Summary
Background and objectives
This study aimed to determine whether opening a new clinic in a remote region would be a cost-effective means of improving care for remote-dwellers with CKD.
Design, setting, participants, & measurements
This study is a cost-utility analysis from a public payer’s perspective over a lifetime horizon, using administrative data from a large cohort of adults with stage 3b-4 CKD in Alberta, Canada. The association between the distance from each simulated patient’s residence and the practice location of the closest nephrologist and clinical outcomes (quality of care, hospitalization, dialysis, and death) were examined. A Markov 6-month cycle economic decision model was analyzed; estimates of the effect of a new clinic were based on the association between residence location, resource use, and outcomes. Costs are reported in 2009 Canadian dollars.
Results
The costs for equipping and operating a clinic for 321 remote-dwelling patients were estimated at $25,000 and $250,000/yr, respectively. The incremental cost-utility ratios (ICURs) ranged from $4000 to $8000/quality-adjusted life-year under most scenarios. However, if reducing distance to nephrologist care does not alter mortality or hospitalization among remote-dwellers, the cost-effectiveness becomes less attractive. All other one-way sensitivity analyses had negligible effects on the ICUR.
Conclusions
Given the low costs of equipping and operating new clinics, and the very attractive ICUR relative to other currently funded interventions, establishing new clinics for remote-dwellers could play an important role in efficiently improving outcomes for patients with CKD. High-quality controlled studies are required to confirm this hypothesis.
Introduction
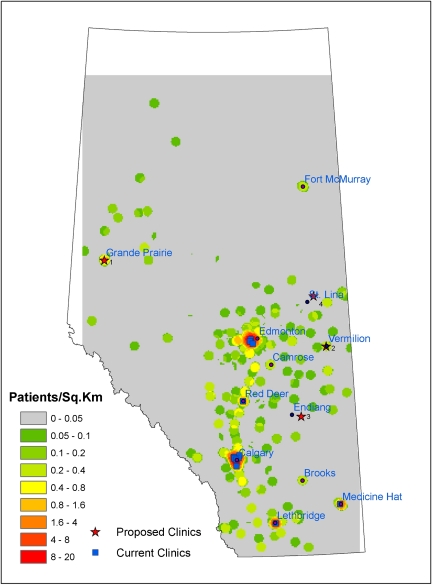
The province of Alberta, located in western Canada, is home to 3.5 million people, spread out across 660,000 km2. Although kidney disease occurs throughout the province, specialized renal care is provided in 17 clinics that are predominantly located in the more densely populated southern half of Alberta (Figure 1). Multiple studies (1–9) have shown that distance from health services is inversely associated with clinical benefits. We recently showed (10) that markers of good-quality care in CKD patients in Alberta decreased with increasing distance from the practice location of the closest nephrologist. Remote-dwellers with CKD were less likely to visit a nephrologist after a CKD diagnosis (estimated GFR <45 ml/min per 1.73 m2); those with concomitant diabetes were less likely to have hemoglobin A1c measured, to have urinary albumin assessed, or to receive recommended treatment with BP medications. Remote-dwellers with CKD were also more likely to die or be hospitalized than otherwise comparable urban-dwellers, regardless of whether diabetes was present.
Figure 1.
Map of Alberta with current and proposed nephrology clinics. There are no data available for the white areas.
Because distance from care providers is a potentially reversible barrier, adding new clinics aimed at providing care to remote-dwellers may improve outcomes—although it is uncertain whether the association between distance from care providers and adverse outcomes is causal. Even if disparities in outcomes are eliminated with decreases in access-to-care distances, it would still be unknown whether establishing a new clinic would be a good use of scarce health care resources. This study evaluated the economic implications and cost-utility of improving the quality of care and outcomes in patients with CKD by simulating the costs and consequences of opening a new nephrology clinic in underserved regions—using the province of Alberta, Canada, as an example.
Materials and Methods
We performed a cost-utility analysis from a public payer’s perspective. This study was reported in accordance with available guidelines (11).
Distance
In our previous work (12), proposed clinic locations were based on minimizing the number of CKD patients residing more than a 120-minute drive from any clinic. We determined that a new clinic in the city of Grand Prairie (a community of about 109,000 people, approximately 400 km from the nearest nephrology clinic) would optimize this objective. Estimates of the effect of a new clinic were based on linking residence location to care delivery and patient outcomes. Adding a clinic in Grand Prairie would shift 876 patients with CKD from a >120-minute drive to a ≤120-minute drive. Driving distance was categorized as ≤30, 30.1–60, 60.1–120, and >120 minutes, as in our previous work (10). The distribution of the 876 stage 3b-4 CKD patients in the respective distance categories would shift from 0%, 0%, 0%, and 100% to 40%, 12%, 48%, and 0%, respectively. The ESRI ArcMap Route Network Analyst (www.esri.com) was used to calculate the driving distances from the patients’ residential postal codes to the nearest current or proposed nephrology clinics. We assumed that patients did not change residences over our timeframe.
