Abstract
Background:
E2104 was designed to evaluate the safety of two different strategies incorporating bevacizumab into anthracycline-containing adjuvant therapy as a precursor to a definitive randomized phase III trial.
Patients and methods:
Patients were sequentially assigned to one of two treatment arms. In addition to dose-dense doxorubicin and cyclophosphamide followed by paclitaxel (Taxol) (ddAC→T), all patients received bevacizumab (10 mg/kg every 2 weeks × 26) initiated either concurrently with AC (Arm A: ddBAC→BT→B) or with paclitaxel (Arm B: ddAC→BT→B). The primary end point was incidence of clinically apparent cardiac dysfunction (CHF).
Results:
Patients enrolled were 226 in number (Arm A 104, Arm B 122). Grade 3 hypertension, thrombosis, proteinuria and hemorrhage were reported for 12, 2, 2 and <1% of patients, respectively. Two patients developed grade 3 or more cerebrovascular ischemia. Three patients in each arm developed CHF. There was no significant difference between arms in the proportion of patients with an absolute decrease in left ventricular ejection fraction of >15% or >10% to below the lower limit of normal post AC or post bevacizumab.
Conclusions:
Incorporation of bevacizumab into anthracycline-containing adjuvant therapy does not result in prohibitive cardiac toxicity. The definitive phase III trial (E5103) was activated with systematic and extensive cardiac monitoring to define the true impact of bevacizumab on cardiac function.
Keywords: angiogenesis, breast cancer, cardiomyopathy, clinical trial, vascular endothelial growth factor
introduction
Breast cancer remains a devastating disease affecting the lives of 194 000 women and resulting in nearly 40 600 deaths in the Unites States each year [1]. Improved early detection and advances in systemic therapy for early-stage disease have led to a small decline in overall breast cancer mortality since 1989 [2]. Further advances will require new therapeutic strategies that are firmly rooted in an understanding of breast cancer biology. Inhibition of the vascular endothelial growth factor (VEGF) provides one such potentially fruitful therapeutic target [3]. Bevacizumab, a monoclonal antibody that recognizes all isoforms of VEGF-A, improves response rate and progression-free survival though not overall survival when combined with initial chemotherapy in patients with metastatic breast cancer-lacking overexpression of the human epidermal growth factor 2 (HER2) [4–7].
Experience gained in patients with advanced disease is meaningful, but even large trials of patients with metastatic disease provide long-term safety data in only a limited number of patients. Feasibility studies in the adjuvant setting are a necessary prerequisite to identify toxic effects that might not be acceptable in an otherwise healthy patient population, thereby limiting exposure and avoiding premature closure of a large adjuvant trial [8–10]. In this case, small trials suggested a potential increase in cardiac toxicity when bevacizumab was combined with chemotherapy regimens that included anthracyclines. Wedam [11] treated 20 patients with inflammatory breast cancer with neoadjuvant docetaxel, doxorubicin, cyclophosphamide, and bevacizumab. Though no patient developed clinically apparent cardiac dysfunction (CHF), two patients had asymptomatic decreases in left ventricular ejection fraction (LVEF) to <40% [11]. Of the 17 sarcoma patients treated with bevacizumab and high-dose doxorubicin (75 mg/m2), 2 developed clinical CHF; an additional four patients had asymptomatic decreases in LVEF [12]. Of the 48 patients with refractory acute myelogenous leukemia, 5 developed either clinical CHF or a decline in LVEF to <40% [13]. In contrast, clinical CHF has not been reported in patients without prior or concurrent anthracycline exposure [14, 15]. E2104 was therefore designed to ensure that the addition of bevacizumab to anthracycline-based adjuvant therapy would not result in prohibitive cardiac toxicity.
patients and methods
patient eligibility
Patients must have had histologically confirmed adenocarcinoma of the breast with involvement of at least one axillary or internal mammary lymph node on routine histological examination with hematoxylin and eosin staining. Patients must have completed definitive breast surgery >28 days and ≤84 days from the start of protocol therapy. Patients with synchronous bilateral breast cancer were eligible if the higher TNM (tumor–node–metastasis) stage tumor met the eligibility criteria for the trial. All patients had to have adequate renal, hepatic, and hematologic function. LVEF above the institutional lower limit of normal was required.
