Abstract
Mood states are associated with alterations in cerebral blood flow and metabolism, yet changes in cerebral structure are typically viewed in the context of enduring traits, genetic predispositions, or the outcome of chronic psychiatric illness. Magnetic resonance imaging (MRI) scans were obtained from two groups of patients with bipolar disorder. In one group, patients met criteria for a current major depressive episode whereas in the other no patient did. No patient in either group met criteria for a current manic, hypomanic, or mixed episode. Groups were matched with respect to age and illness severity. Analyses of gray matter density were performed with Statistical Parametric Mapping software (SPM5). Compared with non-depressed bipolar subjects, depressed bipolar subjects exhibited lower gray matter density in the right dorsolateral and bilateral dorsomedial prefrontal cortices and portions of the left parietal lobe. In addition, gray matter density was greater in the left temporal lobe and right posterior cingulate cortex/parahippocampal gyrus in depressed than in non-depressed subjects. Our findings highlight the importance of mood state in structural studies of the brain—an issue that has received insufficient attention to date. Moreover, our observed differences in gray matter density overlap metabolic areas of change and thus have implications for the conceptualization and treatment of affective disorders.
Keywords: Bipolar disorder, Magnetic resonance imaging, Prefrontal cortex
1. Introduction
Mood disorders are an important group of psychiatric illnesses that inflict substantial morbidity and mortality in 20.8% of the population (Kessler et al., 2005). Research devoted to understanding the neural substrates of mood disorders shows promise in characterizing individual differences among patients and ultimately perhaps in better targeting therapy for illness subgroups. Functional imaging studies, such as positron emission tomography (PET), have documented functional alterations during depression in both cerebral metabolism (Drevets et al., 1997; Ketter et al., 2001; Brooks et al., 2009) and blood flow (Dunn et al., 2005). In bipolar depression, these abnormalities have been found primarily in prefrontal and anterior limbic and paralimbic regions.
Differences associated with bipolar disorder in structural imaging studies, using magnetic resonance imaging (MRI), are commonly interpreted as reflecting trait differences. Studies have focused on volumetric differences between subjects with bipolar disorder and healthy controls and have provided evidence of decreased prefrontal cortical volumes in bipolar disorder patients (Drevets et al., 1997; Sax et al., 1999; López-Larson et al., 2002), although some studies have not found such differences (Strakowski et al., 1993; Zipursky et al., 1997). The reasons for conflicting findings remain to be established, but the consensus is that volumetric differences in prefrontal regions are associated with bipolar disorder (Lyoo et al., 2006). There is conflicting evidence regarding amygdala volume, with some studies reporting larger amygdalae in adult bipolar subjects (Altshuler et al., 2000), some reporting smaller amygdalae (Pearlson et al., 1997), and others finding no significant differences (Swayze et al., 1992; Strakowski et al., 1999); whereas pediatric bipolar subjects more consistently demonstrate decreased amygdala volumes (Chang et al., 2005).
More recent advances in voxel-based morphometry allow for objective assessment of gray matter density. Lyoo et al. (2004) reported decreased gray matter density in 39 subjects with bipolar disorder type I (22 with depression, 17 with mood elevation; 24 medicated,15 unmedicated) in the left anterior cingulate and adjacent left medial prefrontal gyrus. Subsequently, decreased gray matter density has been reported in the anterior cingulate, and dorsolateral and ventrolateral prefrontal cortex in 36 depressed bipolar disorder patients (7 Type I, 29 Type I; 20 medicated,16 unmedicated) (Nugent et al., 2006). There are varying interpretations of whether such alterations in gray matter density are related to developmental abnormalities or neuroplastic changes of repeated stress and glucocorticoid secretion (Nugent et al., 2006). Regardless, both interpretations are consistent with the common thinking that structural alterations of the brain either presage the illness or reflect the injuries of repeated affective or psychotic episodes.
Recently there have been demonstrations of structural plasticity in the absence of cerebral trauma or progressive disease in that gray matter density changes occur in response to cognitive demands. In a striking study reported by Draganski et al. (2004), juggling-naïve healthy young adults were given a baseline MRI scan and then taught to juggle over three months. After the subjects could sustain three-ball juggling for 60 s, they were given a second MRI scan. Draganski et al. found increased gray matter density bilaterally in the mid-temporal region compared with findings in control subjects who were not taught to juggle. After refraining from juggling for 3 months, subjects who juggled exhibited a return to baseline gray matter density.
