Abstract
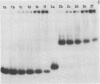
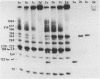
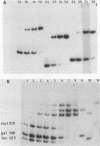
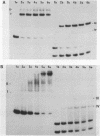
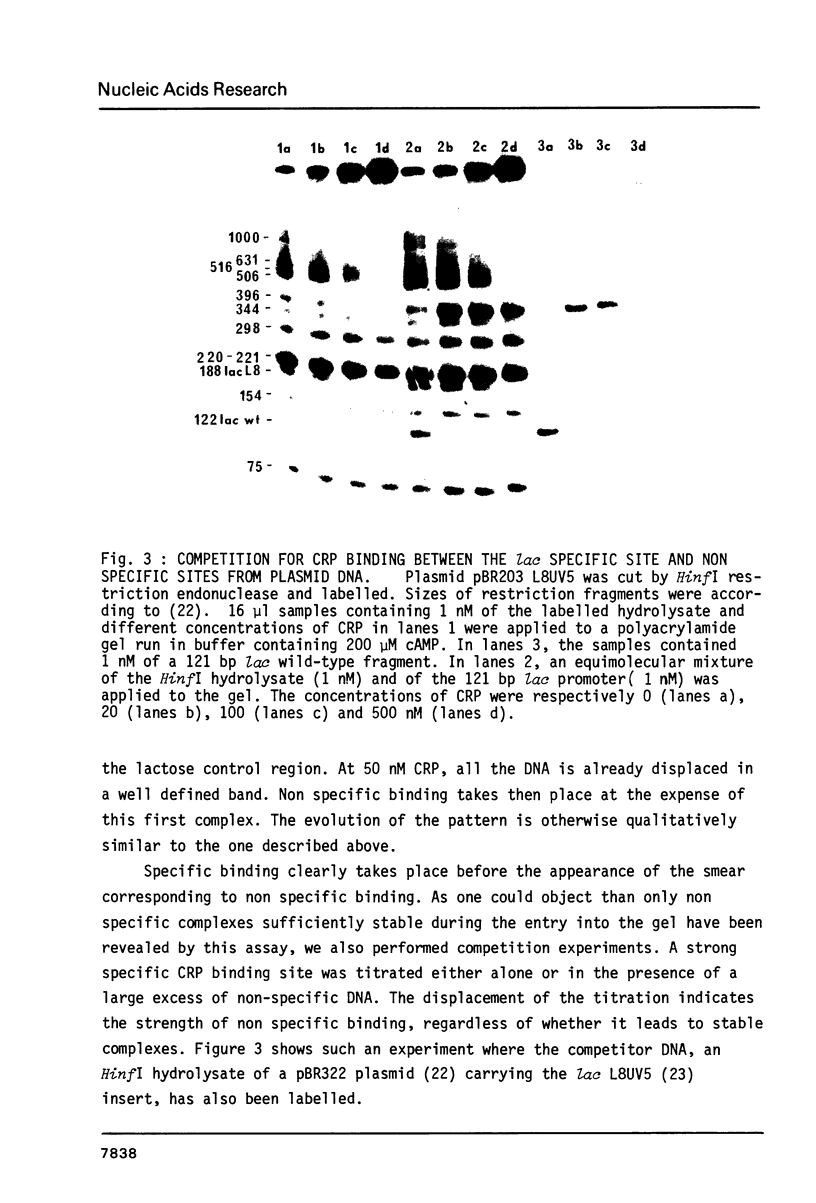
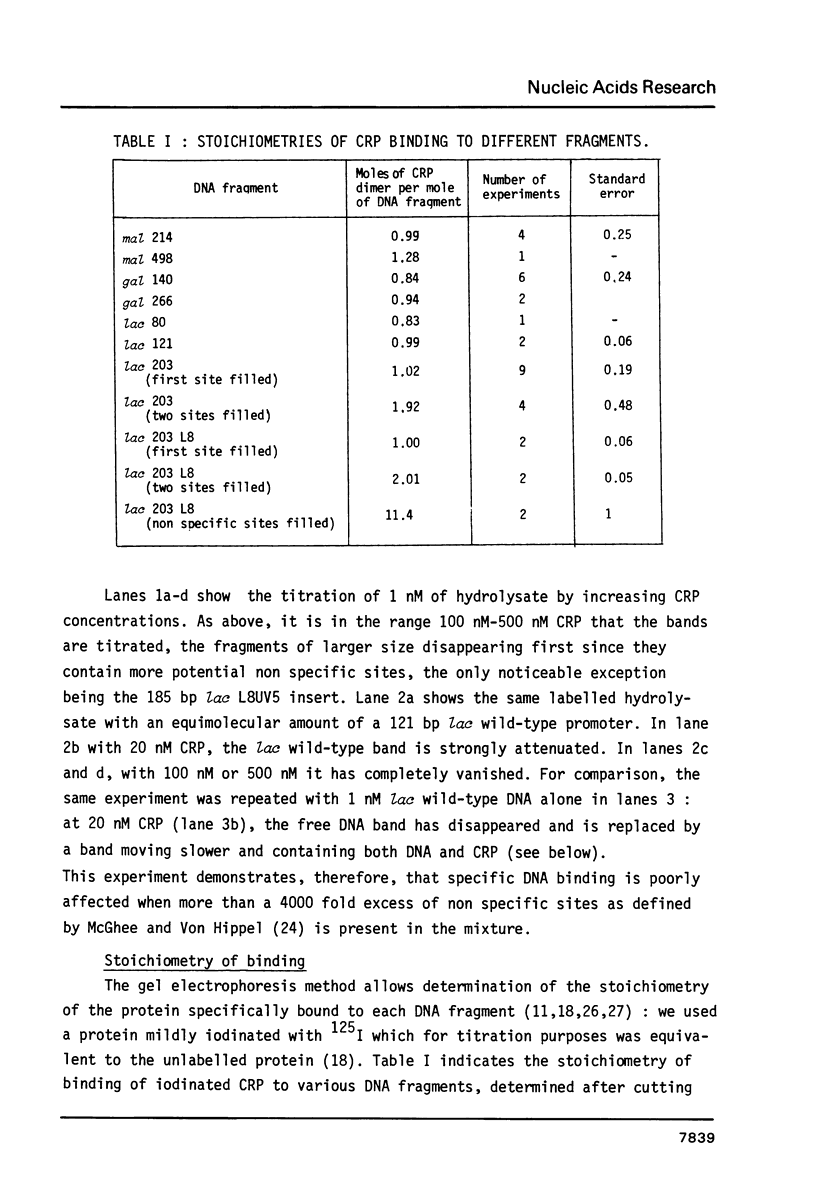
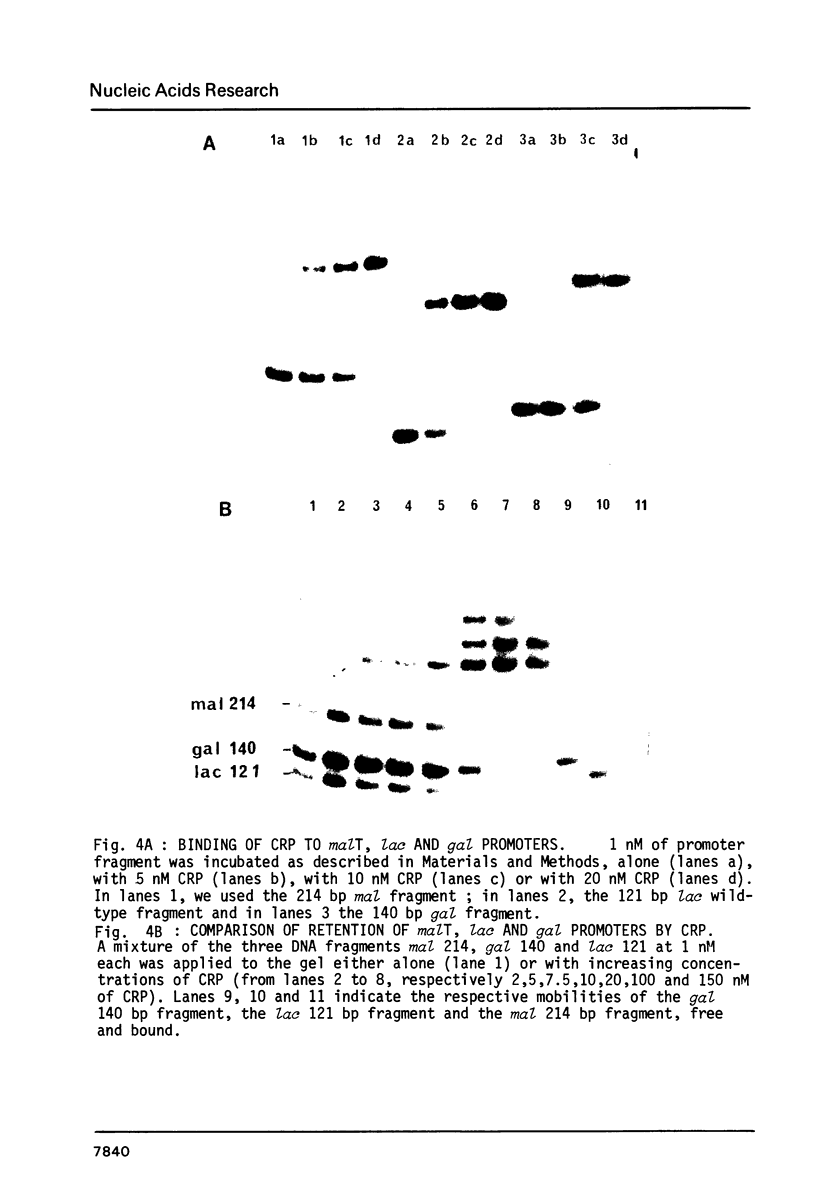
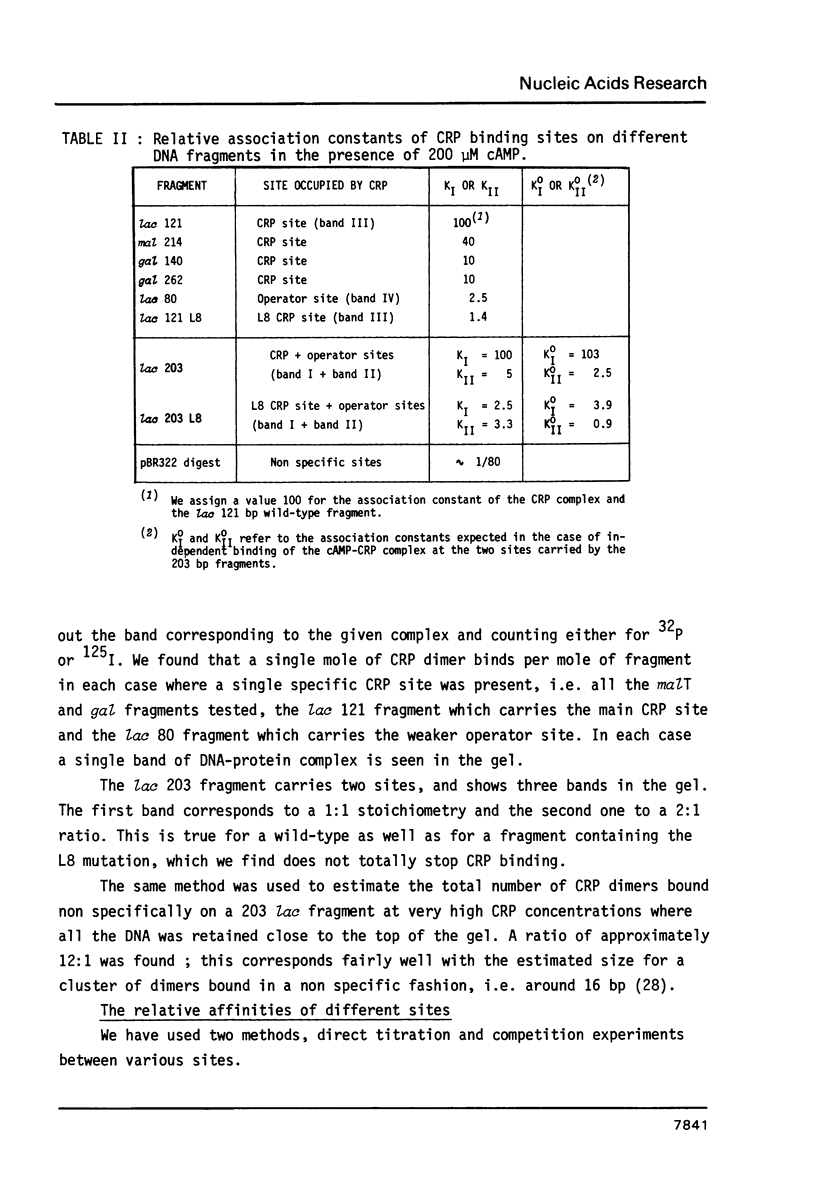
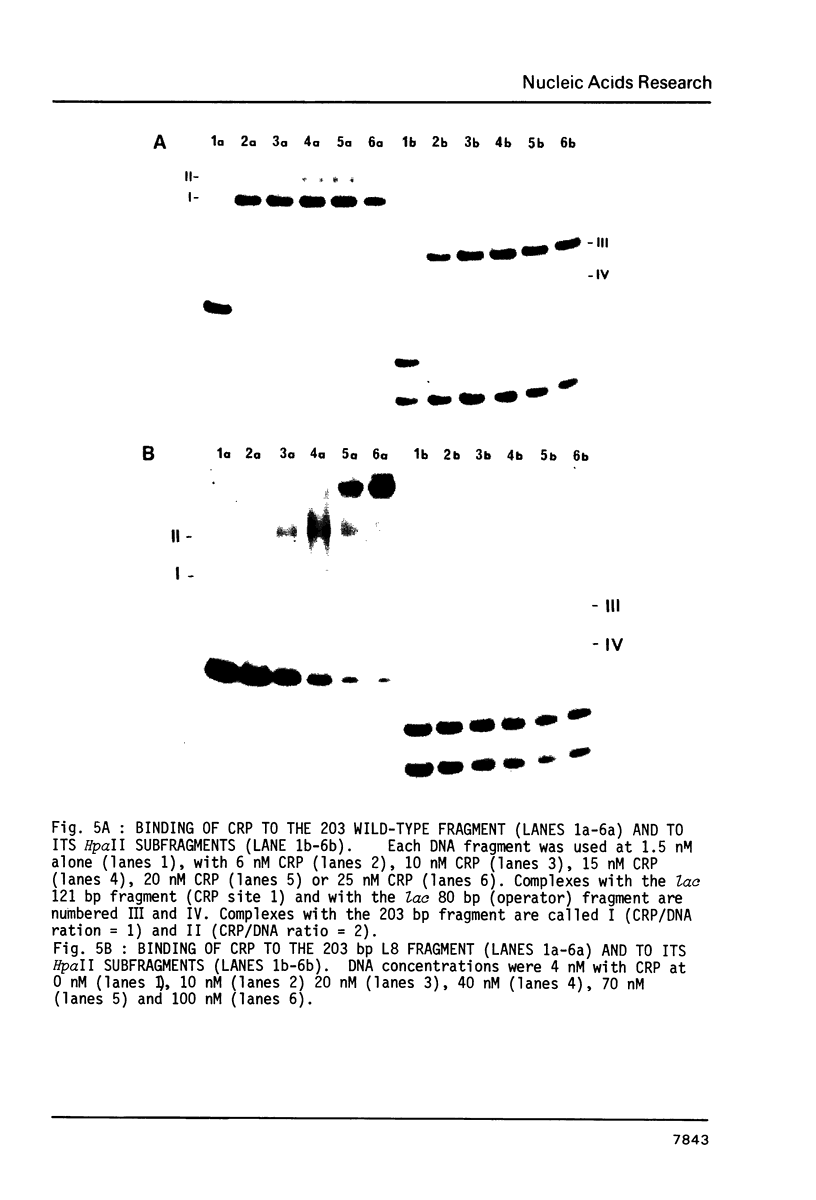
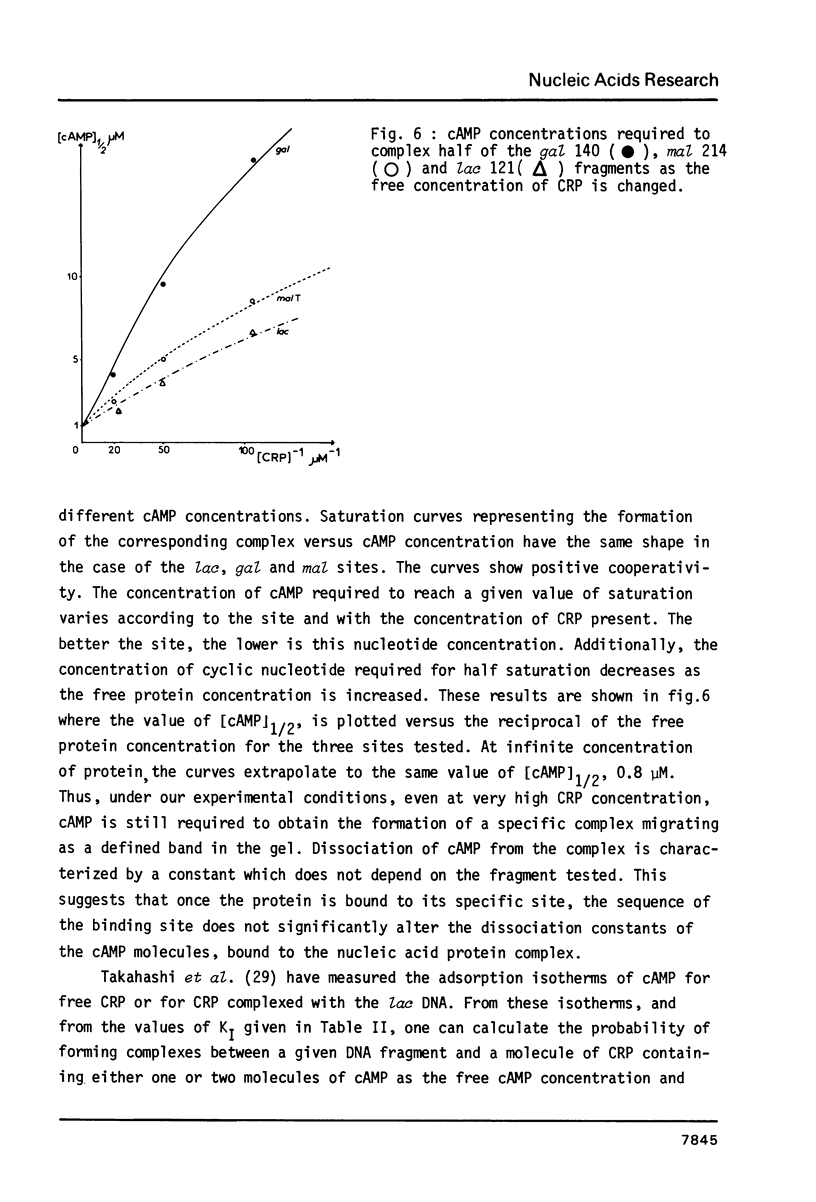
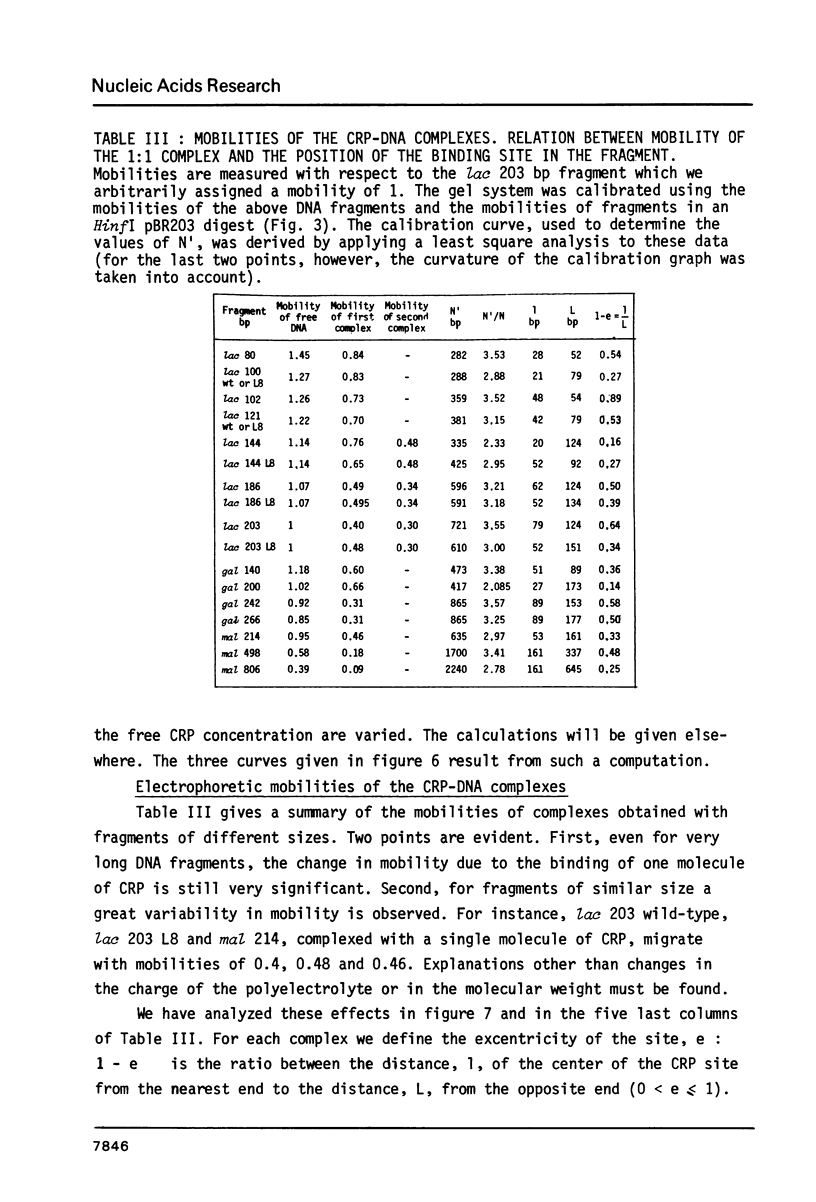
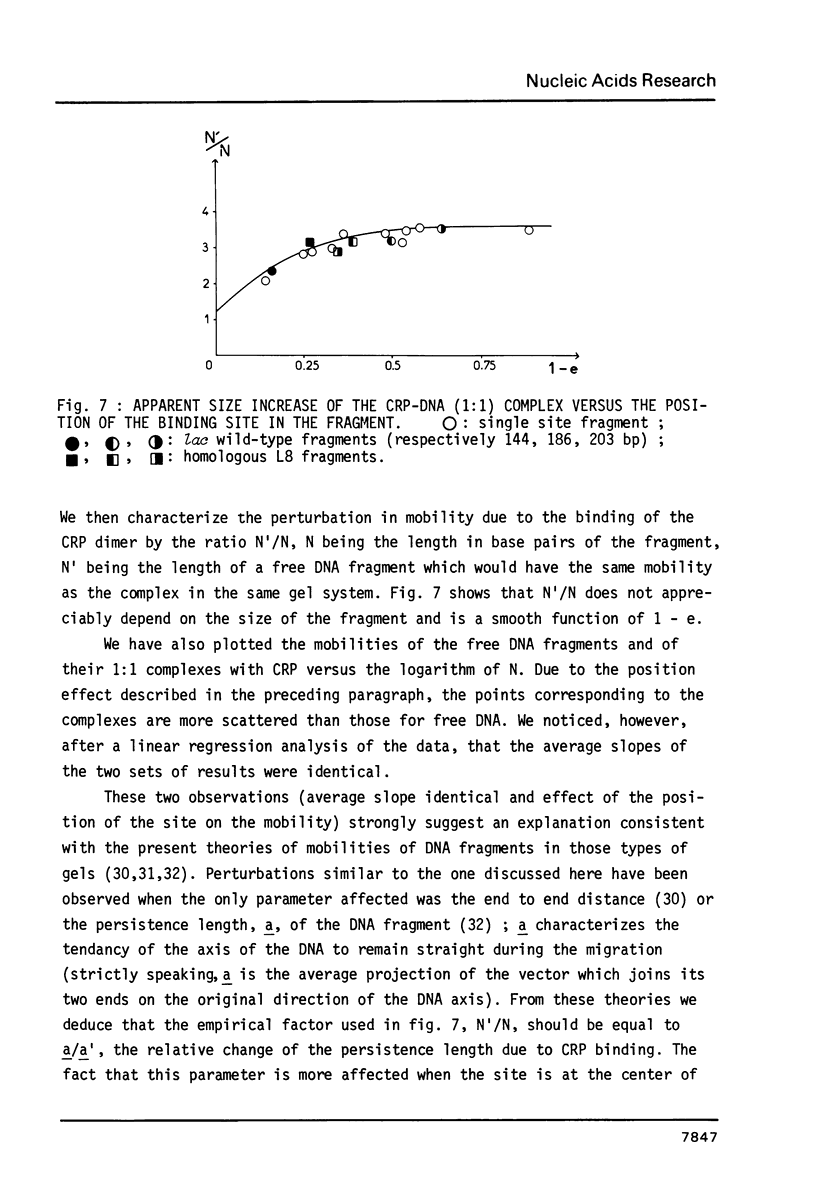
We have determined the stoichiometry of CRP binding to various DNA fragments carrying the lac, malT or gal promoters in the presence of cAMP, using a gel electrophoresis method. In each case, one dimer of CRP binds to the functional CRP site upstream of the transcription start. At the lac promoter, a second CRP dimer can bind to the operator region. Direct binding analysis and competition experiments performed at 200 microM cAMP allow us to measure the affinity of CRP for these different sites and to correlate them with variations in the consensus sequences, already