Patients with HER2 positive disease, defined as 3+ by immunohistochemistry or gene amplification by fluorescence in situ hybridization, were excluded. Patients must not have received prior cytotoxic chemotherapy, hormonal therapy, or radiation for this breast cancer. Prior treatment with an anthracycline, anthracenedione, or taxane for any condition was not allowed. Prior use of tamoxifen or raloxifene for chemoprevention was allowed but must have been discontinued at study entry. Additionally, patients were excluded if they had major surgery within 4 weeks, nonhealing wound or fracture, infection requiring parenteral antibiotics, or clinically significant cardiovascular disease. Therapeutic anticoagulation, regular nonsteroidal anti-inflammatory medication, and aspirin (>325 mg/day) were prohibited, but prophylactic low-dose anticoagulants were permitted.
The study was coordinated by Eastern Cooperative Oncology Group (ECOG) with collaboration by the NCCTG (North Central Cancer Treatment Group) and CALGB (Cancer and Leukemia Group B). Local institutional review boards approved the protocol and patients provided written informed consent before screening.
treatment plan
All patients received dose-dense doxorubicin and cyclophosphamide followed by paclitaxel (ddAC→T) as in the CALGB9741 [16] trial in combination with bevacizumab (10 mg/kg every 2 weeks × 26) initiated either concurrently with AC (Arm A: ddBAC→BT→B) or paclitaxel (Arm B: ddAC→BT→B). Bevacizumab was administered after chemotherapy, initially over 90 min; subsequent infusions were reduced to 60 min, and then 30 min as tolerated. Radiation therapy (RT) was required for all patients treated with breast-conserving surgery; postmastectomy RT was administered at the discretion of the treating physician according to institutional guidelines. Hormonal therapy was recommended for all patients with tumors expressing estrogen and/or progesterone receptors. When indicated, RT and hormonal therapy were to commence within 6 weeks of the completion of chemotherapy and were administered concurrently with bevacizumab.
Chemotherapy dose modifications were mandated for hematologic and nonhematologic toxicity as in C9741 [16]. Bevacizumab therapy was interrupted for proteinuria ≥3500 mg/24 h. Antihypertensive therapy was at the investigator’s discretion. Bevacizumab was permanently discontinued for symptomatic hypertension, nephrotic syndrome, venous thrombosis requiring anticoagulation, arterial thrombosis, serious bleeding, bowel perforation, or wound dehiscence. Chemotherapy dose reduction did not impact bevacizumab dose. However, if a chemotherapy cycle was delayed, bevacizumab therapy was delayed to maintain concurrent administration. If chemotherapy was permanently discontinued due to toxicity, patients could complete therapy with bevacizumab alone.
Bevacizumab was held and cardiac evaluation repeated in 4 weeks in patients for an absolute decrease in LVEF ≥16% or a decrease of 10%–15% to a value less than lower limit of normal (LLN). Bevacizumab was continued but cardiac evaluation repeated in 4 weeks in patients with an LVEF decrease <10% to <LLN. Bevacizumab was permanently discontinued in all patients with symptomatic CHF and those with cardiac assessments requiring bevacizumab to be held at two consecutive or three intermittent time points.
safety assessments
Complete blood counts were assessed before each chemotherapy infusion. Serum chemistry and physical examination were required every other treatment cycle; urine protein : creatinine ratio was assessed approximately every 4 cycles. Adverse events were assessed during and for 3 years post treatment, unless patients initiated non-protocol therapy or had progression of disease. All toxic effects were graded according to the National Cancer Institute—Common Toxicity Criteria (NCI-CTCAE) version 3.0.
primary end point—definition and assessment of clinical CHF
The primary end point was clinical CHF defined as symptomatic decline in LVEF to <LLN or symptomatic diastolic dysfunction. Symptoms considered indicative of CHF included grade 2 or higher dyspnea, grade 1 dyspnea associated with an LVEF <40%, and edema. Auscultation of an S3 gallop, bibasilar rales, or documented cardiomegaly also constituted signs of clinical CHF.
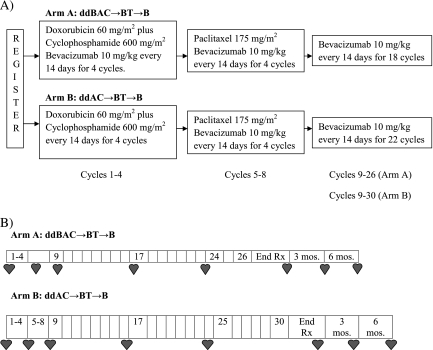
Cardiac assessment with either multiple-gated acquisition scan (MUGA) or echocardiography and a physician-directed cardiac symptoms assessment were carried out within 8 weeks before registration, then day 1 of cycles 5, 9, 17, and within 2 weeks of completing bevacizumab for both arms as well as day 1 of cycle 24 for Arm A and cycle 25 for Arm B. Once off protocol treatment, cardiac assessment continued every 3 months for two additional assessments (Figure 1).