The findings of Draganski et al. (2004) demonstrate gray matter density changes resulting from situations that are far more subtle than depressive episodes. These findings led us to question the assumption that depression is associated with only acute functional, as opposed to acute structural, changes in the brain. This assumption is common, as structural studies of the brain in mood disorders commonly including subjects in a variety of mood states (Lyoo et al., 2004; Nugent et al., 2006). However, mood-state-related structural changes may confound results of studies by contributing to variability related to a mixture of effects of state-related, trait-related, time-related, and medication-related structural changes.
To demonstrate the effects of depression on cerebral structure, we obtained MRI scans from medication-free patients with bipolar disorder who were either depressed or euthymic and well matched on a variety of factors that could account for brain differences. We used the most recent image-processing advances in voxel-based morphometry (VBM) (Ashburner and Friston, 2005).
2. Methods
2.1. Subjects
All 33 subjects were community-dwelling and met Diagnostic and Statistical Manual for Mental Disorders (DSM-IV) criteria for bipolar disorder (type I or II). All subjects were free of psychotropic medications for at least 2 weeks before neuroimaging. Subjects were titrated off their regular medications as part of a washout phase for clinical pharmacotherapy trials. The sample of 17 bipolar disorder subjects (5 type I and 12 type II) who met criteria for a current depressive (but not manic, hypomanic or mixed) episode had an average age of 35.3 (S.D.=10.9) years and comprised 11 females and 6 males. The average age at onset of illness of the depressed group was 17.0 (S.D.=7.4). The sample of 16 euthymic bipolar disorder subjects (7 type I and 9 type II) who did not meet DSM-IV criteria for a current depressive, manic, hypomanic or mixed episode had an average age of 33.9 (S.D.=14.7) years and comprised 5 females and 11 males. The average age at onset of illness of the euthymic group was 18.6 (S.D.=10.6). Demographic information is summarized in Table 1.
Table 1.
Demographic variables.
| Depressed n=17 | Euthymic n=16 | |
|---|---|---|
| Age (years) | 35.3 (10.9) | 33.9 (14.7) |
| Education (years) | 14.9 (1.8) | 15.3 (2.6) |
| Male/female | 6/11 | 11/5 |
| Right-handed | 16 | 14 |
| Caucasian/Hispanic/Native American | 14/1/1 | 12/2/0 |
| Type I/type II | 5/12 | 7/9 |
| Depressive episodes | 3.3 (1.5) | 2.5 (1.8) |
| Age at onset | 17.0 (7.4) | 18.6 (10.6) |
Note: Standard deviations are in parentheses.
This study was approved by the Stanford University Administrative Panel for Human Subjects in Medical Research. Subjects provided both oral and written informed consent prior to participating in any study-related procedures.
2.2. MRI acquisition
MRI scans were performed with a 1.5T General Electric Signa scanner using a standard head coil. A three-dimensional spoiled-gradient echo pulse sequence was used to create 124 1.5-mm-thick contiguous sagittal images (echo time=6 ms, repetition time=9.2–11.2 ms, 256×256 matrix, field of view=24 cm, number of excitations=1, flip angle=45°).
2.3. Image processing
All analyses were performed using Statistical Parametric Mapping software (SPM5) (Wellcome Department of Imaging Science; www.fil.ion.ucl.ac.uk/spm/). Each MR volume was re-oriented into oblique axial slices and then spatially normalized to the Montreal Neurologic Institute (MNI) template and resliced to yield a volume with 2 mm isotropic voxels. Spatial normalization in SPM5 comprises a 12-parameter affine transformation and nonlinear warping with basis functions (Ashburner and Friston, 1999). The images were smoothed with a Gaussian kernel to a full-width at half maximum of 8 mm.
Next, the volumes were segmented into gray matter, white matter, and cerebrospinal fluid using the modified segmentation algorithm in SPM5 and the associated tissue probability maps. The segmented gray matter images were smoothed with a Gaussian kernel to a full-width at half maximum of 12 mmprior to generating statistical parametric maps.
