–925) ng/ml at the start of the study and 322 (IQR 226–448) ng/ml after 12 months. In the high-flux group, the median oxidative LDL values were 577 (IQR, 374–830) ng/ml at the beginning and 311 (IQR, 232–412) ng/ml after 12 months. The absolute median values of the differences between month 0 and month 12 were −291 (IQR, −570 to −32) ng/ml in the low-flux group and −314 (IQR, −565 to −2) ng/ml in the high-flux group (P=0.72).
Patients undergoing low-flux dialysis showed median myeloperoxidase levels of 19 (IQR, 13–34) ng/ml at the time of randomization and 21 (IQR, 15–41) ng/ml after 12 months. The corresponding values for patients using high-flux dialysis were 20 (IQR, 14–42) ng/ml at the start of the study and 21 (IQR, 15–41) ng/ml at month 12. The change in myeloperoxidase did not differ between the two groups (P=0.50).
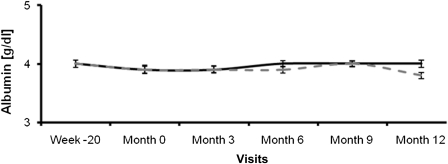
Serum albumin as an indicator for nutritional state and chronic inflammation was documented every 3 months during the main study phase. Both groups started at baseline with a mean value of 3.9 g/dl. At the end of the study, the mean albumin levels were 3.8 g/dl in the low-flux group and 4.0 g/dl in the high-flux group. There was no difference in the change in albumin level between the two groups (P=0.26) (Figure 3).
Figure 3.
Albumin levels (g/dl) of the patients undergoing low-flux (dotted curve) and high-flux (solid curve) hemodialysis.
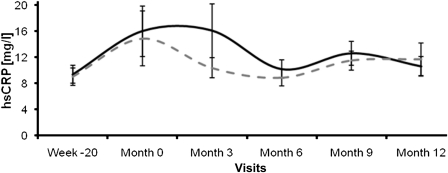
The median hsCRP values at baseline were 5.0 (IQR, 3–11) mg/L in patients undergoing low-flux dialysis and 5.0 (IQR, 4–12) mg/l in patients undergoing high-flux dialysis. After 12 months, hsCRP values were 5.8 (IQR, 3–11) and 5.0 (IQR, 4–12) mg/l, respectively (Figure 4), respectively. Again, the changes in hsCRP were not different between the groups (P=0.74).
Figure 4.
High-sensitivity C-reactive protein levels (mg/L) of the patients undergoing low-flux (dotted curve) and high-flux (solid curve) hemodialysis.
The mean total Malnutrition Inflammation Scores at baseline were 10.6±3.2 in the low-flux group and 10.3±2.9 in the high-flux group. After 12 months, the corresponding values were 11.1±3.3 and 10.6±2.9, respectively. The absolute mean values of the differences between baseline and 12 months were 1.0±3.0 in patients undergoing low-flux dialysis and 0.5±2.2 in patients undergoing high-flux dialysis (P=0.59).
Discussion
The aim of this randomized, multicenter clinical study was to compare the effects of low-flux versus high-flux hemodialysis on hemoglobin concentrations in patients with ESRD treated by hemodialysis. We hypothesized that with high-flux dialysis, additional removal of medium-molecular-weight molecules, which are presumed to exert adverse effects on erythropoiesis, would help improve hemoglobin levels. Potential advantages of high-flux dialysis on inflammatory status, oxidative stress, and nutritional status were the secondary objectives of this study.
The primary endpoint, hemoglobin, remained stable within the range of 11.7–12.0 g/dl in the low-flux group and 11.7–12.1 g/dl in the high-flux group. Of note, these values meet the current recommendations of the European Renal Best Practice Guidelines committee (21). Hemoglobin did not significantly change in either treatment group. Furthermore, the doses of darbepoetin-alfa and iron were not different between and within the groups. Similarly, in a prospective, randomized crossover study in 25 patients undergoing hemodialysis, no association was found between hematocrit levels and the use of high-flux or low-flux dialysis (13). A major limitation of that study was the small sample size and the short observation period (only 8 weeks) (13).
