Summary
Background and objectives
Data collected by the US Renal Data System (USRDS) identify sudden cardiac death (SCD) as the leading cause of death among hemodialysis patients. However, evidence suggests that clinical events captured on the USRDS death notification form may be inaccurate. A new method for classifying SCD was recently developed to enhance the accuracy of SCD classification. This study examined the performance characteristics of this refined definition using a cohort of hemodialysis patients who experienced a witnessed SCD as the reference standard.
Design, setting, participants, & measurements
This is a retrospective cohort study of 363 patients who experienced a witnessed SCD in US Gambro (DaVita) outpatient dialysis clinics. Sensitivity of SCD defined by death notification forms and SCD defined using additional administrative sources was compared. Clinical data recorded near time of death were also examined.
Results
Existing USRDS death notification forms reported 70.8% of witnessed SCD as “cardiac arrest/cause unknown” or “arrhythmia.” The refined definition significantly improved identification to 83.8% of witnessed SCD events (P<0.001). Verified SCD cases that were not identified by either definition were more likely to be reported on the death notification form as death due to myocardial infarction, hyperkalemia, sepsis, malignancy, or unknown cause.
Conclusions
Compared with the death notification form alone, the refined SCD definition significantly improves the sensitivity of reporting of witnessed SCD occurring within outpatient hemodialysis clinics. More accurate reporting of cardiac events by clinicians and refinements to existing death notification forms may further improve recognition and understanding of SCD.
Introduction
Patients with ESRD have a 20% annual mortality rate and an age-specific cardiovascular death rate that is 10–100 times higher than in the general population (1). To better understand the epidemiology of mortality in ESRD patients, reporting of the cause, circumstances, and location of death is mandated for all ESRD patients enrolled in the US Medicare program, and these data are collected and reported by the US Renal Data System (USRDS). According to these data, sudden cardiac death (SCD) accounts for 26% of all-cause mortality among hemodialysis patients, making it the leading cause of death (2). Therefore, understanding and accurately tracking the SCD epidemic are important in reducing cardiovascular death and overall mortality in this population.
A central problem in the study of SCD is the difficulty of accurately defining a SCD event. The medical community lacks consensus on the diagnostic criteria that should be applied to SCD. The highest standard of SCD definition is derived from eyewitness accounts, in which rapid progression from onset of symptoms to death and the lack of noncardiac causes can be determined. Because witnessed accounts are uncommon, operational criteria for SCD include any nontraumatic death occurring in a person previously known to be well (3).
Data on the cause of death for all patients with ESRD are derived from the USRDS Death Notification (form 2746), which is completed by the care providers in the dialysis facility (4). The form includes a list of 70 possible entries for cause of death, but SCD is not listed as a specific option (5). Despite questions about the reliability of data on form 2746 and the use of information gathered from cause of death reporting forms in general, information gathered from this form remains the primary source of data on mortality among Americans with ESRD (4,6,7).
Currently, the USRDS defines SCD as deaths reported due to “cardiac arrest/cause unknown” and “arrhythmia” on form 2746, without further inclusion or exclusion criteria. Sensitivity of the existing USRDS SCD definition has been reported to be between 39% and 67%, using either eyewitness accounts or the review of prospectively collected medical records as reference standards (4,8). To address the shortcomings of the existing death reporting method, the USRDS Cardiovascular Special Studies Center (CVSSC) recently implemented an empirical, more complex methodology to define SCD, utilizing additional available registry data, including death location and diagnosis codes for cardiac arrest (9). It is not known whether this revised definition would be feasible to apply in a clinical setting or if it would improve the accuracy of SCD reporting. Therefore, we examined the effect of the CVSSC definition on the sensitivity of SCD reporting compared with the SCD reporting methods currently used.