Comparators
We compared adding one new clinic to the current standard of care (reference case; Figure 1), assuming that care at the new clinic would be delivered by a nephrologist (residing in a major center and visiting as often as needed to deliver care) and other multidisciplinary health care professionals (residing in the local community). We assumed that adding a new clinic would entail start-up and operational costs (Table 1). Our prior work shows that the distance between a patient’s residence and the closest nephrologist is strongly associated with health care utilization, markers of care quality, and clinical outcomes. Therefore, the residence location of each simulated patient (and thus their calculated distance to the closest nephrology clinic) was used to assess his or her likelihood of process-based and clinical outcomes under each scenario. Specifically, we assumed that adding a clinic (i.e., reducing the distance to the closest nephrologist for simulated patients) would lead to a higher likelihood of receiving urinary albumin measurements, glycosylated hemoglobin testing (in those with diabetes), and the use of statins and angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs). We also assumed that adding a clinic would increase the number of physician claims and their attendant costs (hospitalizations, initiating dialysis, transplantation). The estimated magnitude of these changes was obtained from primary analyses as described below and is reported in Table 2.
Table 1.
Comparator items: Resources and costs
| Item | Unit Valuation ($)a | Quantity |
|---|---|---|
| Patient counts | ||
| patients in a ≤120-min service region | 876 | |
| patients expected to access service | 321 | |
| number of new patients | 135 | |
| Start-up clinic | ||
| examination table | 2200 | 1 |
| thermometer | 750 | 1 |
| glucometer | 1500 | 1 |
| BP cuff | 250 | 1 |
| scale | 500 | 1 |
| automated external defibrillator | 2500 | 1 |
| small fridge | 200 | 1 |
| computer | 1500 | 4 |
| office desk | 600 | 2 |
| chair | 500 | 4 |
| phone handset | 172 | 4 |
| reception desk | 1500 | 1 |
| filing cabinet | 1500 | 2 |
| printer | 181 | 1 |
| fax | 250 | 1 |
| garbage can | 8.45 | 4 |
| recycling bin | 7.99 | 4 |
| total ($) | 22,719 | |
| total per patient ($)b | 70.78 | |
| Ongoing 6-month clinic care (per mo) | ||
| 1200-ft2 space rental | 1300 | 0.5 |
| cleaning service | 845 | 0.5 |
| phone service | 65 | 4 |
| internet service | 60 | 1 |
| wage plus benefits (per yr) | Added staffingc | |
| administrative assistant | 51,900 | 0.3 FTE |
| nurse | 85,912 | 0.3 |
| dietician | 97,349 | 0.1 |
| social worker | 91,352 | 0.1 |
| non-resident nephrologist | ||
| air travel trips | 463/trip | 6 |
| ground travel trips | 230/trip | 0 |
| hotel (d) | 150/d | 24 |
| food (d) | 50/d | 30 |
| small medical and office supplies (6 mo) | 1000/6 mo | 1 |
| total ($) | 49,850 | |
| total per patient ($) | 155.30 | |
| Including physician claims and medications ($)d | ||
| total | 121,324 | |
| total per patient | 377.96 |
FTE, full-time equivalent.
Valuation and numbers of resources were estimated by experts.
Calculated by dividing the total by the number of patients expected to access service.
This would be staffing hours added for the new patients. Physician claim costs are included in Table 3.
Estimated using models adjusting for distance categories (see Table 2).
Table 2.
All model inputs: Stage 3b-4 CKD states
| Model Input | 6-mo Estimate (95% Confidence Interval) | Reference | |||
|---|---|---|---|---|---|
| ≤30 min | 30.1–60 min | 60.1–120 min | >120 min | ||
| Distribution of patients (across distance categories) with current carea | 0 | 0 | 0 | 1 | Ayyalasomayajula 2011 (12) |
| Distribution of patients with Grand Prairie clinic | 0.403 | 0.115 | 0.482 | 0 | Ayyalasomayajula 2011 (12) |
| Transition probability to dialysis care | 0.0012 (0.0009–0.0017) | 0.0010 (0.0007–0.0014) | 0.0008 (0.0005–0.0012) | 0.0007 (0.0005–0.0011) | Rucker 2010 (10) |
| Transition probability to mortality | 0.038 (0.036–0.040) | 0.038 (0.036–0.041) | 0.040 (0.038–0.042) | 0.046 (0.044–0.049) | Rucker 2010 (10) |
| Probability of hospitalization | 0.187 (0.184–0.190) | 0.262 (0.252–0.271) | 0.307 (0.297–0.318) | 0.295 (0.285–0.306) | Rucker 2010 (10) |
| Utility score | 0.86b TTO (0.82–0.89) | 0.86 (0.82–0.89) | 0.86 (0.82–0.89) | 0.86 (0.82–0.89) | Gorodetskaya 2005 (17) |
| Physician claim and medication cost ($)c | 416.80 (411.48–422.19) | 393.78 (377.68–410.58) | 363.45 (348.48–379.06) | 336.71 (322.73–351.30) | Rucker 2010 (10) |
| Hospitalization cost ($) | 10,508.66 (10,275.22–10,742.09) | 8392.71 (7800.00–8985.41) | 7567.95 (7004.28–8131.63) | 7897.87 (7322.69–8473.06) | Rucker 2010 (10) |
Distribution (or proportions) of patients across distance categories for current care are based on patients expected to access the service. Patients expected to access the service were calculated from those who switched from the >120-minute distance category to a category within 120 minutes multiplied by the expected nephrology referral.
