Summary
Background and objectives
Several temporary venous catheterizations are sometimes required for acute renal replacement therapy (RRT) in the intensive care unit (ICU). This study compares first and second catheterizations in the femoral and jugular veins in terms of patient safety.
Design, setting, participants, & measurements
A crossover study from the catheter-dialysis randomized study (Cathedia), which was conducted among 736 critically ill adults requiring RRT, was performed. Catheter insertion complications, catheter-tip colonization, catheter dysfunction and urea reduction ratio (URR) were analyzed considering the crossover and longitudinal designs.
Results
This study analyzed134 patients who underwent two different sites of catheterization, 57 and 77 of whom were initially randomized in the femoral and jugular site, respectively. Using anatomic landmarks, time to insert a femoral catheter was shorter (P=0.01) and more successful (P=0.003) compared with catheterization in the jugular site. Time to catheter-tip colonization at removal was not significantly different between the two sites of insertion (median, 14 days in both groups; hazard ratio, 0.99; 95% confidence interval, 0.61–1.59; P=0.96), as well as time to dysfunction. URRs were analyzed from 395 dialysis sessions (n=48 patients). No significant difference (P=0.49) in mean URR was detected between sessions performed through femoral (n=213; 50.9%) and jugular (n=182; 49.5%) dialysis catheters.
Conclusions
These results validate prior results of this study group and extend external validity to the second catheter used for RRT in the ICU. Femoral and internal jugular acute vascular access sites are both acceptable for RRT therapy in the ICU.
Introduction
Temporary vascular access by central catheter insertion is required for treating ARF by renal replacement therapy (RRT) (1). The major life-threatening complication related to vascular access is infection (2), representing the main cause of nosocomial bloodstream infection in intensive care units (ICU) (3).
Because the use of femoral catheters is associated with increased risk of bloodstream infections compared with internal jugular catheters (4,5), this route has been discouraged for temporary dialysis access (6). However, a parallel experiment from our group (7) showed that this might not be the case in ICU. In addition, the incidences of catheter bloodstream infections (7) or catheter dysfunction (8), as well as urea reduction ratios (URR) (8), were similar between randomized groups. Any unexpected result derived from a single study must be confirmed by other studies before translating evidence base into routine clinical practice.
Another data set from the same Cathedia study included the second catheter inserted at an alternative site within the same patient. The aim of this study was to validate our previous comparisons of vascular access using this new data set in a crossover experiment.
Materials and Methods
Study Design, Settings, and Population
The Cathedia trial was a randomized, controlled trial undertaken in nine university hospitals and three general hospitals in France, between May 2004 and May 2007 (ClinicalTrials.gov Registration NCT00277888). The Cathedia study included 750 patients and compared the risk of catheter infection based on the catheter insertion site (femoral or jugular). The randomization was also stratified by the study center and the mode of RRT (continuous or intermittent hemodialysis).
Eligible Cathedia participants were critically ill adults who were expected to require renal support with RRT in the ICU (7). Inclusion criteria for participation included first central venous catheterization (CVC) for RRT and no contraindication for either jugular or femoral access. Data regarding the second inserted catheter were also collected prospectively with the same case report form.
During RRT management, investigators were asked to complete a second case report form if they chose an alternative site for the second vascular access. A crossover study was thus designed among patients from the Cathedia study who consecutively received a first and a second CVC in the two different sites of insertion (femoral or jugular). Details regarding catheter lengths, types, materials, care, RRT, and dialysis procedures (which were similar between the first and second catheters within each center) were described previously (7,8). The institutional review board at Caen University Hospital approved this study. Informed written consent was obtained from all participants or their proxies.
Endpoints
Catheter-Tip Colonization.
The Brun-Buisson simplified technique (9) of quantitative broth dilution culture was used to define catheter-tip colonization. Catheter-tip colonization was defined as cultures with at least 103 CFU per millimeter of growth. The microbiologists were unaware of the catheter site.
Catheter Dysfunction.
Catheter dysfunction was defined as an inability to attain an adequate blood flow, requiring catheter replacement. Thrombolytic therapy was not permitted in this study.
