Summary
Background and objectives
Anemia in patients with CKD is highly related to impaired erythropoietin (EPO) response, the timing and determinants of which remain unknown.
Design, setting, participants, & measurements
This study measured EPO levels and studied their relation to GFR measured by 51Cr-EDTA renal clearance (mGFR) in 336 all-stage CKD patients not receiving any erythropoiesis-stimulating agent.
Results
In patients with anemia defined by World Health Organization criteria (hemoglobin [Hb] <13 g/dl in men and 12 g/dl in women), EPO response to Hb level varied by mGFR level. EPO and Hb levels were negatively correlated (r=−0.22, P=0.04) when mGFR was >30 ml/min per 1.73 m2, whereas they were not correlated when mGFR was <30 (r=0.09, P=0.3; P for interaction=0.01). In patients with anemia, the ratio of observed EPO to the level predicted by the equation for their Hb level decreased from 0.72 (interquartile range, 0.57–0.95) for mGFR ≥60 ml/min per 1.73 m2 to 0.36 (interquartile range, 0.16–0.69) for mGFR <15. Obesity, diabetes with nephropathy other than diabetic glomerulopathy, absolute iron deficiency, and high C-reactive protein concentrations were associated with increased EPO levels, independent of Hb and mGFR.
Conclusions
Anemia in CKD is marked by an early relative EPO deficiency, but several factors besides Hb may persistently stimulate EPO synthesis. Although EPO deficiency is likely the main determinant of anemia in patients with advanced CKD, the presence of anemia in those with mGFR >30 ml/min per 1.73 m2 calls for other explanatory factors.
Introduction
Erythropoietin (EPO) was first isolated in 1971 (1) and its gene was cloned in 1985 (2). EPO is produced in the kidney and the liver and stimulates the differentiation and proliferation of erythroid progenitors. The subsequent synthesis of recombinant human EPO markedly changed the management of ESRD-related anemia. Recent findings that erythropoiesis-stimulating agents (ESAs) have not improved cardiovascular and renal outcomes in patients with non–end stage CKD (3–5) have raised questions about the timing and degree of EPO deficiency in renal anemia (6). Our knowledge of the underlying cause of impaired EPO production is also incomplete.
A decrease in the endogenous EPO response to anemia is thought to be one of the major mechanisms of CKD anemia. In patients without CKD, EPO and hemoglobin (Hb) levels are negatively correlated, a feedback regulation that tends to be reversed in CKD patients. (7–9) In ESRD patients, the feedback regulation process is blunted, even though it is somewhat preserved after hemorrhage (10,11) and possibly at high altitudes (12). EPO levels remain in the normal range in CKD patients compared with non-anemic healthy controls (7,13,14). CKD patients nonetheless have lower than expected EPO levels for their degree of anemia. The decrease in the endogenous EPO response to anemia has not been quantified in these patients, however. In addition, all studies conducted before 1980 used bioassays that provided higher EPO levels than those measured with the double-antibody sandwich immunoassays currently used. Moreover, although inflammation and iron deprivation are well documented risk factors for ESA resistance in dialysis patients, the determinants of EPO production have not been systematically studied in early stage CKD.
We used reference methods to investigate the timing of EPO deficiency and its determinants according to renal function in 336 CKD patients. In those with anemia, we also quantified the endogenous EPO response to Hb decreases according to GFR level.
Materials and Methods
Study Population
The NephroTest study is a prospective hospital-based cohort, enrolling adult patients with all CKD diagnoses, stages I–V, who are not pregnant and not on dialysis or living with a kidney transplant (15). By the end of 2009, 1294 patients had been referred to two physiology departments to assess CKD progression and complications, including measured GFR (mGFR), according to standard methods. All patients provided written informed consent before inclusion in the cohort. In this study, we analyzed a subgroup of 336 patients who were receiving neither ESA nor intravenous iron and whose endogenous EPO was assayed. They did not differ from those without EPO measures for baseline age, sex, diabetes, median mGFR, or Hb level (data not shown).
Clinical and Biological Information
Demographic, clinical, and laboratory data were collected. The proportion of biopsy-proven nephropathy was 21% among all patients, but <10% among those with diabetes. Diabetes patients without renal biopsies were classified with diabetic glomerulopathy according to clinical criteria, specifically a history of albuminuria >300 mg/g creatininuria and of other microangiopathy (retinopathy and/or neuropathy). The diabetes patients not meeting these criteria were considered to have other types of nephropathy, the most common of which was likely vascular nephropathy. We used Lipschitz's index (16) to define iron status on the basis of transferrin saturation (TSAT) and ferritin as follows: normal, TSAT ≥20%; TSAT <20% and ferritin <40 ng/ml, absolute iron deficiency; TSAT <20% and ferritin ≥40 ng/ml, functional iron deficiency. We primarily used the following World Health Organization (WHO) sex-specific thresholds to define anemia: Hb concentration <12 g/dl for women and <13 g/dl for men. GFR was measured by renal clearance of 51Cr-EDTA as described earlier (17) and was categorized according to Kidney Disease Improving Global Outcomes guidelines (18).
