Abstract
Introduction
Clinical symptoms in lumbar degenerative spondylolisthesis (LDS) vary from predominantly radiating pain to severe mechanical low back pain. We examined whether the outcome of surgery for LDS varied depending on the predominant baseline symptom and the treatment administered [decompression with fusion (D&F) or decompression alone (D)].
Methods
213 consecutive patients (69 ± 9 years; 155f, 58 m) participated. Inclusion criteria were LDS, maximum three affected levels, no previous surgery at the affected level, and D (N = 56) or D&F (N = 157) as the operative procedure. Pre-op and at 12 months’ follow-up (FU), patients completed the multidimensional Core Outcome Measures Index (COMI) including 0–10 leg-pain (LP) and LBP scales. At 12 months’ FU, patients rated global outcome which was then dichotomised into “good” and “poor”.
Results
Pre-operatively, LBP and COMI scores were significantly worse (p < 0.05) in the D&F group than in the D group. The improvement in COMI at 12 months’ FU was significantly greater for D&F than for D (p < 0.001) and was not influenced by the patient’s declared “main problem” at baseline (back pain, leg pain, or neurological disturbances) (p > 0.05). There was a higher proportion (p = 0.01) of “good” outcomes at 12 months’ FU in D&F (86%) than in D (70%). Multiple regression analysis, controlling for possible confounders, revealed treatment group to be the only significant predictor of outcome (adding fusion = better outcome).
Discussion
Our study indicated that LDS patients showed better patient-based outcome with instrumented fusion and decompression than with decompression alone, regardless of baseline symptoms. This may be due to the fact that the underlying slippage as the cause of the stenosis is better addressed with fusion.
Keywords: Back pain, Leg pain, Decompression, Fusion, Degenerative spondylolisthesis
Introduction
Lumbar degenerative spondylolisthesis (LDS) is a well-known lumbar spinal pathology that presents a common problem in our daily spinal practice. It is characterized by displacement of one vertebral body over another due to disc degeneration and facet arthropathy, most commonly combined with various degrees of spinal canal stenosis and/or recessal stenosis at the affected level [18]. LDS occurs most frequently at the level of L4/5 and is more common in women and patients over 60 years of age [1, 6]. The resulting symptoms are usually a combination of stenotic-type radiating buttock and leg pain (LP) and mechanical low back pain (LBP). Conservative management is usually tried first, but if unsuccessful, surgery can be advocated and has demonstrated repeatedly good results in various studies [17, 26]. In earlier years decompression was the most common type of surgical procedure used [2], followed by decompression and uninstrumented fusion [7]. However, today LDS is recognized as an inherent instability of the lumbar spine and therefore the most commonly recommended procedure is a combination of decompression and instrumented lumbar fusion [3, 5, 11, 16]. Since this pathology occurs mainly in elderly patients, comorbidities are commonly present, with increasing perioperative risk in the case of fusion surgery. To avoid these risks, less invasive surgical therapies such as decompression alone have been advocated, especially in the face of predominantly stenotic or radiating pain symptoms [8, 23].
However, questions remain concerning the extent of surgery needed in any individual case and whether the analysis of predominant preoperative symptoms can help in choosing the most appropriate procedure. In clinical practice this decision is often based on the various presenting symptoms of LBP and LP and their relationship to one another, i.e., a patient with mainly symptoms of neural compression due to stenosis may benefit from simple decompression and may forego more extensive fusion surgery despite the underlying slippage that may indicate an inherent “instability”.
Previous studies identified preoperative low back pain as a negative predictor for outcome in lumbar spinal stenosis (LSS) [10] and lumbar disc herniation (LDH) [9] and indicated that predominant LP rather than LBP symptoms led to a more favourable clinical outcome after decompression surgery. However, whether the addition of fusion to the decompression would have eliminated the negative influence of LBP on outcome could not be addressed in those studies.
The present study sought to examine whether the outcome of surgery for lumbar degenerative spondylolisthesis varies depending on the predominant baseline symptom (back pain or leg pain) and the treatment administered (decompression with fusion or decompression alone).
Methods
Inclusion criteria
The study was carried out using the framework of the Spine Society of Europe (SSE) Spine Tango Spine Surgery Registry together with our own local spine surgery outcomes database. It included the prospectively collected data of consecutive patients that had undergone surgery by qualified, specialised spine surgeons in our own Spine Centre, part of an orthopaedic hospital, from March 2004 to May 2008. The patients had to have a good understanding of written German or English or (after 2006) French, Spanish, Italian or Portuguese, have reached 1-year follow-up, and satisfy the study’s surgical admission criteria. The latter made use of the options ticked in relation to the given fields on the Spine Tango surgery form and were as follows: first-time surgery at the level to be operated; maximum three levels to be operated in the lumbar or lumbosacral region of the spine; degenerative disease as the main pathology, with spondylolisthesis specified as the type of degenerative disease; decompression (D) or decompression with fusion (D&F) as the operative procedure.