Materials and Methods
Study Cohort
From among 43,200 US DaVita (formerly Gambro) Healthcare hemodialysis patients who dialyzed between 2002 and 2005, we identified all patients who had experienced a witnessed cardiac arrest within outpatient dialysis facilities. We chose to include only eyewitnessed cardiac arrest events to ensure accurate capture of SCD events and because a significant proportion of all SCD in hemodialysis patients occur within dialysis facilities (10–12). We defined sudden cardiac arrest events as the occurrence of a witnessed sudden pulseless condition in the outpatient dialysis setting that is not clearly attributable to a noncardiac cause. Patients who were enrolled in hospice care or who had a do-not-resuscitate order on file were excluded from consideration. The methods we used to identify the cardiac arrest cohort and the detailed clinical information available for each subject have been published previously and are described in this study (13). We collected data on clinical events that occurred in the dialysis facilities with the Event Reporting Management System database between 2002 and 2005. This data collection system is designed for risk-management purposes to catalog events in outpatient clinics and was utilized throughout the US DaVita (Gambro) Healthcare system during the study time period. Clinics were required to report serious and life-threatening events in this database, and adherence to this policy was monitored with corporate quality assurance programs. Detailed clinical and laboratory data were obtained from the DaVita (Gambro) clinical database. Date of death was extracted from the database or the Social Security Death Index. We screened the database for entries coded “cardiac arrest,” “cardiac arrhythmia,” “hypotension,” or “respiratory arrest.” Three physicians independently adjudicated qualifying events on the basis of chart review. For the diagnosis of sudden cardiac arrest to be accepted, all three physicians had to agree that the event described was cardiac in origin. All available data including patient symptoms, physical exam findings, vital signs, and reports of electrocardiographic findings were reviewed within recorded event narratives. Patients were included if any of the following key events were described in the narrative: deployment of an automated external defibrillator, initiation of Cardiopulmonary resuscitation, documentation of sudden pulselessness, lack of a primary respiratory or neurologic condition initiating the event, or a determination of cardiac arrest after emergency medical services personnel arrived on the scene. To reduce the possibility that inpatient events may have been included from the small minority of outpatient clinics that were attached to a hospital facility, we excluded all events that contained descriptions of care that were inconsistent with outpatient status. A clinical event was entered as a cardiac arrest only if the decision was unanimous. The final adjudicated SCD cohort consisted of all deaths occurring <24 hours after a qualifying sudden cardiac arrest event, and served as the reference standard for sensitivity testing.
To obtain reported causes of death on form 2746 for study participants, we linked patient records with data gathered by the USRDS. We excluded patients who we were unable to link to matching identifiers in the USRDS database. Patients with missing form 2746 data or no cause of death reported on form 2746 were also excluded from further analysis.
Sudden Cardiac Death Definitions
Table 1 outlines the essential components of the “simple” SCD definition that is currently utilized by the USRDS, the proposed “complex” CVSSC SCD criteria, and the reference standard SCD definition we used in this study. The simple SCD definition is derived from reported cause of death on form 2746 and consists of all deaths coded as “cardiac arrest, cause unknown” or “cardiac arrhythmia” without any exclusions. The complex SCD definition was outlined in the 2006 USRDS annual data report and is summarized as follows (9). Location of death (inpatient versus outpatient) is a central element in the criteria, and this information is available on form 2746 (in hospital, outpatient dialysis unit, home, nursing home, and other). For deaths occurring in the hospital setting, an inpatient Medicare claim for ventricular fibrillation or cardiac arrest (International Classification of Diseases, Clinical Modification [ICD-9-CM] codes 427.4 or 427.5) and a primary cause of death due to cardiac disease (death codes 23 and 25–31 on form 2746) is required to classify a SCD. If no inpatient claim data are available, in-hospital SCD can be designated only if the primary cause of death is listed as cardiac arrhythmia. For deaths occurring in the outpatient setting, criteria for SCD are met if there are claim data for ventricular fibrillation or cardiac arrest, and the primary cause of death is listed as due to cardiac disease or “unknown” on form 2746. In the absence of claim data, outpatient SCD is considered only if the primary cause of death is attributed to a cardiac diagnosis. For all deaths, an important element of the complex definition is that all deaths attributed to hyperkalemia, septicemia, and malignant disease are excluded from consideration, as are deaths occurring in the setting of dialysis withdrawal or hospice care.
Table 1.
