Abstract
Objectives
(1) To design a submaximal arm ergometry test (6-minute arm test [6-MAT]), for individuals with spinal cord injury (SCI) and (2) to determine the test-retest reliability and concurrent validity of this test.
Design
Prospective, exploratory, methodological study. To determine test-retest reliability, subjects completed the 6-MAT on 2 days, separated by 1 week. Validity was determined by comparing 6-MAT results with VO2peak.
Setting
Tertiary rehabilitation center.
Participants
30 subjects with SCI (mean age=36.3 years; 83% male).
Intervention
Not applicable.
Main Outcome Measures
Subjects were evaluated on the 6-MAT and a VO2peak test.
Results
All subjects were able to complete the 6-MAT. Test-retest reliability of steady state VO2 and heart rate (HR) during the 6-MAT were excellent; intraclass correlation coefficient (ICC)=0.81 [95% confidence interval (CI)=0.58–0.92], and 0.90 (95%CI=0.75–0.96), respectively. The correlation between VO2peak and 6-MAT VO2 was excellent (r=0.92), while those between VO2peak and 6-MAT HR (r=0.63) and VO2peak and 6-MAT power output (PO) (r=0.73) were good.
Conclusions
This study demonstrated that the 6-MAT has acceptable values for test-retest reliability and validity. The 6-MAT should be further tested for responsiveness to enhance its use as a clinical tool.
Keywords: exercise, rehabilitation, spinal cord injuries, physical fitness, reproducibility of results, paraplegia, quadriplegia, arm ergometry
Introduction
As modern medicine continues to advance, the life expectancy of individuals with spinal cord injury (SCI) has begun to closely resemble that of the general population.1–2 Accordingly, chronic diseases have become more prevalent in this population, and the predominant cause of death has shifted from genitourinary infections to respiratory and cardiovascular diseases.1,3,4 Studies have shown that the prevalence of coronary artery disease in individuals with SCI is at least as high as in the general population.5–6 Consequently, physical fitness needs to be better monitored in individuals with SCI, in part because of its ability to predict cardiovascular disease risk.
Relative to the general population, most individuals with SCI have reduced cardiovascular fitness (VO2peak)7 and a diminished physical work capacity.8 Inactivity and low VO2peak are modifiable risk factors associated with cardiovascular disease.9 By improving VO2peak in individuals with SCI, both the risk for cardiovascular disease10 and physical strain during activities of daily living can be decreased.11 Protocols for assessing VO2peak for individuals with SCI in laboratory settings have been well established,12–13 but costly specialized equipment and trained personnel are required to conduct these tests and thus such assessments are rarely done with people with SCI in rehabilitation or community settings.
Submaximal tests have been used widely in able-bodied populations to estimate cardiovascular fitness,14 but as most involve lower extremity exercise, there are few appropriate tests that can be completed by individuals with bilateral loss of motor function resulting from a SCI. A number of submaximal tests designed for individuals with SCI exist,15–21 but each has limitations to widespread use. An arm ergometer equivalent to the Canadian Aerobic Fitness Test (CAFT)19 was not feasible for individuals with lower limb disabilities because of the high cycling cadences required to complete the test.20 Twelve minute wheeling tests detailed by DiCarlo,15 Dwyer and Davis,16 Franklin et al.,17 and Rhodes et al.,21 all require large 200 metre tracks, which are not typically available in clinical settings. Finally, a three-stage submaximal arm ergometer test was designed to predict VO2peak in individuals with SCI. This multi-stage test required the use of specific regression equations in conjunction with a modified Astrand-Ryhming equation and this calculation was found to be moderately predictive (for men r=0.67, for women r=0.61) of measured VO2peak.18
Despite the studies that have proposed submaximal arm exercise tests, the need remains for a test that is inexpensive, simple to administer in clinics and rehabilitation centres and can be completed by individuals with both paraplegia and tetraplegia. In this study, the 6-minute arm test (6-MAT) is proposed as a new test to assess cardiovascular fitness in individuals with SCI. The 6-MAT involves 6 minutes of submaximal arm ergometry at a constant power output (PO). As steady state physiological responses are typically seen within 2 to 3 minutes of submaximal exercise,22–23 6 minutes was chosen as it would elicit steady state exercise, yet not be so long as to fatigue an untrained individual. One of the challenges to developing a submaximal test in a population with diverse abilities is to be able to individualize the workload to ensure that the task is sufficiently challenging to elicit aerobic responses, but not at the levels of a maximal test. A single stage test was chosen as it would be simple to administer clinically, and require less time from both the clinician and client.
Thus, the first purpose of this study was to develop a submaximal test (6-MAT) which would elicit either a steady state HR of 60–70% of age-predicted maximum HR (for subjects with low level paraplegia) or a rating of 11–15 on Borg’s24 RPE scale (for subjects with tetraplegia or high level paraplegia). The second purpose was to determine the test-retest reliability and concurrent validity of this exercise test. We proposed to establish concurrent validity by quantifying the relationship of the submaximal test with the gold standard of cardiovascular fitness, VO2peak, using a symptom-limited graded exercise test. Concurrent validity quantifies the relationship of one measure to another through the use of correlational statistics.
Methods
Subjects
Adults with SCI were recruited on a volunteer basis. Subjects were recruited from mailings to previous patients of the GF Strong Rehab Centre (provincial centre for spinal cord injury rehabilitation) and by advertisements sent to community support groups. Current clinicians of the GF Strong Rehab Centre were also informed of the study and asked to inform potential clients who may be coming back to the centre for follow-up. Inclusion criteria for participation were as follows: (1) had a traumatic SCI at least 1 year ago, (2) be between 18 and 50 years of age, (3) used a manual or power wheelchair for daily mobility, and (4) able to independently push an arm cycle ergometer. Individuals were excluded from participating in this study if they had a previous myocardial infarction or if they were taking medications that could alter their cardiovascular responses to exercise (e.g. beta-blockers). Eligible subjects gave informed, written consent to participate in this study. Ethical approval for this study was obtained from local university and rehabilitation hospital ethics committees.