The presence of LDS itself was diagnosed based on appropriate radiological imaging (i.e., the normal clinical work-up, as per everyday practice). The individual surgeon’s decision whether to perform D or D&F reflected his/her normal decision-making process used in daily clinical practice, and considered factors such as the patient’s leg pain, back pain, neurological symptoms and radiological findings, as well as their age, general health status, activity level, and willingness to undergo additional fusion.
Questionnaires
Before and 12 months after surgery, patients were requested to complete the multidimensional Core Outcome Measures Index (COMI) questionnaire [12, 13]. On each occasion, the questionnaires were sent to the patients to complete at home, to ensure that the information given was free of care-provider influence. The COMI is a multidimensional index consisting of validated questions covering the domains of pain (leg/buttock and back pain intensity, each measured separately on a 0–10 graphic rating scale), function, symptom-specific well-being, general quality of life, and social and work disability. Patients also indicated by means of a multiple-choice question what they considered their “main/greatest problem” to be: back pain, leg/buttock pain, or neurological disturbances. In addition to these questions answered both before and 12 months after surgery, at the 12-month follow-up there was a further question inquiring about the global outcome of surgery: “how much did the operation help your back problem?”, with five response categories: (1) helped a lot, (2) helped, (3) helped only little, (4) didn’t help, and (5) made things worse. The global outcome was dichotomised into “good” (1 and 2) and “poor” (3, 4, and 5) for the purposes of some of the subsequent analyses.
Comorbidity was assessed with the American Society of Anesthesiologists Physical Status Score (ASA Score), recorded within the Spine Tango Surgery documentation form.
Statistical analyses
Descriptive data are presented as means ± standard deviations (SD). The significance of any differences between treatment groups (D and D&F) in their baseline variables was analysed using unpaired Student’s t tests for continuous data and contingency analyses with Chi-squared and the Fisher’s exact P test for categorical variables.
2-way analysis of variance was used to compare the reduction in COMI score from preoperatively to 12 months’ follow-up in the two treatment groups (D vs. D&F) for each “main problem declared at baseline” group (back pain vs. leg pain vs. neurological disturbances).
Multivariable longitudinal regression analysis was used to predict the 12-month post-operative COMI score. The baseline COMI score, age, gender, comorbidity, and the number of affected levels were first entered (as control variables, since they are recognised potential confounders in analyses of such patients), followed by baseline low back pain intensity and leg pain intensity, and surgical treatment (D or D&F) as potential predictors, using forward conditional selection.
Multivariable logistic regression analysis was used to predict the 12-month outcome category (good or poor, based on the dichotomisation described above), using the same control variables as described above and the categories “main complaint” and surgical treatment as the independent variables of interest.
Statistical significance was accepted at the p < 0.05 level.
Results
In relation to the registry data collected within our Spine Centre, the average compliance rate for the surgeons’ completion of the Surgical Forms after the initial work-in phase was 85% (i.e., 85% percent of all spine surgeries carried out in the Spine Centre had an accompanying Spine Tango Surgery Form). Hence, potentially, up to 15% of eligible patients were not included in the present study (the exact number is unknown, because a completed Tango surgery form was a prerequisite for identifying patients who fulfilled the study’s surgical inclusion criteria).
Of all the patients in our local spine surgery database, 213 patients satisfied the study’s admission criteria. Their baseline data are shown in Table 1. The D&F group was significantly younger and had a significantly higher proportion of women than the D group; the groups did not differ significantly in their ASA comorbidity score. The decompression techniques used (indicated at the discretion of the treating surgeon) mainly included various combinations of laminotomy, flavectomy, lateral recess decompression with partial medial facet resection, and only very limited laminectomies. Fusion procedures included TLIF (50.0%), PLIF (22.8%), and posterolateral or posterior fusion (27.2%) (for the latter, a pedicle screw construct was used in 19%, translaminar screws in 6.3% and other fixation in 1.9%).
Table 1.
