Summary
Background and objectives
Recommendations to decrease the dialysate sodium (DNa) prescription demand analyses of patient outcomes. We analyzed morbidity and mortality at various levels of DNa, simultaneously accounting for interdialytic weight gain (IDWG) and for the mortality risk associated with lower predialysis serum sodium (SNa) levels.
Design, setting, participants, & measurements
We used multiply-adjusted linear mixed models to evaluate the magnitude of IDWG and Cox proportional hazards models to assess hospitalizations and deaths in 29,593 patients from the Dialysis Outcomes and Practice Patterns Study with baseline DNa and SNa as predictors, categorized according to lowest to highest levels.
Results
IDWG increased with higher DNa across all SNa categories, by 0.17% of body weight per 2 mEq/L higher DNa; however, higher DNa was not associated with higher mortality in a fully adjusted model (also adjusted for SNa; hazard ratio [HR]=0.98 per 2 mEq/L higher DNa, 95% confidence interval [CI] 0.95–1.02). Instead, higher DNa was associated with lower hospitalization risk (HR=0.97 per 2 mEq/L higher DNa, 95% CI 0.95–1.00, P=0.04). Additional adjustments for IDWG did not change these results. In sensitivity analyses restricted to study facilities, in which 90%–100% of patients have the same DNa (56%), the adjusted HR for mortality was 0.88 per 2 mEq/L higher DNa (95% CI 0.83–0.94). These analyses represented a pseudo-randomized experiment in which the association between DNa and mortality is unlikely to have been confounded by indication.
Conclusions
In the absence of randomized prospective studies, the benefit of reducing IDWG by decreasing DNa prescriptions should be carefully weighed against an increased risk for adverse outcomes.
Introduction
For patients with ESRD undergoing hemodialysis, the importance of sodium removal by ultrafiltration to control extracellular volume expansion has been stressed since the earliest reports (1). Additional sodium can also be removed by diffusive transport if the dialysate sodium (DNa) level is set below the predialysis serum sodium (SNa) concentration (2,3). Vice versa, higher DNa levels translate into higher dialysate-to-SNa gradients, which are associated with thirst (3,4), interdialytic weight gain (IDWG) (5–7), and hypertension (8,9).
Using a patient’s predialysis SNa as a reference to prescribe individualized or tailored DNa has thus been considered rational (3,10,11), and various prospective interventional trials have found a significant decrease in IDWG by reducing DNa (10,12). As a result of previous guidelines and recommendations (8,9,13) and recent observational studies and commentaries (7,14,15), DNa tailoring may become routine clinical practice.
Data from the Dialysis Outcomes and Practice Patterns Study (DOPPS) and a simultaneous report from the Hemodialysis Study have demonstrated that predialysis SNa concentrations are inversely associated with mortality (16,17). Prior DOPPS analyses were restricted to patients with multiple SNa measurements and intriguingly suggested that, for patients with low mean SNa levels, higher DNa prescriptions are associated with lower mortality risk. In the current study, we have expanded the study population using “baseline” data obtained at entry of patients into the DOPPS and determined the following within various strata of SNa: (1) associations between IDWG and DNa, (2) associations between DNa and outcomes, and (3) the influence of IDWG on outcomes.
Materials and Methods
Data Source
The DOPPS enrolls nationally representative samples of dialysis facilities from 12 different countries and random samples of hemodialysis patients from each participating facility. There have been four study phases (DOPPS 1–4) since the start of the DOPPS in the United States in 1996. Follow-up information is obtained every 4 months and includes laboratory measurements and dates of and diagnoses associated with patient hospitalization and death (18–20).
Both predialysis SNa measurement and the DNa prescription were available for 60% of eligible patients at their entry into DOPPS (baseline SNa). The percentages were 32% in DOPPS 1 (SNa was not recorded during the initial facility round in the United States and Japan), 65% in DOPPS 2, 78% in DOPPS 3, and 89% in DOPPS 4. All patients were included unless they were dialyzing against variable DNa concentrations (sodium modeling, n=6990; 13%), had an SNa value outside 126–150 mEq/L (n=224; <1%), or had a DNa prescription outside 125–155 mEq/L (n=583, 1%). Data from the DOPPS in the United States in phase 4 were not included because of incomplete data on hospitalizations and comorbid conditions. Compared with our previous study on mean SNa and mortality (16), 69% of patients in the present analysis were different. As a result, they represent a distinct study population that is less influenced by the frequency of laboratory measurements, possibly an indicator for patient care.
IDWG
Directly measured data on IDWG—predialysis weight from one hemodialysis session minus postdialysis weight from the previous hemodialysis session—were not uniformly available. Because intradialytic weight loss correlates very strongly with the preceding IDWG (r=0.85–0.88 based on 6124 patients in DOPPS 4), we used the physiologically more meaningful term IDWG to refer to midweek measurements of baseline interdialytic weight loss, expressed as a percentage of “dry” weight or postdialysis weight. This approach is consistent with our previous analyses (21) and other previous reports (22,23).