Urea Reduction Ratio.
Among patients starting with intermittent hemodialysis, the URR was calculated according to the following equation:
 |
Statistical Analysis
Sample size estimation of the primary analysis was reported previously (7). In this secondary analysis, we used available data without post hoc power computation.
Data were expressed as mean ± SD or median (interquartile range) and percentage, depending of the nature of the variable of interest. To determine whether the 1:1 randomization balance of the Cathedia trial was still valid, the chi-squared test was used with a 50-50 proportion to compare patients within the crossover study who had a first CVC inserted in the femoral or jugular site.
Baselines were compared with those of all other patients from the Cathedia study by using either the chi-squared test in cases of qualitative variables, or by using the Student’s or Mann-Whitney tests in cases of continuous variables. Femoral and jugular catheters were compared using the McNemar, Student’s, or Wilcoxon signed-rank tests for dependent samples, as appropriate (10). Catheter colonization and dysfunction curves were estimated by the Kaplan-Meier method for both sites of insertion and were compared by the robust log-rank test, using the sandwich variance estimator, to take into account the crossover design. Hazard ratios (HRs) and robust confidence intervals (CIs) were estimated by a Cox proportional hazard model, after checking the proportionality assumption. The difference in means of URR after jugular and femoral intermittent hemodialysis sessions was estimated by a linear mixed model, within which (1) the longitudinal design was modeled by using a temporal autoregressive structure of order one within each combination of patient and site and (2) the crossover design was modeled by adding an intrasite correlation, for each patient. All analyses were performed using R software (version 2.12.1).
Results
Patient Population
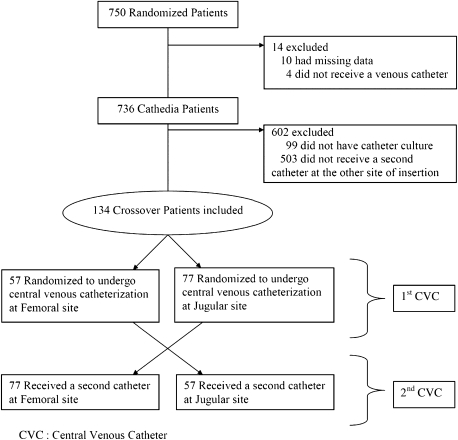
Among the 750 randomized patients of the Cathedia trial, 736 were included in the Cathedia analysis, 134 of whom were included in this study (Figure 1). Fifty-seven patients (42.5%) were first randomized in the femoral group and then received a second CVC at the jugular site; 77 patients (57.5%) received the opposite sequence of catheterization. Those proportions assessed the 1:1 randomization balance (P=0.08; the difference to the 50-50 proportion was not detected as significant).
Figure 1.
Crossover patient flowchart.
To assess the potential selection bias of this crossover study, baselines of the 134 analyzed patients were compared with the 602 remaining patients of the Cathedia study (Table 1). No significant differences were detected except for gender, body temperature, and catheter duration. There were more men in the crossover study (74.6% compared with 65.4%, P=0.04) and patients had a slightly higher body temperature at the time of inclusion in this study (mean, 37.3°C; SD, 2.0) than the remaining patients in the Cathedia trial (mean, 36.7°C; SD 2.2). Moreover, the mean length of catheterization was >1 day longer for the patients analyzed in the crossover study than the other patients of the Cathedia trial (P=0.008), and no significant difference was detected between the proportion of patients who had died by the end of the study (P=0.92).
Table 1.