Sudden cardiac death definitions used in this study
| Criterion | US Renal Data System Simple Definition | Proposed Complex Definition | “Gold Standard” Used in This Study | |
|---|---|---|---|---|
| Location | Any | Outpatient | Inpatient | Outpatient hemodialysis clinic |
| Data source | Form 2746 | Form 2746 and ICD-9-CM codes from Medicare billing data | Form 2746 and ICD-9-CM codes from Medicare billing data | Medical records |
| Reported cause of death | Cardiac arrest, cause unknown, or arrhythmia reported on form 2746 | Primary cardiac cause of death on form 2746; if supportive claim data recorded, “unknown” cause of death also accepted | Primary cause of death due to “cardiac arrhythmia” on form 2746; if supportive claim data, any primary cardiac cause of death | Death occurring <24 h after witnessed cardiac arrest without evidence of noncardiac cause based on eyewitness reports and medical records review |
| Inpatient claims | Not used | Ventricular fibrillation (427.4) and/or cardiac arrest (427.5) | Ventricular fibrillation (427.4) and/or cardiac arrest (427.5) | Not used |
| Exclusions | None | Withdrawal of dialysis; hospice care; primary or secondary cause of death reported as hyperkalemia, septicemia, or malignant disease | Withdrawal of dialysis; hospice care; primary or secondary cause of death reported as hyperkalemia, septicemia, or malignant disease | Withdrawal of dialysis; hospice care; do-not-resuscitate form on file |
ICD-9-CM, International Classification of Diseases, Clinical Modification.
Clinical Demographic, Comorbid Conditions, and Dialysis-Specific Data
Detailed clinical, dialysis, and laboratory data most proximal to the death were obtained from the DaVita (Gambro) clinical database. We recorded the history of pre-existing medical conditions using ICD-9-CM codes recorded in the clinical database, along with data available on the USRDS Medical Evidence Form completed at first initiation of dialysis. Blood samples were drawn by uniform techniques in all DaVita clinics and were measured by automated and standardized methods in a central laboratory. Most laboratory values, including serum albumin, creatinine, potassium, calcium, phosphorus, hemoglobin, parathyroid hormone, and urea nitrogen levels, were measured at least monthly. The most proximal reported value before death was used. Dialysis-specific data were also collected. A full description of variables used for the purpose of this study is described in detail elsewhere (14).
Statistical Methods
Frequency distributions and cross tabulations were used to report causes of death assigned on death notification forms. The sensitivity of each methodology was reported using simple proportions, and the confidence interval (CI) of proportion was calculated using the modified Wald method (15). Differences in sensitivity between the two SCD definitions were reported using the paired t test for comparison of two dependent proportions (16). Agreement between the simple and complex methodology was obtained using the κ statistic, which represents agreement achieved beyond that expected by chance alone. Values for κ can range between −1 and 1; a κ statistic close to 1 represents near perfect agreement, whereas values <0 represent agreement less than chance. A comparison of baseline comorbidity and clinical characteristics of those included and excluded as SCD by the complex method was performed, using the Pearson’s chi-squared or Fisher exact test for categorical values as appropriate and the Wilcoxon rank-sum test for continuous variables. All tests were two sided and were carried out using SAS 9.1 software (SAS Institute, Cary, NC). Results were declared significant at P<0.05.
Results
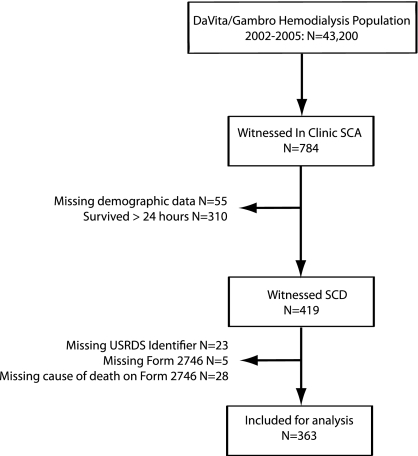
During the 4-year study period, 784 events occurring in outpatient dialysis units were documented in the event reporting system and independently adjudicated as representing witnessed sudden cardiac arrests. The overall event rate was 4.5 events per 100,000 dialysis sessions, or approximately 7 events per 1000 patient-years. After excluding patients who survived more than 24 hours after the event and those without linkable USRDS registry data, we included 363 patients in the final analysis cohort (Figure 1). We used this group of patients who experienced death after a witnessed cardiac arrest as the reference SCD population.
Figure 1.
Study inclusion flowchart.