Measures
Cognitive impairment was assessed with the Cognitive Capacity Screening Examination (CCSE).25–26 No subjects were identified as having a cognitive impairment (as indicated by a score of less than 24 out of 30). The American Spinal Injury Association (ASIA) assessment was used to classify the completeness of each subject’s SCI, as well as to determine their motor and sensory function.27 The physical activity level of all subjects was assessed using the Physical Activity Scale for Individuals with Physical Disabilities (PASIPD).28 The PASIPD is a self-report questionnaire that uses an estimate of the number of days per week and hours per day spent in different leisure, household and occupational activities over the past 7 days. Total scores are calculated as the sum of the average hours per day multiplied by a metabolic equivalent for all 12 items. Independence in wheeled mobility was described using 8 categories of wheeled mobility, modified from categories of functional walking developed by Perry et al.29 The categories in this wheeled mobility scale range from full time power wheelchair users to those who can walk independently for most activities but rely on a manual wheelchair for wheeling long distances (Table 1).
Table 1.
Wheeled Mobility Scale
| Category | Description |
|---|---|
| 1 | Relies fully on power wheelchair |
| 2 | Relies primarily on a power wheelchair for community mobility; uses manual wheelchair for exercise only |
| 3 | Relies primarily on a power wheelchair for community mobility; uses manual wheelchair for some household activities |
| 4 | Relies primarily on manual wheelchair; relies on power wheelchair or assistance with long distances or uphill |
| 5 | Relies fully on manual wheelchair and is independent in all home and community activities |
| 6 | Relies primarily on manual wheelchair; able to walk for exercise only at home |
| 7 | Able to use walking for some household activities, but uses a wheelchair in the community |
| 8 | Can walk independently for most activities; relies on a manual wheelchair for long distances |
Procedures
For both the 6-MAT and cardiovascular fitness test, subjects wore a face mask while respiratory variables (VO2, VCO2, ventilation [VE], respiratory exchange ratio [RER]) were continuously measured by a portable metabolic unit performing breath-by-breath gas analysis (Cosmed K4b2 system)a. Subjects were asked to empty their bladders prior to commencing the exercise tests to minimize any episodes of autonomic dysreflexia. Subjects rated their level of perceived exertion using Borg’s24 15-point rate of perceived exertion (RPE) scale immediately following the tests. Blood pressure (BP) was recorded at rest, immediately following exercise and throughout recovery to ensure it returned to baseline values following the exercise tests. Blood lactate measurements were taken at rest and at the end of the exercise tests using a drop of blood collected from a finger-prick. For subjects with insufficient hand grip, elastic straps were used to secure their hands to the handles of the arm ergometer.
Subjects completed a single, 6-minute stage of submaximal exercise on a standard arm cycle ergometer (Monark Rehab Trainer 881E)b commonly found in rehabilitation settings. An individual PO was initially selected for each subject based on their manual muscle strength, ASIA motor score and physical activity level (as reported in Table 2). These parameters were used following discussions with physical therapists and physicians who worked in spinal cord injury rehabilitation as they felt these were typical variables that were accessible for these clients. The PO was chosen with the aim of eliciting either a steady state HR of 60–70% of age-predicted maximum HR (for subjects with low level paraplegia) or a rating of 11–15 on Borg’s24 RPE scale (for subjects with tetraplegia or high level paraplegia). HR was continually recorded using a chest HR monitor (Polar A3)c. Approximately 1 week after the initial 6-MAT, subjects were invited to return to complete a second 6-MAT. One week after the second test, each subject underwent a VO2peak test on an arm ergometer.
Table 2.
Subject characteristics (n=30)
| Variables | N | Mean | (SD) | Range |
|---|---|---|---|---|
| Sex (M/F) | 25/5 | |||
| Age (years) | 36.3 | 9.3 | 19–49 | |
| Time since injury (years) | 12.0 | 9.8 | 1–34 | |
| Tetraplegia/Paraplegia) | 17/13 | |||
| ASIA Grade (A/B/C/D) | 22/7/0/1 | |||
| ASIA Motor Score (0–100) | 41.2 | 16.7 | 19–75 | |
| PASIPD score | 16.5 | 9.8 | 1.0–38.7 | |
| Wheeled mobility category: | ||||
| 1 | 2 | |||
| 3 | 1 | |||
| 4 | 11 | |||
| 5 | 15 | |||
| 6 | 1 | |||
To measure VO2peak, subjects performed a symptom-limited graded arm cycle ergometer test on an electronically braked arm ergometer (Excaliber)d in the presence of a physician and a kinesiologist. Cardiac stability and HR were monitored by a physician using a 12-lead electrocardiogram (ECG) (Quark C12)a. Subjects initially sat quietly for two minutes while resting values of HR and respiratory variables were collected. Arm cycling began without resistance at a comfortable, self-selected cadence (between 60–80 rpm). Following a brief warm up, PO increased in a step protocol by either 5 or 10 Watts (W)/min (5 W/min for subjects with tetraplegia,30 10 W/min for subjects with paraplegia12). Subjects continued to arm cycle until they reached volitional fatigue (i.e. they were not able to maintain a cycling rate of 30 rpm). American College of Sports Medicine (ACSM) Guidelines9 were used to determine whether the test should be terminated early (e.g., ST-segment depression >2 mm, increasing nervous system symptoms [i.e. blurred vision, dizziness], sustained ventricular tachycardia, chest discomfort).