The smoothed, segmented gray matter images were submitted to voxel-based morphometry using SPM5. In SPM5, two-sample t-tests were used to compare the two groups of scans. Such comparisons use the theory of random Gaussian fields to generate a statistical parametric map of the t statistic across all voxels (Friston et al., 1996). Initial probability levels were set at P<0.005 and a corresponding t threshold of 3.65. Cluster thresholds were set at 50 voxels (400 mm3). MNI coordinates were converted to Talairach (Talairach and Tournoux, 1988) coordinates for reporting purposes using Wake Forest University (WFU) PickAtlas (www.fmri.wfubmc.edu).
3. Results
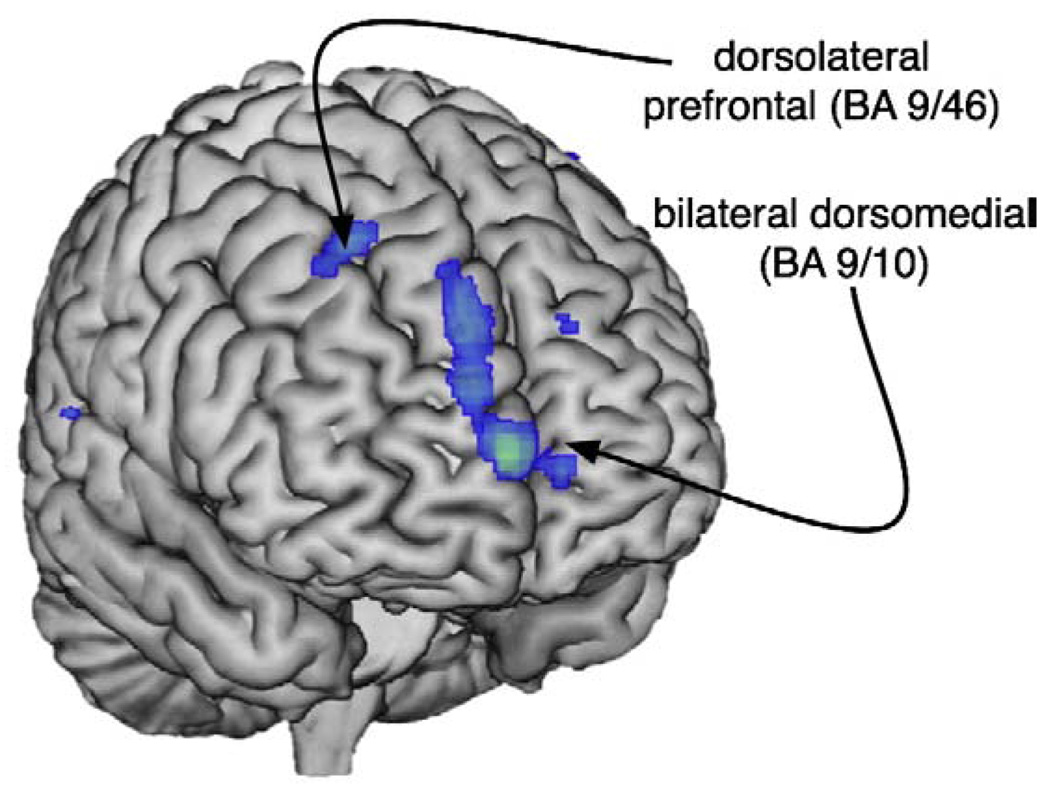
VBM analyses revealed areas in which depressed patients had lower gray matter density than euthymic patients as listed in Table 2 and illustrated in Fig. 1. Depressed patients exhibited lower gray matter density bilaterally in the dorsomedial prefrontal cortex (Brodmann's Area (BA) 9/10) in the medial frontal gyri. In the right hemisphere we also found significantly lower gray matter density in the dorsolateral prefrontal cortex (DLPFC) in the superior frontal gyrus (Table 2). There was no analogous decreased prefrontal density in the left hemisphere, although there was a region of decreased density in the left inferior parietal lobule (BA 4/40).
Table 2.
