Summary
Background and objectives
There are few data on risk factors for sudden cardiac death (SCD) in patients undergoing hemodialysis (HD). The study objective was to identify predictors associated with various causes of death in the Hemodialysis (HEMO) Study and to develop a prediction model for SCD using a competing risk approach.
Design, setting, participants, & measurements
In this analysis of 1745 HEMO participants, all-cause mortality was classified as SCD, non-SCD, and noncardiac death. Predictors for each cause of death were evaluated using cause-specific Cox proportional hazards models, and a competing risk approach was used to calculate absolute risk predictions for SCD.
Results
During a median follow-up of 2.5 years, 808 patients died. Rates of SCD, non-SCD, and noncardiac death were 22%, 17%, and 61%, respectively. Predictors of various causes of death differ somewhat in HD patients. Age, diabetes, peripheral vascular disease, ischemic heart disease, serum creatinine, and alkaline phosphatase were independent predictors of SCD. The 3-year C-statistic for SCD was 0.75 (95% confidence interval, 0.70–0.79), and calibration was good (χ2=1.1; P=0.89). At years 3 and 5 of follow-up, the standard Cox model overestimated the risk for SCD as compared with the competing risk approach on the relative scale by 25% and 46%, respectively, and on the absolute scale by 2% and 6%, respectively.
Conclusions
Predictors of various causes of death differ in HD patients. The proposed prediction model for SCD accounts for competing causes of death. External validation of this model is required.
Introduction
Cardiovascular disease is a major cause of morbidity and mortality in hemodialysis (HD) patients, accounting for more than 40% of deaths (1). The US Renal Data System (USRDS) report attributes the single largest specific cause of death from cardiovascular disease to arrhythmias or cardiac arrest (1). Although certain risk factors associated with sudden cardiac death (SCD) in HD patients are similar to those in the general population, factors unique to kidney failure and the HD procedure play a prominent role but remain poorly understood (2–7).
Several studies have identified risk factors for SCD in HD patients, but few have compared predictors for SCD with other causes of death (2–8). The latter is important given that treatment recommendations may vary depending on the risk factor profile of the patient. For example, an individual at high risk for SCD may benefit from a defibrillator or β-blockers, whereas an individual with an indwelling catheter who is at high risk for noncardiac death may benefit from an antibiotic lock.
There are also limited data on prediction equations for SCD in HD patients, and previous studies have not incorporated competing risk into these equations (9). In the presence of high competing risks as seen in HD populations, competing events may preclude the event of interest and thus the benefit of an intervention. In addition, without incorporation of competing risk, the probability of SCD is overestimated and incorrect conclusions can be drawn. In this study, we evaluated the predictors of SCD, non-SCD, and noncardiac deaths in the Hemodialysis (HEMO) Study using cause-specific Cox proportional hazards models, and a competing risk approach was used to derive absolute risk predictions for SCD.
Materials and Methods
Study Population
The design and methods of the HEMO Study have been described in detail elsewhere (10,11). In brief, the HEMO Study enrolled 1846 patients undergoing long-term HD from 15 clinical centers comprising 72 dialysis units. Exclusion criteria included, but were not limited to, unstable angina, serum albumin level less than 2.6 g/dl, current hospitalization, New York Heart Association class IV congestive heart failure, and active systemic infection. Eligible participants were randomly assigned between May 1995 and February 2001 in a 2×2 factorial design to a standard or a high dialysis dose and to a low-flux or a high-flux dialyzer. Participants were followed until death, kidney transplantation, or the end of the study period in December 2001, whichever came first. The Tufts Medical Center Institutional Review Board approved these retrospective analyses.
Covariates
Baseline demographic data were collected through self-reported questionnaires. Clinical and laboratory data were obtained using standardized protocols (11). Serum creatinine, calcium, and phosphorous levels were obtained from local laboratory measurements, whereas predialysis serum albumin and urea were determined monthly at the HEMO central laboratory. Comorbid conditions were assessed using the Index of Coexisting Disease (ICED) and were ascertained from review of medical records (12). ICED is a standardized method of assessing comorbidity that has been shown to be a valid predictor of mortality (13). ICED aggregated the presence and severity of 19 medical conditions, including peripheral vascular disease, cerebrovascular disease, diabetes, and hypertension. To capture the evidence and additional details of existing or history of cardiac disease, the category for cardiovascular disease was expanded to four categories: ischemic heart disease (IHD), heart failure (HF), arrhythmias, and other heart diseases. Each category of cardiac disease was scored from 0 to 3; 0 indicated no disease in that category, and 3 indicated the presence of moderate or severe manifestations of the disease regardless of treatment. For purposes of this analysis, scores of 1, 2, or 3 were used to denote the presence of baseline disease.