Comparisons between patients from the crossover study and all other patients from the Cathedia trial
| Patients from the Crossover Study (n=134) | All Other Cathedia Patients (n=602) | P Value | |
|---|---|---|---|
| Age, yr, mean (SD) | 65.4 (13.5) | 64.9 (15.2) | 0.70 |
| Male sex | 100 (74.6) | 394 (65.4) | 0.04 |
| Body mass index, mean (SD) | 27.2 (5.0) | 26.6 (5.3) | 0.22 |
| APACHE II score, mean (SD) | 28.6 (9.5) | 27.6 (9.6) | 0.28 |
| No. of organ failures, mean (SD) | 2.5 (1.2) | 2.4 (1.2) | 0.82 |
| Ventilated | 104 (77.6) | 432 (71.8) | 0.17 |
| Days from admission to inclusion, median (IQR) | 1 (0–2) | 1 (0–2) | 0.51 |
| Body temperature, °C, mean (SD) | 37.3 (2.0) | 36.7 (2.2) | 0.01 |
| White blood cell count, cells/µl, median (IQR) | 12.3 (7.4–19.5) | 12.8 (7.7–19.8) | 0.75 |
| Platelet count, 103 cells/ml, median (IQR) | 168.0 (90.0–206.0) | 161.0 (80.5–244.5) | 0.81 |
| Received systemic antibiotics | 88 (65.7) | 364 (60.5) | 0.26 |
| Received catecholamines | 50 (37.3) | 238 (39.5) | 0.63 |
| Immunosuppression | 24 (17.9) | 103 (17.1) | 0.82 |
| Diabetes | 33 (24.6) | 159 (26.4) | 0.67 |
| Days of catheter insertion, mean (SD) | |||
| first CVC | 7.7 (5.0) | 6.1 (6.4) | 0.008a |
| second CVC | 8.0 (6.1) | NA | |
| mean of first and second CVC | 7.9 (5.6) | NA | |
| death | 64 (47.8) | 292 (48.5) | 0.92 |
Data are presented as no. (%) unless otherwise specified. APACHE, acute physiology and chronic health evaluation; CVC, central venous catheterization; IQR, interquartile range; NA, not applicable.
Comparison between the mean of the two first CVC of crossover patients and the first CVC of all other Cathedia patients.
Catheter Characteristics and Catheter Insertion
Paired comparisons between the two different catheter vascular access sites are presented in Table 2. No clinical or significant differences were detected between the two sites except for the time required for insertion and the proportion of failure on one side, which were more important for the jugular site.
Table 2.
Catheter characteristics and follow-up of the 134 crossover patients
| Paired Central Venous Catheters | P Value | ||
|---|---|---|---|
| Femoral | Jugular | ||
| Catheter characteristics | |||
| antiseptic-impregnated catheter | 8 (6.0) | 12 (8.9) | 0.37 |
| ultrasound-guided insertion | 0 (0.0) | 0 (0.0) | NA |
| no. of attempts, median (IQR) | 1.6 (1–2) | 2 (1–2) | 0.41 |
| time required for insertion, min, median (IQR) | 10 (7–15) | 12 (8–19) | 0.01 |
| first attempt of the right side | 85 (63.9) | 84 (62.2) | 0.88 |
| failure on one side | 3 (2.3) | 17 (12.8) | 0.003 |
| Catheter follow-up | |||
| days of insertion, median (IQR) | 7 (4–11) | 7 (4–10) | 0.59 |
| reason for catheter removal | |||
| suspicion of catheter infection | 42 (31.6) | 41 (30.4) | 0.89 |
| catheter dysfunction | 30 (22.6) | 35 (25.9) | 0.46 |
| no longer required | 26 (19.4) | 20 (14.9) | 0.24 |
| systematic | 22 (16.5) | 18 (13.3) | 0.48 |
| death | 12 (9.0) | 17 (12.6) | 0.30 |
| spontaneous catheter withdrawal | 1 (0.8) | 2 (1.5) | 0.56 |
| unknown | 1 (0.8) | 1 (0.7) | 1 |
Data are presented as no. (%) unless otherwise specified. IQR, interquartile range; NA, not applicable.