Coordinates for regions of statistically significant lower gray matter density in depressed relative to euthymic patients.
| Region | Brodmann's area | Hemisphere | Talairach coordinates |
Cluster size | t | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Medial frontal gyrus | 10 | Bilateral | 8 | 64 | 4 | 289 | 4.85 |
| Superior frontal gyrus | 10 | Right | 8 | 61 | 19 | 4.08 | |
| 9 | Right | 10 | 50 | 31 | 4.07 | ||
| Middle frontal gyrus | 8 | Right | 24 | 24 | 47 | 54 | 4.02 |
| Precentral gyrus | 4 | Left | −36 | −23 | 51 | 104 | 3.90 |
| Postcentral gyrus | Left | −44 | −21 | 47 | 3.86 | ||
| Inferior parietal lobule | 40 | Left | −44 | −34 | 55 | 3.55 | |
Note: for all ts: df=30, P<0.005.
Fig. 1.
Anterior surface rendering illustrating (in blue) areas in which depressed bipolar subjects showed lower gray matter density relative to euthymic bipolar subjects.
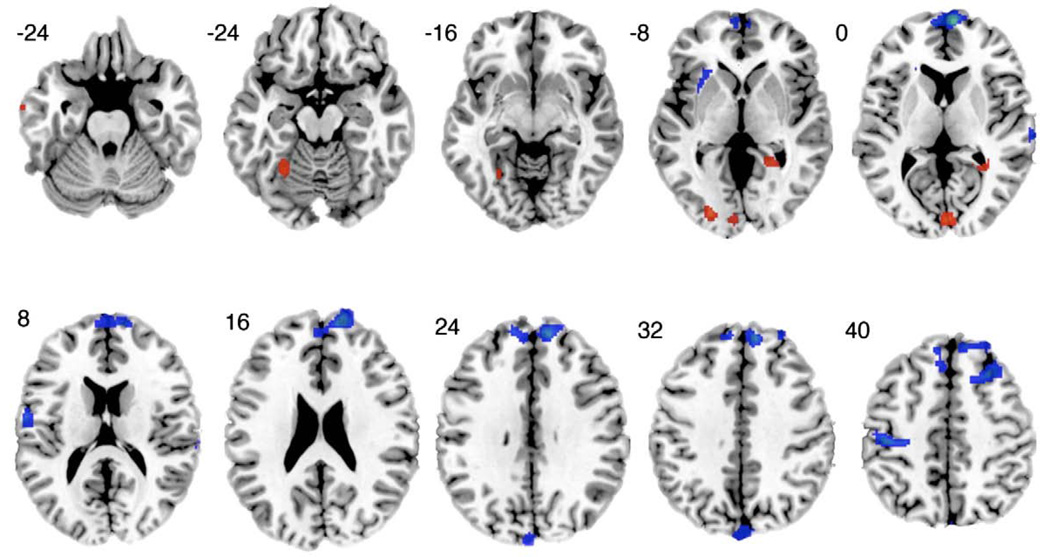
Areas of greater gray matter density among the depressed subjects compared with the euthymic subjects are listed in Table 3 and illustrated in Fig. 2. Greater density was evident in the left DLPFC (BA 10/46), the left temporal lobe (BA 20), and the right posterior cingulate cortex/ parahippocampal gyrus. Additionally, there were areas of greater density bilaterally in the occipital lobe.
Table 3.
Coordinates for regions of statistically significant greater gray matter density in depressed relative to euthymic patients.
| Region | Brodmann's area | Hemisphere | Talairach coordinates |
Cluster size | t | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Fusiform gyrus | 20 | Left | −71 | −5 | −25 | 82 | 4.30 |
| Superior frontal | 11 | Left | −40 | 63 | −18 | 78 | 3.81 |
| gyrus | 11 | Left | −50 | 59 | −18 | 3.19 | |
| Fusiform gyrus | Left | −26 | −53 | −7 | 119 | 3.76 | |
| Cuneus | 18 | Right | 4 | −85 | 9 | 252 | 3.63 |
| Middle occipital | Left | −24 | −85 | 4 | 3.53 | ||
| gyrus | |||||||
| Lingual gyrus | Left | −10 | −91 | 1 | 3.43 | ||
| Middle frontal gyrus | 46 | Left | −48 | 61 | 6 | 58 | 3.56 |
| Inferior frontal | 10 | Left | −51 | 56 | 1 | 3.21 | |
| gyrus | |||||||
| Middle frontal gyrus | 10 | Right | 22 | 46 | 4 | 125 | 3.35 |
| Posterior cingulate/parahippocampal | Right | 34 | −44 | 4 | 3.03 | ||
| gyrus | |||||||
Note: For all ts: df=30, P<0.005.