Baseline demographic, comorbidity, and self-reported clinical data, and surgical procedures used (means ± SD, or % values)
| Variable | Decompression (D) (N = 56) | Decompression and fusion (D&F) (N = 157) | p value |
|---|---|---|---|
| Age (years) | 73.0 ± 8.0 | 67.4 ± 9.4 | <0.0001 |
| Gender | 33 women (59%) | 122 women (78%) | |
| 23 men (41%) | 35 men (22%) | 0.007 | |
| Comorbidity, ASA score (%): | |||
| I | 6 (10.7%) | 18 (11.5%) | |
| II | 27 (48.2%) | 95 (60.5%) | 0.19 |
| III | 23 (41.1%) | 44 (28.0%) | |
| Preoperatively declared “main problem” (%)a: | |||
| Back pain | 12 (22.2%) | 51 (32.7%) | 0.07 |
| Leg pain | 33 (61.1%) | 67 (42.9%) | |
| Neurol. disturbances | 9 (16.7%) | 38 (24.4%) | |
| Low back pain intensitya (0–10 graphic rating scale) | 4.1 ± 3.0 | 5.3 ± 2.9 | 0.01 |
| Leg pain intensitya (0–10 graphic rating scale) | 6.5 ± 2.3 | 6.2 ± 2.7 | 0.51 |
| Intensity of worst pain, back/lega (0–10 graphic rating scale) | 6.7 ± 2.0 | 7.1 ± 2.0 | 0.33 |
| COMI summary scorea (0–10 scale) | 7.0 ± 2.1 | 7.6 ± 1.7 | 0.04 |
Bold values are statistically significant (p value <0.05)
aData from 54/56 patients in group D, and 156/157 patients in group D&F; in 3 patients, patient-rated baseline data were missing (see main text)
A patient-rated questionnaire was completed by 210/213 (98.6%) patients at baseline and 199/213 (93.9%) at 12 months’ follow-up.
Main complaint and baseline symptoms
Patients that had declared that back pain was their “main problem” (N = 63) had a mean baseline LBP score (0–10 scale) of 6.5 (SD 2.3) and LP of 5.0 (SD 3.0); those with leg pain as the declared main problem (N = 100) had a mean LBP score of 4.1 (SD 3.0) and a mean LP of 7.3 (SD 1.9), and those with neurological disturbances (N = 47), a mean LBP score of 5.0 (SD 2.8) and a mean LP of 5.7 (SD 2.5). The LBP intensity was significantly higher (p < 0.01) in those declaring back pain as the main problem than in those whose main problem was either leg pain or neurological disturbances; likewise, LP was significantly higher (p < 0.001) in the group with leg pain as the main problem than in the groups with either back pain or neurological disturbance as the main problem.
Baseline leg pain and back pain in relation to surgical procedure
Baseline values for LP and worst pain (either leg or back) did not differ significantly between the D and D&F groups; however, LBP and COMI scores were slightly but significantly higher (worse status) in the group undergoing D&F than in the D group (p = 0.04; Table 1).
The distribution of the responses regarding the “main problem” did not differ significantly (p = 0.07) between the D and D&F groups, though the D&F group tended to have a higher percentage of patients with LBP and lower percentage with LP as the main problem compared with the D group (Table 1).
Outcomes in relation to surgical procedure
At the 12-month follow-up, the distribution of patient-rated global outcomes differed significantly (p = 0.04) between the D&F and D groups, with better outcomes for D&F: D&F group, 90/145 (62.1%) operation helped back problem a lot, 35/145 (24.1%) helped, 14/145 (9.7%) helped only little, 5/145 (3.4%) did not help, 1/145 (0.7%) made things worse; D group, 23/54 (42.6%) operation helped back problem a lot, 15/54 (27.8%) helped, 10/54 (18.5%) helped only little, 6/54 (11.1%) did not help, 0/54 (0%) made things worse. Hence, in the D&F group, 125/145 (86.2%) patients had a “good” outcome, and 20/145 (13.8%) had a “poor” outcome; in the D group, the figures were 38/54 (70.4%) and 16/54 (29.6%), respectively (group difference, p = 0.01; Table 2).
Table 2.
Perioperative complications and 12-month outcome data in the two surgical treatment groups (D and D&F)
| Variable | Decompression (D) (N = 56) | Decompression and fusion (D&F) (N = 157) | p value |
|---|---|---|---|
| Surgical complications | 7/56 (12.5%; 95% CI, 3.8–21.2%) | 14/157 (8.9%; 95% CI, 4.5–11.8%) | 0.44* |
| General complications | 3/56 (5.4%; 95% CI, 0.0–11.3%) | 13/157 (8.3%; 95% CI, 4.0–12.6%) | 0.57 |
| Reduction in LBP from pre-op to 12 months’ follow-up | 1.7 ± 3.4 | 2.9 ± 2.9 | 0.01 |
| Reduction in LP from pre-op to 12 months’ follow-up | 3.1 ± 3.0 | 3.9 ± 3.4 | 0.13 |
| Reduction in worst pain (leg or back pain) from pre-op to 12 months’ follow-up | 2.5 ± 2.7 | 3.8 ± 2.8 | 0.002 |
| Reduction in COMI score (i.e. improvement) from pre-op to 12 months’ follow-up | 3.1 ± 2.9 | 4.2 ± 2.7 | 0.009 |
| % good outcome at 12 months’ follow-up | 70.4% (95% CI, 58–83%) | 86.2% (95% CI, 80–92%) | 0.01 |
* Fisher’s Exact P test
The reduction in leg pain did not differ significantly between the D and D&F groups; the reductions in back pain and “worst pain” (either back or leg) were, however, significantly greater for the D&F group than for the D group (Table 2).