Catheter-Tip Colonization
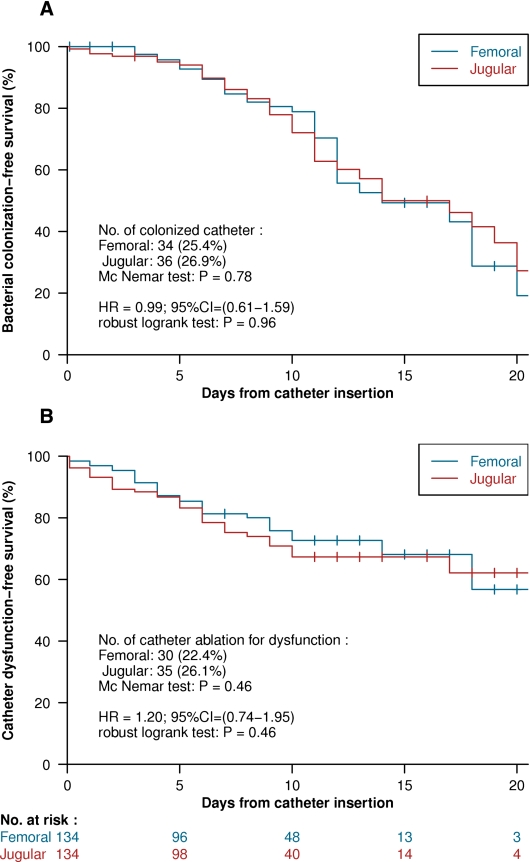
At the time of removal, 34 (25.4%) and 36 (26.9%) catheters were colonized among the femoral and jugular catheters, respectively, of the 134 crossover patients (P=0.78, by McNemar chi-squared test) (Figure 2A). The risk of bacterial colonization at the time of catheter removal was not significantly different between the two insertion sites (HR, 0.99; 95% CI, 0.61–1.59; P=0.96 by robust log-rank test) (Figure 2A). The number of catheter-related bloodstream infections was too low to be compared.
Figure 2.
Kaplan-Meier curves, comparing the two vascular access routes (femoral in blue shape and jugular in red shape), related hazard ratio, 95% confidence interval, and P value of the log rank test, using the “sandwich” robust estimator of the variance, considering the crossover design. (A) Time to catheter colonization estimation. (B) Time to dysfunction estimation.
Catheter Dysfunction
Thirty (22.4%) and 35 (26.1%) catheters were removed for dysfunction among the femoral and jugular catheters, respectively, of the 134 crossover patients (P=0.46, by McNemar chi-squared test) (Figure 2B). The risk of catheter dysfunction was not significantly different between the two insertion sites (HR, 1.20; 95% CI, 0.74–1.95; P=0.46 by robust log-rank test) (Figure 2B).
Urea Reduction Ratio
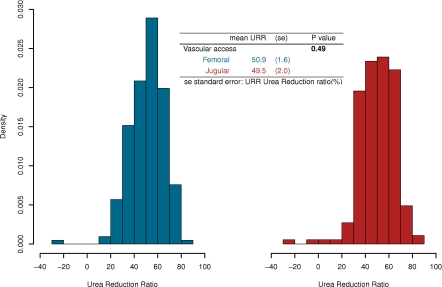
Forty-eight patients underwent an intermittent hemodialysis in each site of catheter insertion. Thus, URR after every intermittent hemodialysis session were computed. Distributions of dialysis URR are represented in Figure 3. We analyzed 395 dialysis sessions, 213 of which were femoral (mean URR, 51.2%; SD, 14.3) and 182 of which were jugular (mean, 50.2%; SD, 15.3). Because a patient could have undertaken more than one dialysis session at each different site of catheterization, a linear mixed model was computed to compare mean URR between those two sites, considering the longitudinal and crossover design of the analysis. The model estimated a mean URR of 50.9% and 49.5% for femoral and jugular intermittent hemodialysis, respectively. The mean difference between the two sites was neither clinically nor significantly different (P=0.49) (Figure 3).
Figure 3.
Histograms of the urea reduction ratio (URR) of dialysis. (Left) Under a femoral catheterization. (Right) Under a jugular catheterization. The result of the linear mixed model comparing the mean URR, depending on the access route, is also presented.