The list of candidate predictors considered for the prediction model included the following: (1) demographic factors (age, sex, race); (2) cardiovascular risk factors (history of diabetes, smoking, systolic and diastolic BP, body mass index); (3) cardiovascular disease (including IHD, HF, arrhythmias, other heart disease, peripheral vascular disease, and cerebrovascular disease); (4) HD-related factors (dialysis vintage, randomly assigned flux and dose groups); and (5) laboratory data (including hematocrit and serum levels of creatinine, potassium, albumin, calcium, phosphorus, and alkaline phosphatase).
Outcome
The primary outcome of the HEMO Study was all-cause mortality. For each death, the principal investigator of each clinical center completed a form that included the cause of death according to the HEMO Study classification and a brief narrative summary describing the events leading to death (14). All death classifications required independent audits by two members of the Outcome Review Committee. SCD was a secondary outcome of interest in the HEMO Study.
Sudden death in the HEMO Study was defined as a witnessed or unwitnessed unexpected death, with preceding duration of symptoms less than 24 hours for witnessed deaths and less than the interval since the last dialysis session for unwitnessed deaths. Sudden death was attributed to IHD if the patient had a history of this condition, to arrhythmias if the patient had a history of arrhythmias in the absence of IHD, and to other heart diseases if the patient had a history of other causes of heart disease in the absence of IHD or arrhythmias. Sudden death due to IHD, arrhythmias, or other heart disease met criteria for SCD. Deaths were subsequently classified into three mutually exclusive groups: SCD, non-SCD, and noncardiac death.
Statistical Analyses
Descriptive analyses were used to summarize baseline characteristics of the study participants according to their final event status (i.e., alive, SCD death, non-SCD death, and noncardiac death). Continuous variables are presented as mean ± SD or median (interquartile range) as appropriate, and categorical variables are given as proportions. Continuous variables were compared using ANOVA or a Kruskal-Wallis test as appropriate, and categorical variables were compared using a χ2 test.
Cause-Specific Cox Hazard Model.
The associations between baseline covariates and various causes of death were assessed using cause-specific Cox proportional hazards models that censored for the respective competing events. Covariates were selected for the analyses according to their biologically plausible potential to act as confounders. Using forward-selection process, we developed multivariable models in which all covariates with a P value of 0.1 or less in univariate analysis for any cause of death were entered in the model selection process. The functional form of continuous predictors and their association with each outcome was explored using restricted cubic splines. To assess the association of predictors on each cause of death and to examine whether these associations differed among the causes of death, we fitted a stratified Cox model by including any covariate that remained significant after the forward-selection process for each cause of death. We tested for differences between the cause-specific hazards ratios for each covariate by including an interaction term between the covariate and the cause of death in a stratified Cox model.
Absolute Risk Prediction Using a Competing Risk Approach.
A competing risk approach was used to calculate absolute risk predictions for SCD (15). Because we were interested in developing a model for SCD, only covariates with a P value of 0.1 or less in the univariate analysis for SCD were included in the model selection process. The standard Cox model and the competing risk approach differ in the way absolute risk predictions are calculated. Whereas predictions from the standard Cox model depend only on the cause-specific hazard of the event of interest and thus overestimate absolute risk in the presence of competing events, the competing risk approach consists of developing a cause-specific hazards models for both the event of interest and the competing event separately, and then combining them according to the cumulative incidence function (16). The competing risk approach calculates the cumulative incidence of SCD as follows:
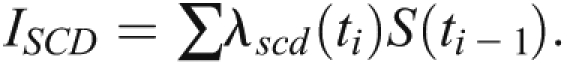 |
The quantities under the summation denote the instantaneous hazard of SCD at event time ti and survival rate from any cause of death past event time ti – 1.
Model Performance and Internal Validation.
Model performance was assessed at 3 years of follow-up for discrimination using the Harrell global C-statistic and for calibration using the modified Hosmer-Lemeshow statistic for survival analysis. The Efron bootstrap resampling technique was used to estimate the optimism in the discrimination statistic and calibration. To account for final model uncertainty, the internal validation technique consisted of repeating the full model-building process described above in 300 bootstrap resamples of equal sample size as our original sample (17). The expected optimism was calculated as the average difference between the performance of models developed in each bootstrap sample when applied to the bootstrap sample and the performance of the same models when applied to the original sample.
An Excel worksheet (Microsoft Corp., Redmond, WA) was created to calculate absolute risk prediction for SCD using the competing risk approach to facilitate application by clinicians.
Analyses were performed using SAS software, version 9.2 (SAS Institute Inc., Cary, NC). Cumulative incidence probabilities were calculated using an SAS macro (18). We considered two-tailed P<0.05 to indicate statistically significant differences.