Fig. 2.
A sequence of axial slices illustrating gray matter density differences in depressed bipolar subjects relative to euthymic bipolar subjects. Notable regions of lower gray matter density, shown in blue, are in the left temporal lobe and the right posterior cingulate and right parahippocampal gyrus. Regions of greater gray matter density, shown in red, are in the left dorsolateral prefrontal, right posterior cingulate, and parahippocampal gyrus, and bilateral occipital lobes. Talairach z-coordinates are provided to the upper left of each slice.
4. Discussion
This study provides evidence of an association between depressed mood and alterations in gray matter density in bipolar disorder. The regions that demonstrated decreased density included bilateral dorsomedial and right dorsolateral prefrontal cortex. Moreover, we found evidence of increased gray matter density in the parahippocampal gyrus. Importantly, all patients had been medication free for at least 2 weeks before the scan, which removed any confounding effects of current or recent psychotropic medication.
Our findings contradict the assumption that mood state is not associated with acute structural cerebral changes. Thus, attempts to elucidate cerebral correlates of depression should include not only metabolic but also structural measures. Historically, structural changes in the human brain have been attributed to developmental changes or neurodegenerative disease. Thus, psychiatric illnesses, such as schizophrenia (Hulshoff Pol et al., 2001) and bipolar disorder (Soares et al., 2005; Nugent et al., 2006) have been associated with focal gray matter density changes that are often attributed to disease course. Only recently has there been increasing appreciation of the potential of other factors such as degree of exposure to the potential neurotrophic properties of psychotropic medications to affect cerebral structure (Moore et al., 2000; Sheline, 2003).
We significantly extend previous demonstrations of neuroplasticity, to show that depressed mood state may be associated with structural changes. The changes in gray matter density that we observed overlap with areas of metabolic change in acute depression. For example, decreased prefrontal metabolism has also been demonstrated in depression (Buchsbaum et al., 1997; Ketter et al., 2001; Brooks et al., 2009). However, we did not detect differences in the subgenual prefrontal cortex, which has been implicated as a structure that is vital to mood-state changes in depression (Mayberg, 1997; Seminowicz et al., 2004). This suggests that some regions may demonstrate metabolic change in the absence of structural changes.
At the least, our findings suggest that mood state should be accounted for in morphometric studies of bipolar disorder. Several prior studies that have reported gray matter density differences in patients with bipolar disorder relative to healthy controls have included depressed patients in their samples. For example, Lyoo et al. (2004) found evidence of decreased gray matter density in the anterior cingulate, left medial frontal gyrus, and right precentral gyrus. Lyoo et al.'s sample of 39 bipolar patients included 17 who were depressed at the time of scanning. More recently, Bearden et al. (2007) explored the effects of lithium on gray matter density in bipolar disorder and found that patients taking lithium exhibited increased gray matter density in the left cingulate and paralimbic association cortices. In Bearden et al.'s study, 30% of the bipolar patients taking lithium met criteria for depression and 50% of those not taking lithium were depressed. The results of the present study raise the question of the extent to which heterogeneous mood states may have influenced the results in these studies.
One possible explanation of our findings can be derived by considering related cerebral networks. The DLPFC has played a significant role in the conceptualization of depression. For example, the hypothesis of reciprocal limbic-cortical function as an explanation of cerebral metabolic changes hinges on reciprocal responses among higher-level cortical regions (e.g., DLPFC) and lower-level limbic regions involved in emotional control (Mayberg et al., 1999). In studies of depression, cortical hypometabolism (in the DLPFC and cingulate cortex) and limbic hypermetabolism (in the amygdalae) support the reciprocal limbic-cortical hypothesis (Liotti and Mayberg, 2001; Ketter et al., 2001). Some researchers have suggested that cognitive therapeutic interventions affect higher level, or DLPFC, changes whereas pharmacological interventions have an effect on lower level, or limbic, activity (Liotti and Mayberg, 2001).