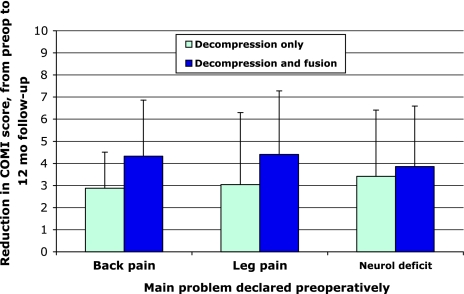
The reduction in COMI score (i.e., the degree of improvement) after 12 months was also significantly greater (p = 0.009) in the D&F group [4.2 (SD 2.7)] than in the D group [3.1 (SD 2.9)] (Table 2). The extent of the reduction did not depend on the main problem at baseline: results were consistently better (greater change in COMI score) for D&F than for D alone, regardless of whether back pain, leg pain or neurological disturbances had been reported as the “main problem” preoperatively (Fig. 1).
Fig. 1.
Reduction in COMI score from preoperatively to 12 months’ follow-up in groups of patients with back pain, leg/buttock pain, or neurological deficit as their declared “main problem” pre-operatively, in relation to the surgical procedure performed
Complications
In the D group, 7/56 patients (12.5%; 95% CI, 3.8–21.2%) had surgical complications [2 bleeding in spinal canal, 2 dura lesion, 1 wound infection, 2 “other” (continuing back pain, wound dehiscence)]; in the D&F group, 14/157 (8.9%; 95% CI, 4.5–11.8%) patients had complications [1 bleeding in spinal canal, 3 bleeding outside spinal canal, 6 dural tears, 2 wound infection, 2 other (necrotic wound)] (Table 2). The difference between the groups was not statistically significant (p = 0.44). The rate of general complications was also similar in the two groups (p = 0.57): in the D group, 3/56 patients (5.4%; 95% CI, 0.0–11.3%) had a general complication (1 cardiovascular, 1 pulmonary, 1 liver/GI) and in the D&F group, 13/157 patients (8.3%; 95% CI, 4.0–12.6%) (1 anaesthesiological, 3 cardiovascular, 1 pulmonary, 1 cerebral, 1 cardiovascular and cerebral, 4 kidney/urinary, 1 liver/GI, 1 other (allergy)) (Table 2).
Multivariable analysis of predictors of outcome
In multivariable regression analysis in which possible confounders were controlled for, surgical treatment group (D vs. D&F) was a significant unique predictor of both the 12-month COMI score (p = 0.03) and of global outcome (p = 0.03) (Tables 3, 4). The odds ratio of 2.6 indicated that the odds of a good outcome with D&F were 2.6 times greater than they were for D alone. The level of back pain or leg pain, and the category for the main problem declared at baseline, had no significant influence on outcome.
Table 3.
Results of the multiple regression analysis to quantify the relative roles of baseline low back pain (LBP) and leg pain (LP) in predicting the COMI score 12 months after surgery (higher score = worse outcome)
| Independent variables | Unstandardized regression coefficients | 95% CI for B | Standardized Coefficients | Sig (p value) | % Explained variance in COMI at 12 months | |
|---|---|---|---|---|---|---|
| B | CI low | CI high | Beta | adj R2 | ||
| (Constant) | −0.085 | −0.085 | 0.964 | |||
| Gender (0F,1 M) | −0.155 | −0.996 | 0.686 | −0.026 | 0.718 | |
| Age | −0.013 | −0.056 | 0.030 | −0.045 | 0.560 | |
| Comorbidity (ASA Score) | 0.780 | 0.163 | 1.397 | 0.187 | 0.014 | |
| No. affected levels | −0.052 | −0.777 | 0.673 | −0.010 | 0.889 | |
| COMI preop | 0.270 | 0.005 | 0.535 | 0.189 | 0.048 | |
| LBP pre-op | 0.075 | −0.064 | 0.214 | 0.082 | 0.291 | |
| LP pre-op | 0.054 | −0.126 | 0.234 | 0.051 | 0.560 | |
| Surgical treatment (0 = D, 1 = D&F) | −0.950 | −1.826 | −0.074 | −0.159 | 0.035 | 9.4% |
After accounting for potential confounders, the surgical treatment undertaken was a significant unique predictor of the 12-month COMI score, with D&F having a better outcome than D alone (p = 0.035). Greater comorbidity (p = 0.014) and higher preoperative COMI (p = 0.048) were also significant predictors of a worse outcome in the multivariable model
Table 4.