Data analysis
Descriptive statistics were calculated for subject characteristics and physiological variables (HR, VO2, RER) during the 6-MAT and VO2peak test. Skewness coefficients were calculated and scatter-plots of variables used in the validity analysis were visually inspected to ensure outlier and influential data points did not compromise the results. The final 30 seconds of the physiological data during the 6-MAT was averaged to get a single representative steady-state value for each subject. The breath-by-breath data obtained during the VO2peak test was averaged at a rate of every 15 seconds to obtain a more accurate measure of VO2peak. The highest value of VO2 (in mL/kg/min) obtained in any 15 second interval during the test was considered to be the VO2peak. Intraclass correlation coefficients (ICC2,1) with 95% confidence interval (CI) and standard error of measurement (SEM) were used to assess the test-retest reliability of steady state VO2 and HR during the 6-MAT. Bland-Altman plots31 were used to reveal the reliability of individual scores on repeated measurement. Pearson product-moment correlations were used to quantify the relationship between VO2peak and steady state VO2, HR and PO during the 6-MAT. Sample size calculations revealed that a sample size of 28 was required for the validity correlations of this study using a two-tailed test with α=0.05, a power of 0.80 and a desired effect size of 0.5.32 An effect size of 0.5 was selected as submaximal assessments (VO2, HR) have shown at least moderate association (r=0.5) with VO2peak in previous investigations with spinal cord injury. 16–18 All statistical analyses were performed using SPSS 11.5 software using a significance level of p ≤ 0.05 (two-tailed).
Results
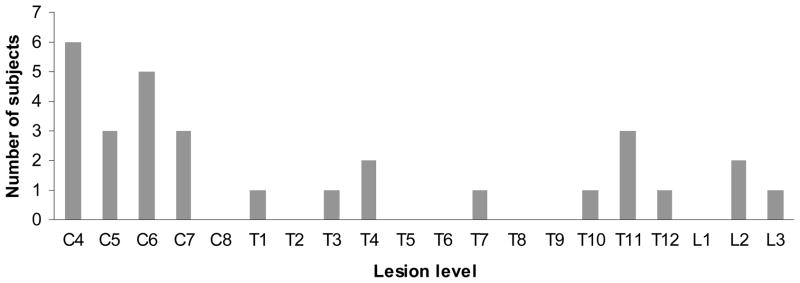
Descriptive data for the subject characteristics can be found in Table 2. A total of 31 subjects volunteered to participate in this study, but 1 subject was excluded after reporting a previous myocardial infarction. Of the remaining 30 subjects that participated in this study, there were 13 with paraplegia, and 17 with tetraplegia. The distribution of lesion levels is presented in Figure 1. All subjects, including those with C4 and C5 lesions, had a minimum manual muscle testing score of grade 4 for wrist extension. Subjects with C4 and C5 lesions had impaired sensation below their stated lesion level; however, muscle function was partially preserved in 1–2 segments below their lesion level, and this allowed them to have adequate wrist extension strength to allow arm cycling. Subjects had a mean (standard deviation [SD]) age of 36.3 (9.3) years and a mean (SD) time since SCI of 12.0 (9.8) years. 83% of the subjects were men, which is comparable to global statistics.32 Subjects in this study ranged from full-time power wheelchair users to individuals that were able to ambulate for exercise.
Figure 1.
Distribution of subject lesion levels
Maximal Test: Peak oxygen consumption
The mean (SD) VO2peak value for all subjects was 18.6 (8.4) mL/kg/min, with the range extending from 6.5 to 38.1 mL/kg/min (Table 3). The peak HR averaged 129 (29) beats/min, and the mean peak PO attained was 60.2 (36.0) W. Subjects in this study had a wide range of fitness levels, and there was overlap in the physiological responses to exercise between subjects with paraplegia and tetraplegia. During both the VO2peak test, and the 6-MAT, 15 subjects required their hands to be secured to the handles of the arm ergometer with elastic straps. No VO2peak tests were terminated early because of adverse effects. One subject with T3 level paraplegia experienced self-reported “mild” autonomic dysreflexia mid-way through the test, which presented as a rapid decrease in HR of 25 beats/min (exercising HR of 152 beats/min decreased to 127 beats/min; within 30 seconds HR returned to 155 beats/min). 7 subjects reported mild light-headedness upon cessation of cycling, and 2 experienced muscle spasms at low POs that briefly interrupted their cycling cadence.
Table 3.
Physiological values during the VO2peak test
| Variables | Mean | SD | Range |
|---|---|---|---|
| Peak PO (W) | 60.2 | 36.0 | 20–160 |
| Peak HR (beats/min) | 129 | 29 | 75–183 |
| Percent HR maximum* | 70.1 | 14.0 | 43.6–97.3 |
| Peak VE (L/min) | 42.8 | 19.5 | 18.0–113.1 |
| VO2peak (mL/kg/min) | 18.6 | 8.4 | 6.5–38.1 |
| VO2peak (L/min) | 1.33 | 0.52 | 0.74–2.81 |
| Peak RER | 1.14 | 0.09 | 0.97–1.34 |
| Blood lactate (mmol/L)† | 6.6 | 2.8 | 2.3–15.1 |
Based on 220-age prediction equation
Only 27 of 30 subjects had their blood lactate tested
Submaximal Test: 6-MAT
All subjects were able to complete the 6-MAT. Four subjects experienced mild muscle spasms during their cycling that briefly interrupted their cycling cadence. The mean (SD) steady state VO2 value was 13.1 (4.2) mL/kg/min, with the range extending from 6.3 to 22.9 mL/kg/min (Table 4). HR averaged 103 (21) beats/min, and the mean PO was 27.8 (17.0) W. Similar to that seen in the VO2peak test, there was overlap between the subjects with paraplegia and tetraplegia for both the PO and physiological responses.
Table 4.