Results
Characteristics of Study Participants
Of the 1846 HEMO participants, 101 (5.5%) were excluded because they lacked complete data on all the predictors. Thus, the sample available for analysis consisted of 1745 participants. Participants excluded for missing data at baseline were younger and more likely to be of nonblack ethnicity. The prevalence of cardiovascular and cerebrovascular disease did not differ between the included and excluded participants. The participants included in these analyses had a mean age of 58 years, and 44% were male. Sixty-three percent of the participants were African American, and 44% had a history of diabetes. The prevalence rates of IHD, HF, arrhythmias, and other heart diseases were 39%, 40%, 31%, and 63%, respectively.
The median duration of follow-up was 2.5 years. A total of 808 patients died. Among these, 319 (39%) and 489 (61%) deaths were attributed to cardiac and noncardiac causes, respectively. One hundred eighty-two (57%) of the cardiac deaths were due to SCD. Participants who died of SCD were more likely to be older, to be male, and to have a higher burden of comorbid conditions (including cardiovascular disease, diabetes, and peripheral vascular disease) compared with those who survived (Table 1).
Table 1.
Baseline characteristics by different causes of death
| Characteristic | Sudden Cardiac Death | Nonsudden Cardiac Death | Noncardiac Deaths | Alive |
|---|---|---|---|---|
| Patients, n (%) | 182 (10) | 137 (8) | 489 (28) | 937 (54) |
| Age (yr) | 62±11 | 64±11 | 63±12 | 53±14 |
| Men, n (%) | 86 (47) | 63 (46) | 212 (43) | 401 (43) |
| Black patients, n (%) | 105 (58) | 72 (53) | 312 (64) | 611 (65) |
| Smoking status, n (%) | ||||
| never | 82 (45) | 63 (46) | 223 (46) | 508 (54) |
| past | 71 (39) | 54 (39) | 179 (37) | 259 (28) |
| current | 29 (16) | 20 (15) | 87 (18) | 170 (18) |
| Body mass index (kg/m2) | 25.0±4.6 | 25.7±5.5 | 24.9±5.5 | 25.8±5.2 |
| Diabetes, n (%) | 115 (63) | 72 (53) | 236 (48) | 347 (37) |
| Ischemic heart disease, n (%) | 117 (64) | 85 (62) | 215 (44) | 266 (28) |
| Heart failure, n (%) | 98 (54) | 78 (57) | 238 (49) | 278 (30) |
| Arrhythmia, n (%) | 78 (43) | 64 (47) | 207 (42) | 195 (21) |
| Cerebrovascular disease, n (%) | 55 (30) | 36 (26) | 109 (22) | 134 (14) |
| Peripheral vascular disease, n (%) | 52 (29) | 31 (23) | 96 (20) | 101 (11) |
| Other heart disease, n (%) | 132 (73) | 102 (74) | 352 (72) | 513 (55) |
| Dialysis vintage (yr) | 1.9 (0.9–5) | 2.4 (1.2–4.7) | 2.5 (1.0–5.1) | 2.0 (0.9–4.2) |
| Predialysis systolic BP (mmHg) | 153±25 | 148±21 | 153±23 | 152±21 |
| Predialysis diastolic BP (mmHg) | 80±13 | 78±13 | 80±13 | 83±13 |
| High-flux hemodialysis group, n (%) | 89 (49) | 57 (42) | 250 (51) | 473 (50) |
| High-Kt/V group, n (%) | 99 (54) | 64 (47) | 237 (48) | 469 (50) |
| Hematocrit (%) | 33.7±4.6 | 33.1±4.3 | 32.8±4.5 | 34.0±4.5 |
| Serum creatinine (mg/dl) | 9.0±2.8 | 9.8±2.6 | 9.5±2.4 | 11.0±3 |
| Serum potassium (mEq/L) | 4.8±0.8 | 4.9±0.8 | 4.8±0.8 | 4.9±0.8 |
| Serum albumin (mg/dl) | 3.5±0.4 | 3.6±0.3 | 3.5±0.4 | 3.7±0.4 |
| Serum calcium (mg/dl) | 9.2±0.9 | 9.4±1.1 | 9.3±1.0 | 9.3±1.0 |
| Serum phosphorus (mg/dl) | 5.8±1.8 | 5.9±1.7 | 5.6±1.9 | 5.8±1.9 |
| Serum alkaline phosphatase (IU/L) | 101 (72–145) | 91 (67–132) | 105 (78–149) | 93 (71–131) |
| Follow-up time (mo) | 25±18 | 24±18 | 29±19 | 40±23 |
Data expressed with a plus/minus sign are the mean ± SD. Data for dialysis vintage and serum alkaline phosphatase are the median (25th–75th percentiles).