Time trade-off estimate assumed to be equivalent for all distance categories.
Of the physician claim and medication costs, 75.2% were due to physician claims, 13.1% to antihypertensive medications (specifically angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers), and 11.7% to lipid-lowering medications (statins, fibrates, nicotinic acid derivatives, bile acid sequestrants, ezetimibe, probucol).
Population
Our target population was CKD patients currently residing further than a >120-minute drive from a nephrology clinic. This was taken from a cohort of 31,452 adult stage 3b-4 CKD patients residing in Alberta (10). From all Albertan patients who had an outpatient serum creatinine measured in 2005, we selected those with estimated GFR <45 ml/min per 1.73 m2, after excluding those requiring renal replacement (RRT) at baseline. These laboratory data were then merged with administrative data from the provincial health care ministry (Alberta Health and Wellness) to obtain information on demographic characteristics, comorbidity and health outcomes (using validated algorithms applied to claims and hospitalization data), and medication use (for patients aged ≥65 years), using methods developed by the Alberta Kidney Disease Network (AKDN) (13). AKDN cohort participants had a mean age of 76 years, 64% were female, and the median GFR was 37 ml/min per 1.73 m2. The proportions of participants with comorbid illnesses were as follows: diabetes mellitus (DM; 32%), chronic obstructive pulmonary disease (COPD; 27%), heart failure (26%), cardiovascular disease (14%), cancer (14%), and peripheral vascular disease (11%).
Of 876 patients residing within 120-minute travel from the proposed Grand Prairie clinic (i.e., the target population for this analysis; all formerly in the remote >120-minute distance category), we estimated that 321 would elect to use the new clinic, on the basis of historical nephrology referral rates from the AKDN cohort. Of these 321 patients, 135 would not have been previously seen by a nephrologist (10). The size of the clinic and the required staffing were based on these data (Table 1). Costs and estimates of effectiveness are attributed to the 321 patients expected to access the new clinic.
Model
An economic decision model was constructed using a Markov process with 6-month cycles (Figure 2). Health states included being alive with stage 3b-4 CKD care in one of four distance categories as described above, dialysis care, transplantation care, and death. All patients initially started in the most remote distance to nephrologist care category (>120 minutes), which could then be modified by the addition of a clinic in a remote region. The model also included hospitalization in the stage 3b-4 CKD distance states (the dialysis and transplantation care states included the costs and consequences of hospitalization). For this analysis, we modeled the effect in a static patient cohort and did not consider increases in the prevalence of stage 3b-4 CKD over time. We also assumed that all estimates of costs and effectiveness remained constant over the entire timeframe. The risk of death and hospitalization among patients who developed kidney failure (dialysis or transplantation) were assumed to be independent of residence location. We used rate data from Statistics Canada (14) to increase the risk of death as the population cohort aged.
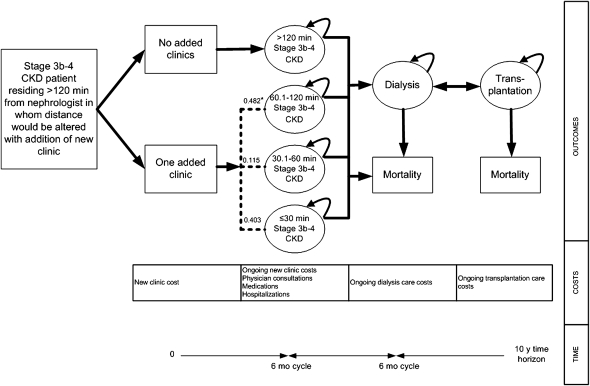
Figure 2.
Economic model. All patients initially started in the greatest distance to nephrologist care category (>120 minutes), which was then modified by the addition of a clinic in a remote region. These patients were than divided across the more proximal distance categories. *Here, we show the distribution of patients in each category for a clinic added in Grand Prairie.
Model outputs were costs, life-years gained, and quality-adjusted life-years (QALYs) gained, and the primary outcome was incremental costs per QALY gained (ICUR). We chose an arbitrary willingness-to-pay (WTP) threshold of $50,000/QALY (15). A strategy that is more costly and leads to worse health outcome (lower QALYs) than the reference case is termed “dominated”; a strategy that results in lower costs but better health outcomes (greater QALYs) is termed “dominant.” We performed the analysis using TreeAge (www.treeage.com). A lifetime horizon was selected. Utilities and costs were discounted by 5% per year as recommended by the Canadian Agency for Drugs and Technologies in Health.