Our findings are in line with the results of a shorter-term study from Locatelli and colleagues (12). In that multicenter randomized, controlled trial the investigators could not detect differences in hemoglobin levels or ESA doses between patients treated for 3 months with a high-flux membrane compared with those treated with a standard (i.e., low-flux) membrane (12). As was seen in our study, patients in the study by Locatelli and colleagues were all adequately dialyzed and had no iron deficiency. It could be argued that it might take >3 months for any beneficial effect of high-flux dialysis on hemoglobin levels to appear; however, our study clearly shows that even with a considerably longer treatment duration of 1 year, no differences between high and low flux were detectable. Furthermore, in a prospective cohort study with a subset of 1207 patients from the Japanese Dialysis Outcomes and Practice Patterns phase II study, high-flux dialysis membranes compared with low-flux membranes had also no effect on anemia or erythropoietin dosage in hemodialysis patients, even during a 2-year follow-up period (11).
However, our negative results are in contrast with some other reports (9,10). In those studies the investigators found a positive effect on hemoglobin levels between patients treated with a high-flux membrane and those undergoing standard dialysis. These studies had several limitations, however. In the randomized study from Ayli and coworkers, the sample size seemed to be rather small (the study included only 48 patients) with a study period of only 6 months (9). In addition, the study patients were anemic (hemoglobin <11 g/dl) and did not respond to ESA therapy (9). In contrast, in our study, 131 patients (63 undergoing low-flux and 68 undergoing high-flux dialysis) completed the trial according to the protocol and were studied for 1 year. The patients were not anemic, had no iron deficiency, and were mostly well nourished.
In the prospective but nonrandomized study from the European Clinical Dialysis Database, a large network of European dialysis centers, patients were switched from low-flux to high-flux dialysis for 6 months (10). Those investigators found favorable effects on hemoglobin levels and inflammation status with high-flux dialysis compared with low-flux dialysis but no effect on ESA dosage (10). Again, the observation period (only 6 months) might have been too short to allow a reliable conclusion to be drawn regarding differences in hemoglobin levels.
The secondary endpoint variables—inflammation, oxidative stress, and nutritional status—were measured by a central laboratory. These measures have a predictive value with regard to cardiovascular mortality in hemodialysis patients (22–24). Comparative analyses did not demonstrate a difference between low-flux dialysis and high-flux dialysis for the levels of myeloperoxidase, oxidative LDL, or hsCRP or for Malnutrition Inflammation Score after 52 weeks of treatment. Evaluation of the Malnutrition Inflammation Score resulted in a relatively low summary score, and most patients were given a single point in almost every evaluated category. This represents a favorable finding for the patients' nutritional and inflammation status. No differences were observed between the start and the end of the main study period or between patients using low-flux and those using high-flux dialysis. Of note, albumin levels were numerically higher in the high-flux group, but the difference was not statistically significant. Intriguingly, in the myeloperoxidase study, diabetic patients with albumin levels <4 g/dl had better survival when they were treated with high-flux dialysis compared with those on low-flux dialysis (25). Furthermore, secondary analysis of the Hemodialysis (HEMO) study demonstrated that high-flux dialysis is associated with a trend toward a smaller decrease in albumin levels over time (26). Our results regarding inflammation and oxidative stress were similar to those of a prospective randomized trial with 40 patients undergoing hemodialysis (27). In that study, high-flux dialysis membranes were not associated with a better inflammatory or oxidative profile than the low-flux dialysis membranes, although the latter significantly improved insulin resistance (27). However, a major limitation in that investigation was the very short observation period (only 2 months) and the relatively small sample size of 40 patients.
In summary, we found no favorable effects with regard to the primary and secondary endpoints for high-flux membranes. However, as mentioned before, there might be a potential benefit for highly selected patients, as seen in the myeloperoxidase study. Of note, in MINOXIS, ultrapure dialysate was used in the majority of patients (77.8% of the low-flux group and 76.5% of the high-flux group), which might have contributed to a decreased inflammatory status and therefore lessened the potential benefit of high-flux dialysis membranes in reducing inflammatory markers.