Predictors for Each Cause of Death
Older age was independently associated with each cause-specific death (Table 2). Diabetes was a significant predictor of SCD but not other deaths. IHD was a predictor of both SCD and non-SCD but not noncardiac deaths, and was significantly different when compared between cardiac and noncardiac deaths. Heart failure was a predictor of non-SCD and noncardiac deaths but not SCD. Lower serum creatinine was independently associated with SCD and noncardiac deaths. White race and lower systolic BP were significant predictors of non-SCD.
Table 2.
Multivariable-adjusted cause-specific hazard ratios
| Variables | Hazard Ratio (95% CI) | P Valuea | ||
|---|---|---|---|---|
| Sudden Cardiac Death | Nonsudden Cardiac Death | Noncardiac Death | ||
| Age | 1.35 (1.11–1.65) | 1.77 (1.39–2.25) | 1.51 (1.34–1.70) | 0.24 |
| Black patients | 0.77 (0.56–1.06) | 0.56 (0.39–0.81) | 0.89 (0.73–1.08) | 0.09 |
| Diabetes | 1.76 (1.25–2.48) | 1.37 (0.94–2.00) | 1.06 (0.87–1.30) | 0.04b |
| Ischemic heart disease | 1.99 (1.43–2.78) | 1.63 (1.11–2.39) | 0.89 (0.73–1.08) | < 0.001b,c |
| Heart failure | 1.21 (0.88–1.65) | 1.50 (1.04–2.17) | 1.25 (1.03–1.51) | 0.63 |
| Arrhythmia | 1.07 (0.77–1.48) | 1.14 (0.79–1.65) | 1.30 (1.07–1.58) | 0.55 |
| Peripheral vascular disease | 1.57 (1.12–2.20) | 1.26 (0.83–1.92) | 1.25 (0.99–1.58) | 0.54 |
| Other heart disease | 1.25 (0.88–1.77) | 1.35 (0.89–2.04) | 1.28 (1.03–1.58) | 0.96 |
| Body mass index | 0.78 (0.66–0.92) | 0.93 (0.77–1.12) | 0.80 (0.72–0.88) | 0.31 |
| Systolic blood pressure | 0.96 (0.83–1.12) | 0.76 (0.64–0.91) | 0.99 (0.90–1.09) | 0.03c,d |
| Serum creatinine | 0.71 (0.58–0.86) | 1.07 (0.86–1.33) | 0.78 (0.69–0.88) | 0.02c,d |
| Serum alkaline phosphatase | 1.18 (1.05–1.32) | 1.18 (1.02–1.37) | 1.08 (0.99–1.17) | 0.37 |
| Serum albumin | 0.87 (0.73–1.03) | 0.80 (0.66–0.99) | 0.76 (0.69–0.84) | 0.44 |
| Serum phosphorus | 1.22 (1.05–1.42) | 1.22 (1.02–1.47) | 1.12 (1.01–1.23) | 0.49 |
Hazard ratios for continuous predictors are given per 1 SD increase. The likelihood ratio test statistic for any differences in the hazards ratios between any two causes of death for any variable in the above model was highly significant (χ2=73.4; degrees of freedom = 28; P<0.001). CI, confidence interval.
P values for testing equality of hazard ratios between the three causes of death in the multivariable cause-specific hazards model. For those statistically different, the differences were compared across each group.
Sudden cardiac death versus noncardiac death (P<0.05).
Nonsudden cardiac versus noncardiac death (P<0.05).
Sudden cardiac death versus nonsudden cardiac death (P<0.05).
Multivariable Model for SCD
For the purpose of easy clinical applicability, a parsimonious model for SCD using the competing risk approach was developed by including only predictors that were found to be significant for SCD (Table 3). Serum phosphorus and body mass index were not statistically significant in univariate analysis (P>0.16 for both) and therefore were not entered into this forward-selection process. Heart failure was also not significant in a model that included IHD (Table 3); however, if IHD was excluded from the model, HF became significant {hazard ratio, 1.53 (95% confidence interval [CI], 1.13–2.06)}.
Table 3.
Multivariable model for sudden cardiac death
| Variable | Hazard Ratio (95% CI) |
|---|---|
| Agea | 1.31 (1.08–1.59) |
| Diabetes | 1.52 (1.11–2.09) |
| Ischemic heart disease | 2.27 (1.65–3.13) |
| Peripheral vascular disease | 1.62 (1.17–2.26) |
| Serum creatininea | 0.66 (0.55–0.79) |
| Serum alkaline phosphatasea | 1.19 (1.06–1.34) |
CI, confidence interval.
Hazard ratios for continuous predictors are given per 1 SD increase.