Statistical Analyses
Descriptive statistics were used to examine the prevalent cross-section of DOPPS 1–4 patients at baseline. Linear regression models were used to analyze (1) the association between the baseline DNa and patient characteristics and (2) IDWG as an outcome evaluated as a function of DNa and SNa. Models for baseline DNa as an outcome variable were adjusted for DOPPS phase and country in addition to all variables listed. (For example, when age is noted as a predictor of DNa, this variable has been adjusted for all other variables.) Adjustments for models with IDWG as an outcome variable are described in the figures. Adjustments for facility clustering were not appropriate for this analysis because many facilities prescribed a single DNa level to most of their patients. Assuming that baseline SNa in each facility would approximately follow a normal distribution (5), higher weight gain would be expected to cluster around facilities with a higher facility DNa, on the basis of evidence that DNa is associated with IDWG in studies at single facilities (5,6) and in prospective studies on DNa reduction (10,12).
Proportional hazards (time-to-event) models (Cox) were used to determine the association between mortality and combinations of DNa (four groups) and SNa (five groups) using a single reference category. Participants were followed until the earliest of death or up to 7 days after departure from the facility for kidney transplantation, change of treatment modality, withdrawal from dialysis, return of renal function, or transfer to another facility. Follow-up time was censored at the end of DOPPS phase for patients who did not depart from the facility. Cox models were adjusted as described in the figures. Similarly adjusted Cox models were used to examine the relationship between time to first hospitalization (excluding vascular access–related procedures) and combinations of DNa and SNa. Proportional hazards were confirmed by testing log(time) interactions and examining log-log survival plots. For calculations, we used MS Excel 2003 and SAS 9.2 for Windows (SAS Institute, Inc, Cary, NC).
Results
Predialysis SNa
In total, 29,593 patients from DOPPS phases 1–4 were eligible for and were included in the present analysis. Median follow-up time was 16.5 (interquartile range, 8.6–24.0) months. Mean (±SD) baseline SNa was 138.2±3.5 mEq/L in the study population and was unequally distributed throughout the DOPPS countries; levels were highest in Japan (139.0±3.2 mEq/L) and lowest in France (137.4±3.5 mEq/L), as previously observed for mean SNa (16).
Correlation of Variables of Interest at Baseline versus Follow-Up
SNa, DNa, and IDWG are recorded every 4 months in DOPPS phases 1, 3, and 4. For patients with at least three measurements of SNa, DNa, and IDWG (n=13,391), follow-up was defined as the mean of all subsequent measurements excluding baseline. Follow-up SNa and IDWG were highly correlated with baseline SNa and IDWG (r=0.59 and r=0.63, respectively). Mean follow-up DNa was within 1 mEq/L of baseline DNa in 86% of patients.
DNa Prescription Practices
As in our previous study of mean SNa (16), the DNa prescription in the present study population showed no correlation with baseline SNa (r=0.02). These data, analyzed at the patient level, suggested that DNa prescriptions were not tailored to the SNa. At the facility level, there was likewise no meaningful correlation between baseline facility mean SNa and mean DNa among 960 facilities (r=0.01).
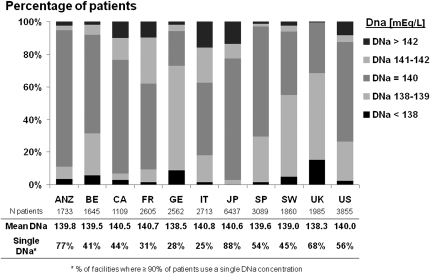
Among facilities with reported SNa and DNa in at least five patients, 56% used the same level of DNa in 90%–100% of their patients, whereas the remaining 44% of facilities used various DNa concentrations. These two practice patterns were distributed unequally across the DOPPS countries (Figure 1) and suggest that many facilities individualize their DNa prescriptions, although not specifically according to SNa. Patient characteristics in facilities with individualized versus nonindividualized DNa are shown in Table 1. In facilities with individualized DNa, we identified several patient characteristics, including hypertension, residual renal function (i.e., urine output >200 mL/d), and male sex, as statistically significant predictors for lower DNa prescription according to linear regression models (Table 2).
Figure 1.
Dialysate sodium (DNa) prescription practice in the Dialysis Outcomes and Practice Patterns Study countries. ANZ, Australia/New Zealand; BE, Belgium; CA, Canada; FR, France; GE, Germany; IT, Italy; JP, Japan; SP, Spain; SW, Sweden; UK, United Kingdom; US, United States.
Table 1.