Steady state physiological values during the 6-MAT
| Variables | Mean | SD | Range |
|---|---|---|---|
| 6-MAT PO (W) | 27.8 | 17.0 | 10–60 |
| HR (beats/min) | 103 | 21 | 61–142 |
| Percent HR maximum* | 56.0 | 10.2 | 35.7–75.7 |
| VE (L/min) | 25.0 | 5.7 | 14.7–39.8 |
| VO2 (mL/kg/min) | 13.1 | 4.2 | 6.3–22.9 |
| VO2 (L/min) | 0.95 | 0.28 | 0.55–1.61 |
| Percent VO2peak | 74.5 | 13.0 | 51.0–97.8 |
| RER | 0.89 | 0.07 | 0.76–1.04 |
| Blood lactate (mmol/L)† | 3.0 | 1.3 | 1.0–6.0 |
Based on 220-age prediction equation
Only 28 of 30 subjects had their blood lactate tested
One of the difficulties of conducting the 6-MAT was determining an appropriate submaximal PO for each subject. In this study, PO for the 6-MAT was selected based on subjects ASIA motor score, manual muscle strength and physical activity level. If the initial PO was too high (i.e. subjects could not complete 6 minutes, RPE > 15, and/or HR > 70% age predicted maximum) or too low (i.e. RPE < 11 and/or HR < 60% age predicted maximum), subjects were given a 15-minute rest period before they attempted a second 6-MAT at an adjusted PO. Subjects HR and VO2 had to return to resting values before testing would recommence. Typically it was evident within the first 2 minutes of arm cycling if the PO selected was too high or low. After testing all subjects with the 6-MAT, manual muscle strength scores and physical activity levels were reviewed to develop clinical guidelines for the selection of appropriate starting PO (Table 5). Using these criteria, 11 of the 13 subjects with paraplegia (85%) and 11 of the 17 subjects with tetraplegia (65%) would have had their PO set appropriately on the first attempt.
Table 5.
PO selection for the 6-MAT
| For individuals with tetraplegia | |||
|---|---|---|---|
| Set PO to 10 W if: | Set PO to 15 W if: | Set PO to 20 W if: | |
| Power wheelchair user OR ≤ grade 4 wrist extension | Manual wheelchair user | Manual wheelchair user AND grade 5 wrist extension AND physically active* | |
| For individuals with paraplegia | |||
|---|---|---|---|
| Set PO to 30 W if: | Set PO to 40 W if: | Set PO to 50 W if: | Set PO to 60 W if: |
| Female: inactive | Female: active OR male: inactive | Female: competitive athlete OR male: active | Male: competitive athelete |
engaged in physical activity at least 3 times per week as assessed by the PASIPD
Reliability of the 6-MAT
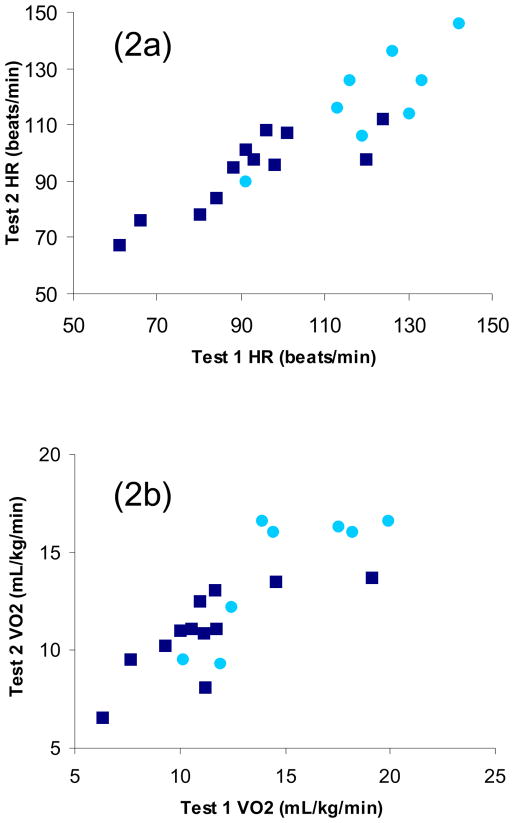
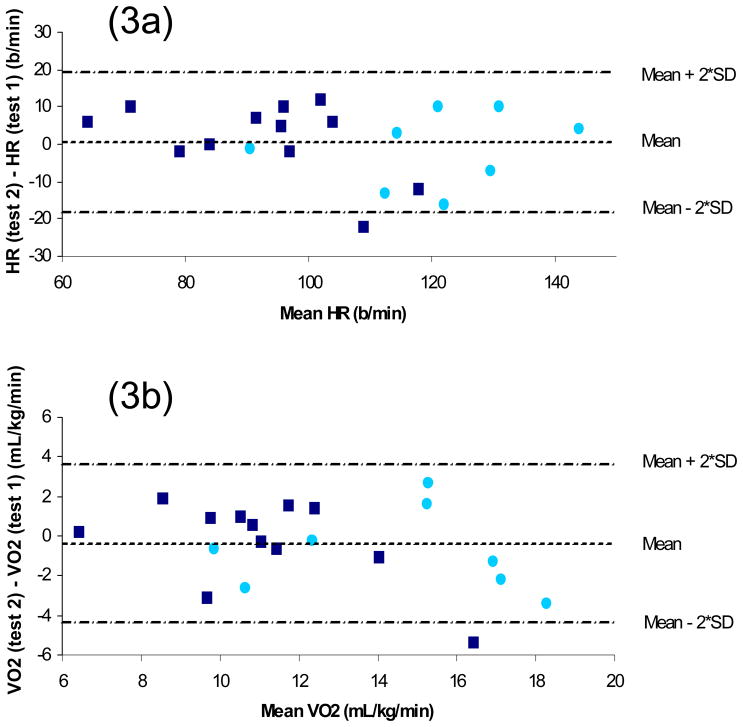
Twenty of the 30 subjects (8 with paraplegia, 12 with tetraplegia) completed the 6-MAT on a second occasion, approximately 1 week after the first test. Steady state ICCs for HR and VO2 were 0.90 (SEM=7.12, 95% CI=0.75–0.96) and 0.81 (SEM=1.62, 95% CI=0.58–0.92), respectively (Figure 2). The 20 subjects who completed the reliability portion of the study were not different from the other 10 subjects with respect to age, ASIA motor score, VO2peak or distribution of tetraplegia and paraplegia. The Bland-Altman method31 showed minimal differences between mean (SD) HR at time 1 (104 [23] beats/min) and time 2 (104 [20] beats/min) for the reliability sample. The mean (SD) difference between time 1 and time 2 was 0 (10) beats/min. As can be seen in Figure 3a, all but 1 data point fell within 2 SD of the mean difference, and the data points are equally distributed above and below the mean difference line (11 above, 8 below, and 1 on the line). The outlying data point was an individual with tetraplegia. Test-retest reliability was recalculated without this outlier, and the ICC increased to 0.92 (95% CI 0.81–0.97). Similarly, during the 6-MAT minimal differences were seen between mean (SD) VO2 at time 1 (12.64 [3.71] mL/kg/min) and time 2 (12.26 [3.10] mL/kg/min) for the reliability sample. The mean (SD) difference between time 1 and time 2 was −0.44 (2.07) mL/kg/min (Figure 3b). Once again, there was only 1 data point outside 2 SD of the mean difference, and the data points are equally distributed above and below the mean difference line (11 above and 9 below). This outlier was once again an individual with tetraplegia, but a different subject. With this outlier removed, the ICC for VO2 increased to 0.86 (95% CI 0.67–0.94).