Model Performance
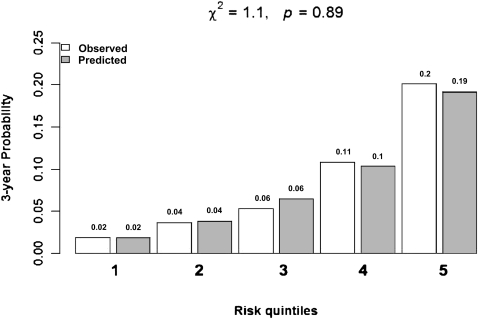
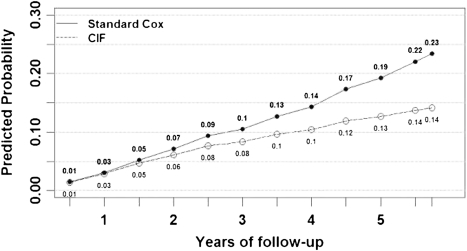
The 3-year C-statistic for the SCD model using the competing risk approach was 0.75 (95% CI, 0.70–0.79). Model fit was good for comparing observed cumulative incidence with predicted cumulative incidence across quintiles of risk (χ2=1.1; P=0.89; Figure 1). As the follow-up time increased, the standard Cox model increasingly overestimated the risk for SCD compared with the competing risk model (Figure 2). The standard Cox model overestimated the probability of SCD by 25% and 46% on the relative scale and 2% and 6% on the absolute scale at 3 and 5 years of follow-up, respectively.
Figure 1.
Three-year model calibration using the competing risk approach.
Figure 2.
Average predicted probabilities for sudden cardiac death using the standard Cox model versus the competing risk approach, with the same covariates in both models. CIF, cumulative incidence function.
All the continuous variables evaluated were noted to have a linear association with SCD except for systolic BP, which had a U-shaped association. Inclusion of a quadratic term for systolic BP was found to be a significant predictor of SCD in the univariate analysis; however, it was not significant after multivariable analysis in the forward or stepwise model. In a backward-selection model, systolic BP and its quadratic term were significant predictors of SCD despite multivariate adjustment. In this backward-selection model, the hazard ratio estimates for other variables were very similar to those in our initial model, and the 3-year C-statistic (0.75 [95% CI, 0.70–0.80]) and model calibration (χ2=0.83; P=0.93) were essentially unchanged. We therefore retained our initial model.
Bootstrap Performance of C-Statistic and Calibration
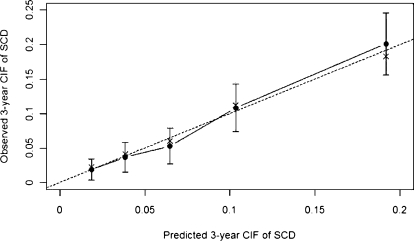
With use of 300 bootstrap resamples, the average 3-year C-statistics of the models developed on the bootstrap samples and when applied to our original sample were 0.77 and 0.75, respectively. Thus, we expect the 3-year predictive discrimination of our model to drop to 0.73 when applied to an external validation dataset. Figure 3 shows that the bias-corrected estimate of the calibration curve at 3-year is good. The apparent predictive accuracy and the bootstrap-corrected estimates are close except for the highest-risk quintile, in which the observed risk is slightly lower than predicted risk.
Figure 3.
Bootstrap estimates of calibration accuracy for 3-year estimate from the final model. Dots correspond to apparent predictive accuracy. × marks the bootstrap-corrected estimate. Error bars are the 95% confidence intervals of the observed cumulative incidence function (CIF) for sudden cardiac death (SCD) at each risk quintile.
Discussion
In this study, we identify predictors for SCD and show that predictors of various causes of death varied among HD patients. We also demonstrate that if competing risk is not taken into account, the probability of SCD will be overestimated by approximately 25%–50%. Finally, we present a prediction model for SCD that incorporates easily obtainable demographic and laboratory variables and has good discrimination and calibration.
Almost two-thirds of cardiac deaths were due to SCD in our study. This is in accordance with findings from the USRDS, in which SCD accounted for 64% of all cardiac deaths. Identifying predictors for various causes of death in the HD population is important because potential interventions may differ depending on the cause of death. In our study, the primary predictors for SCD were diabetes and IHD. The relationship of diabetes to SCD is consistent with that seen in prior studies (19) and is probably related to the fact that diabetes is a powerful risk factor for coronary disease. In the Deutsche Diabetes Dialyse Studie, patients with a hemoglobin A1c level of 8% or greater had a two-fold increased risk for sudden death compared with those with a hemoglobin A1c level less than 6%; however, it remains to be determined whether tighter glycemic control reduces the risk for SCD in clinical trials (20). The finding that IHD was associated with SCD is also consistent with previous reports and is probably due to the fact that IHD provides a substrate for arrhythmias (7,21,22). Peripheral vascular disease was a significant predictor of SCD and may reflect underlying generalized atherosclerosis. The association of serum alkaline phosphatase with SCD is consistent with prior studies and may reflect abnormalities in bone and mineral metabolism as well as vascular calcification, although this remains to be proven (23,24). Finally, lower serum creatinine (a proxy for muscle mass, poor nutritional status, and a catabolic state) is a well recognized risk for death in HD patients (25) and was associated with SCD in our analysis.