Figure 1.
(A) Study schema and treatment regimen. (B) Timing of cardiac assessments. End of treatment (End Rx) cardiac evaluation carried out within 2 weeks of completion of treatment irrespective of the reason treatment was discontinued. Each cardiac evaluation included measurement of left ventricular ejection fraction and physician-directed cardiac symptom assessment.
statistical design and analysis
E2104 was a two-arm nonrandomized phase II trial; accrual to Arm B was to begin immediately upon completion of accrual to Arm A. The sequential design allowed for more rapid completion of Arm A, the treatment strategy proposed for the subsequent definitive phase III trial. Arm B was included to assess the safety of the alternate sequential approach in the event that cardiac toxicity in Arm A was prohibitive. A clinical CHF rate of 3.5% was considered acceptable and a clinical CHF rate of 10% was considered unacceptably high. The design required enrolling 101 patients on each arm. Allowing up to 5% of the patients to not start treatment or to not have adequate cardiac function at baseline, a total of 106 patients were to be entered per arm. Early stopping was planned if at any time, ≥4 cases of clinical CHF were observed in the first 42 assessable patients in either arm; accrual was not interrupted for this interim safety analysis. If >6 cases of clinical CHF were observed among 101 assessable patients in either arm, then the null hypothesis rate of 3.5% was to be rejected. Allowing for the early stopping rule, each arm had 9.8% chance of rejecting the null hypothesis when the true clinical CHF rate was 3.5% and a 90.3% chance of rejecting the null hypothesis when the true clinical CHF rate was 10%. The study was also to be stopped early for excessive rates of grade 3 or higher bleeding (≥1.5%) or grade 3 or higher thrombosis (≥5%) among the first 42 assessable patients in either arm.
Change in LVEF regardless of clinical symptoms was a secondary end point. The proportion of patients with an absolute decrease from baseline of >15% or >10% to below the LLN was calculated within each arm at all time points that LVEF was assessed. If the early stopping criteria was not met on both arms, that same proportion was compared between arms post AC and post bevacizumab using Fisher’s exact test to begin to explore if the addition of bevacizumab to AC increased those rates.
Consistent with the objectives of this study and in recognition of the limitations of the sample size, disease-free and overall survival data were not analyzed.
results
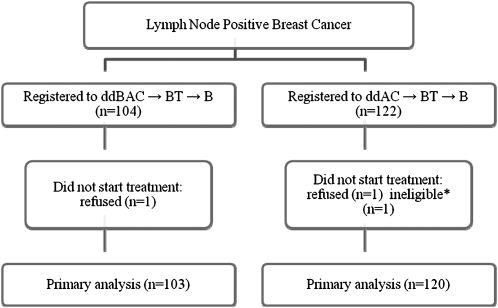
Between October 2005 and November 2006, 226 patients were enrolled. All treated patients (n = 223) were evaluated for toxicity (Figure 2); overall, three patients (1.3%) did not start treatment and were excluded from the toxicity analyses. Baseline demographic and tumor characteristics are summarized in Table 1. Just over 50% of patients completed treatment per protocol; 32% of patients in Arm A and 30% of patients in Arm B discontinued treatment due to adverse events. Other recurring reasons for discontinuation of protocol therapy included patient withdrawal (Arm A = 12%, Arm B = 9%), disease progression (Arm A = 0, Arm B = 2.5%), and unrelated illness (Arm A = 0, Arm B = 1.7%). The majority of early discontinuations, regardless of reason, occurred during maintenance bevacizumab.
Figure 2.
Consort diagram. All treated patients are included in the toxicity analyses. Consistent with the objectives of this study and in recognition of the small sample size, no efficacy analyses were carried out *Metastatic breast cancer.
Table 1.