Effectiveness
Estimates of effectiveness in stage 3b-4 CKD patients were calculated using the AKDN cohort (10) using Cox and Poisson regression (Table 2). The probability of mortality, initiating dialysis, and hospitalization were modified for remote-dwelling CKD patients based on their new distance to nephrologist care with the addition of a clinic. The risk of mortality and hospitalization (over 6 months) increased from 3.8% to 4.6% and 18.7% to 29.5% for distances of ≤120 and >120 minutes from the closest clinic, respectively. Initiation of dialysis was slightly less common among those living further away (0.12% to 0.07%). In other words, one death would be prevented if 125 patients were managed within ≤120 minutes of a clinic, one hospitalization would be prevented if nine patients were managed, and one patient would initiate dialysis if 2000 patients were managed. Estimates were adjusted for age, sex, aboriginal status (registered First Nations and recognized Inuit), baseline GFR, social assistance, low-income subsidy, DM cause of CKD, and comorbidities (hypertension, DM, cancer, cardiovascular disease, myocardial infarction, heart failure, COPD, dementia, HIV/AIDS, liver disease, paraplegia, peptic ulcer disease, peripheral vascular disease, rheumatic disease). Estimates for transplantation, graft failure, and mortality in RRT states were drawn from our previous work in a similar dataset (Table 3) (16).
Table 3.
All model inputs: Dialysis and transplantation health states
| Model Input | 6-mo Estimate (95% Confidence Interval) | References | |
|---|---|---|---|
| Dialysis Care | Transplantation Care | ||
| Transition probability to transplantation care | 0.012 | — | Manns 2010 (16) |
| Transition probability to mortality | 0.100 (0.095–0.105) | 0.024 | CORR 2005 (32); Manns 2010 (16) |
| Transition probability to dialysis care (graft failure) | — | 0.02 (0–0.05) | Manns 2010 (16) |
| Utility score | 0.609 EQ-5D (0.566–0.652) | 0.74 TTO | Manns 2003 (19); Laupacis 1996 (18) |
| Cost, $ | 45,932 (42,731–49,133) | 27,457 pretransplant (23,953–30,963) | Lee 2002 (20); Barnieh 2011 (33) |
| 27,782 during first year (25,146–30,419) | |||
| 11,417 after first year (10,336–12,498) | |||
EQ-5D, EuroQol 5-dimensions; TTO, time trade-off; —, not applicable.
Mean utility scores were based on a focused literature search and were as follows (Tables 2 and 3): patients with stage 3b-4 CKD, 0.86 using time trade-off (TTO) (17); patients with kidney transplants, 0.74 using TTO (18); and patients treated with dialysis, 0.61 using EuroQoL-5D (19). Utilities were assumed to be constant across distance categories due to lack of evidence to the contrary.
Resource Use and Costs
Using a public payer’s perspective, we assumed that adding a new clinic would entail the following costs: start-up costs (medical and office furniture and equipment), ongoing small medical and office supplies, space rental and services (phone, internet, and cleaning), staffing (administrative assistant, nurse, dietician, social worker), flight and ground transportation, and room and board for the nephrologist (Table 1). Start-up and ongoing costs of the clinic were divided across all 321 patients expected to access the new clinic. However, because 186 patients are already followed by nephrologists and would not require new staff (their care would require reallocation of staff to the new location, from their previous location), only the costs of staff needed to see new referrals (n=135) were included in our costing estimates.
Stage 3b-4 CKD patient care costs were calculated from the AKDN cohort (Table 2) using linear regression. Costs included physician claims (nephrologists and other specialists; from only the 214 new referrals), antihypertensive medications (ACE inhibitors and ARBs), and lipid-lowering medications (statins, fibrates, nicotinic acid derivatives, bile acid sequestrants, ezetimibe, probucol). We used the same medication costs estimated in patients aged ≥65 years for those patients aged <65 years. These costs and separate hospitalization costs (over 6 months) decreased with distance from $418 to $337, and $10,510 to $7895, respectively. Thus, costs for preventative care and hospitalization were modified for remote-dwelling CKD patients based on their new distance to nephrologist care with the addition of a clinic. Transplantation and dialysis care costs were drawn from the literature (Table 3) (18,20). All costs are reported in Canadian dollars and are inflated to 2009 costs using the consumer price index for health care goods in Canada. American dollars were converted to Canadian dollars using www.oanda.com.