Demographic and patient characteristics
| Patient Characteristics | All Patients | Facilities Where <90% of Patients Have the Same DNa (Individualized DNa) | Facilities Where 90%–100% of Patients Have the Same DNa (Nonindividualized) |
|---|---|---|---|
| N facilities (N patients)a | 1,062 (29,593) | 425 (13,069 = 44%) | 535 (16,282 = 55%) |
| Male | 60 | 59 | 60 |
| Residual renal function | 44 | 47 | 41 |
| Diabetesb | 37 | 35 | 38 |
| Black race | 6 | 6 | 6 |
| Comorbidities | |||
| hypertension | 81 | 82 | 80 |
| coronary artery disease | 41 | 43 | 40 |
| CVD other | 34 | 37 | 33 |
| congestive heart failure | 30 | 30 | 30 |
| peripheral vascular disease | 26 | 29 | 24 |
| cerebrovascular disease | 16 | 17 | 16 |
| psychiatric disorder | 16 | 18 | 14 |
| cancer | 13 | 14 | 12 |
| lung disease | 11 | 12 | 10 |
| neurologic disease | 10 | 11 | 10 |
| recurrent cellulitis | 8 | 8 | 7 |
| gastrointestinal bleed | 5 | 5 | 5 |
| HIV | 1 | 1 | 1 |
| Vascular access | |||
| AVF | 64 | 62 | 65 |
| catheter | 22 | 24 | 21 |
| graft | 9 | 8 | 9 |
| unknown access | 5 | 5 | 6 |
| Age (yr) | 63.0 ± 14.7 | 63.0 ± 15.0 | 63.0 ± 14.4 |
| Vintage (yr) | 1.9 (0.3–5.5) | 1.6 (0.3–5.0) | 2.1 (0.4–5.9) |
| Body mass index (kg/m2) | 23.6 (20.7–27.2) | 24.3 (21.4–27.8) | 23.1 (20.2–26.7) |
| IDWG (% of body weight) | 3.04 ± 1.91 | 2.95 ± 1.94 | 3.12 ± 1.89 |
| Predialysis SBP (mmHg) | 144.5 ± 24.4 | 141.8 ± 24.4 | 146.6 ± 24.3 |
| Laboratory parameters | |||
| albumin (g/dl) | 3.68 ± 0.53 | 3.70 ± 0.55 | 3.67 ± 0.52 |
| creatinine (mg/dl) | 8.7 ± 3.1 | 8.4 ± 3.0 | 8.9 ± 3.2 |
| ferritin (ng/ml) | 288 (130–538) | 305 (146–549) | 270 (116–527) |
| hemoglobin (g/dl) | 10.9 ± 1.7 | 11.1 ± 1.7 | 10.8 ± 1.7 |
| white blood cells (1000/mm3) | 7.1 ± 2.5 | 7.3 ± 2.5 | 6.9 ± 2.5 |
| predialysis SNa (mEq/L) | 138.2 ± 3.5 | 138.2 ± 3.6 | 138.3 ± 3.5 |
| Prescribed DNa (mEq/L) | 139.9 ± 1.9 | 140.0 ± 2.3 | 139.8 ± 1.4 |
| Calculated GNa (mEq/L) | 1.7 ± 3.9 | 1.8 ± 4.2 | 1.5 ± 3.7 |
Continuous variables expressed as means ±SD, except non–normally distributed variables, which are expressed as median (interquartile range). Categorical variables are expressed as percentages. DNa, dialysate sodium concentration (prescribed); SNa, predialysis serum sodium concentration; GNa, dialysate–to–serum sodium gradient (calculated); IDWG, interdialytic weight gain; SBP, systolic BP; CVD, cardiovascular disease; AVF, arteriovenous fistula.
Columns do not sum because only facilities with sufficient data (n≥5 data points/patients) were included in facility analyses.
Diabetes as comorbid condition or cause of ESRD.
Table 2.
Demographic and patient characteristics associated with DNa
| Predictor Variable | DNa Estimate (95% CI) (mEq/L) | |
|---|---|---|
| Facilities Where <90% of Patients Have the Same DNa (Individualized DNa) | Facilities Where 90%–100% of Patients Have the Same DNa (Nonindividualized) | |
| Male (versus female) | −0.08 (−0.15,0.00)a | 0.00 (−0.04,0.03) |
| Residual renal function (versus no RRF) | −0.17 (−0.26, −0.09)a | −0.02 (−0.07,0.02) |
| Diabetesb | −0.08 (−0.16,0.00) | −0.05 (−0.09, −0.01)a |
| Black race (versus non-black) | −0.15 (−0.32,0.03) | −0.09 (−0.18,0.01) |
| Comorbidities | ||
| hypertension | −0.24 (−0.33, −0.15)a | −0.02 (−0.06,0.03) |
| coronary artery disease | −0.10 (−0.18, −0.02)a | 0.02 (−0.02,0.06) |
| CVD other | 0.07 (−0.01,0.15) | −0.02 (−0.06,0.02) |
| congestive heart failure | 0.03 (−0.06,0.11) | 0.00 (−0.04,0.04) |
| peripheral vascular disease | 0.04 (−0.05,0.13) | −0.04 (−0.09,0.01) |
| cerebrovascular disease | 0.08 (−0.01,0.18) | 0.00 (−0.05,0.05) |
| psychiatric disorder | 0.09 (0.00,0.18) | 0.05 (−0.01,0.10) |
| cancer | 0.04 (−0.07,0.14) | 0.06 (0.01,0.12)a |
| lung disease | 0.05 (−0.05,0.16) | −0.01 (−0.07,0.06) |
| neurologic disease | 0.03 (−0.08,0.14) | −0.03 (−0.10,0.03) |
| recurrent cellulitis | −0.06 (−0.20,0.08) | 0.11 (0.03,0.19)a |
