Summary
Background and objectives
Several adult studies report that patients returning to peritoneal dialysis after allograft failure have increased infection-related morbidity. The impact of allograft failure on infection risk in children is uncertain. We compared peritonitis-free survival between pediatric peritoneal dialysis patients with prior allograft failure and those who were transplant naive.
Design, setting, participants, & measurements
We studied patients, 2–21 years of age, who initiated peritoneal dialysis from January 1, 1992, to December 31, 2007, in the North American Pediatric Renal Trials and Collaborative Studies registry. Demographic characteristics were compared between transplant naive and allograft failure patients using a chi-squared statistic. Peritonitis-free survival was compared between the two groups using Kaplan–Meier estimates. A Cox regression analysis was performed to adjust for covariates, which impact risk of peritonitis.
Results
Of 2829 patients on peritoneal dialysis, 445 had a prior history of allograft failure and 2384 did not (transplant naive). Demographic characteristics including age at dialysis initiation, race, primary renal disease, and era of dialysis initiation were significantly different between the two groups. Peritonitis-free survival was poorer for the allograft failure group. After covariate adjustment, allograft failure showed borderline significance as a factor predictive of peritonitis.
Conclusions
Children initiating peritoneal dialysis after allograft failure may experience a slightly higher infection risk.
Introduction
Short-term renal allograft survival has significantly improved over the past two decades, mainly due to better immunotherapy (1). Nevertheless, chronic allograft nephropathy is still an important issue that compromises long-term graft function and often leads to chronic dialysis.
A study using the Scientific Registry of Transplant Recipients registry reports that 4.9% of 10,748 patients who returned to dialysis after primary allograft failure were <18 years of age (2). The North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) registry reports almost 25% of index transplants in the registry have failed, with the majority (>90%) of these patients returning to dialysis after transplant failure (3).
Several authors report poorer outcomes in adult patients returning to dialysis after allograft failure compared to those who are transplant naive. The largest adult studies have shown increased mortality risk for patients returning to dialysis after transplant failure (2,4). Others describe increased infectious morbidity in peritoneal dialysis (PD) patients after allograft failure. Sasal et al. (5) showed increased infectious morbidity in PD patients with prior allograft failure compared with an age- and diabetes-matched cohort of transplant naive PD patients. Several authors report greater morbidity due to infection in PD patients maintained on immunosuppression after transplant failure (6–8). In contrast, a large study using data from the Australia and New Zealand Dialysis and Transplant Registry found no difference in peritonitis-free survival between transplant failure and transplant naive patients initiating PD (9). However, these studies only pertain to adult patients.
We previously reported comparable mortality risk between pediatric patients commencing dialysis after allograft failure and those who are transplant naive (10). However, the impact of allograft failure on infectious morbidity in children returning to PD is uncertain. Peritonitis is a common complication of PD in children, which can lead to morbidity and treatment failure. We hypothesize that pediatric patients initiating PD after allograft failure will have greater infection risk, similar to adult patients. To the best of our knowledge, the impact of allograft failure on peritonitis-free survival has not previously been evaluated in the pediatric setting.
Materials and Methods
Data Source
Data for our study were obtained from the NAPRTCS registry. Statistical analysis was provided by the EMMES Corporation, the Data Coordinating Center of NAPRTCS.
Participants
We identified patients in the NAPRTCS registry who initiated PD from January 1, 1992, to December 31, 2007 (n=3495). We excluded patients who had a history of two or more renal transplants. To reduce bias, patients <2 years of age were excluded due to underrepresented number of patients with a failed allograft. The remaining patients were eligible for our study (n=2829).
Definitions and Outcomes
The study population was divided into two groups: patients with previous failed allograft (n=445) and those who were transplant naive (n=2384). Allograft failure was defined in the registry by a patient commencing PD. Patients were followed through their ensuing dialysis therapy and censored at first peritonitis episode, when dialysis was terminated (due to transplant, return of renal function, or death) or if the patient was lost to follow-up. Immunosuppression status was determined at 30 days after dialysis initiation.
Statistical Analyses
Categorical variables were compared using the chi-squared statistic. Annualized peritonitis rates were determined for the transplant naive and transplant failure patients. Time to first peritonitis was analyzed using the Kaplan–Meier method and a comparison made using the log-rank statistic. A Cox proportional hazards regression analysis was performed to determine hazard ratios (HRs) for covariates affecting time to first peritonitis. Covariates used for adjusted analysis include age at dialysis initiation, sex, race, primary renal disease, era of dialysis initiation, immunosuppression, and prior transplant status. All statistical analyses were performed using the SAS System for Windows, version 9.2 (SAS Institute, Cary, NC).