EPO Measurement and Responsiveness to Hb Level
Endogenous EPO levels were determined in serum (100 µl) with the Quantikine IVD EPO double-antibody sandwich ELISA method from R&D Systems (Minneapolis, MN).
To assess the response of endogenous EPO to Hb levels, we used a method previously developed and validated in non-CKD patients with anemia (19–21). We predicted the EPO value for a given Hb level for all patients with WHO-defined anemia according to the following equation: predicted log10EPO (p-log10EPO) = 4.46 − (0.274 × Hb in g/dl), so that predicted EPO (p-EPO) = 10(p-logEPO) in international units per liter (19). We then calculated the ratio of observed/predicted EPO in these patients by dividing the observed endogenous EPO levels by those predicted by the equation. Unlike other studies (19), however, we divided crude rather than log-transformed EPO values to express results as a percentage of EPO deficiency.
Statistical Analyses
EPO values, because they were not normally distributed, were log-transformed in the regression analyses. The relationships between EPO, Hb, and mGFR levels were studied in three steps. We first analyzed EPO response to decreased Hb according to mGFR level, independent of anemia status. For this purpose, we assessed the correlation between Hb and log10EPO and tested the interactions with renal function level for different mGFR thresholds: 15, 30, and 45 ml/min per 1.73 m2. We then studied the correlations between endogenous EPO and Hb levels according to WHO-defined anemia status and mGFR in two classes: <30 or ≥30 ml/min per 1.73 m2. Finally, we studied Pearson correlations between EPO and mGFR levels in patients with and without anemia. In a sensitivity analysis, we repeated this step after classifying patients according to anemia defined with only one cut-off value (12 g/dl) irrespective of sex.
Other factors associated with EPO levels were first studied with bivariate analyses that used Kruskal–Wallis correlations for qualitative variables and Pearson correlations for quantitative variables. Interactions with mGFR, anemia status, or sex in the relationships between the factors studied and EPO level were systematically tested. We then conducted a multivariate regression analysis of determinants of EPO levels, using three nested models that we compared according to maximum likelihood criteria. Model 1 included age in years, center, and a combined variable derived from previous step findings: no anemia, anemia and mGFR ≥30 ml/min per 1.73 m2, anemia, and mGFR <30. Models 2 and 3 sequentially added other risk factors identified from the bivariate analysis, namely, body mass index (BMI) and diabetes with glomerular or other types of nephropathy in model 2, and iron index and C-reactive protein (CRP) in model 3. We studied log10EPO and expressed the results in percentages of EPO change in the exposed compared with the reference category. All analyses were performed with SAS 9.1 software (SAS Institute, Cary, NC).
Results
Patient Characteristics
Mean age was approximately 60 years, and patients were predominantly men and of European origin (Table 1). Vascular nephropathy was the most common type of CKD. Two-thirds of the patients had CKD stage IIIB or IV. WHO-defined anemia (Hb <13 g/dl in men and Hb <12 g/dl in women) was present in 55% of men and 63% of women. EPO levels ranged from 1.9 to 47.8 IU/L.
Table 1.
Patient characteristics
| Characteristic | Value |
|---|---|
| Total no. of patients | 336 |
| Age (yr) | 58.8±14.6 |
| Male sex | 246 (73) |
| African origin | 25 (7.4) |
| Diabetes | |
| with diabetic glomerulopathy | 46 (13.6) |
| with other nephropathy types | 43 (12.8) |
| Kidney disease | |
| diabetic nephropathy | 46 (13.6) |
| glomerular nephropathy | 57 (16.9) |
| vascular nephropathy | 94 (27.8) |
| polycystic kidney disease | 8 (2.3) |
| interstitial nephritis | 32 (9.5) |
| unknown | 99 (29.4) |
| BMI (kg/m2) | 26.1±4.2 |
| mGFR (ml/min per 1.73 m2) | 33.6 (22.9–46.1) |
| ≥60 | 35 (10.4) |
| 45–60 | 52 (15.4) |
| 30–45 | 117 (34.8) |
| 15–30 | 103 (30.6) |
| <15 | 29 (8.6) |
| PCR (mg/mmol) | 45.3 (17.7–142.5) |
| Serum albumin (g/L) | 40.2±5.2 |
| Hemoglobin (g/dl) | 12.4±1.4 |
| men | 12.7±1.4 |
| women | 11.6±1.3 |
| EPO (IU/L) | 9.1 (6.7–13.01) |
| Oral iron therapy | 43 (12.8) |
| ACEI/ARBs | 265 (78.8) |
Data are expressed as mean ± SD, median (interquartile range), or n (%). BMI, body mass index; mGFR, GFR measured by 51Cr-EDTA renal clearance; PCR, ratio of urinary protein to creatinine; EPO, measured erythropoietin; ACEI/ARBs, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers.