Figure 2.
Figure 2a Scatter-plot comparing HR during 6-MAT test 1 and test 2; 2b Scatter-plot comparing VO2 during 6-MAT test 1 and test 2 (light circle, subjects with paraplegia; dark square, subjects with tetraplegia)
Figure 3.
Figure 3a Bland Altman plot of difference in 6-MAT HR between time 1 and time 2 versus average HR from time 1 and time 2; 3b Bland Altman plot of difference in 6-MAT VO2 between time 1 and time 2 versus average VO2 from time 1 and time 2 (light circle, subjects with paraplegia; dark square, subjects with tetraplegia)
Validity of the 6-MAT
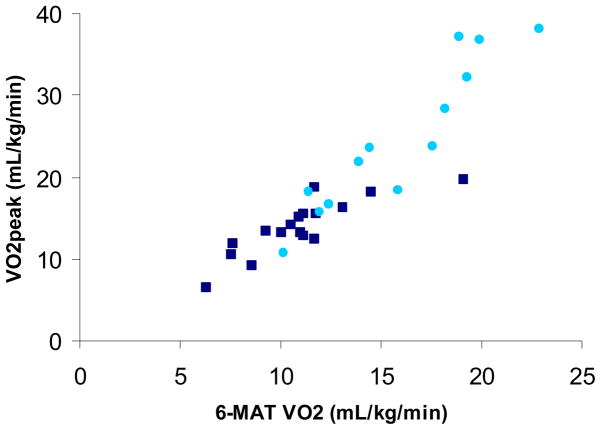
Upon visual inspection of the bi-variate scatterplots, there was considerable overlap in values between subjects with tetraplegia and paraplegia (Figure 4). Given the overlap of values between subjects with tetraplegia and paraplegia, in addition to the similar coefficients (slope and intercept) of the bi-variate scatterplots between the subjects with tetraplegia and paraplegia, all subjects were pooled. Using Pearson’s correlations, an excellent, positive, linear correlation was found between the 6-MAT VO2 and VO2peak (r=0.92) (Figure 4), while good correlations were found between 6-MAT PO and VO2peak (r=0.73) and 6-MAT HR and VO2peak (r=0.63).
Figure 4.
Scatter-plot comparing VO2 during the 6-MAT and VO2peak (r=0.92) (light circle, subjects with paraplegia; dark square, subjects with tetraplegia)
Discussion
Demographic variables (age, sex, time since injury, level of injury) were comparable to global statistics.33 As we excluded individuals who were ambulatory or could not use a manual wheelchair, the majority of our participants had lower cervical injuries (C4–C7) with a smaller representation of thoracic and lumbar injuries. Such a distribution reflects the large representation of lower cervical injuries among all spinal cord injury levels reported by the National Spinal Cord Statistical Centre Database.34 We expected the majority of participants to have ASIA A and B injuries (as represented in the sample), however, we did not have any ASIA C injuries which is a limitation to the generalizability of the sample. The PASIPD is a fairly new measurement tool used for the evaluation of physical activity level of individuals with physical disabilities, so limited data is available for comparison. The mean PASIPD score of the subjects in this study was comparable to that reported by Washburn et al.28 in individuals with a variety of disabilities (including SCI).
As this study only recruited wheelchair users that were able to independently cycle an arm ergometer, it is appropriate that the majority of subjects were described by categories 4 and 5 of the wheeled mobility scale (independent manual wheelchair users who do and do not require assistance with wheeling long distances or uphill). No subjects were described by categories 7 or 8, as these categories describe primarily ambulators, and not individuals that mainly use a wheelchair.