The corticolimbic hypothesis provides an explanation of how our observed gray matter changes could contribute to depressive episodes. The orbitofrontal cortex, where we observed bilateral decreases in density, has extensive reciprocal connections with the amygdala, thalamus, and the subgenual prefrontal cortex (SGPFC) and is often associated with emotional homeostasis (Mega et al.,1997). If structural changes in gray matter density in the orbitofrontal cortex disrupted emotional homeostasis, such changes might generate a cascade of functional abnormalities in other regions, such as the limbic system and, in particular, the anterior cingulate and subgenual prefrontal cortex.
Interestingly, the medial orbitofrontal cortex (BA 11) enjoys a reciprocal relation with the SGPFC and efferent connections from the parahippocampal gyrus (Adler et al., 2006). This network is critical for affective domains partially because of integral connections with the amygdala. One hypothesis posits that decreased inhibitory feedback between medial, dorsal, and inferior frontal cortex and elements of the anterior limbic network contribute to the emotional dysregulation observed in bipolar disorder (Haznedar et al., 2005). Our study revealed decreased gray matter density in both the superior frontal gyrus and the parahippocampal gyrus, but no evidence of density changes in the amygdala. As noted previously, there is conflicting evidence regarding amygdala volumes in adults with bipolar disorder. Differences in age, chronicity, and treatment could account for variation in amygdala volume in adult patients with bipolar disorder, and thus may have contributed to the absenceof amygdala density changes associated with depression.
Key data that highlight the importance of networks involving the anterior limbic system illustrate relations between cerebral metabolism and treatment response. For example, successful pharmacological treatment of depression can be associated with a normalization of cerebral metabolism in the DLPFC and the anterior cingulate (Kennedy et al., 2001). Response to paroxetine in unipolar depression increases cerebral metabolism in the dorsolateral, ventrolateral, and medial aspects of prefrontal cortex as well as anterior cingulate and parahippocampal regions (Kennedy et al., 2001). Pharmacological response data are consistent with findings from deep brain stimulation studies, in which subcortical tract stimulation results in normalized cerebral blood flow in the dorsolateral and subgenual prefrontal cortices (Mayberg et al., 2005). In addition, patients with bipolar disorder who were treated with lithium exhibited increased gray matter density in the bilateral cingulate cortex (Bearden et al., 2007).
4.1. Study limitations
There are several characteristics of the current study that limit its generalizablity. First, our design was between subjects, so there remains a possibility that even though groups were closely matched, they differed on some other unmeasured aspect. One such factor that could have influenced our results is lifetime exposure to various psychotropic medications with varying neurotrophic properties. Given the substantial interindividual differences in treatment of bipolar disorder, controlling for this across subjects is a substantial challenge that could be avoided by utilizing a within-subject design. Thus a more compelling demonstration of reversible neuroplasticity would demand a within-subjects design controlling for lifetime exposure to psychotropic medications, preferably with gray matter changes associated with a change from euthymia to depression and back to euthymia. Of course, such a study would be fraught with additional challenges based on time effects and the likely contaminating effects of medication or therapy interventions to address the depression.
Although our depressed and euthymic groups were matched on several variables, the gender balance differed in that the depressed group was predominantly female and the euthymic group was predominantly male. Research regarding gender differences in gray matter density in bipolar disorder is lacking, but some research suggests such differences may exist. For example, adolescent females with bipolar disorder were found to have larger gray matter volumes in lateral and medial orbitofrontal cortices than their male counterparts (Najt et al., 2007). To the extent that such results hold in adult bipolar disorder patients, they would have worked against our pattern of results, as the depressed group (which was mostly female) exhibited lower prefrontal density than the euthymic group (which was mostly male). Even so, we are unable to define the precise role that gender may have played in our findings.
Finally, our study may underestimate the extent of gray matter changes associated with depression. Insufficient sensitivity may have arisen from limited sample size and the small size of regions of specific interest. Thus, if there are mood-state-dependent changes in gray matter density, we may not have detected them because of insufficient power.
4.2. Conclusions
The association between gray matter density and mood state extends our knowledge of changes associated with depression beyond altered blood flow and metabolism. Moreover, the presence of mood-state-dependent gray matter variations suggests a possible mechanism through which longer-term changes in structural volumes could occur. Should our findings of mood-state-related changes in gray matter density extend to other mood states, such as mania, structural changes could provide a partial explanation of the dramatic clinical mood and functional alterations observed in mood disorders and may need to be accounted for in studies of functional changes during depression. For example, when regional abnormalities of N-acetylaspartate, choline, and glutamate/glutamine are considered (Winsberg et al., 2000), alterations in gray matter density may be one part of a constellation of profound, albeit unmeasured, cortical changes in depression that need to be considered. Further studies are needed to better characterize structural cerebral changes with depression and their relationships to the functional cerebral and dramatic clinical changes seen during depressive episodes.