Of note, several traditional cardiovascular risk factors, such as smoking and cholesterol, were not associated with SCD. This finding is consistent with results from the Choices for Healthy Outcomes in Caring for ESRD Study (26). We noted no relationship between HF and SCD when IHD was included in the model. The exact reason for this is unknown but may be due to the fact that IHD incorporated all the risk for HF in this population. Other possibilities include the fact that HF is a relatively nonspecific diagnosis and may be due to volume overload rather than intrinsic structural heart disease. In addition, we are unable to determine whether the HF was due to systolic or diastolic dysfunction because echocardiographic data were not collected.
Heart failure, IHD, nonblack race, low systolic BP, and higher serum alkaline phosphatase level were identified as significant predictors of non-SCD. The protective association of black race, and the risk associated with low systolic BP, is consistent with data in other dialysis studies (27–30). SCD and non-SCD therefore have similar predictors, and we acknowledge the potential for misclassification between these two causes of death, particularly given that classification depends partly on location of death and presence of a witness rather than a distinct pathophysiologic process. Predictors of noncardiac deaths included low body mass index, low serum albumin, and low creatinine. These are markers of nutritional status, catabolic state, or increased inflammation and have been shown to be associated with increased all-cause mortality in HD patients (31–34).
Our study proposes a simple predictive model for SCD using easily obtainable variables. The model has good discrimination and calibration, and the results were consistent with internal bootstrapping. Furthermore, we have demonstrated that the standard Cox model, which ignores other competing risks for deaths, overestimated the risk for SCD by 25% and 46% on the relative scale at 3 and 5 years, respectively.
These results have several potential implications. First, identifying predictors of various causes of death may help improve understanding of the pathophysiology of the disease. Second, knowledge of predictors or prediction equations for SCD and other outcomes may lead to different interventions in a particular patient. Third, appreciation of the risk factor profile in a particular patient may lead to identification of patients most suitable for a clinical trial targeting a specific intervention.
To our knowledge, this is the first study to compare predictors of different causes of death in HD patients. Another strength of the study is that we have developed a prediction model for SCD that takes into account competing causes of death. Finally, the HEMO Study was a randomized clinical trial in which risk factors and outcomes were carefully ascertained. Because each death was adjudicated by a committee, the causes of death may be more accurate than those provided by administrative data (35).
This study also had several limitations. The HEMO Study participants were subject to exclusion criteria, such as unstable angina or New York Heart Association class IV congestive heart failure; therefore, generalization of our findings to the HD population in the United States should be done with caution. Data regarding ejection fraction, left ventricular hypertrophy, and QT interval on electrocardiography, known risk factors for SCD (particularly in the general population), were not available for all patients (36–38). In addition, cardiac biomarkers, such as troponins or natriuretic peptides, and markers of inflammation, such as IL-6, were not collected (26,39,40). High-sensitivity C-reactive protein was available in only a subset of participants and was therefore not included. However, it is important to note that these variables are also not routinely obtained in clinical practice for inclusion in a prediction equation. We used only baseline variables to determine the association with SCD and did not include changes in clinical measures over time. Thus, we were unable to adjust for dialysis-related factors (i.e., dialysate potassium concentration) more proximate to the time of death (41). We acknowledge that because HD patients are a “captive audience” who are seen three times a week, classification of SCD may differ from definitions in the general population. Furthermore, the definition may lead to an HD patient who does not have known heart disease being classified as having SCD and to an HD patient with cardiovascular disease who had an unwitnessed death due to cerebrovascular accident being classified as having SCD. Despite these limitations, the HEMO Study is one of the few studies that a priori defined SCD as an outcome of interest; the incidence of SCD in our study is in accordance with findings from the USRDS. Finally, although we internally validated the model using bootstrapping techniques and accounted for over-optimism, external validation was not performed.
In conclusion, to provide appropriate intervention, select patients for future trials, and evaluate new treatments for SCD, it is important to identify HD patients at high risk for SCD. We have shown that predictors of various causes of death differ to some extent in HD patients. We have also developed a predictive model for SCD using a competing risk approach. This model requires external validation.