Patient demographics and disease characteristics
| Arm A (N = 103) | Arm B (N = 120) | Total (N = 223) | |
| n (%) | n (%) | n (%) | |
| Median age (years) | 50 (33–76) | 50 (27–76) | 50 (27–76) |
| ECOG PS 0 | 96 (93) | 102 (85) | 198 (89) |
| Median tumor size (cm) | 2.4 (0.5–9.0) | 2.3 (0.2–12.3) | 2.3 (0.2–12.3) |
| Median number of involved lymph nodesa | 3 (0–23) | 4 (1–53) | 3 (0–53) |
| Surgery and radiation | |||
| BCSb | 1 (1) | 1 (1) | 2 (1) |
| BCS + RT | 33 (32) | 46 (38) | 79 (35) |
| Mastectomy | 22 (21) | 23 (19) | 45 (20) |
| Mastectomy + RT | 47 (46) | 50 (42) | 97 (43) |
| Histological gradec | |||
| I | 8 (8) | 12 (10) | 20 (9) |
| II | 45 (46) | 49 (42) | 94 (44) |
| III | 46 (46) | 56 (48) | 102 (47) |
| ER and PgR status | |||
| ER−/PgR− | 24 (23) | 33 (28) | 57 (26) |
| ER+ and/or PgR+ | 79 (77) | 87 (73) | 166 (74) |
Includes all treated patients. BCS, breast-conserving surgery; ECOG, Eastern Cooperative Oncology Group; ER, estrogen receptor; PgR, progesterone receptor; RT, radiation therapy.
One patient with node negative disease was classified as ineligible. She was treated per protocol and thus is included in the safety analyses.
Reasons for no RT include patient refusal and disease recurrence.
Grade was unknown in four and three patients on each arm respectively
Overall, three patients in each arm [Arm A-2.9%, 95% confidence interval (CI): 0.6% to 8.3%; Arm B-2.5%, 95% CI: 0.5% to 7.13%] developed clinical CHF (Table 2). Clinical CHF occurred throughout the planned treatment course and was not clearly related to RT; no obvious risk factors were identified. CHF appeared largely reversible with four of six patients reporting recovery of LVEF to within the normal range at a subsequent time point. Sequential assessment of cardiac function is summarized in Table 3. There was no significant difference between arms in the proportion of patients with an absolute decrease in LVEF of >15% or >10% to below the LLN post AC (7% versus 4%, P = 0.23; Fisher’s exact test) or post bevacizumab (15% versus 12%, P = 0.64; Fisher’s exact test) in Arms A and B, respectively.
Table 2.
Patients with clinical CHF
| Age | Baseline LVEF (%) | Time of CHF | LVEF at CHF (%) | Primary | RT | CHF before RT | Follow-up LVEF (%) | HTN | |
| Arm A | 76 | 55 | 6 monthsa | 25 | left | yes | no | NA | G2 at baseline |
| 40 | 59 | C5 | 40 | left | no | — | 57 at 9 months | G1 at C4 | |
| 57 | 54 | C24 | 30 | left | yes | no | 34 at End Rx | G1 at C9 | |
| Arm B | 49 | 57 | C5 | 45 | left | yes | yes | 57 at 9 | — |
| 42 | 60 | 9 monthsb | 40 | right | no | — | 60 at 18 months | G2 at C1, C7 and G1 at C5, C6, C16 | |
| 48 | 70 | C9 | 45 | left | yes | yes | 55 at 15 months | — |
CHF, cardiac dysfunction; End Rx, end of treatment; LVEF, Left ventricular ejection fraction; RT, radiation therapy; HTN, hypertension; NA, not available.
Post registration. Patient completed six cycles of therapy, CHF first documented ∼3 months after treatment was discontinued.
Post registration. Patient completed 17 cycles of therapy. CHF first documented ∼2 months after treatment was discontinued.
Table 3.