Acknowledgments
An earlier version of this report was presented at the Society for Biological Psychiatry's 62nd Annual Scientific Convention and Program, San Diego, CA, May 17–19, 2007. This research was supported in part by the Stanley Foundation Research Awards Program (TK, PW), the National Alliance for Research in Schizophrenia and Depression (TK, PW, SH), Abbott Laboratories (TK, PW, SH), the National Institutes of Health (JCB, AR, TK), the Medical Research Service of the Veterans Affairs Palo Alto Health Care System (JOB, AR), and by the Department of Veterans Affairs Sierra-Pacific Mental Illness Research, Education, and Clinical Center (JOB, AR). The authors acknowledge the assistance of Wanda Sykes during the analyses and Stephanie Woodard for comments on the manuscript. The authors declare that they have no competing interests.
References
- Adler CM, DelBello MP, Strakowski SM. Brain network dysfunction in bipolar disorder. CNS Spectrums. 2006;11:312–320. doi: 10.1017/s1092852900020800. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, Wilkins J, Gerner R, Mintz J. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biological Psychiatry. 2000;48:147–162. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Human Brain Mapping. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Thompson PM, Dalwani M, Hayashi KM, Lee AD, Nicoletti M, Trakhtenbroit M, Glahn DC, Brambilla P, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Soares JC. Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biological Psychiatry. 2007;62:7–16. doi: 10.1016/j.biopsych.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks JO, III, Wang PW, Bonner JC, Rosen AC, Hoblyn JC, Hill SJ, Ketter TA. Decreased prefrontal, anterior cingulate, insula, and ventral striatal metabolism in medication-free depressed outpatients with bipolar disorder. Journal of Psychiatric Research. 2009;43:181–188. doi: 10.1016/j.jpsychires.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum MS, Someya T, Tu JC, Tang CY, Bunney WE. Neuroimaging bipolar illness with positron emission tomography and magnetic resonance imaging. Psychiatric Annals. 1997;27:489–495. [Google Scholar]
- Chang K, Barnea-Goraly N, Karchemskiy A, Simeonova DI, Barnes P, Ketter T, Reiss AL. Cortical magnetic resonance imaging findings in familial pediatric bipolar disorder. Biological Psychiatry. 2005;58:197–203. doi: 10.1016/j.biopsych.2005.03.039. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Dunn RT, Willis MW, Benson BE, Repella JD, Kimbrell TA, Ketter TA, Speer AM, Osuch EA, Post RM. Preliminary findings of uncoupling of flow and metabolism in unipolar compared with bipolar affective illness and normal controls. Psychiatry Research. 2005;140:181–198. doi: 10.1016/j.pscychresns.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. Neuroimage. 1996;4:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Roversi F, Pallanti S, Baldini-Rossi N, Schnur DB, Licalzi EM, Tang C, Hof PR, Hollander E, Buchsbaum MS. Fronto-thalamo-striatal gray and white matter volumes and anisotropy of their connections in bipolar spectrum illnesses. Biological Psychiatry. 2005;57:733–742. doi: 10.1016/j.biopsych.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Mandl RC, van Haren NE, Koning H, Collins DL, Evans AC, Kahn RS. Focal gray matter density changes in schizophrenia. Archives of General Psychiatry. 2001;58:1118–1125. doi: 10.1001/archpsyc.58.12.1118. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Krüger S, Mayberg HS, Meyer JH, McCann S, Arifuzzman AI, Houle S, Vaccarino FJ. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. American Journal of Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketter TA, Kimbrell TA, George MS, Dunn RT, Speer AM, Benson BE, Willis MW, Danielson A, Frye MA, Herscovitch P, Post RM. Effects of mood and subtype on cerebral glucose metabolism in treatment-resistant bipolar disorder. Biological Psychiatry. 2001;49:97–109. doi: 10.1016/s0006-3223(00)00975-6. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS. The role of functional neuroimaging in the neuropsychology of depression. Journal of clinical and experimental neuropsychology : official journal of the International Neuropsychological Society. 2001;23:121–136. doi: 10.1076/jcen.23.1.121.1223. [DOI] [PubMed] [Google Scholar]