Sensitivity Analyses
We performed a number of sensitivity analyses. First, this model assumes that disparities in outcomes would be eliminated if the distances that patients travel to access care were equivalent. In one-way analyses, for each of the five effectiveness and resource use parameters influenced by distance for stage 3b-4 CKD patients, we assumed no change in the parameter because the distance to a nephrologist was decreased by the addition of a new clinic. This assumes a self-selection bias or a competing risk of outcome that is not affected by distance to care. Second, in a threshold analysis, we explored how small the probability of mortality would need to be in the remote category (in the comparator arm) in order for the ICUR to be increased to our WTP threshold. We also looked at measured reductions in mortality: 75% (0.040), 50% (0.042), 25% (0.044), and no change (0.046). Third, using a societal perspective, we considered driving, parking, and workforce productivity costs for stage 3b-4 CKD patients. Fourth, we varied our discount rate to be the Canadian government’s real interest rate (21) for 2009 (i.e., 0.65% per year). Fifth, probabilistic sensitivity analysis was conducted using Monte Carlo simulation and an estimated distribution of each parameter for 1000 simulations. Probabilities and utilities were assigned β distributions and costs were assigned γ distributions. A cost-effectiveness acceptability curve was generated to represent the probability that adding a new CKD clinic would be cost-effective over a range of WTP thresholds. Finally, along with an exhaustive set of one-way sensitivity analyses, we also considered a number of scenarios exploring the following items: alternative clinic locations, a second clinic, not reallocating staffing costs, varying the number of referrals, and the comorbidity profile in remote patients (Supplemental Methods, Results, and Tables 1–6).
Results
Model Validity
Following a published guideline (22), we ensured that our economic model had face, technical, and internal validity. Internal validity was assessed by comparing 3-year (approximate median follow-up) observations of death and progression to dialysis in the statistical model with 3-year predictions in the economic model. Ninety-nine percent of simulated patients had died after 18.5 years.
Clinic Costing
We estimated that equipping a stage 3b-4 CKD clinic with one examination room, one shared office, and one reception area would cost approximately $22,719 (Table 1). Total 6-month operating costs excluding physician claims and medications were $49,850; 60% of operating costs were attributable to staffing and 21% to rent and cleaning services. Including physician claims and medications increased 6-month operating costs to $121,324. Estimated costs for alternative locations were similar (Supplemental Table 1).
Reference Case Analysis
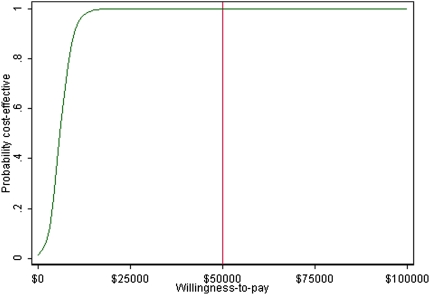
Adding a clinic in Grand Prairie to manage stage 3b-4 CKD patients increased mean QALYs gained from 8.995 to 9.792 over 10 years and incurred an additional $3803/patient, resulting in an ICUR of $4774/QALY (Table 4). The cost-utility acceptability curve shows that there is a 100% probability that adding one clinic would be cost-effective at a WTP threshold of $50,000/QALY (Figure 3).
Table 4.
Results: Reference case and sensitivity analyses
| Analysis | Comparator/Change | Total Cost ($) | Incremental Cost ($) | Total QALYs | Incremental QALYs | ICUR ($/QALY) |
|---|---|---|---|---|---|---|
| Primary | Reference case | 29,525.25 | — | 8.995 | — | — |
| Adding 1 clinic: Grand Prairie | 33,328.51 | 3803.26 | 9.792 | 0.797 | 4774.01 | |
| Physician claim and medication cost:† No change | Adding 1 clinic | 32,738.83 | 3213.58 | 9.792 | 0.797 | 4033.82 |
| Hospitalization cost: No change | Adding 1 clinic | 31,451.74 | 1926.49 | 9.792 | 0.797 | 2418.21 |
| Transition probability to dialysis care: No change | Adding 1 clinic | 32,671.05 | 3145.80 | 9.807 | 0.812 | 3875.05 |
| Transition probability to mortality: No change (0% reduction) | Adding 1 clinic: 0.046 | 30,652.91 | 1127.66 | 8.983 | −0.012 | Dominated |
| Transition probability to mortality: Threshold (5% reduction) | Adding 1 clinic: 0.0456 | 30,792.89 | 1267.65 | 9.025 | 0.030 | 41,923.86a |
| Transition probability to mortality: 25% reduction | Adding 1 clinic: 0.044 | 31,367.11 | 1841.86 | 9.198 | 0.203 | 9089.71 |
| Transition probability to mortality: 50% reduction | Adding 1 clinic: 0.042 | 32,118.75 | 2593.50 | 9.424 | 0.428 | 6053.50 |
| Transition probability to mortality: 75% reduction | Adding 1 clinic: 0.040 | 32,911.05 | 3385.80 | 9.662 | 0.667 | 5079.15 |
| Probability of hospitalization: No change | Adding 1 clinic | 38,444.92 | 8919.68 | 9.792 | 0.797 | 11,196.34 |
| Societal perspective: Stage 3b-4 CKD states only | Revised reference case | 40,740.10 | — | 8.995 | — | — |
| Adding 1 clinic | 38,206.26 | −2533.84 | 9.792 | 0.797 | Dominant | |
| Discount rate: Real interest rate | Revised reference case | 34,428.01 | — | 10.430 | — | — |
| Adding 1 clinic | 41,000.13 | 6572.12 | 11.572 | 1.142 | 5754.76 |
ICUR, incremental cost-utility ratio; QALY, quality-adjusted life-year; —, not applicable.