Sequential LVEF assessmentsa
| Arm A | Arm B | |
| Median baseline LVEF (%) | 65 (50–82) | 63 (53–78) |
| Cycle 5 | N = 94 | N = 113 |
| Median LVEF (%) | 63 (36–87) | 61 (45–80) |
| LVEF decrease >10%, n (%) | 13 (14) | 7 (6) |
| LVEF decrease >15% or >10% to <LLN, n (%) | 7 (7) | 4 (4) |
| Cycle 9 | N = 82 | N = 94 |
| Median LVEF | 62 (42–81) | 60.5 (46–77) |
| LVEF decrease >10%, n (%) | 10 (12) | 10 (11) |
| LVEF decrease >15% or >10% to <LLN, n (%) | 4 (5) | 3 (3) |
| Cycle 17 | N = 61 | N = 82 |
| Median LVEF (%) | 61 (35–79) | 59 (45–80) |
| LVEF decrease >10%, n (%) | 14 (23) | 13 (16) |
| LVEF decrease >15% or >10% to <LLN, n (%)b | 9 (15) | 4 (5) |
| Cycle 24/25 | N = 56 | N = 69 |
| Median LVEF (%) | 58.5 (30–78) | 60 (39–78) |
| LVEF decrease >10%, n (%) | 7 (13) | 12 (17) |
| LVEF decrease >15% or >10% to <LLN, n (%) | 2 (4) | 2 (3) |
| End of treatment | N = 72 | N = 86 |
| Median LVEF (%) | 60 (34–73) | 60 (41–80) |
| LVEF decrease >10%, n (%) | 14 (19) | 18 (21) |
| LVEF decrease >15% or >10% to <LLN, n (%) | 11 (15) | 10 (12) |
| Post treatment 1 | N = 53 | N = 56 |
| Median LVEF (%) | 60 (25–78) | 59.5 (38–73) |
| LVEF decrease >10%, n (%) | 6 (11) | 8 (14) |
| LVEF decrease >15% or >10% to <LLN, n (%) | 4 (8) | 4 (7) |
| Post treatment 2 | N = 26 | N = 34 |
| Median LVEF (%) | 66 (37–85) | 59.5 (45–76) |
| LVEF decrease >10%, n (%) | 4 (15) | 5 (15) |
| LVEF decrease >15% or >10% to <LLN, n (%) | 1(4) | 2 (6) |
CHF, cardiac dysfunction; LLN, lower limit of normal; LVEF, left ventricular ejection fraction.
LVEF listed for each time point was compared with baseline LVEF irrespective of LVEF at any prior time point. Reasons for missing LVEF assessments over time include treatment discontinuation, site error, patient noncompliance or refusal, and disease progression.
There is a significant difference between groups at this time point. Of the 13 patients with LVEF decrease >15% or >10% to <LLN at this time point, 1 developed clinical CHF and 12 reported a subsequent LVEF within the normal range.
Grade 3 hypertension was reported for 12% of patients and was generally managed with medical therapy; no patient experienced grade 4 hypertension (Table 4). Proteinuria was common but rarely clinically significant. Bevacizumab clearly increased minor mucosal bleeding, principally grade 1/2 epistaxis; grade 3 bleeding was reported by only one patient. Grade 4 hemorrhage was not reported. The overall incidence of thromboembolic events was infrequent; however, two patients experienced cerebrovascular ischemia and one patient developed cardiac ischemia. The 1.3% rate of arteriothrombotic events is similar to that reported with chemotherapy and bevacizumab regimens in the metastatic setting.
Table 4.
Toxicity
| Arm A (n = 103) | Arm B (n = 120) | |||||
| Grade | 1,2 (%) | 3 (%) | 4 (%) | 1,2 (%) | 3 (%) | 4 (%) |
| Anemia | 48 | 1 | 1 | 46 | 4 | 0 |
| Neutropenia | 15 | 13 | 17 | 8 | 7 | 20 |
| Febrile neutropenia | — | 4 | — | — | 5 | — |
| Thrombocytopenia | 25 | 2 | — | 26 | 2 | 1 |
| Nausea | 81 | 5 | — | 88 | — | — |
| Mucositis (oral) | 60 | 3 | — | 51 | 3 | — |
| Fatigue | 77 | 11 | — | 81 | 7 | — |
| Increased AST/ALT | 21/25 | -/2 | 1/- | 31/25 | 1/2 | 1/- |
| Sensory neuropathy | 65 | 7 | 1 | 70 | 8 | — |
| Arthralgia/joint pain | 63 | 5 | — | 70 | 10 | — |
| Myalgia/muscle pain | 55 | 7 | — | 69 | 5 | — |
| Dyspnea | 30 | 2 | 1 | 33 | 3 | — |
| Hypertension | 35 | 13 | — | 33 | 11 | — |
| Headache | 50 | 2 | — | 43 | 9 | — |
| Left ventricular diastolic dysfunction | — | 1 | — | — | 2 | — |
| Left ventricular systolic dysfunctiona | 18 | 4 | — | 17 | 3 | — |
| Proteinuria | 9 | 2 | — | 20 | 2 | — |
| Thrombosis/embolism | 2 | 1 | — | 1 | 2 | — |
| Hemorrhage (nose) | 39 | — | — | 35 | — | — |
| Hemorrhage (rectal) | 7 | 1 | — | 4 | — | — |
| Allergic reaction | 4 | 4 | — | 3 | — | — |
| CNS ischemia | — | — | 1 | — | 1 | — |
| Leukoencephalopathy | 1 | — | — | — | — | — |
Worst toxicity reported per patient based on CTCAE version 3.0. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CHF, cardiac dysfunction; CNS, central nervous system; LVEF, left ventricular ejection fraction.