A portable metabolic system was used to measure VO2. Some studies35, 36 have reported excellent reliability, but small systematic biases in VO2 values of a portable system compared to a standard metabolic cart. These differences are modest and others have concluded that this small discrepancy was acceptable for measuring VO2 across a wide range of exercise intensities.37 Such small systematic biases would not affect the reliability or the correlations attained in this study. The VO2peak values found in this group of SCI subjects were similar to others previously reported in the literature38–39 indicating that this sample is representative of the general population of individuals with SCI. This study included individuals with diverse activity levels ranging from sedentary individuals using power wheelchairs to international calibre wheelchair athletes. Using normative categories that were developed from the 20th percentiles of VO2peak values from 146 men with SCI,39 the subjects from this study were categorized as follows: for subjects with tetraplegia, 1 poor, 1 fair, 6 average, 6 good and 3 excellent; for subjects with paraplegia, 2 poor, 4 fair, 3 average, 1 good and 3 excellent. The VO2peak values used by Janssen et al.39 to develop the normative categories are from only male subjects, with 40% of them being athletes. It is likely that the ‘normative’ VO2peak values used to categorize individuals are higher than the true values of the general population of individuals with SCI, thus VO2peak classification of subjects in this study may be underestimated.
All subjects were able to complete 6 minutes of arm ergometry exercise at an individually selected submaximal PO. During the 6-MAT, subjects exercised at an average of 74.5% of VO2peak and at 56% of their age-predicted maximum HR, indicating that the exercise was aerobic. Blood lactate immediately following the 6-MAT averaged 3.0 mmol/L. The onset of blood lactate accumulation, or the transition to anaerobic exercise, is said to take place when blood lactate concentrations rise above 4.0 mmol/L,40 so our value of 3.0 mmol/L provides further confirmation that aerobic exercise was occurring during the 6-MAT.
Based on the description of acceptable reliability values presented by Andresen41 and Fleiss42, HR and VO2 measured during the 6-MAT have excellent test-retest reliability. Visual inspection of the Bland-Altman plots reveals fairly equal distribution of test-retest differences above and below 0, suggesting minimal bias with repeated testing. For both HR and VO2, only 1 of the 20 subjects in the reliability sample fell outside 2 SD of the mean difference, indicating limited test-retest variation for both variables.31 Both HR and VO2 have well established relationships with PO, so it is not surprising that reliability was high.
The 6-MAT was designed as a submaximal cardiovascular fitness test, and thus was validated against the gold standard of cardiovascular fitness testing, the VO2peak test. The correlation between VO2peak and 6-MAT VO2 was excellent (r=0.92), indicating that those subjects with a high submaximal VO2, had a high VO2peak. Submaximal PO and VO2peak had a lower correlation (r=0.73). It is not surprising that this correlation was weaker, as up to 9 subjects cycled at the same PO for the 6-MAT, and all did not have the same absolute VO2peak. It is not possible to require the same PO from each participant because of their varying abilities. The correlation between submaximal HR and VO2peak was also lower (r=0.63). The 6-MAT was designed to have subjects cycle while maintaining a constant PO at 60–70% of their age-predicted maximum HR or at an RPE level of 11–15. As this target HR value is based solely on age, 2 individuals of the same age but different cardiovascular fitness levels would both be exercising at a similar submaximal HR. The same submaximal HR would then be associated with potentially different VO2peak values. Thus the correlation between 6-MAT HR and VO2peak was expected to be significant, but not necessarily high. Similarly, the popular submaximal endurance assessment using the 6 Minute Walk Test has been shown to have moderate association with VO2peak in a number of populations.43, 44
The use of HR reserve, rather than % of age-predicted maximal HR or RPE to determine submaximal PO was considered, but to do this, subjects would have had to have a VO2max test first to determine their maximal HR. As the 6-MAT test is designed for clinical use where maximal testing may not be possible, the use of RPE and % of age-predicted maximal HR would be more clinically feasible.
Although there was considerable overlap in VO2peak values between subjects with tetraplegia and paraplegia, many of the subjects with tetraplegia had lower VO2peak values. This is likely due to a combination of factors including: decreased muscle innervation in the trunk and upper extremities of those with tetraplegia, the absence of the lower extremity skeletal muscle pump that aids in venous return to the heart45, and impairment to the sympathetic nervous system and thus a limited ability to increase HR and cardiac output especially in those with injuries of T5 and higher.46 Subjects with impaired sympathetic nervous system function (those with injuries of T5 and higher, n=21) had lower VO2peak values, but their test-retest reliability and correlations with VO2peak were similar to those with injuries below T5 (n=9). However, the proportion of subjects with injuries below T9 was small and a larger, more equally distributed sample would help to determine specific influences associated with injury level.
The 6-MAT is a practical test that can be administered to individuals of all fitness levels. Unlike the modified arm CAFT19, all subjects recruited for this study were able to complete the 6-MAT. Functionally, all subjects had a minimum manual muscle testing score of grade 4 for wrist extension. The equipment required to conduct the 6-MAT is minimal (arm ergometer, HR monitor, RPE scale), and all pieces are available in most rehab settings. For the 6-MAT to be used clinically, clinicians first need to determine the appropriate PO for an individual to be exercising at using the guidelines provided in Tables 5. Baseline outcome variables would be determined by recording the client’s exercising HR during the final 30 seconds of the 6-MAT, and taking their RPE at the end of the test. At a later time (i.e. after an intervention aimed at increasing cardiovascular fitness) the 6-MAT should be re-administered at the same PO that was used pre-intervention. A decrease in HR and/or RPE may indicate an increase in VO2peak, while an increase in HR and/or RPE may indicate a decrease in VO2peak.
Conclusion
In summary, the steady state HR and VO2 responses obtained during the 6-MAT are reliable, and the concurrent validity of the 6-MAT for assessing cardiovascular fitness in individuals with SCI is excellent. It would be useful to test the 6-MAT for responsiveness in the future to enhance its use as a clinical tool for the measurement of cardiovascular fitness in individuals with SCI.
Acknowledgments
We gratefully acknowledge the contributions of Chihya Hung, Amira Tawashy and Kelly Hiller for their help with subject recruitment and data collection. This study was supported by the Rick Hansen Man-in-Motion Foundation Research Fund, the Canadian Institute of Health Research (MSH-63617), the Michael Smith Foundation for Health Research, and the Natural Sciences and Engineering Research Council of Canada.