Disclosures
C.H. served as a consultant for Amgen, Abbott, Affymax, and Fibrogen; has an equity interest in Cambridge Heart and Boston Scientific; and has received research support from Johnson & Johnson and the National Institutes of Diabetes and Digestive and Kidney Diseases/National Institutes of Health.
An Excel worksheet to predict absolute risk of SCD using the competing risk approach is available at http://160.109.101.132/icrhps/prodserv/default.asp.
Acknowledgments
The participation by patients and the staff in the HEMO Study is greatly appreciated. We thank the Institute for Clinical Research and Health Policy Studies at Tufts Medical Center for helping us host the Web-based calculator.
The study was funded by National Institutes of Health Grants T32 DK07777 and K24 DK078204.
An abstract representing this work was published at the American Society of Nephrology Annual Meeting in Denver, Colorado, in 2010.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Expect the Unexpected: Sudden Cardiac Death in Dialysis Patients,” on pages 8–11.
References
- 1.U.S. Renal Data System: USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2008 [Google Scholar]
- 2.Bleyer AJ, Hartman J, Brannon PC, Reeves-Daniel A, Satko SG, Russell G: Characteristics of sudden death in hemodialysis patients. Kidney Int 69: 2268–2273, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bleyer AJ, Russell GB, Satko SG: Sudden and cardiac death rates in hemodialysis patients. Kidney Int 55: 1553–1559, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK: Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 12: 2131–2138, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Green D, Roberts PR, New DI, Kalra PA: Sudden cardiac death in hemodialysis patients: an in-depth review. Am J Kidney Dis 57: 921–929, 2011 [DOI] [PubMed] [Google Scholar]
- 6.Herzog CA: Cardiac arrest in dialysis patients: approaches to alter an abysmal outcome. Kidney Int Suppl S197–S200, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Herzog CA, Mangrum JM, Passman R: Sudden cardiac death and dialysis patients. Semin Dial 21: 300–307, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Karnik JA, Young BS, Lew NL, Herget M, Dubinsky C, Lazarus JM, Chertow GM: Cardiac arrest and sudden death in dialysis units. Kidney Int 60: 350–357, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Drechsler C, Wanner C, Ritz E, Winkler K, Cessie Sl, Dekker F, März W, Krane V: Prediction of sudden cardiac death in hemodialysis patients. Presented at the World Congress of Nephrology, Milan, Italy, May 22–26, 2009.
- 10.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, Allon M, Bailey J, Delmez JA, Depner TA, Dwyer JT, Levey AS, Levin NW, Milford E, Ornt DB, Rocco MV, Schulman G, Schwab SJ, Teehan BP, Toto R. Hemodialysis (HEMO) Study Group: Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 347: 2010–2019, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Greene T, Beck GJ, Gassman JJ, Gotch FA, Kusek JW, Levey AS, Levin NW, Schulman G, Eknoyan G: Design and statistical issues of the hemodialysis (HEMO) study. Control Clin Trials 21: 502–525, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Miskulin DC, Athienites NV, Yan G, Martin AA, Ornt DB, Kusek JW, Meyer KB, Levey AS. Hemodialysis (HEMO) Study Group: Comorbidity assessment using the Index of Coexistent Diseases in a multicenter clinical trial. Kidney Int 60: 1498–1510, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Athienites NV, Miskulin DC, Fernandez G, Bunnapradist S, Simon G, Landa M, Schmid CH, Greenfield S, Levey AS, Meyer KB: Comorbidity assessment in hemodialysis and peritoneal dialysis using the index of coexistent disease. Semin Dial 13: 320–326, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Cheung AK, Sarnak MJ, Yan G, Berkoben M, Heyka R, Kaufman A, Lewis J, Rocco M, Toto R, Windus D, Ornt D, Levey AS. HEMO Study Group: Cardiac diseases in maintenance hemodialysis patients: Results of the HEMO Study. Kidney Int 65: 2380–2389, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Pencina MJ, D’Agostino RB, Sr, Larson MG, Massaro JM, Vasan RS: Predicting the 30-year risk of cardiovascular disease: the framingham heart study. Circulation 119: 3078–3084, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolbers M, Koller MT, Witteman JC, Steyerberg EW: Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology 20: 555–561, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Harrell FE, Jr, Lee KL, Mark DB: Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15: 361–387, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Rosthøj S, Andersen PK, Abildstrom SZ: SAS macros for estimation of the cumulative incidence functions based on a Cox regression model for competing risks survival data. Comput Methods Programs Biomed 74: 69–75, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Genovesi S, Valsecchi MG, Rossi E, Pogliani D, Acquistapace I, De Cristofaro V, Stella A, Vincenti A: Sudden death and associated factors in a historical cohort of chronic haemodialysis patients. Nephrol Dial Transplant 24: 2529–2536, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Drechsler