- López-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biological Psychiatry. 2002;52:93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Kim MJ, Stoll AL, Demopulos CM, Parow AM, Dager SR, Friedman SD, Dunner DL, Renshaw PF. Frontal lobe gray matter density decreases in bipolar I disorder. Biological Psychiatry. 2004;55:648–651. doi: 10.1016/j.biopsych.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Sung YH, Dager SR, Friedman SD, Lee JY, Kim SJ, Kim N, Dunner DL, Renshaw PF. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disorder. 2006;8:65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. Journal of Neuropsychiatry and Clinical Neuroscience. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Mega MS, Cummings JL, Salloway S, Malloy P. The limbic system: an anatomic, phylogenetic, and clinical perspective. Journal of Neuropsychiatry and Clinical Neuroscience. 1997;9:315–330. doi: 10.1176/jnp.9.3.315. [DOI] [PubMed] [Google Scholar]
- Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK, Menji HK. Lithium-induced increase in human brain grey matter. Lancet. 2000;356:1241–1242. doi: 10.1016/s0140-6736(00)02793-8. [DOI] [PubMed] [Google Scholar]
- Najt P, Nicoletti M, Chen HH, Hatch JP, Caetano SC, Sassi RB, Axelson D, Brambilla P, Keshavan MS, Ryan ND, Birmaher B, Soares JC. Anatomical measurements of the orbitofrontal cortex in child and adolescent patients with bipolar disorder. Neuroscience Letter. 2007;413:183–186. doi: 10.1016/j.neulet.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Milham MP, Bain EE, Mah L, Cannon DM, Marrett S, Zarate CA, Pine DS, Price JL, Drevets WC. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. Neuroimage. 2006;30:485–497. doi: 10.1016/j.neuroimage.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Barta PE, Powers RE, Menon RR, Richards SS, Aylward EH, Federman EB, Chase GA, Petty RG, Tien AY. Ziskind-Somerfeld Research Award 1996. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biological Psychiatry. 1997;41:1–14. doi: 10.1016/s0006-3223(96)00373-3. [DOI] [PubMed] [Google Scholar]
- Sax KW, Strakowski SM, Zimmerman ME, DelBello MP, Keck PE, Hawkins JM. Frontosubcortical neuroanatomy and the continuous performance test in mania. American Journal of Psychiatry. 1999;156:139–141. doi: 10.1176/ajp.156.1.139. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, Rafi-Tari S. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biological Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- Soares JC, Kochunov P, Monkul ES, Nicoletti MA, Brambilla P, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Lancaster J, Fox P. Structural brain changes in bipolar disorder using deformation field morphometry. Neuroreport. 2005;16:541–544. doi: 10.1097/00001756-200504250-00004. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Wilson DR, Tohen M, Woods BT, Douglass AW, Stoll AL. Structural brain abnormalities in first-episode mania. Biological Psychiatry. 1993;33:602–609. doi: 10.1016/0006-3223(93)90098-x. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Archives of General Psychiatry. 1999;56:254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- Swayze VW, Andreasen NC, Alliger RJ, Yuh WT, Ehrhardt JC. Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biological Psychiatry. 1992;31:221–240. doi: 10.1016/0006-3223(92)90046-3. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. New York: Theime Medical Publishers, Inc; 1988. Co-planar Stereotactic Atlas of the Human Brain. [Google Scholar]
- Winsberg ME, Sachs N, Tate DL, Adalsteinsson E, Spielman D, Ketter TA. Decreased dorsolateral prefrontal N-acetyl aspartate in bipolar disorder. Biological Psychiatry. 2000;47:475–481. doi: 10.1016/s0006-3223(99)00183-3. [DOI] [PubMed] [Google Scholar]
- Zipursky RB, Seeman MV, Bury A, Langevin R, Wortzman G, Katz R. Deficits in gray matter volume are present in schizophrenia but not bipolar disorder. Schizophrenia Research. 1997;26:85–92. doi: 10.1016/s0920-9964(97)00042-x. [DOI] [PubMed] [Google Scholar]