proposed. The order is lac greater than malT greater than gal greater than lac operator greater than lac L8 much greater than non specific sites. No strong coupling exists between the two lac sites when on the same fragment. Conversely, we have studied, at constant CRP concentrations, the cAMP levels required to obtain half maximal binding to a particular DNA site : the required cAMP level increases inversely as the affinity for CRP. These variations may account for the differential activation of various cAMP sensitive operons in vivo. Anomalies in the migrations of the 1:1 complexes between CRP and DNA have been analysed and related to the size and to the position of the CRP site in the fragment. The electrophoretic mobility of the complexes depends not only on the size of the fragment but on the position of the CRP site : the mobility is lower when CRP binds near the center of the fragment. This effect is due to a clear change in the persistence length of the DNA induced by CRP binding. We suggest that, upon binding, the protein introduces a local bend (or a kink) in the DNA structure.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner M. J., Spitz E., Rickenberg H. V. Cyclic adenosine 3',5'-monophosphate in Escherichia coli. J Bacteriol. 1973 Jun;114(3):1068–1073. doi: 10.1128/jb.114.3.1068-1073.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby S., Dreyfus M. Segment-specific mutagenesis of the regulatory region in the Escherichia coli galactose operon: isolation of mutations reducing the initiation of transcription and translation. Gene. 1983 Jan-Feb;21(1-2):121–131. doi: 10.1016/0378-1119(83)90154-3. [DOI] [PubMed] [Google Scholar]
- Busby S., Irani M., Crombrugghe B. Isolation of mutant promoters in the Escherichia coli galactose operon using local mutagenesis on cloned DNA fragments. J Mol Biol. 1982 Jan 15;154(2):197–209. doi: 10.1016/0022-2836(82)90060-2. [DOI] [PubMed] [Google Scholar]
- Debarbouille M., Cossart P., Raibaud O. A DNA sequence containing the control sites for gene malT and for the malPQ operon. Mol Gen Genet. 1982;185(1):88–92. doi: 10.1007/BF00333795. [DOI] [PubMed] [Google Scholar]