Footnotes
COSMED K4b2, COSMED USA, Inc., 2211 North Elston Avenue, Suite 305 Chicago, IL 60614.
Monark Rehab Trainer, Model 881E; Monark Exercise AB; 43282 Varberg, Sweden.
Polar Electro Inc, 370 Crossways Park Dr, Woodbury, NY 11797-2050.
Excalibur; Lode BV, Zernikepark 16, 9747 AN Groningen, The Netherlands.
SPSS Inc, 233 S Wacker Dr, 11th Floor, Chicago, IL 60606.
References
- 1.DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–19. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- 2.Krause JS, Devivo MJ, Jackson AB. Health status, community integration, and economic risk factors for mortality after spinal cord injury. Arch Phys Med Rehabil. 2004;85:1764–73. doi: 10.1016/j.apmr.2004.06.062. [DOI] [PubMed] [Google Scholar]
- 3.Frankel HL, et al. Long-term survival in spinal cord injury: a fifty year investigation. Spinal Cord. 1998;36:266–74. doi: 10.1038/sj.sc.3100638. [DOI] [PubMed] [Google Scholar]
- 4.Soden RJ, et al. Causes of death after spinal cord injury. Spinal Cord. 2000;38:604–10. doi: 10.1038/sj.sc.3101080. [DOI] [PubMed] [Google Scholar]
- 5.Yekutiel M, Brooks ME, Ohry A, Yarom J, Carel R. The prevalence of hypertension, ischaemic heart disease and diabetes in traumatic spinal cord injured patients and amputees. Paraplegia. 1989;27:58–62. doi: 10.1038/sc.1989.9. [DOI] [PubMed] [Google Scholar]
- 6.Bauman WA, et al. Coronary artery disease: metabolic risk factors and latent disease in individuals with paraplegia. Mt Sinai J Med. 1992;59:163–68. [PubMed] [Google Scholar]
- 7.Davis GM. Exercise capacity of individuals with paraplegia. Med Sci Sports Exerc. 1993;25:423–32. [PubMed] [Google Scholar]
- 8.Hopman MTE, Dueck C, Monroe M, Philips WT, Skinner JS. Limits to maximal performance in individuals with spinal cord injury. Int J Sports Med. 1998;19:98–103. doi: 10.1055/s-2007-971889. [DOI] [PubMed] [Google Scholar]
- 9.Franklin BA, Whaley MH, Howley ET, editors. American College of Sports Medicine’s guidelines for exercise testing and prescription. 6. Baltimore: Lippincott Williams and Wilkens; 2000. [Google Scholar]
- 10.Dearwater SR, Laporte RE, Robertson RJ, Brenes G, Adams LL, Becker D. Activity in the spinal-cord injured patient: an epidemiologic analysis of metabolic parameters. Med Sci Sports Exerc. 1986;18:541–4. [PubMed] [Google Scholar]
- 11.Janssen TW, van Oers CA, Rozendaal EP, Willemsen EM, Hollander AP, van der Woude LH. Changes in physical strain and physical capacity in men with spinal cord injuries. Med Sci Sports Exerc. 1996;28:551–9. doi: 10.1097/00005768-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Martel G, Noreau L, Jobin J. Physiological responses to maximal exercise on arm cranking and wheelchair ergometer with paraplegics. Paraplegia. 1991;29:447–56. doi: 10.1038/sc.1991.61. [DOI] [PubMed] [Google Scholar]
- 13.Walker R, Powers S, Stuart MK. Peak oxygen uptake in arm ergometry: effects of testing protocol. Brit J Sports Med. 1986;20:25–6. doi: 10.1136/bjsm.20.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noonan V, Dean E. Submaximal exercise testing: clinical application and interpretation. Phys Ther. 2000;80:782–807. [PubMed] [Google Scholar]
- 15.DiCarlo SE. Effect of arm ergometry training on wheelchair propulsion endurance of individuals with quadriplegia. Phys Ther. 1988;68:40–4. doi: 10.1093/ptj/68.1.40. [DOI] [PubMed] [Google Scholar]
- 16.Dwyer GB, Davis RW. The relationship between a twelve minute wheelchair push test and VO2peak in women wheelchair athletes. Sports Med, Training and Rehab. 1997;8:1–11. [Google Scholar]
- 17.Franklin BA, Swantek KI, Grais SL, Johnstone KS, Gordon S, Timmis GC. Field test estimation of maximal oxygen consumption in wheelchair users. Arch Phys Med Rehabil. 1990;71:574–8. [PubMed] [Google Scholar]
- 18.Kofsky PR, Davis GM, Shephard RJ, Jackson RW, Keene GCR. Field testing: assessment of physical fitness of disabled adults. Eur J Appl Physiol. 1983;51:109–20. doi: 10.1007/BF00952543. [DOI] [PubMed] [Google Scholar]
- 19.Longmuir PE, Shephard RJ. A simple upper body analogue of the Canadian Home Fitness Test for the assessment of mobility-impaired adults. Can J Rehab. 1993;7:133–41. [Google Scholar]
- 20.Longmuir PE, Shephard RJ. Reliability and validity of a modified Canadian Aerobic Fitness Test for individuals with mobility impairments. Adapted Physical Activity Quarterly. 1995;12:161–75. [Google Scholar]
- 21.Rhodes EC, McKenzie DC, Coutts KD, Rogers AR. A field test for the prediction of aerobic capacity in male paraplegics and quadraplegics. Can J Appl Spt Sci. 1981;6:182–6. [PubMed] [Google Scholar]
- 22.Hagberg JM, Mullen JP, Nagle FJ. Oxygen consumption during constant-load exercise. J Appl Physiol. 1978;45:381–4. doi: 10.1152/jappl.1978.45.3.381. [DOI] [PubMed] [Google Scholar]