Relations between EPO, Hb, and MGFR levels
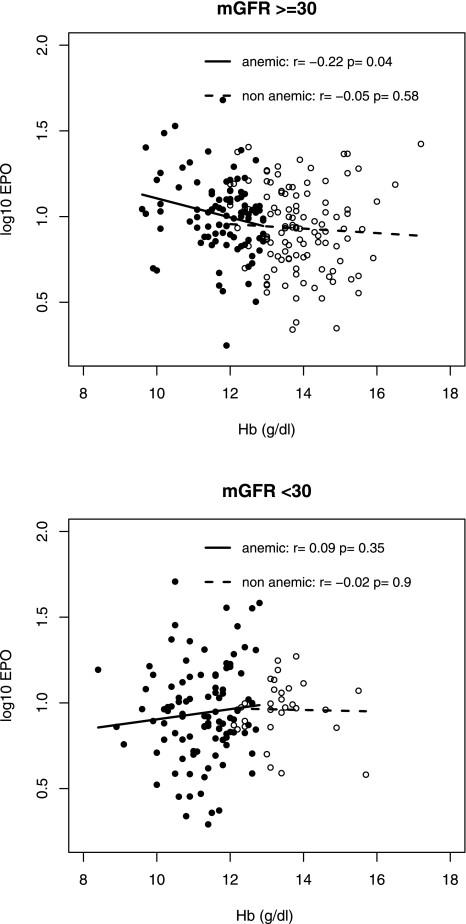
Endogenous EPO response to Hb level varied according to mGFR level. The strongest interaction between Hb and mGFR in the relationship with EPO was observed for an mGFR threshold of 30 ml/min per 1.73 m2 (P=0.01 for interaction); interaction was not statistically significant for mGFR of 45 or 15 ml/min per 1.73 m2 (P=0.72 and P=0.18). EPO and Hb levels were negatively correlated (r=−0.2, P=0.04) in patients with mGFR values ≥30 ml/min per 1.73 m2, but not in those with mGFR levels <30 (r=0.07, P=0.32). Analysis by anemia status, however, showed that this pattern was limited to patients with anemia (Figure 1).
Figure 1.
Relations between erythropoietin (log10) and hemoglobin (Hb) levels by anemia status according to the World Health Organization definition and measured GFR (mGFR) level. Open circles, nonanemic patients; solid circles, anemic patients.
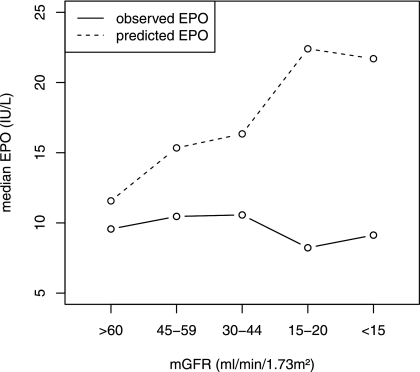
Moreover, there was no correlation between EPO and mGFR levels in patients with or without anemia (r=0.08 and r=0.01, respectively, both not significant). In anemic patients, predicted EPO values were consistently greater than observed EPO at every mGFR (Figure 2). The observed/predicted EPO ratio fell markedly with mGFR decline, from 0.72 [interquartile range, 0.57–0.95] for mGFR ≥60 ml/min per 1.73 m2 to 0.36 [interquartile range, 0.16–0.69] for mGFR <15, and the relative EPO deficits ranged from 28% to 64% for CKD stages I–II and V.
Figure 2.
Observed and predicted erythropoietin (EPO) by measured GFR (mGFR) levels in patients with anemia according to the World Health Organization definition.
Other Factors Associated with EPO Level
EPO was significantly higher in patients who were 60–69 years of age, were overweight or obese, or had absolute iron deficiency, diabetes with nephropathy other than diabetic glomerulopathy, or CRP >8 mg/L compared with their counterparts without these conditions (Table 2). EPO levels were not related to sex, geographic origin, treatment with renin angiotensin system inhibitors, urine protein/creatinine ratio, vitamin D, or folate levels. There was a significant interaction (P=0.04) between serum albumin and mGFR. Higher EPO levels with lower albumin values were observed only in patients with mGFR ≥30 ml/min per 1.73 m2. No other significant interaction was seen with mGFR, sex, or anemia (all P≥0.2). The mean Hb level was significantly higher in overweight or obese patients than in those with normal BMI (12.6±1.5 versus 12.2±1.3 g/dl; P=0.03), but did not significantly differ with CRP level (≤8 or >8 mg/L: 12.5±1.5 versus 12.1±1.5 g/dl; P=0.16).
Table 2.