Results of the multiple logistic regression analysis to identify predictors of a “good” global outcome 12 months after surgery (good = 1, poor = 0)
| Independent variables | Unstandardized regression coefficients | Signif. | Odds ratio | 95% CI for odds ratio | ||
|---|---|---|---|---|---|---|
| B | SE | p value | (Exp (B)) | Lower | Upper | |
| Gender (0F,1 M) | 0.398 | 0.454 | 0.380 | 1.489 | 0.612 | 3.623 |
| Age | −0.007 | 0.024 | 0.762 | 0.993 | 0.948 | 1.040 |
| Comorbidity (ASA Score) | −0.199 | 0.334 | 0.552 | 0.819 | 0.426 | 1.578 |
| No. affected levels | 0.008 | 0.390 | 0.983 | 1.008 | 0.469 | 2.167 |
| Main problem LP (vs. LBP) | −0.556 | 0.465 | 0.231 | 0.573 | 0.231 | 1.426 |
| Main problem neurol deficit (vs. LBP) | −0.138 | 0.593 | 0.816 | 0.871 | 0.272 | 2.787 |
| Surgical treatment (0 = D, 1 = D&F) | 0.947 | 0.430 | 0.028 | 2.578 | 1.110 | 5.988 |
After accounting for potential confounders, the surgical treatment undertaken was the only significant predictor of a good outcome (p = 0.021)
Discussion
In the treatment of LDS, surgical management seems to be superior to conservative management [25, 26], and the combination of decompression and instrumented fusion seems to be associated with better results than decompression alone [3, 14]. However, many patients also benefit from decompression alone and there are no clear indications as to what kind of surgery is best in any given patient [22].
In the present study we anticipated that, in the absence of major low back symptoms (and in the presence of mainly radiating pain), decompression alone would result in at least as good an outcome as decompression combined with fusion. However, instead, we found that patients with LDS had a better outcome after decompression combined with fusion than after decompression alone, regardless of their predominant symptom at baseline. In other words, adding fusion to decompression resulted in systematically better results all round.
A possible explanation for this finding is that performing simple decompression without fusion does not sufficiently help even the patient with predominant stenotic symptoms, since the underlying cause of the stenosis, i.e. the inherent instability is still not treated. Another reason may be that, due to the slippage, more stenosis is present that would require more extensive resection, which is not always possible without creating more instability, and this therefore cannot be overcome by simple decompression. In this sense, LDS differs from simple spinal stenosis, in that the slippage is the primary cause of the stenosis and not simple degenerative changes leading to canal compromise. Fox et al. looked at a series of patients with decompression for spinal stenosis with and without fusion and reported a progression in spondylolisthesis after decompression alone in 73% of patients with LDS compared with 31% without, again demonstrating the ongoing process of instability that might be enhanced by decompression alone [4].
The findings of the present study concur with those reported by Martin et al. in systematically reviewing the literature comparing different types of surgical treatment for LDS [14]. They looked at studies that had compared D versus F, and instrumented F versus non-instrumented F in LDS, citing only relevant RCTs and comparative observational studies. He identified two RCTs and six comparative observational studies that had examined the outcome of D&F versus D alone in LDS. Inclusion criteria were, among others, no previous surgery, minimum 1-year follow-up, and minimum of five patients per treatment group for comparison. Outcome was categorized into clinical outcome, reoperation rate and fusion rate. However, only few of the studies included in the review had actually used patient-based outcome measures in their evaluation. Further, and in contrast to the present study, none of the observational studies declared using a consecutive series of all eligible patients. Three papers from Japan used JAO scores [15, 21, 27] and one used ODI and SF 36 [5]. To pool the data, an attempt was made to dichotomise each clinical outcome into “satisfactory” or “not satisfactory”, since the various outcome instruments were not comparable. All studies had their limitations and did not identify or quantify preoperative symptoms, and only one study gave detailed criteria for their selection of treatment [21]. Nonetheless, the review concluded that spinal fusion appeared to lead to a better clinical outcome than decompression alone, although no conclusion could be made about the clinical benefit of instrumenting a spinal fusion.
Interestingly, despite the assumption that the less extensive procedure of decompression alone is safer than decompression and fusion, in the present study, if anything, there were slightly (though not significantly) more intra/perioperative surgical complications (i.e., directly related to the surgery itself, such as haematoma, dural tear, etc.) in the D group (12.5%) than in the D&F group (8.9%), although the confidence intervals were rather wide and the study was not powered (or intended) to draw definitive conclusions in relation to this issue. Possible reasons for the slightly higher rate of intra/perioperative complications in the D group might include the greater age and slightly greater comorbidity of the patients, and the fact that decompression surgery without fusion is carried out with less resection of the bony elements and hence in a more limited space than in D&F, where (because fusion is being added anyway) the exposure is usually more generous and resection can be performed more liberally without the risk of causing increased instability. Adding screws per se to the otherwise very similar surgery (decompression) did not seem to increase surgical risk in the present study; this may be because most of the surgeons involved were also very experienced in deformity surgery and hence less likely to incur complications during screw placement. The additional fusion may turn out to be associated with a greater rate of late surgical complications (e.g., non-union, implant failure, adjacent segment degeneration), but this was not the subject of the present study. Neither the surgical nor general complication rates were in any way statistically associated with the overall global outcome (p > 0.75; details not shown), and hence any slight group differences were unlikely to constitute a confounding factor in the outcome analyses. The surgical complication rate reported here overall (for both groups together) is not dissimilar to that of 8.5% recently reported for a large series of patients undergoing various types of surgery for degenerative spondylolisthesis [20].