| gastrointestinal bleed | 0.05 (−0.10,0.20) | −0.03 (−0.11,0.05) |
| HIV | 0.87 (0.39,1.36)a | −0.15 (−0.42,0.12) |
| Vascular access | ||
| catheter (versus AVF) | 0.00 (−0.10,0.09) | −0.04 (−0.09,0.02) |
| graft (versus AVF) | 0.08 (−0.06,0.21) | −0.03 (−0.10,0.04) |
| unknown access (versus AVF) | −0.13 (−0.30,0.05) | 0.06 (−0.03,0.15) |
| Age (per 5 yr older) | 0.03 (0.02,0.04)a | −0.01 (−0.02,0.00)a |
| Vintage (per 1 yr longer) | 0.01 (0.01,0.02)a | 0.00 (0.00,0.01)a |
| Body mass index (per 5 units higher) | 0.05 (0.02,0.09)a | 0.02 (0.00,0.04)a |
| Facility characteristics | ||
| facility % Kt/V <1.2 (per 20% more) | −0.06 (−0.11, −0.02)a | −0.13 (−0.15, −0.10)a |
| facility % phosphorus <5.5 (per 20% more) | −0.24 (−0.30, −0.18)a | 0.01 (−0.02,0.04) |
| facility % catheter use (per 20% more) | −0.03 (−0.08,0.02) | −0.01 (−0.04,0.02) |
| Laboratory parameters | ||
| albumin (per 1 g/dl higher) | −0.02 (−0.09,0.06) | −0.13 (−0.17, −0.09)a |
| creatinine (per 1 mg/dl higher) | 0.00 (−0.02,0.01)a | 0.00 (−0.01,0.00) |
| ferritin (per 100 ng/ml higher) | 0.01 (0.00,0.01) | 0.00 (0.00,0.01) |
| hemoglobin (per 1 g/dl higher) | 0.03 (0.01,0.05) | 0.01 (0.00,0.02) |
| white blood cells (per 1000/mm3 higher) | 0.00 (−0.01,0.02) | −0.01 (−0.02,0.00)a |
| predialysis SNa (per 1 mEq/L higher) | 0.02 (0.01,0.03)a | 0.00 (0.00,0.01) |
CI, confidence interval; DNa, dialysate sodium concentration (prescribed); SNa, predialysis serum sodium concentration; RRF, residual renal function; CVD, cardiovascular disease; AVF, arteriovenous fistula.
Statistical significance.
Diabetes as comorbid condition or cause of ESRD.
IDWG and Predialysis Systolic BP
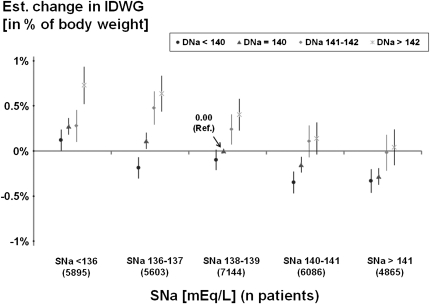
IDWG was slightly lower in facilities with individualized versus nonindividualized DNa (Table 1). When IDWG was treated as an outcome variable and DNa as a predictor in linear regression models, DNa prescriptions >142 mEq/L (mean, 144.2 mEq/L) were consistently associated with the highest IDWG, even when analyzed in different strata of SNa (Figure 2). Compared with patients with DNa <140 mEq/L, IDWG in patients with DNa >142 mEq/L was higher by 0.55% of total body weight (95% confidence interval [CI], 0.45%, 0.66%). Expressed continuously, IDWG increased by 0.17% of postdialysis body weight per 2 mEq/L higher DNa (95% CI, 0.15%, 0.20%), independent of SNa. Moreover, IDWG decreased by 0.10% of postdialysis body weight per 2 mEq/L higher SNa (95% CI, −0.11%, −0.09%), independent of DNa, suggesting that higher IDWG may lead to a slight dilution of the predialysis SNa.
Figure 2.
Interdialytic weight gain by dialysate sodium (DNa) and predialysis serum sodium (SNa). Linear regression model using a single reference point (SNa category: 138–139 mEq/L; DNa category: 140 mEq/L) and adjusted for Dialysis Outcomes and Practice Patterns Study phase, country, age, sex, body mass index, diabetes, and 13 other comorbid conditions. Test for SNa × DNa interaction was not significant (P=0.22). Overall tests for trend were significant for both SNa (P<0.0001) and DNa (P<0.0001). Mean interdialytic weight gain for the single reference group was 3.12% of body weight. Data are shown with 95% confidence intervals. Est., estimated.
Average predialysis systolic BP (SBP) was 4.8 mmHg lower in facilities with individualized DNa versus nonindividualized DNa (Table 1). With predialysis SBP as an outcome and DNa as a predictor in linear regression models, predialysis SBP decreased by 0.88 mmHg per 2 mEq/L higher DNa (95% CI, −1.21, −0.56 mmHg). In an analysis restricted to facilities with individualized DNa, predialysis SBP was 1.50 mmHg lower per 2 mEq/L higher DNa (95% CI, −1.90 to −1.10 mmHg). With analysis restricted to facilities with nonindividualized DNa, however, predialysis SBP was 0.66 mmHg higher per 2 mEq/L higher DNa (95% CI, 0.05, 1.28).