Grade 3 left ventricular systolic dysfunction includes decreases in LVEF to 20%–40% and/or symptoms of CHF.
The expected noncardiac chemotherapy-related toxic effects were comparable to those reported in C9741 [16]. Myelosuppression was common but neutropenic fever or serious infectious complications were infrequently reported. Sensory neuropathy, myalgia, and arthralgia were reported by nearly three out of four patients but reached grade 3 severity in ≤10% of patients. There were no treatment-related deaths [17].
discussion
This pilot trial was conducted to ensure that the addition of bevacizumab to sequential anthracycline- and taxane-containing adjuvant therapy did not expose patients with potentially curable disease to unacceptable cardiac toxicity. Previous adjuvant trials, including C9741, have reported cardiac toxicity based on a modified version of the NCI–CTC criteria alone. However, as the true impact of an asymptomatic decline in LVEF remains unclear and there is variability in assessment of LVEF by both echocardiography [18] and MUGA [19, 20], we chose ‘clinical’ CHF as the most relevant safety end point. In practice, risk must always be viewed in the context of benefit. Designing clinical trials when benefit is necessarily unknown requires arbitrary decisions regarding the level of risk that would be acceptable. We based our assumptions on the prior experience with trastuzumab [21]. As such, we agreed a priori that a rate of clinical CHF of 3.5% would allow the definitive randomized trial to proceed. Using that definition, the results of E2104 support the conduct of the ongoing E5103 adjuvant trial, including the extensive cardiac monitoring requirements.
Heightened awareness of the potential for cardiac toxicity and serial monitoring may have increased reports of clinical CHF, a form of ascertainment bias. However, similar results have been reported in other pilot trials conducted independently [22]. McArthur et al. [23] treated 80 patients with ddAC followed by nanoparticle paclitaxel; bevacizumab was initiated concurrently with chemotherapy and continued for 1 year. One patient developed clinical CHF and six had asymptomatic declines in LVEF requiring treatment to be held [23]. Serial assessment of serum troponin did not predict either CHF or change in LVEF [24]. Yardley et al. [25] reported preliminary results of a pilot study adding bevacizumab to one of three different adjuvant chemotherapy regimens: (i) doxorubicin + cyclophosphamide followed by docetaxel (AC→D), (ii) docetaxel + doxorubicin + cyclophosphamide (TAC), or (iii) docetaxel + carboplatin + trastuzumab (TCH—HER2+ patients only). All chemotherapy regimens were administered every 3 weeks with granulocyte growth factor prophylaxis; bevacizumab was initiated with chemotherapy and continued for 1 year. Of patients who had completed four cycles of therapy, 1/53 in Arm A and 2/52 in Arm B developed CHF [25]. Mayer et al. [26] treated four sequential 40-patient cohorts with residual disease after standard neoadjuvant chemotherapy; 95% had received prior anthracycline therapy. All patients received with bevacizumab for 1 year as monotherapy, with metronomic chemotherapy for 6 months or with capecitabine for 18 weeks (two cohorts with differing capecitabine schedules). Two patients developed clinical CHF at 4 and 11 months; one patient with a history of mantle RT for lymphoma died of progressive CHF. At 6 months, 11 patients experienced a drop in LVEF of 10%–14%; LVEF declined ≥15% in two patients [26].
Our study has important limitations. E2104 was not designed to provide a discrete point estimate of the impact of bevacizumab on cardiac function. Rather, we sought to exclude an unacceptable risk and to inform the monitoring strategy for the planned definitive trial. Larger studies with a chemotherapy alone arm will further define the true impact of bevacizumab. In addition, we necessarily focused on acute events. Limited data are available on long-term cardiac safety of adjuvant anthracycline chemotherapy in breast cancer patients. However, recent analysis of the Surveillance, Epidemiology, and End Results Medicare database found an adjusted hazard ratio for CHF of 1.26 (95% CI = 1.12–1.42) for women aged 66–70 treated with anthracycline-based compared with non-anthracycline-based chemotherapy [27]. The difference in rates of CHF continued to increase through >10 years of follow-up. Though E5103 was recently amended to collect 2-year post-registration cardiac events, the true impact may not be known for decades. Finally, declining compliance with LVEF assessments throughout the study period (Table 3) may result in an underestimate of the rate of asymptomatic LVEF decline. However, with the attention to cardiac toxicity and extensive monitoring required in this trial, it seems less likely to have significantly impacted the detection of clinical CHF.