Our study has several limitations. First, the population size was relatively small, although to date the MINOXIS trial is the largest randomized, controlled trial investigating hemoglobin levels in conjunction with markers of oxidative stress and inflammation. The primary and secondary endpoints did not differ between study groups, but the effect sizes were minimal. Therefore, clinically relevant differences are not expected to be detected by an increase in sample size. Finally, not all patients completed the study. However, the drop-out rate was similar in both groups. Randomization to high- or low-flux dialysis in our study did not result in differences in inflammation, oxidative stress, or any changes in hemoglobin levels. These data do not support the hypothesis of a beneficial association of enhanced convective toxin removal and improved patient outcome. Altogether, the effect of a high-flux membrane is much less than might be expected from other studies.
Disclosures
None.
Acknowledgments
We thank all patients who participated in the MINOXIS Study. We are grateful to all investigators, study nurses, and collaborators involved in patient recruitment and sample and data handling.
A.S. is supported by a European Renal Association–European Dialysis and Transplant Association long-term fellowship. The study was supported by Amgen GmbH, Munich, Germany, and Fresenius Medical Care Deutschland GmbH, Bad Homburg, Germany, with a grant to the institution.
MINOXIS study investigators: Peter Steinbeck, Dialysezentrum; Michael Henk, Dialysepraxis-Rheinbach; Martina Graupner, Gemeinschaftspraxis; Jörg Stammkötter, Dialysepraxis; Thomas Klein, Gemeinschaftspraxis; Carsten Brockmann, Dialysezentrum Bad Bevensen; Steffen Hengst, PHV Alsfeld/Lauterbach; Johannes Bunia, Dialysezentrum; Gottfried Walker, Dialysezentrum-Pirmasens; Kai Meßtorff, Dialysepraxis Stade–Zentrum; Georg Fuchs, Dialysezentrum Neckarsulm; Joachim Lippert, Dialysepraxis Cochem; Karsten Schumann, Dialysezentrum Lünen-Brambauer; Andreas Morawietz, M. Dialyse Schwerin-Lankow; Dieter Brückner, KfH Dortmund; Hans-Joachim Müller, Städtisches Klinikum Fulda; Jörg Schletter, Gemeinschaftspraxis; Alexander Schischma, Gemeinschaftspraxis; and Stefan Hofebauer, Dialysepraxis.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Voormolen N, Grootendorst DC, Urlings TA, Boeschoten EW, Sijpkens YW, Huisman RM, Krediet RT, Dekker FW: Prevalence of anemia and its impact on mortality and hospitalization rate in predialysis patients. Nephron Clin Pract 115: c133–c141, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Auer J, Oliver DO, Winearls CG: The quality of life of dialysis patients treated with recombinant human erythropoietin. Scand J Urol Nephrol Suppl 131: 61–65, 1990 [PubMed] [Google Scholar]
- 3.Delano BG: Improvements in quality of life following treatment with r-HuEPO in anemic hemodialysis patients. Am J Kidney Dis 14[Suppl 1]: 14–18, 1989 [PubMed] [Google Scholar]
- 4.Ofsthun N, Labrecque J, Lacson E, Keen M, Lazarus JM: The effects of higher hemoglobin levels on mortality and hospitalization in hemodialysis patients. Kidney Int 63: 1908–1914, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Avram MM, Blaustein D, Fein PA, Goel N, Chattopadhyay J, Mittman N: Hemoglobin predicts long-term survival in dialysis patients: A 15-year single-center longitudinal study and a correlation trend between prealbumin and hemoglobin. Kidney Int Suppl S6–S11, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D: Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. J Am Soc Nephrol 16: 2180–2189, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Eschbach JW: The anemia of chronic renal failure: Pathophysiology and the effects of recombinant erythropoietin. Kidney Int 35: 134–148, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Ayli D, Ayli M, Azak A, Yüksel C, Kosmaz GP, Atilgan G, Dede F, Abayli E, Camlibel M: The effect of high-flux hemodialysis on renal anemia. J Nephrol 17: 701–706, 2004 [PubMed] [Google Scholar]