The baseline characteristics of the study cohort are listed in Table 2. The median age was 69 years and the median duration of dialysis was 2.4 years. Approximately half of the cohort were men, and 46% were white. The USRDS-reported comorbid illnesses were similar to those reported for the entire prevalent US hemodialysis population: 34% were reported to have coronary artery disease at dialysis initiation, 46% had a diagnosis of congestive heart failure, and 55% had diabetes. Less than 6% were reported to have HIV/AIDS or a diagnosis of cancer at dialysis initiation.
Table 2.
Baseline characteristics of the study cohort
| Characteristic | Value |
|---|---|
| Study participants, N | 363 |
| Median age, yr (IQR) | 69 (59, 78) |
| Male participants, % | 55.4 |
| Caucasian, % | 46 |
| Median dialysis vintage, yr (IQR) | 2.44 (0.99, 5.0) |
| Coronary artery disease, % | 34.5 |
| History of congestive heart failure, % | 46.2 |
| History of arrhythmia, % | 13.7 |
| Chronic obstructive pulmonary disease, % | 8.2 |
| Diabetes, % | 55.2 |
| HIV/AIDS, % | 1.4 |
| Cancer, % | 4.2 |
| Recorded place of death on form 2746, % | |
| hospital | 70.5 |
| dialysis unit | 21.5 |
| home | 1.1 |
| nursing home | 4.9 |
| missing | 1.9 |
IQR, interquartile range.
Reported Cause of Death and Performance of SCD Definitions
A total of 24 different primary causes of death were recorded for the study participants, and the most commonly reported primary causes of death recorded for the study participants are shown in Table 3. The simple SCD definition utilizes only cause of death information to identify SCD events (primary cause of death due to “cardiac arrest” or “cardiac arrhythmia”), and these criteria correctly identified 70.8% of patients in the study cohort (95% CI, 65.9–75.2). Among the 106 patients missed by the simple SCD classification, acute myocardial infarction was the recorded cause of death most frequently listed (48.1%), followed by atherosclerotic heart disease (13.2%), and cardiomyopathy (7.6%). We found that 8.5% of missed patients were categorized as “other cause of death” or “unknown.”
Table 3.
Recorded causes of death on form 2746 for study participantsa
| Characteristic | Proportion of All Participants with Data, % |
|---|---|
| Primary cause of death (n=363) | |
| cardiac arrest, cause unknown | 55.9 |
| cardiac arrhythmia | 14.9 |
| myocardial infarction, acute | 14.1 |
| atherosclerotic heart disease | 3.9 |
| other or unknown | 2.5 |
| cardiomyopathy | 2.2 |
| Secondary cause of death (n=154) | |
| atherosclerotic heart disease | 32.5 |
| cardiomyopathy | 26.0 |
| other | 6.5 |
| valvular heart disease | 5.2 |
| cardiac arrest, cause unknown | 3.9 |
| cardiac arrhythmia | 3.9 |
| myocardial infarction | 3.9 |
| malignant disease | 1.6 |
| hyperkalemia | 1.3 |
Other reported causes of death with cumulative frequency <1% are not listed.
We then examined the complex CVSSC SCD definition, which increased the percentage of study participants identified as SCD to 83.8% (95% CI, 79.6–87.2). The improvement in sensitivity was primarily caused by the addition of qualifying deaths attributed to myocardial infarction. Despite this, myocardial infarction continued to be the most commonly reported cause of death among those missed by the CVSSC SCD definition (29%), because supportive inpatient claim data for ventricular fibrillation or cardiac arrest were lacking. An additional 21% excluded deaths were listed as deaths due to hyperkalemia, malignancy, or sepsis. The assignment of cause of death to malignancy and sepsis is surprising because no patients were hospitalized, receiving hospice therapy, or had a do-not-resuscitate order on file at the time of cardiac arrest.
In a comparison of the two methodologies, the CVSSC definition increased identification of SCD by 12.9% (95% CI, 8.9–17) and this difference was statistically significant (P<0.0001). We compared the overall agreement between the two methodologies using the κ statistic. The overall agreement was moderate, with a κ statistic of 0.52 (95% CI, 0.42–0.62).