Although the group mean results were superior for D&F than for D alone there were clearly a number of patients that did have a good outcome with D alone. To the authors’ knowledge, no studies have been able to identify factors that are predictive of success with D alone. We had anticipated that baseline symptoms would give an indication as to the extent of surgery required, but our working hypothesis could not be confirmed. Further, with the exception of comorbidity status none of the variables entered into the multiple regression analyses were able to predict the outcome of surgery beyond the actual surgical procedure used. Few studies have examined predictors of outcome in LDS. Pearson et al. looked at radiographic predictors such as Meyerding grade, disc height, and a-p translation in functional radiographs in patients with LDS, treated either conservatively or surgically. However, none of these were significantly predictive of outcome [17]. Rousseau specifically looked at outcome predictors in LDS, but only in a limited number of patients (24, of whom only 18 completed the evaluation) treated with fusion and decompression, and identified high scores of preoperative leg pain and the use of interbody fusion during surgery as favourable for outcome [19]. It would appear that much work remains to be done to identify predictors of outcome of surgery for LDS, and, in particular, to identify those patients who would benefit from decompression alone.
A number of shortcomings of the present study are worthy of mention. There is some uncertainty regarding the definition of buttock pain and the “location” to which it is best classified as belonging—back or leg. In the present study it was classified together with leg pain; the reasons for this have been discussed extensively in a previous study [10]. Another problem concerns the reliability of the assessment of “the main problem” (back or leg pain), an issue that has been raised in the literature before [24]. We were nonetheless able to demonstrate a reasonable association between our “main complaint” categories and the associated mean leg pain and back pain scores (see “Results”, Main complaint and baseline symptoms), which at least provided some evidence for the validity of the “main complaint” question used. Another shortcoming is the fact that we did not randomize the patients to the respective treatment groups (D vs. D&F), but their group membership instead reflected our daily practice and clinical expertise in choosing the appropriate procedure. This can lead to bias and unknown confounding factors when comparing the groups. All patients were diagnosed with LDS and the main indication for surgery was failed conservative treatment and various presentations of LBP and buttock/leg pain. The decision to decompress only or to add an instrumented fusion lay with the surgeon and the patient and was based on a number of factors. In some instances age and comorbidities were the reason to obviate the anticipated additional risk of fusion surgery, and in other instances the patient refused the additional fusion. Not surprisingly, the decompression only group had a trend for a higher preoperative LP score and significantly lower back pain and COMI scores, was significantly older, and had slightly but not significantly more comorbidity (as given by the ASA score) than the D&F group. Nonetheless, in the statistical analysis, these factors were controlled for by means of multivariable approaches. Although the indications for decompression alone were not uniform, the assessment of the predominant symptoms and all the outcome measures was uniform, owing to our use of systematic data collection methods. Unfortunately, the total number of patients was not sufficient to perform valid subgroup analyses in relation to the different surgical procedures used. This should be done in a future study using data from the whole SSE Spine Tango registry.
In summary, given the evidence, it would appear advisable to perform a fusion together with decompression in patients with LDS. There will always be a group of patients where fusion may not be necessary and we still consider it a worthwhile goal to try to identify these. We attempted to identify a subset of patients in our decompression-only group that did benefit from this less extensive surgical procedure by carrying out a secondary analysis of this subgroup compared with the subgroup receiving D only that did poorly (detailed results not shown). However, there were no significant sub-group differences for any of the baseline factors examined, except for a slightly worse quality of life in those with a poor outcome; hence the most likely predictors—age, baseline symptoms, and comorbidity—do not appear to help in making decisions about the likely outcome with decompression only. The question still remains whether there is a “stable” subgroup of patients with LDS that are more comparable to simple spinal stenosis patients without spondylolisthesis and who may benefit from simple decompression alone. Further studies looking at radiological markers as predictors for stability versus instability in LDS may be helpful in identifying such a subgroup.
Conclusion
Whilst mindful of the limitations of observational studies, our results suggest that LDS patients show better patient-based outcomes with instrumented fusion and decompression than with decompression alone, regardless of baseline symptoms.
Acknowledgments
We are grateful to Gordana Balaban, Dave O’Riordan, Julian Amacker, Kirsten Clift, and Sara Preziosa, for their excellent work collecting the data
Conflict of interest None of the authors has any potential conflict of interest.