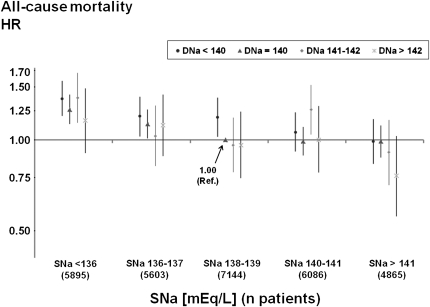
Mortality Associated with Predialysis SNa and DNa
We confirmed in the present study population that predialysis SNa was associated inversely with all-cause mortality (hazard ratio [HR], 0.94 per 2 mEq/L higher SNa [95% CI, 0.93, 0.96]; P<0.0001), as observed by 16) and 17). Because higher IDWG has been associated with a higher mortality risk (21,24), it might be hypothesized that higher DNa also would be associated with higher mortality. However, higher DNa concentrations were not associated with higher mortality across all subgroups of baseline SNa (Figure 3). Instead, the fully adjusted overall mortality HR (also adjusted for predialysis SNa) was lower with higher DNa (HR, 0.98 per 2 mEq/L higher DNa), without reaching statistical significance (95% CI, 0.95, 1.02; P=0.29). Of note, additional adjustments for IDWG and predialysis SBP did not systematically affect the overall mortality risk associated with various DNa prescriptions or the corresponding significance levels (Supplementary Figure 1: mortality HR, 0.98 per 2 mEq/L higher DNa [95% CI, 0.95, 1.01]). Moreover, the relation between DNa and mortality did not vary by level of IDWG (P for interaction = 0.79).
Figure 3.
Adjusted all-cause mortality risk by dialysate sodium (DNa) and predialysis serum sodium (SNa). Cox model using a single reference point (predialysis SNa category: 138–139 mEq/L; DNa category: 140 mEq/L), stratified by phase and region and adjusted for age, race, sex, vintage, body mass index, diabetes, 13 other comorbid conditions, residual renal function, vascular access, serum albumin, hemoglobin, serum creatinine, ferritin, white blood cell count, and facility clustering. Test for SNa × DNa interaction was not significant (P=0.92). Overall tests for trend were significant for SNa (P<0.0001) but not for DNa (P=0.29). Data are shown with 95% confidence intervals. HR, hazard ratio.
Sensitivity Analyses of Mortality Risk
Residual Renal Function.
In the preceding mortality analysis, the model was adjusted for residual renal function. To further determine whether patients without residual renal function might have a worse outcome because of higher IDWG, we restricted the analysis to patients with vintage >1 year and no urine volume >200 mL/d (n=13,331). In this analysis, the mortality HRs were 0.94 per 2 mEq/L higher SNa (95% CI, 0.92, 0.97) and 0.99 per 2 mEq/L higher DNa (95% CI, 0.94, 1.04), consistent with our overall model.
Individualized versus Nonindividualized DNa.
When the analysis was restricted to patients in facilities with nonindividualized DNa, DNa was significantly associated with lower all-cause mortality (HR, 0.88 per 2 mEq/L higher DNa [95% CI, 0.83, 0.94]). By contrast, in facilities with individualized DNa, the mortality HR was 1.04 per 2 mEq/L higher DNa (95% CI, 1.00, 1.08).
Cardiovascular Mortality.
With analysis restricted to deaths from cardiovascular disease (CVD), the CVD mortality HR was 0.96 per 2 mEq/L higher DNa (95% CI, 0.90, 1.03). In facilities with nonindividualized DNa, the CVD mortality risk was significantly associated with lower CVD-mortality (HR, 0.81 per 2 mEq/L higher DNa [95% CI, 0.69, 0.94]). By contrast, in facilities with individualized DNa, the CVD mortality HR was 1.04 per 2 mEq/L higher DNa (95% CI, 0.97, 1.12).
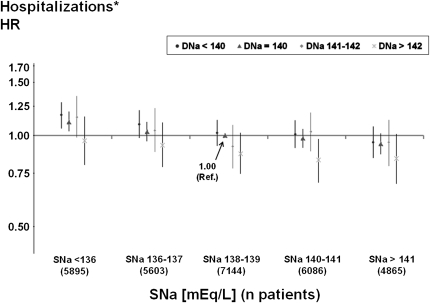
Hospitalization Risk Associated with DNa and Predialysis SNa
DNa was inversely associated with the risk for first hospitalization from any cause, excluding vascular access procedures, in adjusted Cox models. This finding was consistent through all categories of baseline SNa (Figure 4). Overall, the hospitalization risk was 3% lower per 2 mEq/L higher DNa (HR, 0.97 [95% CI, 0.95, 1.00]; P=0.04), independent of SNa, and 3% lower per 2 mEq/L higher SNa (HR, 0.97 [95% CI, 0.96, 0.98]), independent of DNa. Adjusting the models for IDWG and predialysis SBP did not systematically affect the overall morbidity risk associated with various DNa prescriptions or the corresponding significance levels (Supplementary Figure 2: hospitalization HR, 0.97 per 2 mEq/L higher DNa [95% CI, 0.95, 1.00]; P=0.04).
Figure 4.