- 10.Merello Godino JI, Rentero R, Orlandini G, Marcelli D, Ronco C: Results from EuCliD (European Clinical Dialysis Database): Impact of shifting treatment modality. Int J Artif Organs 25: 1049–1060, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama H, Kawaguchi T, Wada T, Takahashi Y, Higashi T, Yamazaki S, Fukuhara S, Akiba T, Akizawa T, Asano Y, Kurokawa K, Saito A. J-DOPPS Research Group: Biocompatibility and permeability of dialyzer membranes do not affect anemia, erythropoietin dosage or mortality in Japanese patients on chronic non-reuse hemodialysis: A prospective cohort study from the J-DOPPS II study. Nephron Clin Pract 109: c100–c108, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Locatelli F, Andrulli S, Pecchini F, Pedrini L, Agliata S, Lucchi L, Farina M, La Milia V, Grassi C, Borghi M, Redaelli B, Conte F, Ratto G, Cabiddu G, Grossi C, Modenese R: Effect of high-flux dialysis on the anaemia of haemodialysis patients. Nephrol Dial Transplant 15: 1399–1409, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Opatrný K, Jr, Reischig T, Vienken J, Eiselt J, Vít L, Opatrná S, Sefrna F, Racek J, Brown GS: Does treatment modality have an impact on anemia in patients with chronic renal failure? Effect of low- and high-flux biocompatible dialysis. Artif Organs 26: 181–188, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K, McAllister CJ, Lehn RS, Lee GH, Nissenson AR, Kopple JD: Effect of malnutrition-inflammation complex syndrome on EPO hyporesponsiveness in maintenance hemodialysis patients. Am J Kidney Dis 42: 761–773, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Bárány P, Divino Filho JC, Bergström J: High C-reactive protein is a strong predictor of resistance to erythropoietin in hemodialysis patients. Am J Kidney Dis 29: 565–568, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann U, Fischereder M, Marx M, Schweda F, Lang B, Straub RH, Krämer BK: Induction of cytokines and adhesion molecules in stable hemodialysis patients: Is there an effect of membrane material? Am J Nephrol 23: 442–447, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Stenvinkel P: Anaemia and inflammation: What are the implications for the nephrologist? Nephrol Dial Transplant 18[Suppl 8]: viii17-22, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Locatelli F, Canaud B, Eckardt KU, Stenvinkel P, Wanner C, Zoccali C: Oxidative stress in end-stage renal disease: An emerging threat to patient outcome. Nephrol Dial Transplant 18: 1272–1280, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Locatelli F, Andrulli S, Memoli B, Maffei C, Del Vecchio L, Aterini S, De Simone W, Mandalari A, Brunori G, Amato M, Cianciaruso B, Zoccali C: Nutritional-inflammation status and resistance to erythropoietin therapy in haemodialysis patients. Nephrol Dial Transplant 21: 991–998, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH: A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 38: 1251–1263, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Locatelli F, Aljama P, Canaud B, Covic A, De Francisco A, Macdougall IC, Wiecek A, Vanholder R. Anaemia Working Group of European Renal Best Practice (ERBP): Target haemoglobin to aim for with erythropoiesis-stimulating agents: A position statement by ERBP following publication of the Trial to reduce cardiovascular events with Aranesp therapy (TREAT) study. Nephrol Dial Transplant 25: 2846–2850, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Kovesdy CP, Kalantar-Zadeh K: Review article: Biomarkers of clinical outcomes in advanced chronic kidney disease. Nephrology (Carlton) 14: 408–415, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wanner C, Metzger T: C-reactive protein a marker for all-cause and cardiovascular mortality in haemodialysis patients. Nephrol Dial Transplant 17[Suppl 8]: 29–32, discussion 39–40, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Brennan ML, Hazen SL: Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am J Kidney Dis 48: 59–68, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Locatelli F, Martin-Malo A, Hannedouche T, Loureiro A, Papadimitriou M, Wizemann V, Jacobson SH, Czekalski S, Ronco C, Vanholder R. Membrane Permeability Outcome (MPO) Study Group: Effect of membrane permeability on survival of hemodialysis patients. J Am Soc Nephrol 20: 645–654, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R. Hemodialysis (HEMO) Study Group: Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347: 2010–2019, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Chu PL, Chiu YL, Lin JW, Chen SI, Wu KD: Effects of low- and high-flux dialyzers on oxidative stress and insulin resistance. Blood Purif 26: 213–220, 2008 [DOI] [PubMed] [Google Scholar]