Location of Death and Performance of SCD Definitions
In the study cohort, 72% of all deaths were reported as occurring in the hospital, and 6.8% of deaths were reported to have occurred at home or in a nursing facility. The simple definition correctly identified 69% of the reported hospital events as SCD, whereas the complex definition identified 79% of these events (10.2% increase in sensitivity; 95% CI, 6–15; P<0.0001).
Characteristics of Study Participants Missed by the Complex CVSSC Definition
Table 4 compares the characteristics of 59 (16.2%) SCD patients who were missed by the complex CVSSC SCD definition with the 304 (83.3%) who were correctly identified. There were no significant differences in demographic data and comorbid conditions. Patients who were missed had significantly higher last recorded serum potassium levels compared with those who were identified (median, 5.0 versus 4.6 mEq/L; P=0.01), but otherwise there were no significant differences in last recorded laboratory values or other clinical factors. Examination of reported cause of death between the two groups revealed a significantly greater proportion of deaths reported as myocardial infarction, hyperkalemia, other cause of death or unknown cause of death, and malignancy/sepsis among SCD patients who were missed.
Table 4.
Characteristics of patients included versus excluded as sudden cardiac death by the US Renal Data System complex definition
| Included | Excluded | P Value | |
|---|---|---|---|
| Study participants, n | 304 | 59 | |
| Age, yr (median) | 68 (60.5–78) | 69 (55–78) | 0.40 |
| Dialysis vintage, yr | 2.47 (1.05–5.11) | 2.20 (0.94–4.03) | 0.40 |
| Male gender, % | 56.2 | 50.8 | 0.40 |
| Black race, % | 39.5 | 32.2 | 0.30 |
| Last recorded laboratory values, n (median) | |||
| serum potassium | 4.6 (4.1–5.1) | 5.0 (4.3–5.4) | 0.01 |
| serum phosphorus | 5.1 (4.1–6.5) | 5.7 (4.4–6.8) | 0.05 |
| serum calcium | 9.2 (8.7–9.8) | 9.2 (8.4–10) | 0.90 |
| parathyroid hormone | 208 (104–371) | 204 (114–458) | 0.60 |
| hemoglobin | 11.5 (10.7–12.4) | 11.4 (10.1–12.3) | 0.70 |
| creatinine | 6.8 (5.4–8.7) | 7.1 (5.5–8.8) | 0.30 |
| albumin | 3.6 (3.3–3.9) | 3.6 (3.2–3.9) | 0.60 |
| urea reduction ratio | 0.71 (0.66–0.76) | 0.73 (0.69–0.76) | 0.10 |
| Primary cause of death reported on form 2746, n (%) | |||
| cardiac arrest, cause unknown | 197 (64.8) | 6 (10.2) | <0.0001 |
| cardiac arrhythmia | 52 (17.1) | 2 (3.4) | 0.02 |
| myocardial infarction | 34 (11.2) | 17 (28.8) | 0.0001 |
| atherosclerotic heart disease | 11 (3.6) | 3 (5.1) | 0.40 |
| cardiomyopathy | 8 (2.6) | 0 (0) | 0.60 |
| other or unknown cause of death | 1 (0.33) | 8 (13.6) | 0.0001 |
| malignancy or sepsis reported as primary or secondary cause of death | 0 (0) | 7 (11.9) | <0.0001 |
| hyperkalemia reported as primary or secondary cause of death | 0 (0) | 5 (8.5)a | <0.0001 |
Data in parentheses represent interquartile range.
The mean last recorded serum potassium levels for these patients was 5.1 mEq/L (SD 1.2).
Discussion
Patients with advanced CKD, and those maintained on hemodialysis in particular, have long been known to suffer an extraordinary risk of experiencing SCD. To improve the lives of these patients, clinicians must make the sensitive and accurate detection and reporting of SCD events a high priority. In this study, we examined the sensitivity of two registry-defined methods of SCD reporting. The method currently utilized by the USRDS for SCD rate reporting identified 70.8% of SCD events classified by eyewitness reports. Using additional registry data that refine the definition of SCD significantly improved identification to 83.8% of our study cohort. The remaining 16.2% of patients who suffered a confirmed SCD but were excluded by the new criteria were more likely to have myocardial infarction, hyperkalemia, sepsis/malignancy, and “unknown” or “other” recorded as the primary cause of death.