Adjusted hospitalization risk by dialysate sodium and predialysis serum sodium. *Hospitalizations of all-cause but non–vascular access-related conditions were analyzed. Cox model using a single reference point (predialysis serum sodium [SNa] category: 138–139 mEq/L; dialysate sodium [DNa] category: 140 mEq/L), stratified by phase and region and adjusted for age, race, sex, vintage, body mass index, diabetes, 13 other comorbid conditions, residual renal function, vascular access, serum albumin, hemoglobin, serum creatinine, ferritin, white blood cell count, and facility clustering. Test for SNa × DNa interaction was not significant (P=0.61). Overall tests for trend were significant for both SNa (P<0.0001) and DNa (P=0.04). Data are shown with 95% confidence intervals. HR, hazard ratios.
Sensitivity Analyses of Hospitalization Risk
Restricting analyses to patients in facilities with nonindividualized DNa or individualized DNa prescriptions yielded consistent inverse correlations of DNa with hospitalization risk (HR, 0.97 per 2 mEq/L higher DNa in each analysis). The risk for hospitalization due to fluid overload (recorded only in DOPPS 2–4) was also lower with higher DNa (HR, 0.94 per 2 mEq/L higher [95% CI, 0.84, 1.05]).
Dialysate-to-SNa Gradient and Mortality
Our analyses of DNa within various strata of SNa did not identify an increased mortality risk associated with higher DNa. However, an additional technique was used to investigate whether the dialysate-to-SNa gradient (GNa) might be associated with mortality beyond SNa alone. First, we observed that the GNa was directly associated with mortality risk (HR, 1.05 per 2 mEq/L higher GNa [95% CI, 1.03, 1.06]). Then we created a random “faux DNa” for each patient, normally distributed about a mean of 140 mEq/L, with an SD of 1.85 mEq/L (as observed in this study). We created a “faux gradient” by subtracting the patient’s observed SNa from the faux DNa. The mortality results were essentially the same for faux GNa (HR, 1.05 per 2 mEq/L higher faux GNa [95% CI, 1.03, 1.06]) compared with the actual GNa and were consistent across five iterations, strongly suggesting that the association between the gradient and mortality is primarily attributable to the SNa component and not the DNa component of the gradient.
Discussion
In the present study, we have shown that higher DNa prescriptions are associated with increased IDWG of the order of 0.17% of postdialysis weight (or 0.12 kg for a 70-kg patient) per 2 mEq/L higher DNa, but not with a higher risk for hospitalization or death. Instead, patients dialyzed with higher DNa concentrations had a significantly lower risk for hospitalization and, in facilities where all or almost all patients used the same DNa, a significantly lower risk for death.
Previous studies have found that greater dialysate-to-SNa concentration gradients are associated with higher mortality, and our analyses by gradient confirm this observation (25). Because high gradients are typically observed in patients with low SNa who have an associated higher mortality risk, it was important to analyze the components of the gradient: SNa and DNa. Figure 3 supports the hypothesis that SNa explains the association of mortality with higher sodium concentration gradients by showing that mortality does not increase with DNa within each SNa group. Our additional analyses using a random variable DNa to create a faux gradient also indicate that the association between GNa and mortality depends on the mortality risk associated with lower SNa.
Encouragement to decrease the DNa concentration seem logical because thirst and IDWG can be reduced. However, the incremental IDWG, adjusted for SNa, was relatively small and our analyses did not identify an influence of IDWG on morbidity and mortality associated with DNa. Furthermore, viewing IDWG as a principal determinant of dialysis mortality may be questioned, for the following reasons:
Only patients with very high levels of IDWG (>5.7% of body weight) have an elevated risk (21).
The magnitude of the adjusted mortality risk previously identified with very large IDWG did not exceed 1.25 (24).
IDWG is not synonymous with chronic fluid overload. When measured by bioimpedance (26) or inferred from slopes of intradialytic relative plasma volume (27), there are markedly higher mortality risks for overhydration (HR, 1.7) (26) or hypervolemia (HR, 2.1) (27) than for IDWG (21,24).
The mortality risk associated with higher DNa was significantly lower in facilities that did not individualize their DNa prescriptions but was higher in facilities that used individualized DNa. The former analysis represents a pseudo-randomized experiment in which a normally distributed range of patient SNa levels are subjected to the same DNa. The latter result is likely confounded by indication because sicker and older patients were treated with higher DNa (Table 2).
We obtained similarly divergent associations between predialysis SBP and DNa in facilities with individualized versus nonindividualized DNa. Predialysis SBP was higher with higher DNa prescriptions in nonindividualized DNa-facilities, suggesting that in the pseudo-randomized experiment of facilities with nonindividualized DNa, we could identify the physiologic effect of higher DNa on BP, demonstrated in small prospective clinical trials of reduced or individualized DNa (12,28,29). In contrast, the finding of relatively higher SBP associated with lower DNa prescriptions in individualized DNa facilities is likely confounded by indication because hypertension is associated with prescription of lower DNa (Table 2).
This study is observational; thus, we can only speculate as to why higher DNa does not appear to be harmful. A recent publication demonstrated that lower urinary sodium excretion is associated with higher CVD mortality (30), consistent with previous reports (31–34), and questioned the assumption that reductions in dietary salt intake could substantially reduce CVD events and medical costs (35). However, these observations may not be transferrable to patients undergoing hemodialysis. The observed benefits of higher DNa may be due to improved intradialytic cardiovascular stability, which may reduce the risks associated with intradialytic hypotension (36), myocardial stunning (37,38), and endotoxemia (39).