While the rate of CHF was not prohibitive, the overall rate of discontinuation before completion of planned therapy (nearly 50%) was somewhat surprisingly high. Most patients stopped due to an adverse event but no single adverse event predominated and toxicity rarely reached grade 3 severity. In comparison, ∼25% to 30% of patients in the adjuvant trastuzumab trials discontinued trastuzumab before completing planned therapy [28], while <20% of patients are non-adherent with anastrazole [29] within the first year of therapy. This may reflect a difference in willingness of patients and treating physicians to accept even mild toxic effects in a pilot trial setting. As many of the bevacizumab-specific toxic effects have a constant cumulative risk over time [30], further attrition should be expected in trials using a longer duration of therapy.
While it is tempting to post hoc conclude that the level of discontinuation means the regimen is ‘not feasible’, such a global feasibility end point was not defined prospectively. At the extreme, therapy that cannot be administered cannot be effective. However, calculation of the impact of early discontinuation on the ability of a subsequent trial to determine benefit requires knowledge of both the benefit and the impact of duration of therapy—factors that remain unknowable. Subsequent pilot trials should consider including detailed investigation of patient-reported outcomes to further define the reasons for treatment discontinuation. Such information could guide the design of subsequent trials, as well as the development of strategies to mitigate toxicity and enhance adherence.
funding
Supported in part by Public Health Service Grants (CA23318, CA66636, CA21115, CA49883, CA25224, and CA77651) from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services.
disclosure
KDM and ADS are consultants to and receive research funding from Genentech/Roche. EAP receives research funding from Genentech/Roche. The remaining authors have no declared conflicts of interest. E2104 was conducted under a corporate research and development agreement between Genentech and the NCI. Genentech provided bevacizumab and partial funding but did not participate in study design or data collection. Analysis was conducted by ECOG. The lead author made the decision to publish and wrote the manuscript, which was reviewed by all authors and submitted to NCI and Genentech for comment. The authors vouch for the completeness and accuracy of the data.
Acknowledgments
This study was coordinated by the Eastern Cooperative Oncology Group (Robert L. Comis, MD). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute. E2104 was conducted under a corporate research and development agreement between Genentech and the NCI. Genentech provided bevacizumab and partial funding but did not participate in study design or data collection. Analysis was conducted by ECOG. The lead author made the decision to publish and wrote the manuscript, which was reviewed by all authors and submitted to NCI and Genentech for comment. The authors vouch for the completeness and accuracy of the data.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Parker S, Tong T, Bolden S, et al. Cancer statistics, 1997. CA Cancer J Clin. 1997;47:5–27. doi: 10.3322/canjclin.47.1.5. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 4.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 5.Miles DW, Chan A, Dirix LY, et al. Phase III study of bevacizumab plus docetaxel compared with placebo plus docetaxel for the first-line treatment of human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol. 2010;28:3239–3247. doi: 10.1200/JCO.2008.21.6457. [DOI] [PubMed] [Google Scholar]
- 6.Robert N, Dieras V, Glaspy J, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab (B) for first-line treatment of HER2-negative locally recurrent or metastatic breast cancer (MBC) J Clin Oncol. 2009;27 doi: 10.1200/JCO.2010.28.0982. 42s (Abstr 1005) [DOI] [PubMed] [Google Scholar]
- 7.O'Shaughnessy J, Miles D, Gray R, et al. A meta-analysis of overall survival data from three randomized trials of bevacizumab (BV) and first-line chemotherapy as treatment for patients with metastatic breast cancer (MBC) J Clin Oncol. 2010;28 115s (Abstr 1005) [Google Scholar]
- 8.Miller KD, Gradishar W, Schuchter L, et al. A randomized phase II pilot trial of adjuvant marimastat in patients with early-stage breast cancer. Ann Oncol. 2002;13:1220–1224. doi: 10.1093/annonc/mdf199. [DOI] [PubMed] [Google Scholar]