Results
Descriptive Data
The demographic characteristics of the transplant naive (n=2384) and allograft failure (n=445) groups are shown in Table 1. Patient characteristics including age at dialysis initiation, race, primary renal disease, era of dialysis initiation, and immunosuppression were significantly different between the two groups (P<0.05). The transplant naive group was generally younger in age compared with the allograft failure group. A greater percentage of white patients and a lower percentage of Hispanic patients were characteristic of the allograft failure group compared with the transplant naive group. Primary renal disease was different between the groups without a clear pattern of distribution. Sex was similar between the two groups.
Table 1.
Demographic characteristics for pediatric PD patients in the North American Pediatric Renal Trials and Collaborative Studies registry by prior transplant status
| Patient Characteristics | Transplant Naive | Transplant Failure | Chi-Squared P Value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| All patients | 2384 | 100 | 445 | 100 | |
| Age at PD initiation (yr) | |||||
| 2–5 | 387 | 16.2 | 39 | 8.8 | <0.001 |
| 6–12 | 924 | 38.8 | 133 | 29.9 | |
| 13–17 | 973 | 40.8 | 208 | 46.7 | |
| 18+ | 100 | 4.2 | 65 | 14.6 | |
| Race | |||||
| white | 1159 | 48.6 | 254 | 57.1 | <0.001 |
| black | 482 | 20.2 | 97 | 21.8 | |
| Hispanic | 574 | 24.1 | 64 | 14.4 | |
| other | 169 | 7.1 | 30 | 6.7 | |
| Sex | |||||
| male | 1261 | 52.9 | 236 | 53 | 0.96 |
| female | 1122 | 47.1 | 209 | 47 | |
| missing | 1 | 0 | 0 | 0 | |
| Primary disease | |||||
| obstructive uropathy | 259 | 10.9 | 49 | 11 | 0.02 |
| FSGS | 392 | 16.4 | 84 | 18.9 | |
| renal dysplasia | 272 | 11.4 | 76 | 17.1 | |
| reflux nephropathy | 93 | 3.9 | 12 | 2.7 | |
| other | 1106 | 46.4 | 197 | 44.3 | |
| missing | 262 | 11 | 27 | 6.1 | |
| Era of dialysis initiation | |||||
| 1992–1994 | 552 | 23.2 | 147 | 33 | <0.001 |
| 1995–1997 | 633 | 26.6 | 101 | 22.7 | |
| 1998–2000 | 481 | 20.2 | 64 | 14.4 | |
| 2001–2003 | 375 | 15.7 | 80 | 18 | |
| 2004–2007 | 343 | 14.4 | 53 | 11.9 | |
| Immunosuppression | |||||
| yes | 217 | 9.1 | 217 | 48.8 | <0.001 |
| no/missing | 2167 | 90.9 | 228 | 51.2 | |
PD, peritoneal dialysis.
Outcome Data
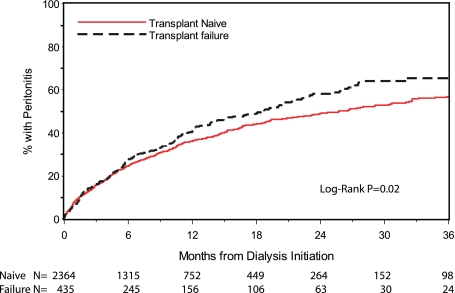
The annualized peritonitis rates were 0.59 and 0.66 for the transplant naive and transplant failure group, respectively (Table 2). Peritonitis-free survival curves for the transplant naive and allograft failure groups by Kaplan–Meier estimates are shown in Figure 1. Time to first peritonitis was significantly shorter in the allograft failure group compared with the transplant naive group (log rank P=0.02).
Table 2.
Annualized peritonitis rate for pediatric PD patients in the North American Pediatric Renal Trials and Collaborative Studies registry by prior transplant status
| Transplant Status | Number of Peritonitis Episodes | Follow-Up (yr) | Annualized Peritonitis Rate | 95% Confidence Interval |
|---|---|---|---|---|
| Naive | 1906 | 3237 | 0.59 | 0.56–0.62 |
| Allograft failure | 500 | 758 | 0.66 | 0.60–0.72 |
Figure 1.
Peritonitis-free survival curves for the transplant naive and allograft failure groups by Kaplan–Meier estimates.