Other study limitations include the lack of quality control of the delivered dialysate concentration and our inability to identify which assay was used by various DOPPS facilities to determine predialysis SNa. However, our previous analysis at a single center showed that the difference between measured and prescribed DNa concentrations was on average very close to zero, and its distribution was not skewed (5). Regarding sodium assays, recommendations from the International Federation of Clinical Chemistry Expert Panel on the conversion of sodium levels obtained by direct potentiometry should ensure that these values correspond to indirect potentiometry, which is typically used in centralized laboratories (40), and thus most likely by most DOPPS facilities.
In conclusion, higher DNa prescriptions were associated with higher IDWG but also with a 3% lower risk for hospitalization for every 2 mEq/L higher DNa. In facilities that did not individualize DNa prescriptions, all-cause mortality risk was 12% lower with every 2 mEq/L higher DNa. These findings were independent of predialysis SNa. Thus, the benefit of reducing IDWG by decreasing DNa does not seem to translate into improved outcomes and, in the absence of randomized prospective studies, should be balanced carefully against a potentially increased risk for morbidity and death.
Disclosures
For the present analysis, M.H. received a research grant from the Else Kröner-Fresenius Foundation without restrictions on publications and has been a visiting scientist at Arbor Research Collaborative for Health.
Supplementary Material
Acknowledgments
Heather Van Doren provided editorial assistance for this manuscript, and Jinyao Zhang was consulted for additional statistical support (both employees of Arbor Research Collaborative for Health).
The DOPPS is administered by Arbor Research Collaborative for Health and is supported by scientific research grants from Amgen (since 1996), Kyowa Hakko Kirin (since 1999, in Japan), Genzyme (since 2009), and Abbott (since 2009), without restrictions on publications.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.05440611/-/DCSupplemental.
See related editorial, “Dialysate Sodium and the Milieu Intérieur,” on pages 5–7.
References
- 1.Scribner BH, Buri R, Caner JE, Hegstrom R, Burnell JM: The treatment of chronic uremia by means of intermittent hemodialysis: A preliminary report. 1960. J Am Soc Nephrol 9: 719–726, discussion 719–726, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Flanigan MJ: How should dialysis fluid be individualized for the chronic hemodialysis patient? Sodium. Semin Dial 21: 226–229, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Lindley EJ: Reducing sodium intake in hemodialysis patients. Semin Dial 22: 260–263, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Flanigan MJ: Role of sodium in hemodialysis. Kidney Int Suppl 76: S72–S78, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Hecking M, Kainz A, Hörl WH, Herkner H, Sunder-Plassmann G: Sodium setpoint and sodium gradient: influence on plasma sodium change and weight gain. Am J Nephrol 33: 39–48, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Keen ML, Gotch FA: The association of the sodium “setpoint” to interdialytic weight gain and blood pressure in hemodialysis patients. Int J Artif Organs 30: 971–979, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Munoz Mendoza J, Sun S, Chertow GM, Moran J, Doss S, Schiller B: Dialysate sodium and sodium gradient in maintenance hemodialysis: A neglected sodium restriction approach? Nephrol Dial Transplant 26: 1281–1287, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal R: Management of hypertension in hemodialysis patients. Hemodial Int 10: 241–248, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Charra B: Fluid balance, dry weight, and blood pressure in dialysis. Hemodial Int 11: 21–31, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Santos SF, Peixoto AJ: Revisiting the dialysate sodium prescription as a tool for better blood pressure and interdialytic weight gain management in hemodialysis patients. Clin J Am Soc Nephrol 3: 522–530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flanigan M: Dialysate composition and hemodialysis hypertension. Semin Dial 17: 279–283, 2004 [DOI] [PubMed] [Google Scholar]
- 12.de Paula FM, Peixoto AJ, Pinto LV, Dorigo D, Patricio PJ, Santos SF: Clinical consequences of an individualized dialysate sodium prescription in hemodialysis patients. Kidney Int 66: 1232–1238, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Jindal K, Chan CT, Deziel C, Hirsch D, Soroka SD, Tonelli M, Culleton BF. Canadian Society of Nephrology Committee for Clinical Practice Guidelines: Hemodialysis clinical practice guidelines for the Canadian Society of Nephrology. J Am Soc Nephrol 17[Suppl 1]: S1–S27, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Penne EL, Sergeyeva O: Sodium gradient: A tool to individualize dialysate sodium prescription in chronic hemodialysis patients? Blood Purif 31: 86–91, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Lomonte C, Basile C: Do not forget to individualize dialysate sodium prescription. Nephrol Dial Transplant 26:1126–1128, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Hecking M, Karaboyas A, Saran R, Sen A, Hörl WH, Pisoni RL, Robinson BM, Sunder-Plassmann G, Port FK: Predialysis serum sodium level, dialysate sodium, and mortality in maintenance hemodialysis patients: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2011. September 22 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Waikar SS, Curhan GC, Brunelli SM: Mortality associated with low serum sodium concentration in maintenance hemodialysis. Am J Med 124: 77–84, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner MH, Wolfe RA: The Dialysis Outcomes and Practice Patterns Study (DOPPS): Design, data elements, and methodology. Am J Kidney Dis 44[Suppl 2]: 7–15, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Young EW, Goodkin DA, Mapes DL, Port FK, Keen ML, Chen K, Maroni BL, Wolfe RA, Held PJ: The Dialysis Outcomes and Practice Patterns Study (DOPPS): An international hemodialysis study. Kidney Int 57: S74–S81, 2000 [Google Scholar]