- DiLauro R., Taniguchi T., Musso R., de Crombrugghe B. Unusual location and function of the operator in the Escherichia coli galactose operon. Nature. 1979 Jun 7;279(5713):494–500. doi: 10.1038/279494a0. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Johnson P. Nucleotide sequence changes produced by mutations in the lac promoter of Escherichia coli. J Mol Biol. 1977 Mar 25;111(1):65–75. doi: 10.1016/s0022-2836(77)80132-0. [DOI] [PubMed] [Google Scholar]
- Epstein W., Rothman-Denes L. B., Hesse J. Adenosine 3':5'-cyclic monophosphate as mediator of catabolite repression in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2300–2304. doi: 10.1073/pnas.72.6.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M. G., Crothers D. M. CAP and RNA polymerase interactions with the lac promoter: binding stoichiometry and long range effects. Nucleic Acids Res. 1983 Jan 11;11(1):141–158. doi: 10.1093/nar/11.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. Stoichiometry of catabolite activator protein/adenosine cyclic 3',5'-monophosphate interactions at the lac promoter of Escherichia coli. Biochemistry. 1982 Nov 23;21(24):6032–6036. doi: 10.1021/bi00267a001. [DOI] [PubMed] [Google Scholar]
- Guiso N., Blazy B. Regulatory aspects of the cyclic AMP receptor protein in Escherichia coli K-12. Biochem Biophys Res Commun. 1980 May 14;94(1):278–283. doi: 10.1016/s0006-291x(80)80217-8. [DOI] [PubMed] [Google Scholar]
- Joseph E., Bernsley C., Guiso N., Ullmann A. Multiple regulation of the activity of adenylate cyclase in Escherichia coli. Mol Gen Genet. 1982;185(2):262–268. doi: 10.1007/BF00330796. [DOI] [PubMed] [Google Scholar]
- Kolb A., Buc H. Is DNA unwound by the cyclic AMP receptor protein? Nucleic Acids Res. 1982 Jan 22;10(2):473–485. doi: 10.1093/nar/10.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb A., Busby S., Herbert M., Kotlarz D., Buc H. Comparison of the binding sites for the Escherichia coli cAMP receptor protein at the lactose and galactose promoters. EMBO J. 1983;2(2):217–222. doi: 10.1002/j.1460-2075.1983.tb01408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman L. S., Frisch H. L. Why does the electrophoretic mobility of DNA in gels vary with the length of the molecule? Biopolymers. 