- 23.Whipp BJ, Wasserman K. Oxygen uptake kinetics for various intensities of constant-load work. J Appl Physiol. 1972;33:351–6. doi: 10.1152/jappl.1972.33.3.351. [DOI] [PubMed] [Google Scholar]
- 24.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–8. [PubMed] [Google Scholar]
- 25.Jacobs JW, Bernhard MR, Delgado A, Strain JJ. Screening for organic mental syndromes in the medically ill. Ann Intern Med. 1977;86:40–6. doi: 10.7326/0003-4819-86-1-40. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman DM, Weinberger M, Strain JJ, Jacobs JW. Detection of cognitive deficits by a brief mental status examination: The cognitive capacity screening examinations, a reappraisal and a review. Gen Hosp Psychiatry. 1979;1:247–55. doi: 10.1016/0163-8343(79)90026-4. [DOI] [PubMed] [Google Scholar]
- 27.Marino RJ, et al. International standards for neurological classification of spinal cord injury. J Spinal Cord Med. 2003;26:S50–6. doi: 10.1080/10790268.2003.11754575. [DOI] [PubMed] [Google Scholar]
- 28.Washburn RA, Zhu W, McAuley E, Frogley M, Figoni SF. The physical activity scale for individuals with physical disabilities: development and evaluation. Arch Phys Med Rehabil. 2002;83:193–200. doi: 10.1053/apmr.2002.27467. [DOI] [PubMed] [Google Scholar]
- 29.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–9. doi: 10.1161/01.str.26.6.982. [DOI] [PubMed] [Google Scholar]
- 30.Lasko-McCarthey P, Davis JA. Protocol dependency of VO2max during arm cycle ergometry in males with quadriplegia. Med Sci Sports Exerc. 1991;23:1097–101. [PubMed] [Google Scholar]
- 31.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–10. [PubMed] [Google Scholar]
- 32.Portney LG, Watkins MP. Foundations of Clinical Research, Applications to Practice. 2. Upper Saddle River, NJ: Prentice Hall; 2000. [Google Scholar]
- 33.Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord injury epidemiology. J Neurotraum. 2004;21:1355–70. doi: 10.1089/neu.2004.21.1355. [DOI] [PubMed] [Google Scholar]
- 34.National Spinal Cord Injury Statistical Centre. 2005 Annual Statistical Report for the Model Spinal Cord Injury Care Systems. Burmingham, Alabama: 2005. [Google Scholar]
- 35.Pinnington HC, Wong P, Tay J, Green D, Dawson B. The level of accuracy and agreement in measures of FEO2, FECO2 and VE between the Cosmed K4b2 portable, respiratory gas analysis system and a metabolic cart. J Sci Med Sport. 2001;4:324–35. doi: 10.1016/s1440-2440(01)80041-4. [DOI] [PubMed] [Google Scholar]
- 36.Duffield R, Dawson B, Pinnington HC, Wong P. Accuracy and reliability of a Cosmed K4b2 portable gas analysis system. J Sci Med Sport. 2004;7:11–22. doi: 10.1016/s1440-2440(04)80039-2. [DOI] [PubMed] [Google Scholar]
- 37.McLaughlin JE, King GA, Howley ET, Bassett DR, Jr, Ainsworth BE. Validation of the COSMED K4 b2 portable metabolic system. Int J Sports Med. 2001;22:280–4. doi: 10.1055/s-2001-13816. [DOI] [PubMed] [Google Scholar]
- 38.Coutts KD, Rhodes EC, McKenzie DC. Maximal exercise responses of tetraplegics and paraplegics. J Appl Physiol: Respirat Environ Exercise Physiol. 1983;55:479–82. doi: 10.1152/jappl.1983.55.2.479. [DOI] [PubMed] [Google Scholar]
- 39.Janssen TWJ, Dallmeijer AJ, Veeger HEF, van der Woude LHV. Normative values and determinants of physical capacity in individuals with spinal cord injury. J Rehabil Res Devel. 2002;39:29–39. [PubMed] [Google Scholar]
- 40.Yoshida T, Chida M, Ichioka M, Suda Y. Blood lactate parameters related to aerobic capacity and endurance performance. Eur J Appl Physiol Occup Physiol. 1987;56:7–11. doi: 10.1007/BF00696368. [DOI] [PubMed] [Google Scholar]
- 41.Andresen EM. Criteria for assessing the tools of disability outcomes research. Arch Phys Med Rehabil. 2000;81:S15–20. doi: 10.1053/apmr.2000.20619. [DOI] [PubMed] [Google Scholar]
- 42.Fleiss JL. Statistical methods for rates and proportions. Wiley; New York: 1981. [Google Scholar]
- 43.Opasich C, Pinna GD, Mazza A, Febo O, Riccardi R, Riccardi PG, Capomolla S, Forni G, Cobelli F, Tavazzi L. Six-minute walking performance in patients with moderate-to-severe heart failure; is it a useful indicator in clinical practice? Eur Heart J. 2001;22:488–96. doi: 10.1053/euhj.2000.2310. [DOI] [PubMed] [Google Scholar]
- 44.Pang MY, Eng JJ, Dawson AS. Relationship between ambulatory capacity and cardiorespiratory fitness in chronic stroke: influence of stroke-specific impairments. Chest. 2005;127:495–501. doi: 10.1378/chest.127.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hopman MTE, Verheijen PHE, Binkhorst RA. Volume changes in the legs of paraplegic subjects during arm exercise. J Appl Physiol. 1993;75:2079–83. doi: 10.1152/jappl.1993.75.5.2079. [DOI] [PubMed] [Google Scholar]
- 46.Teasell RW, Arnold JM, Krassioukov A, Delaney GA. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehabil. 2000;81:506–16. doi: 10.1053/mr.2000.3848. [DOI] [PubMed] [Google Scholar]