- 20.Robinson BM, Port FK: Caring for dialysis patients: international insights from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Identifying best practices and outcomes in the DOPPS. Semin Dial 23: 4–6, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Saran R, Bragg-Gresham JL, Rayner HC, Goodkin DA, Keen ML, Van Dijk PC, Kurokawa K, Piera L, Saito A, Fukuhara S, Young EW, Held PJ, Port FK: Nonadherence in hemodialysis: associations with mortality, hospitalization, and practice patterns in the DOPPS. Kidney Int 64: 254–262, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Leggat JE, Jr, Orzol SM, Hulbert-Shearon TE, Golper TA, Jones CA, Held PJ, Port FK: Noncompliance in hemodialysis: Predictors and survival analysis. Am J Kidney Dis 32: 139–145, 1998 [DOI] [PubMed] [Google Scholar]
- 23.Tang HL, Wong SH, Chu KH, Lee W, Cheuk A, Tang CM, Kong IL, Fung KS, Tsang WK, Chan HW, Tong KL: Sodium ramping reduces hypotension and symptoms during haemodialysis. Hong Kong Med J 12: 10–14, 2006 [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, Fonarow GC: Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation 119: 671–679, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sergeyeva O, Usvyat L, Kotanko P, Levin N: Positive intradialytic sodium gradients relate to increasing intradialytic weight gain, hospitalization rate, and mortality in chronic hemodialysis patients. World Congress of Nephrology poster. NDT Plus 2(suppl 2):iii650, 2009 [Google Scholar]
- 26.Wizemann V, Wabel P, Chamney P, Zaluska W, Moissl U, Rode C, Malecka-Masalska T, Marcelli D: The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant 24: 1574–1579, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agarwal R: Hypervolemia is associated with increased mortality among hemodialysis patients. Hypertension 56: 512–517, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambie SH, Taal MW, Fluck RJ, McIntyre CW: Online conductivity monitoring: Validation and usefulness in a clinical trial of reduced dialysate conductivity. ASAIO J 51: 70–76, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Manlucu J, Gallo K, Heidenheim PA, Lindsay RM: Lowering postdialysis plasma sodium (conductivity) to increase sodium removal in volume-expanded hemodialysis patients: a pilot study using a biofeedback software system. Am J Kidney Dis 56: 69–76, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Stolarz-Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerová J, Richart T, Jin Y, Olszanecka A, Malyutina S, Casiglia E, Filipovský J, Kawecka-Jaszcz K, Nikitin Y, Staessen JA. European Project on Genes in Hypertension (EPOGH) Investigators: Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA 305: 1777–1785, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Alderman MH, Madhavan S, Cohen H, Sealey JE, Laragh JH: Low urinary sodium is associated with greater risk of myocardial infarction among treated hypertensive men. Hypertension 25: 1144–1152, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Cohen HW, Hailpern SM, Alderman MH: Sodium intake and mortality follow-up in the Third National Health and Nutrition Examination Survey (NHANES III). J Gen Intern Med 23: 1297–1302, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alderman MH, Cohen H, Madhavan S: Dietary sodium intake and mortality: The National Health and Nutrition Examination Survey (NHANES I). Lancet 351: 781–785, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Cohen HW, Hailpern SM, Fang J, Alderman MH: Sodium intake and mortality in the NHANES II follow-up study. Am J Med 119: 275.e7–14, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, Goldman L: Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 362: 590–599, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tislér A, Akócsi K, Borbás B, Fazakas L, Ferenczi S, Görögh S, Kulcsár I, Nagy L, Sámik J, Szegedi J, Tóth E, Wágner G, Kiss I: The effect of frequent or occasional dialysis-associated hypotension on survival of patients on maintenance haemodialysis. Nephrol Dial Transplant 18: 2601–2605, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Breidthardt T, McIntyre CW: Dialysis-induced myocardial stunning: the other side of the cardiorenal syndrome. Rev Cardiovasc Med 12: 13–20, 2011 [DOI] [PubMed] [Google Scholar]
- 38.McIntyre CW: Haemodialysis-induced myocardial stunning in chronic kidney disease a new aspect of cardiovascular disease. Blood Purif 29: 105–110, 2010 [DOI] [PubMed] [Google Scholar]
- 39.McIntyre CW, Harrison LE, Eldehni MT, Jefferies HJ, Szeto CC, John SG, Sigrist MK, Burton JO, Hothi D, Korsheed S, Owen PJ, Lai KB, Li PK: Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol 6: 133–141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holbek CC: Understanding the different values in electrolyte measurements. 2002. Available at http://acutecaretesting.org Accessed August 20, 2010
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.