Willingness to pay $50,000.
Figure 3.
Cost-effective acceptability curve: One added clinic versus none. The red vertical line represents a willingness-to-pay threshold of $50,000.
Sensitivity Analyses
If estimates of effectiveness and costs were assumed to be constant across distance categories (i.e., estimates from the remote category were applied to all distance categories), most of the ICURs decreased (became more attractive) including dialysis and all stage 3b-4 CKD patient costs with the exceptions of mortality and hospitalization. If the probability of hospitalization remained at 0.295 for all categories, then the ICUR would increase (became less attractive) to $11,196/QALY. If the probability of mortality remained at 0.046 (0% reduction) for all distance categories, then the strategy of adding a clinic would be dominated (more costly with less benefit) by current care. In a threshold analysis, we found that a probability of 0.0456 for mortality (a 5% reduction) in the remote category would suffice to maintain an ICUR <$50,000/QALY. Measured reductions in mortality for all distance categories found $5079/QALY, $6054/QALY, and $9090 ICURs at 75%, 50%, and 25% reductions, respectively.
When we included driving, parking, and workforce productivity costs for stage 3b-4 CKD patients, using a societal perspective, the reference case was dominated by adding a clinic. All scenario analyses did not importantly affect our results (Supplemental Table 4). Changes to other costs and measures of effectiveness had negligible effects on the ICUR (Supplemental Tables 5 and 6). Thus, our results were robust to a wide range of one-way sensitivity analyses for both cost and effectiveness parameters.
Discussion
Introducing a new nephrology clinic in a remote location such as Grand Prairie, Alberta (or in one of the alternative remote locations we considered), would cost approximately $250,000/yr for operating costs and $25,000 in initial equipment costs. Assuming that improving access to nephrology care by reducing travel time would lead to improved outcomes and lower hospitalization rates, as suggested by observational studies (1–9), establishing this additional clinic would represent good value for money, with ICURs ranging from $4000 to $8000/QALY in most scenarios. However, if reducing distance to specialized care does not reduce the risk of hospitalization or mortality, the cost-effectiveness of establishing a new clinic may be considerably less attractive—although it still might be economically attractive by current standards.
Our previous work shows that remote-dwellers with CKD are less likely to access health services than patients who live closer to nephrologists (10). Remote-dwellers with kidney failure initiate dialysis less frequently, and remote-dwellers with less advanced CKD are less likely to see nephrologists, to be prescribed potentially beneficial medications and to receive other recommended elements of care. Perhaps as a consequence, remote-dwellers with CKD also are more likely to die and to be hospitalized than patients living closer to a nephrologist. In our analysis, we assumed that this association is causal and that reducing the distance that patients must travel to visit a nephrologist will decrease the likelihood of mortality and hospitalizations. Although appealing, this hypothesis remains to be proven. Remote patients might self-select less aggressive care because of different preferences, or they may face circumstances such as higher-risk occupations and recreational activities (23) that differ from urban-dwellers (leading to worse health outcomes that are not directly related to CKD and would not be expected to improve with better access to a nephrologist). Although we adjusted for measured characteristics that differ by residence location (such as aboriginal status, diabetes, and other comorbidity), the possibility for residual confounding remains.
Using a simple search in MEDLINE, we did not find formal economic evaluations introducing clinics in more remote communities. As such, both the costing and the cost-utility analysis work are novel. Because improving care of remote- and rural-dwellers continues to be a high priority for governments around the world (24–26), our results are relevant for decision makers, particularly given that this clinic seems cost-effective by accepted standards, and are markedly lower than for other interventions in common use for patients with (27,28) and without CKD (29–31).
Our study has several important strengths, including the use of a single large administrative database, a comprehensive set of sensitivity analyses, and precise costing estimates. However, our study also has certain limitations that should be considered when interpreting results. First, we took the perspective of a public payer, meaning that societal costs (such as workforce productivity, patient-borne costs, and caregiver time) were not included in the primary analysis. However, as we show in our sensitivity analysis, including these costs would lead to further reduced ICURs and thus our results are conservative. Second, we could not externally validate our work as no prior studies have demonstrated the efficacy of new nephrology clinics for improving outcomes in remote-dwellers with CKD. Third, we could not adjust for unmeasured potential confounders (23) and our simulated study needs to be tested in a real-world setting. Given the broad reach of the intervention, a minimum of two communities would need to be selected: one to receive a temporary nephrology clinic and the other to act as an external control (perhaps using a pre/post design using hospitalization as a short-term surrogate for mortality). Fourth, our model does not account for the time it would take to get a new clinic operating at full capacity. This lag would be difficult to estimate in practice; however, we expect our results to reflect the potential benefits of establishing a new clinic once a steady state had been established. The effect of such a lag on individual patients would likely be reduced by our selection of a lifetime horizon. Finally, we were unable to assign an economic value to the intangible benefit of improved equity inherent in reducing the travel time needed for remote-dwellers to access care. Because the Canada Health Act guarantees reasonable access to medical care for all Canadians (regardless of residence location), this benefit of establishing a new clinic is important not only to decision makers in Canada, but also to decision makers in other countries with remote communities.