Recent consensus guidelines defined SCD as “death from an unexpected circulatory arrest, usually due to a cardiac arrhythmia occurring within an hour of the onset of symptoms” (17, p 394). However, clinical information collected around the time of an unexpected death in hemodialysis patients is very often limited, and a significant proportion of sudden deaths are unwitnessed (18). Although adjudication of cause of death may be difficult in these cases, clinicians and clinic leaders must be responsible for identifying SCD events as accurately as possible. Misapplication of a SCD diagnosis may have several detrimental consequences. First, efforts to improve the rate of the “true” cardiac events may not be detected if a diagnosis of SCD is not stringent. Second, an inaccurate classification of SCD could obscure the influence of an exposure or process that contributes to nonsudden or noncardiac deaths. Finally, inaccuracies in SCD reporting within large registry reports, such as the USRDS, hamper accurate assessment of the relative contribution of SCD to overall mortality in this vulnerable population.
The primary data source on the cause of death for hemodialysis patients in the United States is the ESRD Death Notification (form 2746), and completion of this report is required by law. Factors related to the ways in which this form is completed give rise to concerns about the validity of the data it contains. First, the form may be completed up to 30 days after the death, and details surrounding the event may be unavailable, forgotten, or passed over in this time period. Second, although a physician must sign the form, there is no requirement that the person completing the report must be the patient’s treating nephrologist, or even a clinician. Indeed, in a previous study, one-quarter of all 2746 death forms were completed by nonphysicians who may have had limited knowledge of the patient’s overall condition (4). Even if the treating nephrologist completed the form, he or she is often not the physician caring for the patient at the hospital or emergency room at the time of death. Because direct knowledge of circumstances surrounding an event is crucial to defining a SCD, these limitations generate concern regarding the validity of adjudicating SCD events using these data. An example from our study of either lack of knowledge about death circumstances or user error is that 6.8% of deaths in our study were recorded on form 2746 as having occurred in a nursing home or at home, even though the available clinical data clearly demonstrated that all deaths occurred in the dialysis clinic or hospital setting.
An inherent problem with identifying SCD from registry data is that form 2746 does not include a specific option to enter SCD as the cause of death. Two methodologies for SCD identification using the current form 2746 have been described. The first is a simple definition that included all deaths reported as cardiac arrhythmia and cardiac arrest. The shortcomings of this simplistic definition are apparent because according to this definition, a cardiac arrest occurring in the setting of a withdrawal from dialysis or hospice therapy would be considered a SCD, whereas a sudden unexpected outpatient death due to myocardial infarction would not. Because of these problems, the USRDS CVSSC applied a more complex definition, combining other data elements contained in the form with claim code data from billing records. Of the two methods examined in this study, we found that the complex methodology significantly improved SCD detection. Therefore, the complex method should be preferred in epidemiologic and other investigations of SCD using USRDS data as the primary source of information.
Although the complex method increased identification of SCD, it fell short in several areas. First, the majority of missed SCDs were classified as deaths due to ischemic heart disease (“myocardial infarction” or “atherosclerotic heart disease”). Although not all deaths due to ischemic heart disease are sudden, a myocardial infarction may certainly be a proximal cause of SCD. Although we could not verify the diagnosis of myocardial infarction in our study cohort, it is likely that many of the deaths attributed to myocardial infarction were based on clinical impressions rather than specific supporting data (e.g., electrocardiographic [ECG] changes or cardiac biomarkers). Similarly, it is difficult to support a diagnosis of “atherosclerotic heart disease” as a principal cause of death, especially because accumulating evidence suggests that ischemic events in ESRD patients are often not related to atheromatous coronary lesions (19). To improve specificity of cardiac deaths, we suggest that only deaths with clear evidence of myocardial infarction, such as supporting ECG, cardiac enzyme, or autopsy data, be considered myocardial infarction deaths on form 2746. In the absence of a specific SCD coding option, deaths due to sudden cardiac arrest in which there is suspicion but no direct evidence of myocardial ischemia should instead be coded as “cardiac arrest, cause unknown.”