Crude analysis of factors associated with erythropoietin (EPO) levels
| Factor | n | EPO (UI/ml), median (IQR) | P |
|---|---|---|---|
| Sex | 0.2 | ||
| men | 245 | 9.3 (6.7–13.5) | |
| women | 91 | 8.9 (6.7–11.9) | |
| Age (yr) | 0.01 | ||
| <50 | 91 | 8.5 (5.7–11.5) | |
| 50–59 | 76 | 8.9 (6.9–11.6) | |
| 60–69 | 73 | 10.5 (7.2–16.1)a | |
| 70–max | 96 | 9.2 (7.0–12.7) | |
| African origin | 0.3 | ||
| no | 296 | 8.9 (6.7–12.6) | |
| yes | 25 | 9.9 (6.1–13.6) | |
| Body mass index (kg/m2) | 0.005 | ||
| <25 | 136 | 8.6 (5.9–11.4) | |
| 25–30 | 138 | 9.3 (6.7–13.1)b | |
| 30–max | 62 | 10.6 (7.6–15.7)c | |
| Diabetes | 0.002 | ||
| no | 247 | 8.8 (6.1–12.2) | |
| with diabetic glomerulopathy | 46 | 8.6 (6.8–12.1) | |
| with other nephropathy types | 43 | 11.4 (8.7–16.2)c | |
| Iron index | 0.02 | ||
| normal | 253 | 8.9 (6.3–12.7) | |
| absolute iron deficiency | 16 | 14.9 (9.0–19.8)a | |
| functional iron deficiency | 67 | 8.9 (7.0–11.8) | |
| CRP | 0.006 | ||
| ≤8 g/L | 289 | 8.9 [6.3-12.7] | |
| >8 g/L | 40 | 10.8 [7.7-14.6] | |
| Serum albumin (g/L) | |||
| in patient with mGFR ≥30 ml/min per 1.73 m2 | 0.003 | ||
| ≥40 | 124 | 8.5 (5.9–12.6) | |
| <40 | 80 | 10.9 (7.6–14.0) | |
| in patients with mGFR <30 ml/min per 1.73 m2 | 0.6 | ||
| ≥40 | 61 | 9.4 (6.5–13.0) | |
| <40 | 71 | 8.3 (6.3–12.2) | |
| PCR (mg/mmol) | 0.2 | ||
| <30 | 140 | 8.9 (5.9–12.7) | |
| 30–300 | 165 | 9.8 (7.05–13.5) | |
| 300-max | 31 | 7.6 (6.1–10.5) | |
| PTH (pg/ml) | 0.3 | ||
| <60 | 145 | 8.9 (6.7–11.9) | |
| ≥60 | 191 | 9.2 (6.7–14.1) | |
| 1-25OH2 vitamin D (pg/ml) | 0.09 | ||
| <20 | 214 | 9.6 (7.0–13.5) | |
| ≥20 | 121 | 8.7 (5.6–11.8) | |
| Folate (ng/ml) | 0.5 | ||
| <8 | 141 | 9.4 (6.7–15.4) | |
| ≥8 | 175 | 8.9 (6.2–12.4) | |
| ACE inhibitors/ARBs | 0.6 | ||
| no | 71 | 9.4 (7.1–12.0) | |
| yes | 265 | 8.9 (6.5–13.2) |
EPO, erythropoietin; IQR, interquartile range; mGFR, GFR measured by 51Cr-EDTA renal clearance; iron index, transferrin saturation (TSAT) ≥20% indicates normal iron profile, TSAT<20% and ferritin <40 ng/ml indicate absolute iron deficiency, TSAT <20% and ferritin ≥40 ng/ml indicate functional iron deficiency; CRP, C-reactive protein; BMI, body mass index; PCR, urinary protein/creatinine ratio; PTH, parathyroid hormone; ACE inhibitors/ARBs, angiotensin-converting enzyme inhibitors/angiotensin receptor blockers.
P<0.01.
P<0.05.
P<0.001.
Multivariate Analysis of Factors Associated with Change in EPO Levels
In view of the interaction observed between mGFR and anemia status, we created a combined variable for multivariate analyses (Table 3). After adjusting for covariates, EPO levels were 17.3% higher in patients with WHO-defined anemia and mGFR ≥30 ml/min per 1.73 m2 than in the reference group without anemia, whereas they were similar in those with anemia and mGFR <30 ml/min per 1.73 m2 (Table 3). The fully adjusted EPO increase ranged from 22% to 55% and was associated with overweight, obesity, diabetes with nephropathy types other than diabetic glomerulopathy, absolute iron deficiency, and CRP >8 mg/L. Including these variables significantly improved the log likelihood of the fully adjusted model compared with that with anemia and mGFR alone to explain EPO changes. Serum albumin and EPO levels were no longer associated after adjusting for the covariates. Similar results were obtained throughout the analysis when we used a single cut-off value of 12 g/dl for both sexes. EPO levels for patients with anemia and mGFR ≥ 30 ml/min/per 1.73 m2 was 13% higher than in those without anemia (P=0.04), and relations with other factors were unchanged.
Table 3.