In summary, we found that establishing a new clinic to care for remote-dwellers with CKD in the province of Alberta is associated with ICURs that are generally considered to represent good value for money (ranging from $4000 to $8000/QALY). These results depend on the assumption that better access to care will improve clinical outcomes, as suggested by observational studies. High-quality controlled studies assigning communities to having an added renal insufficiency clinic or current care are required to confirm this hypothesis. Given the low costs of equipping a clinic ($25,000) and operating a clinic ($250,000/yr) and the very attractive ICUR relative to other currently funded interventions, establishing new clinics for remote-dwellers could play an important role in improving outcomes for people with CKD.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Meng Lin for effectiveness estimates, Janice McKenzie for resource estimates, and Jeff Foster for cost estimates.
Support for this work was provided in part through an interdisciplinary team grant from the Alberta Heritage Foundation for Medical Research. The foundation was not involved in the interpretation of results or the drafting of this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07350711/-/DCSupplemental.
References
- 1.Tonelli M, Klarenbach S, Rose C, Wiebe N, Gill J: Access to kidney transplantation among remote- and rural-dwelling patients with kidney failure in the United States. JAMA 301: 1681–1690, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Tonelli M, Manns B, Culleton B, Klarenbach S, Hemmelgarn B, Wiebe N, Gill JS. Alberta Kidney Disease Network: Association between proximity to the attending nephrologist and mortality among patients receiving hemodialysis. CMAJ 177: 1039–1044, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tonelli M, Hemmelgarn B, Culleton B, Klarenbach S, Gill JS, Wiebe N, Manns B. Alberta Kidney Disease Network: Mortality of Canadians treated by peritoneal dialysis in remote locations. Kidney Int 72: 1023–1028, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Tonelli M, Klarenbach S, Manns B, Culleton B, Hemmelgarn B, Bertazzon S, Wiebe N, Gill JS. Alberta Kidney Disease Network: Residence location and likelihood of kidney transplantation. CMAJ 175: 478–482, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tonelli M, Hemmelgarn B, Kim AK, Bertazzon S, Klarenbach S, Manns B, Wiebe N, Culleton B, Gill JS. Alberta Kidney Disease Network: Association between residence location and likelihood of kidney transplantation in Aboriginal patients treated with dialysis in Canada. Kidney Int 70: 924–930, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Smith KB, Humphreys JS, Wilson MG: Addressing the health disadvantage of rural populations: How does epidemiological evidence inform rural health policies and research? Aust J Rural Health 16: 56–66, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Rosenblatt RA, Baldwin LM, Chan L, Fordyce MA, Hirsch IB, Palmer JP, Wright GE, Hart LG: Improving the quality of outpatient care for older patients with diabetes: Lessons from a comparison of rural and urban communities. J Fam Pract 50: 676–680, 2001 [PubMed] [Google Scholar]
- 8.Rose G, Duerksen F, Trepman E, Cheang M, Simonsen JN, Koulack J, Fong H, Nicolle LE, Embil JM: Multidisciplinary treatment of diabetic foot ulcers in Canadian Aboriginal and non-Aboriginal people. Foot Ankle Surg 14: 74–81, 2008 [DOI] [PubMed] [Google Scholar]
- 9.McLean G, Guthrie B, Sutton M: Differences in the quality of primary medical care services by remoteness from urban settlements. Qual Saf Health Care 16: 446–449, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rucker D, Hemmelgarn BR, Lin M, Manns BJ, Klarenbach SW, Ayyalasomayajula B, James MT, Bello A, Gordon D, Jindal KK, Tonelli M: Quality of care and mortality are worse in chronic kidney disease patients living in remote areas. Kidney Int 79: 210–217, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Drummond MF, Jefferson TO. The BMJ Economic Evaluation Working Party: Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 313: 275–283, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayyalasomayajula B, Wiebe N, Hemmelgarn BR, Bello A, Manns B, Klarenbach S, Tonelli M: A novel technique to optimize facility locations of new nephrology services for remote areas. Clin J Am Soc Nephrol 6: 2157–2164, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemmelgarn BR, Clement F, Manns BJ, Klarenbach S, James MT, Ravani P, Pannu N, Ahmed SB, MacRae J, Scott-Douglas N, Jindal K, Quinn R, Culleton BF, Wiebe N, Krause R, Thorlacius L, Tonelli M: Overview of the Alberta Kidney Disease Network. BMC Nephrol 10: 30, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Statistics Canada: Table 102-0504: Life expectancy and deaths, deaths and mortality rates, by age group and sex, Canada, provinces and territories, annual, 1974 to 2007 (table). CANSIM (database), using E_STAT (distributor). Last updated February 2010. Available at: http://estat.statcan.ca/cgi-win/CNSMCGI.EXE?CANSIMFILE=EStat\English\CII_1_E.htm. Accessed July 12, 2011.