Results from the multivariate logistic regression analysis are shown in Table 3. After covariate adjustment, the association between allograft failure and time to first peritonitis for patients on PD was not statistically significant (HR 1.19, confidence interval [CI] 0.99–1.42, P=0.06). Immunosuppression was not linked to peritonitis (HR 1.06, CI 0.88–1.27, P=0.56). Factors associated with peritonitis were black race and initiating dialysis during earlier years. Patients of black race were 44% more likely to develop peritonitis compared with those of white race (HR 1.44, CI 1.23–1.68, P<0.001). Compared with the reference group from the era 1992–1994, patients initiating PD in recent years were less likely to develop peritonitis; those initiating dialysis between 2001 and 2003 had a 32% decreased risk of peritonitis (HR 0.68, CI 0.55–0.84, P=0.004), and those initiating dialysis in the era 2004–2007 had a 44% decreased risk of peritonitis (HR 0.56, CI 0.44–0.71, P≤0.001).
Table 3.
Covariate-adjusted hazard ratios for peritonitis
| Covariates | Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| Age (yr) at PD initiation (reference group: >13 y) | |||
| 2–5 | 1.16 | 0.96–1.40 | 0.12 |
| 6–12 | 1.07 | 0.93–1.23 | 0.35 |
| Race (reference group: white) | |||
| black | 1.44 | 1.23–1.68 | <0.001 |
| Hispanic | 1.03 | 0.87–1.23 | 0.72 |
| other | 0.93 | 0.71–1.21 | 0.58 |
| Sex (reference group: male) | |||
| female | 0.94 | 0.82–1.07 | 0.33 |
| Primary disease (reference group: obstructive uropathy) | |||
| FSGS | 1.05 | 0.83–1.33 | 0.69 |
| renal dysplasia | 1.18 | 0.92–1.52 | 0.18 |
| reflux nephropathy | 0.89 | 0.61–1.31 | 0.55 |
| other | 0.97 | 0.82–1.24 | 0.97 |
| Era of dialysis initiation (reference group: 1992–1994) | |||
| 1995–1997 | 1.02 | 0.86–1.21 | 0.81 |
| 1998–2000 | 0.87 | 0.72–1.05 | 0.15 |
| 2001–2003 | 0.68 | 0.55–0.84 | 0.004 |
| 2004–2007 | 0.56 | 0.44–0.71 | <0.001 |
| Immunosuppression (reference group: none) | |||
| Immunosuppression | 1.06 | −2.15 | 0.56 |
| Transplant status (reference group: transplant naive) | |||
| transplant failure | 1.19 | 0.99–1.42 | 0.06 |
Discussion
Infection outcomes in children returning to PD after renal allograft failure have not previously been evaluated. The results of this study, using a large pediatric database, showed a difference in peritonitis risk in children with previous allograft failure compared with their transplant naive counterparts in the unadjusted analysis. This difference disappeared in the adjusted analysis, which may be due to differences in demographic characteristics between the two groups, which were controlled for in the multivariate analysis.
Several authors provide evidence supporting the role of immunosuppression in increasing infection risk in adults. We did not find immunosuppression to be an independent risk factor for peritonitis. Transplant naive patients who were on immunosuppression for their primary renal disease could have affected this outcome. Also, our study was limited by incomplete data reporting to the registry in regard to immunosuppressive therapy, which likely affected our results. Therefore, the possible role of immunosuppression in increased susceptibility to infection on PD should not be discounted. The relationship of immunosuppression and dialysis morbidity has been studied in adults, but there are no reports of this in the pediatric literature. A consistent clinical approach for immunosuppression dose, length of therapy, and weaning method in children returning to dialysis after transplant failure is lacking, thereby making a retrospective study in this area difficult. A prospective study will be necessary to effectively compare dialysis morbidity outcomes between children on immunosuppression and those who are not.
In our study, we found that black race confers increased risk of peritonitis, which has been described as a risk factor for peritonitis in a previous NAPRTCS report by Furth et al. (11). Black race as a risk factor for peritonitis has also been shown in adult patients (12). Hijazi et al. (13) reported African-American race as a negative prognostic indicator for mortality in children on dialysis; however, they did not look at race as a risk factor for peritonitis. Similarly in our previous report, we also found black race to have a 50% increased risk of mortality (10). Closer study is warranted to gain more detailed information and to understand better the relationship between ethnicity and dialysis-related morbidity and mortality. Consistent with other reports, we did not find patient age to be associated with peritonitis risk (11,14).
Our study is one of the first pediatric reports on PD infection risk after allograft failure. The strengths of our study include the use of NAPRTCS, a large pediatric database that encompasses data for nearly two decades. NAPRTCS uniquely represents a diverse, international patient population, providing data from across the United States and from Canada, Mexico, and Costa Rica. In spite of these strengths, our study does have weaknesses such as the limitations posed by an observational study using registry data. Data collection by the registry is voluntary, and all data are not available for every patient; most notably, there was limited information on immunosuppression. Our study was also subject to preexisting guidelines for how patient information is defined and reported to the registry, which precluded availability of more detailed and specific data.