Multivariate analysis of factors associated with erythropoietin levels in 336 patients
| Factor | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| % Change | P | P-LRT | % Change | P | P-LRT | % Change | P | P-LRT | |
| Anemia*mGFR status | |||||||||
| no anemia | Ref | — | 0.10 | Ref | — | 0.03 | Ref | — | 0.05 |
| anemia and mGFR ≥30 | 15.7 | 0.04 | 20.1 | 0.01 | 17.3 | 0.03 | |||
| anemia and mGFR <30 | 0.3 | 0.9 | 2.6 | 0.7 | 0.0 | 0.9 | |||
| BMI (kg/m2) | 0.008 | 0.005 | |||||||
| <25 | Ref | — | Ref | — | |||||
| 25–30 | 15.3 | 0.03 | 15.8 | 0.02 | |||||
| ≥30 | 29.8 | 0.002 | 30.5 | 0.001 | |||||
| Diabetes | |||||||||
| no | Ref | — | 0.03 | Ref | — | 0.01 | |||
| with diabetic glomerulopathy | −6.4 | 0.4 | −5.5 | 0.5 | |||||
| with other nephropathy types | 23.8 | 0.02 | 26.0 | 0.01 | |||||
| Iron index | |||||||||
| normal | Ref | — | 0.006 | ||||||
| absolute iron deficiency | 55.2 | 0.001 | |||||||
| functional iron deficiency | −0.1 | 0.9 | |||||||
| CRP | |||||||||
| ≤8 mg/L | Ref | — | 0.02 | ||||||
| >8 mg/L | 21.8 | 0.03 | |||||||
| Log likelihood | 1.05 | 9.6; P< 0.01versus model 1 | 19.0; P<0.001 versus model 1 | ||||||
Model 1 adjusted for age in years, center (P=0.41), combined variable anemia × mGFR status (<30 ml/min per 1.73 m2 or ≥30 ml/min per 1.73 m2). Model 2 adjusted for age in years, center (P=0.43), combined variable anemia × mGFR status, body mass index, diabetes. Model 3 adjusted for age in years, center (P=0.41), combined variable anemia × mGFR status, body mass index, diabetes, iron index, and C-reactive protein. P is the P value of each category compared with the reference category; P-LRT is the P value of the variable in the model. mGFR, GFR measured by 51Cr-EDTA renal clearance; BMI, body mass index; CRP, C-reactive protein; iron index, transferrin saturation (TSAT) ≥20% indicates normal iron profile; TSAT<20% and ferritin <40 ng/ml indicate absolute iron deficiency; TSAT <20% and ferritin ≥40 ng/ml indicates functional iron deficiency.
Discussion
This study confirms the existence of an interaction between hemoglobin, GFR, and EPO in non–end stage CKD, but its contribution and novelty lie in four key points. First, in contrast with earlier studies, this analysis was conducted by anemia status, which enabled us to show that there was no relation between EPO concentration and mGFR levels in patients without anemia, whereas EPO deficiency increased as mGFR declined in those with anemia. Second, the most original finding indicates that several factors other than Hb and GFR levels may stimulate EPO production in CKD. Third, this study also quantified relative EPO deficiency throughout the mGFR range; it showed the importance of this deficiency as early as stages I–II, as well as its aggravation as mGFR declined, reaching 64% at stage V. Finally, the quality of patient phenotype, for both GFR and EPO measurements, resulted in a most accurate assessment of the EPO deficiency in non–end stage CKD.
In both normal and disease conditions, serum EPO must be interpreted according to Hb level (8). Under non-anemic conditions, there is no need for higher EPO because Hb concentration and oxygen supply to tissues are still sufficient. In contrast, in patients with anemia, serum EPO is expected to be inversely correlated with Hb as a result of the feedback process described in the introduction (7,8). Impairment of this physiologic feedback process in CKD was first described in dialysis patients (7). Fehr et al. (9) first suggested that it began below a GFR of 40 ml/min per 1.73 m2, but they did not stratify the analysis by anemia status. In this study, we examined the interaction between Hb and GFR according to anemia status and found a slightly lower GFR threshold in patients with anemia (30 ml/min per 1.73m2); this is likely explained by the use of Cockcroft-Gault estimated GFR in the study by Fehr et al. (9), whereas we used mGFR.
We further showed that despite relative EPO deficiency very early in the course of CKD, physiologic response to anemia was somewhat preserved in patients with GFR >30 ml/min per 1.73 m2. Conversely, at <30 ml/min per 1.73 m2, patients with anemia had severe EPO deficiency, with lower EPO levels than patients without anemia. At this stage, declining EPO concentration is likely to explain a major part of CKD anemia.
In addition to Hb and mGFR levels, a number of other factors were related to EPO concentration. First, we found that absolute iron deficiency was associated with an increased EPO level. This association was previously described in non-CKD patients and in dialysis patients (22–25), and was also expected on the basis of recent findings on hepcidin as well as the involvement of EPO in iron metabolism (26). EPO reduces circulating hepcidin in humans (27) and directly affects the intestinal handling of iron in rat models (28). Inversely, absolute iron deficiency is associated with increased EPO levels and early blockage of erythroid differentiation (29). Transferrin receptor 2, recently described as a sensor of body iron levels, may mediate EPO change with absolute iron deficiency (30).
A novel finding is that higher EPO levels were associated with increased BMI. Several studies have shown no excess risk of anemia in patients with obesity despite increased inflammation and functional iron deficiency related to the hepcidin increase (15,31,32). We previously described a 40% lower risk of anemia in obese patients in the Nephrotest cohort (15). This apparently protective effect of obesity on anemia may result from the positive relation we observed between BMI and EPO levels. Further research is needed to investigate whether sleep apnea syndrome and its consequent chronic hypoxia, common in obese patients, are a plausible trigger for the EPO increase.