References
- 1.Cummins J, Lurie JD, Tosteson TD, Hanscom B, Abdu WA, Birkmeyer NJ, Herkowitz H, Weinstein J. Descriptive epidemiology and prior healthcare utilization of patients in the Spine Patient Outcomes Research Trial’s (SPORT) three observational cohorts: disc herniation, spinal stenosis, and degenerative spondylolisthesis. Spine (Phila Pa 1976) 2006;31:806–814. doi: 10.1097/01.brs.0000207473.09030.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Epstein NE, Epstein JA, Carras R, Lavine LS. Degenerative spondylolisthesis with an intact neural arch: a review of 60 cases with an analysis of clinical findings and the development of surgical management. Neurosurgery. 1983;13:555–561. doi: 10.1227/00006123-198311000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Fischgrund JS, Mackay M, Herkowitz HN, Brower R, Montgomery DM, Kurz LT. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine (Phila Pa 1976) 1997;22:2807–2812. doi: 10.1097/00007632-199712150-00003. [DOI] [PubMed] [Google Scholar]
- 4.Fox MW, Onofrio BM, Hanssen AD. Clinical outcomes and radiological instability following decompressive lumbar laminectomy for degenerative spinal stenosis: a comparison of patients undergoing concomitant arthrodesis versus decompression alone. J Neurosurg. 1996;85:793–802. doi: 10.3171/jns.1996.85.5.0793. [DOI] [PubMed] [Google Scholar]
- 5.Ghogawala Z, Benzel EC, Amin-Hanjani S, Barker FG, 2nd, Harrington JF, Magge SN, Strugar J, Coumans JV, Borges LF. Prospective outcomes evaluation after decompression with or without instrumented fusion for lumbar stenosis and degenerative Grade I spondylolisthesis. J Neurosurg Spine. 2004;1:267–272. doi: 10.3171/spi.2004.1.3.0267. [DOI] [PubMed] [Google Scholar]
- 6.Jacobsen S, Sonne-Holm S, Rovsing H, Monrad H, Gebuhr P. Degenerative lumbar spondylolisthesis: an epidemiological perspective: the Copenhagen Osteoarthritis Study. Spine (Phila Pa 1976) 2007;32:120–125. doi: 10.1097/01.brs.0000250979.12398.96. [DOI] [PubMed] [Google Scholar]
- 7.Katz JN, Lipson SJ, Lew RA, Grobler LJ, Weinstein JN, Brick GW, Fossel AH, Liang MH. Lumbar laminectomy alone or with instrumented or noninstrumented arthrodesis in degenerative lumbar spinal stenosis. Patient selection, costs, and surgical outcomes. Spine (Phila Pa 1976) 1997;22:1123–1131. doi: 10.1097/00007632-199705150-00012. [DOI] [PubMed] [Google Scholar]
- 8.Kelleher MO, Timlin M, Persaud O, Rampersaud YR. Success and failure of minimally invasive decompression for focal lumbar spinal stenosis in patients with and without deformity. Spine (Phila Pa 1976) 2010;35:E981–E987. doi: 10.1097/BRS.0b013e3181c46fb4. [DOI] [PubMed] [Google Scholar]
- 9.Kleinstuck FS, Fekete T, Jeszenszky D, Mannion AF, Grob D, Lattig F, Mutter U, Porchet F (2011)The outcome of decompression surgery for lumbar herniated disc is influenced by the level of concomitant pre-operative low back pain. Eur Spine J (in press) [DOI] [PMC free article] [PubMed]
- 10.Kleinstuck FS, Grob D, Lattig F, Bartanusz V, Porchet F, Jeszenszky D, O’Riordan D, Mannion AF. The influence of preoperative back pain on the outcome of lumbar decompression surgery. Spine (Phila Pa 1976) 2009;34:1198–1203. doi: 10.1097/BRS.0b013e31819fcf35. [DOI] [PubMed] [Google Scholar]
- 11.Kornblum MB, Fischgrund JS, Herkowitz HN, Abraham DA, Berkower DL, Ditkoff JS. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine (Phila Pa 1976) 2004;29:726–733. doi: 10.1097/01.BRS.0000119398.22620.92. [DOI] [PubMed] [Google Scholar]
- 12.Mannion AF, Elfering A, Staerkle R, Junge A, Grob D, Semmer NK, Jacobshagen N, Dvorak J, Boos N. Outcome assessment in low back pain: how low can you go? Eur Spine J. 2005;14:1014–1026. doi: 10.1007/s00586-005-0911-9. [DOI] [PubMed] [Google Scholar]
- 13.Mannion AF, Porchet F, Kleinstück F, Lattig F, Jeszenszky D, Bartanusz V, Dvorak J, Grob D. The quality of spine surgery from the patient’s perspective: part 1. The Core Outcome Measures Index (COMI) in clinical practice. Eur Spine J. 2009;18:367–373. doi: 10.1007/s00586-009-0942-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin CR, Gruszczynski AT, Braunsfurth HA, Fallatah SM, O’Neil J, Wai EK. The surgical management of degenerative lumbar spondylolisthesis: a systematic review. Spine (Phila Pa 1976) 2007;32:1791–1798. doi: 10.1097/BRS.0b013e3180bc219e. [DOI] [PubMed] [Google Scholar]