1982 May;21(5):995–997. doi: 10.1002/bip.360210511. [DOI] [PubMed] [Google Scholar]
- Lis J. T., Schleif R. Different cyclic AMP requirements for induction of the arabinose and lactose operons of Escherichia coli. J Mol Biol. 1973 Sep 5;79(1):149–162. doi: 10.1016/0022-2836(73)90276-3. [DOI] [PubMed] [Google Scholar]
- Lumpkin O. J. Mobility of DNA in gel electrophoresis. Biopolymers. 1982 Nov;21(11):2315–2316. doi: 10.1002/bip.360211116. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Theoretical aspects of DNA-protein interactions: co-operative and non-co-operative binding of large ligands to a one-dimensional homogeneous lattice. J Mol Biol. 1974 Jun 25;86(2):469–489. doi: 10.1016/0022-2836(74)90031-x. [DOI] [PubMed] [Google Scholar]
- Piovant M., Lazdunski C. Different cyclic adenosine 3',5'-monophosphate requirements for induction of beta-galactosidase and tryptophanase. Effect of osmotic pressure on intracellular cyclic adenosine 3,5-monophosphate concentrations. Biochemistry. 1975 May 6;14(9):1821–1825. doi: 10.1021/bi00680a003. [DOI] [PubMed] [Google Scholar]
- Queen C., Rosenberg M. A promoter of pBR322 activated by cAMP receptor protein. Nucleic Acids Res. 1981 Jul 24;9(14):3365–3377. doi: 10.1093/nar/9.14.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer F., Kolb A., Buc H. Point mutations change the thermal denaturation profile of a short DNA fragment containing the lactose control elements. Comparison between experiment and theory. EMBO J. 1982;1(1):99–105. doi: 10.1002/j.1460-2075.1982.tb01131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A. Cyclic AMP receptor proteins interacts with lactose operator DNA. Nucleic Acids Res. 1981 Jan 24;9(2):277–292. doi: 10.1093/nar/9.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanblatt S. H., Revzin A. Two catabolite activator protein molecules bind to the galactose promoter region of Escherichia coli in the presence of RNA polymerase. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1594–1598. doi: 10.1073/pnas.80.6.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M., Blazy B., Baudras A. An equilibrium study of the cooperative binding of adenosine cyclic 3',5'-monophosphate and guanosine cyclic 3',5'-monophosphate to the adenosine cyclic 3',5'-monophosphate receptor protein from Escherichia coli. Biochemistry. 1980 Oct 28;19(22):5124–5130. doi: 10.1021/bi00563a029. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Blazy B., Baudras A., Hillen W. On the origin of selectivity in recognition by cyclic adenosine 3',5'-monophosphate receptor protein of its specific binding site of the lactose promoter region. J Mol Biol. 1983 Jul 15;167(4):895–899. doi: 10.1016/s0022-2836(83)80118-1. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Blazy B., Baudras A. Non-specific interactions of CRP from E. coli with native and denatured DNAs: control of binding by cAMP and cGMP and by cation concentration. Nucleic Acids Res. 1979 Nov 24;7(6):1699–1712. doi: 10.1093/nar/7.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]