Diabetes is a risk factor for anemia, which has led some authors (33,34) to hypothesize that EPO response may be blunted in diabetes patients with microangiopathy compared with patients without diabetes. Despite a higher rate of anemia in Nephrotest patients with diabetes (15), our results do not support this hypothesis. EPO levels were indeed similar between patients with diabetic glomerulopathy and those without diabetes. In contrast, EPO levels were higher in diabetes patients with nephropathy other than diabetic glomerulopathy, the most common of which was vascular nephropathy. This finding, which requires confirmation by others, suggests that hypoxia may play a role in this association.
Finally, we found a positive association between inflammation and EPO level, independent of Hb and mGFR levels. Wagner et al. (35) recently found the same association in diabetes patients with CKD. Experimentally, it is well established that EPO production is downregulated by some cytokines that also influence EPO action (36–38). In clinical studies, however, patients with chronic inflammation have preserved EPO feedback to changes in their Hb level (39–41). Their EPO level is stimulated somewhat less than it is in the presence of absolute iron deficiency, which has the strongest effect on EPO production. To note, both our results and those of Wagner et al. (35) suggest that inflammation may stimulate EPO independent of Hb level. It is unlikely that the variations of the different pro- and anti-inflammatory cytokines explain the variations in EPO levels. Other pro-ischemic factors may participate in EPO stimulation.
The major strengths of our study include its use of mGFR as well as its large sample size of well phenotyped patients with a wide range of renal function and types of kidney disease compared with previous studies. Misclassification of patients according to renal function is therefore unlikely. This study also had the power to analyze a number of determinants and to test potential interactions with renal function in their relation with EPO, which has facilitated understanding of the underlying mechanisms. The higher number of men than women in this cohort reflects the well established higher risk of CKD in men, but the lack of interaction with sex in these associations means that these results can be generalized to both sexes.
This study also has limitations. Clinical criteria were used to discriminate vascular nephropathy from diabetic nephropathy in most patients with diabetes, which may have resulted in some misclassification. Other limitations are mainly related to its cross-sectional design, which prevents causal inferences. For example, whether EPO deficiency precedes decreased Hb and causes anemia in the early stage of CKD or whether Hb decline is caused by other mechanisms and is insufficiently corrected by EPO cannot be answered. Because the EPO level was measured only once for each patient, the robustness of the correlations may be affected by the interassay variability of the EPO level within any individual. This study clearly showed, however, distinct patterns of relations between EPO and Hb levels according to whether mGFR was <30 or ≥30 ml/min per 1.73 m2. This interaction suggests that EPO plays a role in different phases of CKD anemia. Similarly, the association between EPO levels and the other factors we studied should be interpreted with caution. Nonetheless, the absence of interaction between these factors and mGFR, in contrast to Hb, suggests that these factors have a persistent effect on EPO level throughout the range of mGFR.
Altogether, our findings may shed some light on the surprising and disappointing results from recent ESA trials, particularly the Trial to Reduce Cardiovascular Events with Aranesp Therapy (5) conducted in CKD patients with diabetes. The higher EPO levels found in our study associated with diabetes with nephropathy types other than diabetic glomerulopathy may be a missing link. These patients may predominantly have vascular nephropathy and have enhanced EPO production, despite their similar anemia rates (15). This finding suggests resistance to EPO, which has recently been linked to mortality (42). ESA should thus be administered cautiously to patients with enhanced EPO concentration, because they may benefit less and experience more adverse effects. Endogenous EPO response to anemia may need to be considered in assessing the potential benefit of an ESA prescription.
In conclusion, CKD anemia is associated with an early relative EPO deficiency, which increases from 28% to 64% as renal function declines. In patients with mGFR >30 ml/min per 1.73 m2, the presence of anemia calls for explanations other than EPO deficiency, although it is likely the main determinant of anemia in patients with advanced CKD. Nonetheless, the stimulation of EPO synthesis by several factors in addition to Hb complicates the view of EPO response to decreased Hb in populations with CKD.
Disclosures
M.F. has received consulting or lecture fees or research funds from Affymax, Genzyme, Hoffmann-La Roche, Novartis, Sandoz, Shire, Takeda, and Vifor International. M.F. has been employed by Amgen since January 1, 2011, but was a full-time academic associate professor during the time of study conception, data collection, data analysis, and preparation of the manuscript. N.C. has received consulting or lecture fees or research funds from Amgen, Roche, Sandoz, and Shire. L.M. has received consulting or lecture fees or research funds from Hospal, Gambro, Hoffmann-La Roche, and Vifor.
Acknowledgments
The NephroTest CKD cohort study is supported by the following grants: INSERM GIS-IReSP AO 8113LS TGIR (B.S.), French Ministry of Health AOM 09114 (M.F.), INSERM AO 8022LS (B.S.), Agence de la Biomédecine R0 8156LL (B.S.), AURA (M.F.), and Roche 2009-152-447G (M.F.). The NephroTest initiative was also sponsored by unrestricted grants from F. Hoffman-La Roche Ltd (L.M.).