- 15.Laupacis A, Feeny D, Detsky AS, Tugwell PX: How attractive does a new technology have to be to warrant adoption and utilitzation? Tentative guidelines for using clinical and economic evaluations. CMAJ 146: 473–481, 1992 [PMC free article] [PubMed] [Google Scholar]
- 16.Manns B, Hemmelgarn B, Tonelli M, Au F, Chiasson TC, Dong J, Klarenbach SW. Alberta Kidney Disease Network: Population based screening for chronic kidney disease: Cost effectiveness study. BMJ 341: c5869, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorodetskaya I, Zenios S, McCulloch CE, Bostrom A, Hsu CY, Bindman AB, Go AS, Chertow GM: Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int 68: 2801–2808, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Laupacis A, Keown P, Pus N, Krueger H, Ferguson B, Wong C, Muirhead N: A study of the quality of life and cost-utility of renal transplantation. Kidney Int 50: 235–242, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Manns B, Johnson JA, Taub K, Mortis G, Ghali WA, Donaldson C: Quality of life in patients treated with hemodialysis or peritoneal dialysis: What are the important determinants? Clin Nephrol 60: 341–351, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Lee H, Manns B, Taub K, Ghali WA, Dean S, Johnson D, Donaldson C: Cost analysis of ongoing care of patients with end-stage renal disease: The impact of dialysis modality and dialysis access. Am J Kidney Dis 40: 611–622, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Claxton K, Paulden M, Gravelle H, Brouwer W, Culyer AJ: Discounting and decision making in the economic evaluation of health-care technologies. Health Econ 20: 2–15, 2011 [DOI] [PubMed] [Google Scholar]
- 22.McCabe C, Dixon S: Testing the validity of cost-effectiveness models. Pharmacoeconomics 17: 501–513, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Canadian Population Health Initiative: How healthy are rural Canadians? An assessment of their health status and health determinants. Toronto, Canadian Institute for Health Information, 2006. Available at: http://secure.cihi.ca/cihiweb/products/rural_canadians_2006_report_e.pdf Accessed June 24, 2011.
- 24.Commission on the Future of Health Care in Canada: Rural and remote communities. In: Building on values—the future of health care in Canada. Ottawa, Canada, Government of Canada, 2002 pp 159–170. Available at: http://dsp-psd.pwgsc.gc.ca/Collection/CP32-85-2002E.pdf Accessed June 28, 2011.
- 25.Rural Panel Research Institute Health Panel: Securing high quality health care in rural America: The impetus for change in the Affordable Care Act. Rockville, Maryland, Federal Office of Rural Health Policy, Health Resources and Services Administration, US Department of Health and Human Services, 2010. Available at: http://www.rupri.org/Forms/HealthPanel_ACA_Dec2010.pdf Accessed June 28, 2011.
- 26.National Rural Health Alliance Inc: Australia's health system needs re-balancing: A report on the shortage of primary care services in rural and remote areas. Deakin West, ACT, Australia, National Rural Health Alliance Inc, 2011. Available at: http://nrha.ruralhealth.org.au/cms/uploads/publications/nrha-final-full-complementary-report.pdf Accessed June 28, 2011.
- 27.Clement FM, Klarenbach S, Tonelli M, Wiebe N, Hemmelgarn B, Manns BJ: An economic evaluation of erythropoiesis-stimulating agents in CKD. Am J Kidney Dis 56: 1050–1061, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Manns B, Klarenbach S, Lee H, Culleton B, Shrive F, Tonelli M: Economic evaluation of sevelamer in patients with end-stage renal disease. Nephrol Dial Transplant 22: 2867–2878, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Bischof M, Briel M, Bucher HC, Nordmann A: Cost-effectiveness of drug-eluting stents in a US Medicare setting:a cost-utility analysis with 3-year clinical follow-up data. Value Health 12: 649–656, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Chabot I, Rocchi A: How do cost-effectiveness analyses inform reimbursement decisions for oncology medicines in Canada? The example of sunitinib for first-line treatment of metastatic renal cell carcinoma. Value Health 13: 837–845, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, Fry-Smith A, Burls A: A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess 10: iii–iv, xi–xiii, 1–229, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Canadian Organ Replacement Registry: Treatment of end-stage organ failure in Canada 2002 and 2003. Ottawa, Canada, Canadian Institutes of Health Information, 2005. Available at: http://secure.cihi.ca/cihiweb/products/CORR_Annual_Report_0203_e.pdf Accessed June 17, 2011.
- 33.Barnieh L, Manns BJ, Klarenbach S, McLaughlin K, Yilmaz S, Hemmelgarn BR: A description of the costs of living and standard criteria deceased donor kidney transplantation. Am J Transplant 11: 478–488, 2011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.