- 15.Matsudaira K, Yamazaki T, Seichi A, Takeshita K, Hoshi K, Kishimoto J, Nakamura K. Spinal stenosis in grade I degenerative lumbar spondylolisthesis: a comparative study of outcomes following laminoplasty and laminectomy with instrumented spinal fusion. J Orthop Sci. 2005;10:270–276. doi: 10.1007/s00776-005-0887-7. [DOI] [PubMed] [Google Scholar]
- 16.Okuda S, Oda T, Miyauchi A, Haku T, Yamamoto T, Iwasaki M. Surgical outcomes of posterior lumbar interbody fusion in elderly patients. Surgical technique. J Bone Joint Surg Am. 2007;89(Suppl 2 Pt.2):310–320. doi: 10.2106/JBJS.G.00307. [DOI] [PubMed] [Google Scholar]
- 17.Pearson AM, Lurie JD, Blood EA, Frymoyer JW, Braeutigam H, An H, Girardi FP, Weinstein JN. Spine patient outcomes research trial: radiographic predictors of clinical outcomes after operative or nonoperative treatment of degenerative spondylolisthesis. Spine (Phila Pa 1976) 2008;33:2759–2766. doi: 10.1097/BRS.0b013e31818e2d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Postacchini F, Perugia D. Degenerative lumbar spondylolisthesis. Part I: etiology, pathogenesis, pathomorphology, and clinical features. Ital J Orthop Traumatol. 1991;17:165–173. [PubMed] [Google Scholar]
- 19.Rousseau MA, Lazennec JY, Bass EC, Saillant G. Predictors of outcomes after posterior decompression and fusion in degenerative spondylolisthesis. Eur Spine J. 2005;14:55–60. doi: 10.1007/s00586-004-0703-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sansur CA, Reames DL, Smith JS, Hamilton DK, Berven SH, Broadstone PA, Choma TJ, Goytan MJ, Noordeen HH, Knapp DR, Hart RA, Zeller RD, Donaldson WF, Polly DW, Perra JH, Boachie-Adjei O, Shaffrey CI. Morbidity and mortality in the surgical treatment of 10, 242 adults with spondylolisthesis. J Neurosurg Spine. 2010;13:589–593. doi: 10.3171/2010.5.SPINE09529. [DOI] [PubMed] [Google Scholar]
- 21.Satomi K, Hirabayashi K, Toyama Y, Fujimura Y. A clinical study of degenerative spondylolisthesis. Radiographic analysis and choice of treatment. Spine (Phila Pa 1976) 1992;17:1329–1336. doi: 10.1097/00007632-199211000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Sengupta DK, Herkowitz HN. Degenerative spondylolisthesis: review of current trends and controversies. Spine (Phila Pa 1976) 2005;30:S71–S81. doi: 10.1097/01.brs.0000155579.88537.8e. [DOI] [PubMed] [Google Scholar]
- 23.Toyoda H, Nakamura H, Konishi S, Dohzono S, Kato M, Matsuda H (2011) Clinical outcome of microsurgical bilateral decompression via unilateral approach for lumbar canal stenosis: minimum five-year follow-up. Spine (Phila Pa 1976) 36(5):410–415 [DOI] [PubMed]
- 24.Wai EK, Howse K, Pollock JW, Dornan H, Vexler L, Dagenais S. The reliability of determining “leg dominant pain”. Spine J. 2009;9:447–453. doi: 10.1016/j.spinee.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Weinstein JN, Lurie JD, Tosteson TD, Hanscom B, Tosteson AN, Blood EA, Birkmeyer NJ, Hilibrand AS, Herkowitz H, Cammisa FP, Albert TJ, Emery SE, Lenke LG, Abdu WA, Longley M, Errico TJ, Hu SS. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356:2257–2270. doi: 10.1056/NEJMoa070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinstein JN, Lurie JD, Tosteson TD, Zhao W, Blood EA, Tosteson AN, Birkmeyer N, Herkowitz H, Longley M, Lenke L, Emery S, Hu SS. Surgical compared with nonoperative treatment for lumbar degenerative spondylolisthesis. four-year results in the Spine Patient Outcomes Research Trial (SPORT) randomized and observational cohorts. J Bone Joint Surg Am. 2009;91:1295–1304. doi: 10.2106/JBJS.H.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yone K, Sakou T, Kawauchi Y, Yamaguchi M, Yanase M. Indication of fusion for lumbar spinal stenosis in elderly patients and its significance. Spine (Phila Pa 1976) 1996;21:242–248. doi: 10.1097/00007632-199601150-00016. [DOI] [PubMed] [Google Scholar]