Peritonitis is a frequent complication in children on PD and can lead to significant morbidity and treatment failure. Although allograft failure was not a statistically significant factor, it may be a clinically relevant factor in the development of peritonitis. Although continued immune suppression could be a contributory factor, other potential factors like the inflammatory milieu may play a role. This is however speculative. Notably, transplant failure has been linked to infectious morbidity in the adult PD population. This link between transplant failure and dialysis morbidity is an important issue because children with ESRD will likely require several transplants and sequences of dialysis over their lifetimes. Many of these pediatric patients transition to adult nephrology care, and the relationship between transplant failure and infection risk may prove to be a greater issue over time.
After allograft failure, PD is the preferred dialysis modality for suitable patients, as it is a home-based therapy and does not require in-center dialysis sessions. Our results did not find allograft failure to be a significant factor affecting peritonitis. However, due to conglomeration of potential risk factors in this group of patients (e.g., chronic inflammation and ongoing immunosuppression and perhaps just older vintage of patients), larger studies with more granular data may demonstrate other novel risk factors for peritonitis in this unique patient population. A better understanding of these risk factors would improve and optimize medical care for children on dialysis. This information would also allow a greater understanding of how pediatric risk factors compare with those seen in the adult dialysis population, which is vital to developing an effective approach in managing adolescent dialysis patients transitioning to adult nephrology care.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D: Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 342: 605–612, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Rao PS, Schaubel DE, Jia X, Li S, Port FK, Saran R: Survival on dialysis post-kidney transplant failure: Results from the Scientific Registry of Transplant Recipients. Am J Kidney Dis 49: 294–300, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Martz K, Stablein D: North American Pediatric Renal Trials and Collaborative Studies 2008 Annual Report: Renal Transplantation, Dialysis, Chronic Renal Insufficiency, Rockville, MD, EMMES Corporation, 2008, Section 5, page 1 [Google Scholar]
- 4.Meier-Kriesche HU, Kaplan B: Death after graft loss: A novel endpoint for renal transplantation. Transplant Proc 33: 3405–3406, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Sasal J, Naimark D, Klassen J, Shea J, Bargman JM: Late renal transplant failure: An adverse prognostic factor at initiation of peritoneal dialysis. Perit Dial Int 21: 405–410, 2001 [PubMed] [Google Scholar]
- 6.Gregoor PJ, Kramer P, Weimar W, van Saase JLCM: Infections after renal allograft failure in patients with or without low-dose maintenance immunosuppression. Transplantation 63: 1528–1530, 1997 [DOI] [PubMed] [Google Scholar]
- 7.Smak Gregoor PJH, Zietse R, van Saase JLCM, op de Hoek CT, IJzermans JN, Lavrijssen AT, de Jong GM, Kramer P, Weimar W: Immunosuppression should be stopped in patients with renal allograft failure. Clin Transplant 15: 397–401, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Andrews PA, Warr KJ, Hicks JA, Cameron JS: Impaired outcome of continuous ambulatory peritoneal dialysis in immunosuppressed patients. Nephrol Dial Transplant 11: 1104–1108, 1996 [PubMed] [Google Scholar]
- 9.Badve SV, Hawley CM, McDonald SP, Mudge DW, Rosman JB, Brown FG, Johnson DW. ANZDATA Registry PD Working Committee: Effect of previously failed kidney transplantation on peritoneal dialysis outcomes in the Australian and New Zealand patient populations. Nephrol Dial Transplant 21: 776–783, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Chen A, Martz K, Kershaw D, Magee J, Rao PS: Mortality risk in children after renal allograft failure: A NAPRTCS study. Pediatr Nephrol 25: 2517–2522, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Furth SL, Donaldson LA, Sullivan EK, Watkins SL. North American Pediatric Renal Transplant Cooperative Study: Peritoneal dialysis catheter infections and peritonitis in children: A report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Nephrol 15: 179–182, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Nessim SJ, Bargman JM, Austin PC, Nisenbaum R, Jassal SV: Predictors of peritonitis in patients on peritoneal dialysis: Results of a large, prospective Canadian database. Clin J Am Soc Nephrol 4: 1195–1200, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hijazi R, Abitbol CL, Chandar J, Seeherunvong W, Freundlich M, Zilleruelo G: Twenty-five years of infant dialysis: A single center experience. J Pediatr 155: 111–117, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Bordador EB, Johnson DW, Henning P, Kennedy SE, McDonald SP, Burke JR, McTaggart SJ. Australian and New Zealand Dialysis and Transplant Registry: Epidemiology and outcomes of peritonitis in children on peritoneal dialysis in Australasia. Pediatr Nephrol 25: 1739–1745, 2010 [DOI] [PubMed] [Google Scholar]