C, Krane V, Ritz E, März W, Wanner C: Glycemic control and cardiovascular events in diabetic hemodialysis patients. Circulation 120: 2421–2428, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Huikuri HV, Castellanos A, Myerburg RJ: Sudden death due to cardiac arrhythmias. N Engl J Med 345: 1473–1482, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Myerburg RJ, Interian A, Jr, Mitrani RM, Kessler KM, Castellanos A: Frequency of sudden cardiac death and profiles of risk. Am J Cardiol 80[5B]: 10F–19F, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Lomashvili KA, Garg P, Narisawa S, Millan JL, O’Neill WC: Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification. Kidney Int 73: 1024–1030, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schoppet M, Shanahan CM: Role for alkaline phosphatase as an inducer of vascular calcification in renal failure? Kidney Int 73: 989–991, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Fink JC, Burdick RA, Kurth SJ, Blahut SA, Armistead NC, Turner MS, Shickle LM, Light PD: Significance of serum creatinine values in new end-stage renal disease patients. Am J Kidney Dis 34: 694–701, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Parekh RS, Plantinga LC, Kao WH, Meoni LA, Jaar BG, Fink NE, Powe NR, Coresh J, Klag MJ: The association of sudden cardiac death with inflammation and other traditional risk factors. Kidney Int 74: 1335–1342, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32[Suppl 3]: S112–S119, 1998 [DOI] [PubMed] [Google Scholar]
- 28.Port FK, Hulbert-Shearon TE, Wolfe RA, Bloembergen WE, Golper TA, Agodoa LY, Young EW: Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis 33: 507–517, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Shoji T, Tsubakihara Y, Fujii M, Imai E: Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int 66: 1212–1220, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, Van Stone J, Levey A, Meyer KB, Klag MJ, Johnson HK, Clark E, Sadler JH, Teredesai P: “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int 54: 561–569, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Iseki K, Kawazoe N, Fukiyama K: Serum albumin is a strong predictor of death in chronic dialysis patients. Kidney Int 44: 115–119, 1993 [DOI] [PubMed] [Google Scholar]
- 32.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD: Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int 63: 793–808, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Leavey SF, McCullough K, Hecking E, Goodkin D, Port FK, Young EW: Body mass index and mortality in ‘healthier’ as compared with ‘sicker’ haemodialysis patients: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 16: 2386–2394, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Beddhu S, Cheung AK, Larive B, Greene T, Kaysen GA, Levey AS, Rocco M, Sarnak M, Toto R, Eknoyan G; Hemodialysis (HEMO) Study Group: Inflammation and inverse associations of body mass index and serum creatinine with mortality in hemodialysis patients. J Ren Nutr 17: 372–380, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rocco MV, Yan G, Gassman J, Lewis JB, Ornt D, Weiss B, Levey AS. Hemodialysis Study Group The National Institutes of Health-funded Hemodialysis. Health Care Financing Administration: Comparison of causes of death using HEMO Study and HCFA end-stage renal disease death notification classification systems. Am J Kidney Dis 39: 146–153, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Haigney MC, Zareba W, Gentlesk PJ, Goldstein RE, Illovsky M, McNitt S, Andrews ML, Moss AJ, the Multicenter Automatic Defibrillator Implantation Trial II investigators: QT interval variability and spontaneous ventricular tachycardia or fibrillation in the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J Am Coll Cardiol 44: 1481–1487, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Krane V, Heinrich F, Meesmann M, Olschewski M, Lilienthal J, Angermann C, Störk S, Bauersachs J, Wanner C, Frantz S, for the German Diabetes and Dialysis Study Investigators: Electrocardiography and outcome in patients with diabetes mellitus on maintenance hemodialysis. Clin J Am Soc Nephrol 4: 394–400, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paoletti E, Specchia C, Di Maio G, Bellino D, Damasio B, Cassottana P, Cannella G: The worsening of left ventricular hypertrophy is the strongest predictor of sudden cardiac death in haemodialysis patients: a 10 year survey. Nephrol Dial Transplant 19: 1829–1834, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Fonarow GC, Horwich TB: Combining natriuretic peptides and necrosis markers in determining prognosis in heart failure. Rev Cardiovasc Med 4[Suppl 4]: S20–S28, 2003 [PubMed] [Google Scholar]
- 40.Iliou MC, Fumeron C, Benoit MO, Tuppin P, Calonge VM, Moatti N, Buisson C, Jacquot C: Prognostic value of cardiac markers in ESRD: Chronic Hemodialysis and New Cardiac Markers Evaluation (CHANCE) study. Am J Kidney Dis 42: 513–523, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Pun PH, Lehrich RW, Honeycutt EF, Herzog CA, Middleton JP: Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int 79: 218–227, 2011 [DOI] [PubMed] [Google Scholar]