Discussion
The risks of catheter infection and dysfunction were similar between jugular and femoral vein access sites among the 134 patients who received both catheters alternatively and randomly. In addition, the URR were comparable between routes. The results of this crossover design confirm those we found using a parallel design (7,8). Our findings are in accordance with new guidelines from the Centers for Disease Control and Prevention (11) for preventing central catheter infection, but contrast with the National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative Clinical Practice Guidelines for Vascular Access (6).
Our sample, by design, only selected patients who were still alive at the end of the first catheterization, who still required a second catheter for RRT, and for whom an alternative site was chosen. Although this constitutes selection bias, it embraces clinical practice situations in which our results are applicable. In addition, baseline characteristics were similar between patients included and not included in this crossover analysis. Gender and body temperature were marginally different between samples. Although both factors were not associated with catheter-tip colonization in our study (12), men carry more microorganisms on the skin than do women (13). The first site and thus the order of catheterization were randomized, which increases internal validity of the results.
Femoral venous access was faster and more successful than jugular access. For this reason, the femoral route is usually considered as the emergency route to gain vascular access. It should be noted that ultrasound guidance was not used for jugular catheterization. Ultrasound guidance has been shown to reduce catheterization failures and complications, for jugular central catheterization in particular, and should therefore be used (14–16).
Our results confirm that despite its proximity to the groin, the femoral route does not expose patients to a higher risk of catheter-related infection than the internal jugular route when catheterization occurred in the ICU with standard infection control practices. The main route for catheter-tip colonization for such indwelling time is extraluminal (17) from a patient’s skin flora. The local density of microorganisms is therefore of major importance and the jugular site may also pose problems. After neck movements, oral secretions may contaminate the dressing. Another potential problem associated with temporary catheterization is vein stenosis (18), particularly when the jugular site is chosen in the presence of pacemaker leads (19). After colonization of the central catheter, the pacemaker leads can also become infected (20). In this context, the femoral route also seems preferable.
In this analysis, each patient served as his or her own control. Prior studies conducted in the same setting considered this design to compare the effect of catheter site (21) or catheter purpose (22) (RRT versus drug administration) on the risk of catheter infection. For example, Deshpande et al. (21) found a higher incidence density of jugular (48 per 1000 catheter-days) compared with femoral (8 per 1000 catheter-days) catheter-tip colonizations among 24 patients who received catheters at both sites. Although very large, this difference was not significant (P=0.06). Souweine et al. (22) also reported a higher incidence of dialysis catheter-tip colonization for the jugular site (18.6 per 1000 catheter-days, n=45) compared with the femoral site (3.6 per 1000 catheter-days, n=85), although the difference was not significant (P=0.15). Our results, which are based on a larger sample size and a stronger internal validity, do not support this trend favoring the femoral route over the internal jugular route for RRT vascular access in ICU.
Catheter dysfunction and URR were not different between sites, possibly because most of the femoral catheters were >20 cm long, in contrast with prior studies (23–28), and a more central position of the tip reduces dysfunctions and improves the flow by limiting recirculation rates. In addition, all previous studies were observational (23–28).
The catheter duration was 7 days, in accordance with previous studies (22,29,30). The median indwelling time reported in this study is 2 days longer than the 5 days reported in the overall Cathedia cohort (7). Therefore, this result extends generalizability of our previous findings to longer temporary catheterization. This is important because in clinical practice, ARF may last up to 6 weeks and may consequently require multiple temporary venous accesses at multiple sites. The tunneled catheters may avoid the possible complications of the multiple venous accesses at multiple sites (30); however, according to a meta-analysis by Randolph et al. (31), routine tunneling of short-term catheters to reduce catheter infection in ICU is not recommended.
This work has some limitations. First, because of the low number of events observed, colonization was studied rather than catheter-related bloodstream infection, which may limit clinical relevancy. Second, only the first and second dialysis catheters were included. Third, catheter insertion used anatomic landmarks, whereas the use of ultrasound-guided insertion is known to reduce the risk of mechanical complications, particularly for the internal jugular route (15). Fourth, although cutaneous antisepsis and catheter care were controlled, dialysis prescriptions were not standardized between sites, which may bias URR comparisons. However, dialysis sessions were managed for each pairwise comparison by the same healthcare staff and within the same patient in random order. Finally, despite the high statistical power provided by the crossover design, this analysis only included 18.2% of the original cohort. Consequently, we were not able to explore subgroups according to the body mass index, side of catheterization, or dialysis prescriptions.