- 9.Miller KD, Saphner TJ, Waterhouse DM, et al. A randomized phase II feasibility trial of BMS-275291 in patients with early stage breast cancer. Clin Cancer Res. 2004;10:1971–1975. doi: 10.1158/1078-0432.ccr-03-0968. [DOI] [PubMed] [Google Scholar]
- 10.Bryant J, Smith R, Margolese R, et al. Increased gallbladder adverse events associated with octreotide pa LAR in patients with breast cancer. Proc Am Soc Clin Oncol. 2001;20 50a (Abstr 197) [Google Scholar]
- 11.Wedam SB, Low JA, Yang SX, et al. Antiangiogenic and antitumor effects of bevacizumab in patients with inflammatory and locally advanced breast cancer. J Clin Oncol. 2006;24:769–777. doi: 10.1200/JCO.2005.03.4645. [DOI] [PubMed] [Google Scholar]
- 12.D'Adamo DR, Anderson SE, Albritton K, et al. Phase II study of doxorubicin and bevacizumab for patients with metastatic soft-tissue sarcomas. J Clin Oncol. 2005;23:7135–7142. doi: 10.1200/JCO.2005.16.139. [DOI] [PubMed] [Google Scholar]
- 13.Karp JE, Gojo I, Pili R, et al. Targeting vascular endothelial growth factor for relapsed and refractory adult acute myelogenous leukemias: therapy with sequential 1-beta-d-arabinofuranosylcytosine, mitoxantrone, and bevacizumab. Clin Cancer Res. 2004;10:3577–3585. doi: 10.1158/1078-0432.CCR-03-0627. [DOI] [PubMed] [Google Scholar]
- 14.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 15.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 16.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 17.Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99:1232–1239. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 18.McGowan JH, Cleland JG. Reliability of reporting left ventricular systolic function by echocardiography: a systematic review of 3 methods. Am Heart J. 2003;146:388–397. doi: 10.1016/S0002-8703(03)00248-5. [DOI] [PubMed] [Google Scholar]
- 19.Massardo T, Jaimovich R, Lavados H, et al. Comparison of radionuclide ventriculography using SPECT and planar techniques in different cardiac conditions. Eur J Nucl Med Mol Imaging. 2007;34:1735–1746. doi: 10.1007/s00259-007-0472-8. [DOI] [PubMed] [Google Scholar]
- 20.Hiscock SC, Evans MJ, Morton RJ, et al. Investigation of normal ranges for left ventricular ejection fraction in cardiac gated blood pool imaging studies using different processing workstations. Nucl Med Commun. 2008;29:103–109. doi: 10.1097/MNM.0b013e3282f20e45. [DOI] [PubMed] [Google Scholar]
- 21.Perez EA, Suman VJ, Davidson NE, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26:1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yardley DA. Integrating bevacizumab into the treatment of patients with early-stage breast cancer: focus on cardiac safety. Clin Breast Cancer. 2010;10:119–129. doi: 10.3816/CBC.2010.n.016. [DOI] [PubMed] [Google Scholar]
- 23.McArthur H, Rugo H, Nulsen B, et al. Cardiac safety of adjuvant bevacizumab (B) plus dose-dense doxorubicin/cyclophosphamide (AC) followed by nanoparticle albumin-bound paclitaxel (nab-P) in patients with early stage breast cancer. Breast Cancer Res Treat. 2008 doi: 10.1158/1078-0432.CCR-10-1969. 288s (Abstr 4104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McArthur H, Rugo H, Nulsen B, et al. Troponins do not predict cardiotoxicity in a pilot study of adjuvant bevacizumab (B) plus dose-dense doxorubicin/cyclophosphamide (AC) followed by nanoparticle Albumin-Bound Paclitaxel (nab-P) Cancer Res. 2009;69(Supp 24) 675s. [Google Scholar]
- 25.Yardley D, Hart L, Waterhouse D, et al. Preliminary safety results: addition of bevacizumab to 3 docetaxel regimens as adjuvant therapy for early stage breast cancer, Cancer Res. 2009;69(Supp 2) doi: 10.1007/s10549-013-2764-y. 289s (Abstr 4107) [DOI] [PubMed] [Google Scholar]
- 26.Mayer E, Nohria A, Miller K, et al. Cardiovascular safety of adjuvant bevacizumab for breast cancer. J Clin Oncol. 2010;28(Suppl) 84s (Abstr 571) [Google Scholar]
- 27.Pinder MC, Duan Z, Goodwin JS, et al. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25:3808–3815. doi: 10.1200/JCO.2006.10.4976. [DOI] [PubMed] [Google Scholar]
- 28.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 29.Partridge AH, LaFountain A, Mayer E, et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556–562. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 30.Langmuir V, Cobleigh M, Herbst R, et al. Successful long-term therapy with bevacizumab (Avastin) in solid tumors. Proc Am Soc Clin Oncol. 2002;21:9a. [Google Scholar]