Another factor that contributed to the inability to identify SCD was the relatively large proportion of all deaths that were not assigned a specific cause of death on form 2746. We found that 6.6% of all patients were classified as “unknown” or “other cause of death” as the primary or secondary cause of death. A prior study found more than twice as many codes for unknown cause of death on form 2746 compared with cause determined by adjudication in a controlled clinical trial (4). Even though we made a concerted effort using multiple data sources to adjudicate cause of death in our study, all of the resources we used would have also been available to clinicians completing cause of death forms. It is likely that a more careful review of the available data would have increased the accuracy of form 2746 data, and would have allowed for less frequent use of “unknown” or “other” cause of death categories that do not contribute meaningful data for epidemiologic studies.
Finally, the role of hyperkalemia in adjudicating possible SCD is worthy of special consideration, particularly in this patient population. Because the diagnosis of SCD requires the absence of a noncardiac cause, both the simple and CVSSC definitions excluded all deaths attributed to hyperkalemia. ESRD patients are often exposed to elevated serum potassium levels; depending on the clinical circumstance, this could lead to cardiac arrest in the absence of a primary cardiac condition or it may play a secondary role in triggering a SCD. Although in most cases we could not determine the serum potassium level at the time of cardiac arrest and therefore could not determine if hyperkalemia played a primary role in the event, the most proximal recorded serum potassium was 5.1 mEq/L for those patients whose deaths were reported as being caused by hyperkalemia. The level of serum potassium at which the cause of death should be directly attributed to hyperkalemia is difficult to determine and decidedly arbitrary, and the relative contribution of disordered potassium homeostasis to the epidemic of sudden death in ESRD patients is unknown. We previously reported in a matched case-control study that a modestly elevated serum potassium concentration of 5.1 mEq/L was associated with the lowest risk of SCD compared with lower levels, and other reports also confirm that CKD patients seem to be protected against the effects of hyperkalemia on mortality (14,20). These observations suggest that in hemodialysis patients, it is not prudent to automatically attribute hyperkalemia as the cause of death when the last measured serum potassium concentration is outside the range observed in the general population. Instead, we suggest that the assignment of death due to hyperkalemia be reserved for cases in which characteristic detrimental effects of elevated serum potassium can be demonstrated (e.g., characteristic ECG changes).
Our study has important limitations. First, because our study included only patients with confirmed SCD, we were not able to examine the specificity or other discriminatory characteristics of the refined SCD definition. A prior study of 228 hemodialysis patients who died reported a specificity of 90.7% for the simple SCD definition (8); however, the specificity of the complex CVSSC definition has not been examined. Second, we purposely selected a cohort of patients who experienced an event in the outpatient dialysis clinic, and this limits the generalizability of our findings. Because all deaths were directly witnessed (either in the dialysis clinic or on arrival at the hospital setting), it is unclear how the refined SCD definition performs in incidents of SCD that are either unwitnessed or occur outside of healthcare facilities. However, previous reports suggest that a significant proportion (11%–18%) of sudden cardiac arrest events in hemodialysis patients occur within outpatient dialysis clinics (9,10,12,21). Importantly, utilizing witnessed sudden cardiac arrest events afforded us greater confidence in the reliability of our SCD adjudication, which was central to the purpose of this study.
In conclusion, this study emphasizes that clinicians must be aware that SCD is a common occurrence in hemodialysis patients, and accurate reporting of these cardiac events can be a critical component of improving the quality of care of this vulnerable population. We determined that a more complex method of defining SCD using registry data enhances the sensitivity of detection and is an important refinement. Confounding concurrent conditions such as myocardial infarction and hyperkalemia increase the likelihood of a missed SCD using registry data. Revision of form 2746 to provide greater specificity to these death categories may improve the ability to distinguish these events from SCD. Dialysis clinicians should be encouraged to more thoroughly investigate circumstances and conditions surrounding patient deaths, and should be vigilant to avoid over-reporting of deaths due to “unknown” and “other” causes.
Disclosures
None.
Acknowledgments
This work was supported by grant funding from Satellite Health Care to P.H.P.
The data reported here were supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Expect the Unexpected: Sudden Cardiac Death in Dialysis Patients,” on pages 8–11.