In conclusion, femoral and internal jugular routes are both equally safe and functional for RRT vascular access in ICU. Whether these data apply outside the ICU is speculative. Because of easier placement in the absence of ultrasound guidance, these data add to the evidence that femoral access can be used but with optimal insertion and sterile technique in the nonobese, bedbound, ICU patient, and that the alternative internal jugular route may be preferable once the patient starts to mobilize.
Disclosures
None.
Acknowledgments
We gratefully acknowledge the dedication of the nursing and medical staff members of all the participating centers and the generosity of the study participants or family members, without whom this study could not have been completed.
This study was funded by the Centre Hospitalier Universitaire de Caen and was supported by an unrestricted academic grant from the French Health Ministry (Programme Hospitalier de Recherche Clinique National 2003).
Data Monitoring Task Force: A. Gauneau (clinical research assistant, CHU de Caen), V. Léon (clinical research assistant, CHU de Caen), J.J. Dutheil (clinical research assistant, CHU de Caen), E. Vastel (clinical research assistant, CHU de Caen), F. Chaillot (administrator, CHU de Caen).
Participating Centers and Cathedia Investigators: Centre Hospitalier Universitaire de Caen, Réanimations Médicale: J.J. Parienti (principal investigator), M. Ramakers, D. du Cheyron, C. Daubin, P. Charbonneau, N. Terzi, B. Bouchet, S. Chantepie, A. Seguin, S. Chevalier, D. Guillotin, X. Valette, T. Dessieux, W. Grandin, S. Lammens, C. Buleon, V. Pottier, C. Quentin, S. Thuaudet, C. Le Hello. Réanimations Chirurgicale: A. Ouchikhe (principal investigator), D. Samba, C. Jehan, G. Viquesnel, G. Leroy, E. Frostin, C. Eustratiades, M.O. Fischer, M.R. Clergeau, F. Michaux. Centre Hospitalier Universitaire Cochin-Port-Royal, Assistance Publique- Hôpitaux de Paris, Réanimation Médicale: J.P. Mira (principal investigator), S. Marqué, M. Thirion, S. Buyse, E. Clapson, J.D. Chiche, A. Soummer, B. Planquette, C. Baklini, D. Grimaldi, T. Braun, O. Huet, S. Perbet, J. Medrano, N. Verroust, A. Mathonnet, N. Joram, E. Lopez, D. Grimaldi, G. Colin. Centre Hospitalier Universitaire Lariboisière, Assistance Publique- Hôpitaux de Paris, Réanimation Médicale: B. Megarbane (principal investigator), F.J. Baud, N. Deye, D. Résière, G. Guerrier, J. Theodore, S. Rettab, P. Brun, S. Karyo, S. Delerme, A. Abdelwahab, J.M. Ekhérian, I. Malissin, A. Mohebbi-Amoli. Centre Hospitalier Universitaire de Clermont-Ferrand, Réanimation Médicale et Néphrologie: N. Gazui (principal investigator), B. Souweine, F. Thiollière, A. Lautrette, J. Liotier. Centre Hospitalier Universitaire Sainte Marguerite, Assistance Publique- Hôpitaux de Marseille, Réanimation Médicale: J.M. Forel (principal investigator), S. Gayet, N. Embriaco, D. Demory, J. Allardet-Servent, F. Michel. Centre Hospitalier Universitaire Garches, Assistance Publique- Hôpitaux de Paris, Réanimation Médicale: A. Polito (principal investigator), D. Annane, V. Maxime. Centre Hospitalier General, Argentueil, Réanimation Médicale: M. Thirion (principal investigator), E. Bourgeois, I. Rennuit, R. Hellmann, J. Beranger. Fondation Hôpital Saint-Joseph, Paris, Réanimation Médicale: B. Misset (principal investigator), V. Willems, F. Philippart, A. Tabah. Centre Hospitalier Universitaire d’Amiens, Service de Néphrologie Réanimation Médicale: B. de Cagny (principal investigator), M. Slama, N. Airapetian, J. Maizel, B. Gruson, A. Sarraj, F. Lengelle. Centre Hospitalier Universitaire Croix Rousse, Hospices Civils de Lyon: C. Guérin (principal investigator). Centre Hospitalier Général, Pau: P. Badia (principal investigator). Centre Hospitalier Général, Saint-Malo: L. Auvray (principal investigator). All centers are in France.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Schetz M: Vascular access for HD and CRRT. Contrib Nephrol 156: 275–286, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Mermel LA: Prevention of intravascular catheter-related infections. Ann Intern Med 132: 391–402, 2000 [DOI] [PubMed] [Google Scholar]