The NephroTest study group collaborators include C. Gauci, P. Houillier, E. Letavernier, P. Urena, G. Maruani, M. Vallet, J.P.H. Rougier, E. Rondeau, P. Ronco, E. Plaisier, H. Fessi, C. Descamps, R. de La Faille, S. Dautheville, E. Daugas, C. d Auzac, M. A. Costa, and J. Bouet.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Goldwasser E, Kung CKH: Purification of erythropoietin. Proc Natl Acad Sci USA 68: 697–698, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shoemaker CB, Mitsock LD: Murine erythropoietin gene: Cloning, expression, and human gene homology. Mol Cell Biol 6: 849–858, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A. CREATE Investigators: Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D. CHOIR Investigators: Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R. TREAT Investigators: A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Fishbane S, Miyawaki N, Szczech LA: Hypothesis: An erythropoietin honeymoon phase exists. Kidney Int 78: 646–649, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Erslev AJ: Erythropoietin. N Engl J Med 324: 1339–1344, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Beguin Y, Clemons GK, Pootrakul P, Fillet G: Quantitative assessment of erythropoiesis and functional classification of anemia based on measurements of serum transferrin receptor and erythropoietin. Blood 81: 1067–1076, 1993 [PubMed] [Google Scholar]
- 9.Fehr T, Ammann P, Garzoni D, Korte W, Fierz W, Rickli H, Wüthrich RP: Interpretation of erythropoietin levels in patients with various degrees of renal insufficiency and anemia. Kidney Int 66: 1206–1211, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Walle AJ, Wong GY, Clemons GK, Garcia JF, Niedermayer W: Erythropoietin-hematocrit feedback circuit in the anemia of end-stage renal disease. Kidney Int 31: 1205–1209, 1987 [DOI] [PubMed] [Google Scholar]
- 11.Ross RP, McCrea JB, Besarab A: Erythropoietin response to blood loss in hemodialysis patients in blunted but preserved. ASAIO J 40: M880–M885, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Brookhart MA, Schneeweiss S, Avorn J, Bradbury BD, Rothman KJ, Fischer M, Mehta J, Winkelmayer WC: The effect of altitude on dosing and response to erythropoietin in ESRD. J Am Soc Nephrol 19: 1389–1395, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGonigle RJ, Wallin JD, Shadduck RK, Fisher JW: Erythropoietin deficiency and inhibition of erythropoiesis in renal insufficiency. Kidney Int 25: 437–444, 1984 [DOI] [PubMed] [Google Scholar]
- 14.Radtke HW, Claussner A, Erbes PM, Scheuermann EH, Schoeppe W, Koch KM: Serum erythropoietin concentration in chronic renal failure: Relationship to degree of anemia and excretory renal function. Blood 54: 877–884, 1979 [PubMed] [Google Scholar]
- 15.Moranne O, Froissart M, Rossert J, Gauci C, Boffa JJ, Haymann JP, M’rad MB, Jacquot C, Houillier P, Stengel B, Fouqueray B. NephroTest Study Group: Timing of onset of CKD-related metabolic complications. J Am Soc Nephrol 20: 164–171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipschitz DA, Cook JD, Finch CA: A clinical evaluation of serum ferritin as an index of iron stores. 1974. Nutrition 8: 443–447, discussion 448, 1992 [PubMed] [Google Scholar]
- 17.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P: Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol 16: 763–773, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G: Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67: 2089–2100, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Belonje AMS, Voors AA, van der Meer P, van Gilst WH, Jaarsma T, van Veldhuisen DJ: Endogenous erythropoietin and outcome in heart failure. Circulation 121: 245–251, 2010 [DOI] [PubMed] [Google Scholar]
- 20.van der Meer P, Lok DJ, Januzzi JL, de la Porte PW, Lipsic E, van Wijngaarden J, Voors AA, van Gilst WH, van Veldhuisen DJ: Adequacy of endogenous erythropoietin levels and mortality in anaemic heart failure patients. Eur Heart J 29: 1510–1515, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Opasich C, Cazzola M, Scelsi L, De Feo S, Bosimini E, Lagioia R, Febo O, Ferrari R, Fucili A, Moratti R, Tramarin R, Tavazzi L: Blunted erythropoietin production and defective iron supply for erythropoiesis as major causes of anaemia in patients with chronic heart failure. Eur Heart J 26: 2232–2237, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Bray GL, Taylor B, O’Donnell R: Comparison of the erythropoietin response in children with aplastic anemia, transient erythroblastopenia, and iron deficiency. J Pediatr 120: 528–532, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Joosten E, Van Hove L, Lesaffre E, Goossens W, Dereymaeker L, Van Goethem G, Pelemans W: Serum erythropoietin levels in elderly inpatients with anemia of chronic disorders and iron deficiency anemia. J Am Geriatr Soc 41: 1301–1304, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Theurl I, Mattle V, Seifert M, Mariani M, Marth C, Weiss G: Dysregulated monocyte iron homeostasis and erythropoietin formation in patients with anemia of chronic disease. Blood 107: 4142–4148, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Teruel JL, Marcen R, Navarro JF, Villafruela JJ, Fernandez Lucas M, Rivera M, Ortuño J: Influence of body iron stores on the serum erythropoietin concentration in hemodialyzed patients. Am J Nephrol 14: 95–98, 1994 [DOI] [PubMed] [Google Scholar]