References
- 1.Foley RN: Clinical epidemiology of cardiac disease in dialysis patients: Left ventricular hypertrophy, ischemic heart disease, and cardiac failure. Semin Dial 16: 111–117, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Annual Data Report, US Renal Data System, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010 [Google Scholar]
- 3.Engdahl J, Holmberg M, Karlson BW, Luepker R, Herlitz J: The epidemiology of out-of-hospital ‘sudden’ cardiac arrest. Resuscitation 52: 235–245, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Rocco MV, Yan G, Gassman J, Lewis JB, Ornt D, Weiss B, Levey AS. Hemodialysis Study GroupThe National Institutes of Health-funded Hemodialysis. Health Care Financing Administration: Comparison of causes of death using HEMO Study and HCFA end-stage renal disease death notification classification systems. Am J Kidney Dis 39: 146–153, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Death Notification ESRD: In: SERVICES, H. A. H. (Ed.) Baltimore, 2004. [Google Scholar]
- 6.Perneger TV, Klag MJ, Whelton PK: Cause of death in patients with end-stage renal disease: Death certificates vs registry reports. Am J Public Health 83: 1735–1738, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauer MS, Blackstone EH, Young JB, Topol EJ: Cause of death in clinical research: Time for a reassessment? J Am Coll Cardiol 34: 618–620, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Bleyer AJ, Hartman J, Brannon PC, Reeves-Daniel A, Satko SG, Russell G: Characteristics of sudden death in hemodialysis patients. Kidney Int 69: 2268–2273, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Annual Data Report US Renal Data System, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2006 [Google Scholar]
- 10.Karnik JA, Young BS, Lew NL, Herget M, Dubinsky C, Lazarus JM, Chertow GM: Cardiac arrest and sudden death in dialysis units. Kidney Int 60: 350–357, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Lafrance JP, Nolin L, Senécal L, Leblanc M: Predictors and outcome of cardiopulmonary resuscitation (CPR) calls in a large haemodialysis unit over a seven-year period. Nephrol Dial Transplant 21: 1006–1012, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Pun PH, Lehrich RW, Smith SR, Middleton JP: Predictors of survival after cardiac arrest in outpatient hemodialysis clinics. Clin J Am Soc Nephrol 2: 491–500, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Lehrich RW, Pun PH, Tanenbaum ND, Smith SR, Middleton JP: Automated external defibrillators and survival from cardiac arrest in the outpatient hemodialysis clinic. J Am Soc Nephrol 18: 312–320, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Pun PH, Lehrich RW, Honeycutt EF, Herzog CA, Middleton JP: Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int 79: 218–227, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Agresti A, Coull BA: Approximate is better than “exact” for interval estimation of binomial proportions. Am Stat 52: 119–126, 1998 [Google Scholar]
- 16.Wild CJ, Seber GAF: Comparing two proportions from the same survey. Am Stat 47: 178–181, 1993 [Google Scholar]
- 17.Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M, Gregoratos G, Klein G, Moss AJ, Myerburg RJ, Priori SG, Quinones MA, Roden DM, Silka MJ, Tracy C, Smith SC, Jr, Jacobs AK, Adams CD, Antman EM, Anderson JL, Hunt SA, Halperin JL, Nishimura R, Ornato JP, Page RL, Riegel B, Blanc JJ, Budaj A, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. American College of Cardiology/American Heart Association Task ForceEuropean Society of Cardiology Committee for Practice GuidelinesEuropean Heart Rhythm AssociationHeart Rhythm Society: ACC/AHA/ESC 2006 Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation 114: e385–e484, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Ritz E, Wanner C: The challenge of sudden death in dialysis patients. Clin J Am Soc Nephrol 3: 920–929, 2008 [DOI] [PubMed] [Google Scholar]
- 19.McIntyre CW, Burton JO, Selby NM, Leccisotti L, Korsheed S, Baker CS, Camici PG: Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol 3: 19–26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Einhorn LM, Zhan M, Hsu VD, Walker LD, Moen MF, Seliger SL, Weir MR, Fink JC: The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med 169: 1156–1162, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Young BA: Prevention of sudden cardiac arrest in dialysis patients: Can we do more to improve outcomes? Kidney Int 79: 147–149, 2011 [DOI] [PubMed] [Google Scholar]