- 3.McGee DC, Gould MK: Preventing complications of central venous catheterization. N Engl J Med 348: 1123–1133, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Oliver MJ, Callery SM, Thorpe KE, Schwab SJ, Churchill DN: Risk of bacteremia from temporary hemodialysis catheters by site of insertion and duration of use: A prospective study. Kidney Int 58: 2543–2545, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Lorente L, Henry C, Martín MM, Jiménez A, Mora ML: Central venous catheter-related infection in a prospective and observational study of 2,595 catheters. Crit Care 9: R631–R635, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vascular Access Work Group: Clinical practice guidelines for vascular access. Am J Kidney Dis 48[Suppl 1]: S248–S273, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Parienti JJ, Thirion M, Mégarbane B, Souweine B, Ouchikhe A, Polito A, Forel JM, Marqué S, Misset B, Airapetian N, Daurel C, Mira JP, Ramakers M, du Cheyron D, Le Coutour X, Daubin C, Charbonneau P. Members of the Cathedia Study Group: Femoral vs jugular venous catheterization and risk of nosocomial events in adults requiring acute renal replacement therapy: A randomized controlled trial. JAMA 299: 2413–2422, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Parienti JJ, Mégarbane B, Fischer MO, Lautrette A, Gazui N, Marin N, Hanouz JL, Ramakers M, Daubin C, Mira JP, Charbonneau P, du Cheyron D. Cathedia Study Group: Catheter dysfunction and dialysis performance according to vascular access among 736 critically ill adults requiring renal replacement therapy: A randomized controlled study. Crit Care Med 38: 1118–1125, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Brun-Buisson C, Abrouk F, Legrand P, Huet Y, Larabi S, Rapin M: Diagnosis of central venous catheter-related sepsis. Critical level of quantitative tip cultures. Arch Intern Med 147: 873–877, 1987 [PubMed] [Google Scholar]
- 10.Mills EJ, Chan AW, Wu P, Vail A, Guyatt GH, Altman DG: Design, analysis, and presentation of crossover trials. Trials 10: 27, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad II, Randolph AG, Rupp ME, Saint S. Healthcare Infection Control Practices Advisory Committee: Guidelines for the prevention of intravascular catheter-related infections. Am J Infect Control 39[Suppl 1]: S1–S34, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Parienti JJ, Dugué AE, Daurel C, Mira JP, Mégarbane B, Mermel LA, Daubin C, du Cheyron D. Members of the Cathedia Study Group: Continuous renal replacement therapy may increase the risk of catheter infection. Clin J Am Soc Nephrol 5: 1489–1496, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reichel M, Heisig P, Kampf G: Identification of variables for aerobic bacterial density at clinically relevant skin sites. J Hosp Infect 78: 5–10, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Slama M, Novara A, Safavian A, Ossart M, Safar M, Fagon JY: Improvement of internal jugular vein cannulation using an ultrasound-guided technique. Intensive Care Med 23: 916–919, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Nadig C, Leidig M, Schmiedeke T, Höffken B: The use of ultrasound for the placement of dialysis catheters. Nephrol Dial Transplant 13: 978–981, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Karakitsos D, Labropoulos N, De Groot E, Patrianakos AP, Kouraklis G, Poularas J, Samonis G, Tsoutsos DA, Konstadoulakis MM, Karabinis A: Real-time ultrasound-guided catheterisation of the internal jugular vein: A prospective comparison with the landmark technique in critical care patients. Crit