- 26.Nemeth E: Targeting the hepcidin-ferroportin axis in the diagnosis and treatment of anemias. Adv Hematol 2010: 750643, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashby DR, Gale DP, Busbridge M, Murphy KG, Duncan ND, Cairns TD, Taube DH, Bloom SR, Tam FW, Chapman R, Maxwell PH, Choi P: Erythropoietin administration in humans causes a marked and prolonged reduction in circulating hepcidin. Haematologica 95: 505–508, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Srai SK, Chung B, Marks J, Pourvali K, Solanky N, Rapisarda C, Chaston TB, Hanif R, Unwin RJ, Debnam ES, Sharp PA: Erythropoietin regulates intestinal iron absorption in a rat model of chronic renal failure. Kidney Int 78: 660–667, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Bullock GC, Delehanty LL, Talbot AL, Gonias SL, Tong WH, Rouault TA, Dewar B, Macdonald JM, Chruma JJ, Goldfarb AN: Iron control of erythroid development by a novel aconitase-associated regulatory pathway. Blood 116: 97–108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forejtnikovà H, Vieillevoye M, Zermati Y, Lambert M, Pellegrino RM, Guihard S, Gaudry M, Camaschella C, Lacombe C, Roetto A, Mayeux P, Verdier F: Transferrin receptor 2 is a component of the erythropoietin receptor complex and is required for efficient erythropoiesis. Blood 116: 5357–5367, 2010 [DOI] [PubMed] [Google Scholar]
- 31.Ausk KJ, Ioannou GN: Is obesity associated with anemia of chronic disease? A population-based study. Obesity (Silver Spring) 16: 2356–2361, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Tussing-Humphreys LM, Nemeth E, Fantuzzi G, Freels S, Guzman G, Holterman AX, Braunschweig C: Elevated systemic hepcidin and iron depletion in obese premenopausal females. Obesity (Silver Spring) 18: 1449–1456, 2010 [DOI] [PubMed] [Google Scholar]
- 33.Biaggioni I, Robertson D, Krantz S, Jones M, Haile V: The anemia of primary autonomic failure and its reversal with recombinant erythropoietin. Ann Intern Med 121: 181–186, 1994 [DOI] [PubMed] [Google Scholar]
- 34.Bosman DR, Winkler AS, Marsden JT, Macdougall IC, Watkins PJ: Anemia with erythropoietin deficiency occurs early in diabetic nephropathy. Diabetes Care 24: 495–499, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Wagner M, Alam A, Zimmermann J, Rauh K, Koljaja-Batzner A, Raff U, Wanner C, Schramm L: Endogenous erythropoietin and the association with inflammation and mortality in diabetic chronic kidney disease. Clin J Am Soc Nephrol 6: 1573–1579, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Faquin WC, Schneider TJ, Goldberg MA: Effect of inflammatory cytokines on hypoxia-induced erythropoietin production. Blood 79: 1987–1994, 1992 [PubMed] [Google Scholar]
- 37.Fandrey J, Jelkmann WE: Interleukin-1 and tumor necrosis factor-alpha inhibit erythropoietin production in vitro. Ann N Y Acad Sci 628: 250–255, 1991 [DOI] [PubMed] [Google Scholar]
- 38.Congote LF, Sadvakassova G, Dobocan MC, Difalco MR, Li Q: Erythropoietin-dependent endothelial proteins: Potential use against erythropoietin resistance. Cytokine 51: 113–118, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Cazzola M, Ponchio L, de Benedetti F, Ravelli A, Rosti V, Beguin Y, Invernizzi R, Barosi G, Martini A: Defective iron supply for erythropoiesis and adequate endogenous erythropoietin production in the anemia associated with systemic-onset juvenile chronic arthritis. Blood 87: 4824–4830, 1996 [PubMed] [Google Scholar]
- 40.Corazza F, Beguin Y, Bergmann P, André M, Ferster A, Devalck C, Fondu P, Buyse M, Sariban E: Anemia in children with cancer is associated with decreased erythropoietic activity and not with inadequate erythropoietin production. Blood 92: 1793–1798, 1998 [PubMed] [Google Scholar]
- 41.Spivak JL, Barnes DC, Fuchs E, Quinn TC: Serum immunoreactive erythropoietin in HIV-infected patients. JAMA 261: 3104–3107, 1989 [PubMed] [Google Scholar]
- 42.Solomon SD, Uno H, Lewis EF, Eckardt KU, Lin J, Burdmann EA, de Zeeuw D, Ivanovich P, Levey AS, Parfrey P, Remuzzi G, Singh AK, Toto R, Huang F, Rossert J, McMurray JJ, Pfeffer MA. Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) Investigators: Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med 363: 1146–1155, 2010 [DOI] [PubMed] [Google Scholar]