Care 10: R162, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mermel LA: What is the predominant source of intravascular catheter infections? Clin Infect Dis 52: 211–212, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Sticherling C, Chough SP, Baker RL, Wasmer K, Oral H, Tada H, Horwood L, Kim MH, Pelosi F, Michaud GF, Strickberger SA, Morady F, Knight BP: Prevalence of central venous occlusion in patients with chronic defibrillator leads. Am Heart J 141: 813–816, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Asif A, Carrillo R, Garisto JD, Lopera G, Ladino M, Barakat U, Eid N, Salman L: Epicardial cardiac rhythm devices for dialysis patients: Minimizing the risk of infection and preserving central veins [published online ahead of print August 27, 2010]. Semin Dial doi:10.1111/j.1525-139X.2010.00757.x [DOI] [PubMed] [Google Scholar]
- 20.Carrillo RG, Garisto JD, Salman L, Merrill D, Asif A: Contamination of transvenous pacemaker leads due to tunneled hemodialysis catheter infection: A report of 2 cases. Am J Kidney Dis 55: 1097–1101, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Deshpande KS, Hatem C, Ulrich HL, Currie BP, Aldrich TK, Bryan-Brown CW, Kvetan V: The incidence of infectious complications of central venous catheters at the subclavian, internal jugular, and femoral sites in an intensive care unit population. Crit Care Med 33: 13–20, discussion 234–235, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Souweine B, Liotier J, Heng AE, Isnard M, Ackoundou-N’Guessan C, Deteix P, Traoré O: Catheter colonization in acute renal failure patients: Comparison of central venous and dialysis catheters. Am J Kidney Dis 47: 879–887, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Leblanc M, Fedak S, Mokris G, Paganini EP: Blood recirculation in temporary central catheters for acute hemodialysis. Clin Nephrol 45: 315–319, 1996 [PubMed] [Google Scholar]
- 24.Little MA, Conlon PJ, Walshe JJ: Access recirculation in temporary hemodialysis catheters as measured by the saline dilution technique. Am J Kidney Dis 36: 1135–1139, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Liangos O, Rao M, Ruthazer R, Balakrishnan VS, Modi G, Pereira BJ, Jaber BL: Factors associated with urea reduction ratio in acute renal failure. Artif Organs 28: 1076–1081, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Naumovic RT, Jovanovic DB, Djukanovic LJ: Temporary vascular catheters for hemodialysis: A 3-year prospective study. Int J Artif Organs 27: 848–854, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Hryszko T, Brzosko S, Mazerska M, Malyszko J, Mysliwiec M: Risk factors of nontunneled noncuffed hemodialysis catheter malfunction. A prospective study. Nephron Clin Pract 96: c43–c47, 2004 [DOI] [PubMed] [Google Scholar]
- 28.du Cheyron D, Bouchet B, Bruel C, Daubin C, Ramakers M, Charbonneau P: Antithrombin supplementation for anticoagulation during continuous hemofiltration in critically ill patients with septic shock: A case-control study. Crit Care 10: R45, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Souweine B, Traore O, Aublet-Cuvelier B, Badrikian L, Bret L, Sirot J, Gazuy N, Laveran H, Deteix P: Dialysis and central venous catheter infections in critically ill patients: Results of a prospective study. Crit Care Med 27: 2394–2398, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Klouche K, Amigues L, Deleuze S, Beraud JJ, Canaud B: Complications, effects on dialysis dose, and survival of tunneled femoral dialysis catheters in acute renal failure. Am J Kidney Dis 49: 99–108, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Randolph AG, Cook DJ, Gonzales CA, Brun-Buisson C: Tunneling short-term central venous catheters to prevent catheter-related infection: A meta-analysis of randomized, controlled trials. Crit Care Med 26: 1452–1457, 1998 [DOI] [PubMed] [Google Scholar]