Abstract
Background
Transforaminal epidural injection of steroids is used to treat lumbar radicular pain. However, there are only a few well-designed randomized, controlled studies on the effectiveness of steroid injection.
Study design
Hence, this study aims to assess the effectiveness of steroid injection to treat lumbar radicular pain using a meta-analysis of transforaminal epidural injection therapy for low back and lumbar radicular pain. The comparison was based on the mean difference in the Visual Analogue Score (VAS) and Oswestry Disability Index (ODI) from baseline to the specified followed up.
Methods
The available literature of lumbar transforaminal epidural injections in managing low back and radicular pain was reviewed. Data sources included relevant literature of the English language identified through searches of PubMed and EMBASE from 1966 to 2009, and manual searches of the bibliographies of known primary and review articles. Finally, the search included the Current Controlled Trials Register and the Cochrane Database of Controlled Trials.
Results
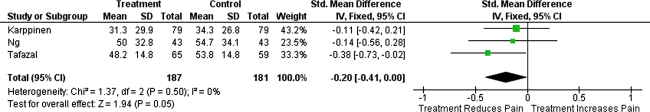
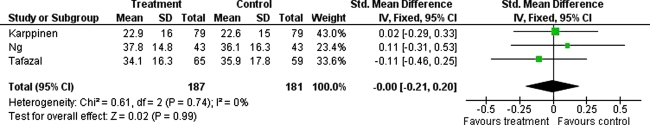
The initial search identified 126 papers. After screening, five randomised controlled trials (RCTs) were studied for analysis and only three of these had followed-up patients systematically with pain and disability outcome scores to 3 months and of these, only one had follow up to 12 months. A total of 187 patients (‘treatment group’ receiving local anaesthetic/steroid injection) were compared with 181 patients (‘control’ group, receiving local anaesthetic only or saline injection). Improvement in pain (standardised mean difference in VAS 0.2 in favour of ‘treatment’; 95%CI: −0.41 to 0.00, p = 0.05, I squared 0%) but not disability (standardised mean difference in ODI 0; 95%CI: −0.21 to 0.20, p = 0.99, I squared 0%) was observed between ‘treatment’ and ‘control’ groups; these differences were not significant. Additionally, the one study following patients to 12 months did not find any significant difference in VAS and ODI between treatment and control groups.
Conclusion
The current meta-analysis shows that transforaminal epidural steroid injections, when appropriately performed, should result in an improvement in pain, but not disability. The three RCTs that followed patients to 3 months (and the single study to 12 months) have found no benefit by the addition of steroids. The limitations of this study include the paucity of the available literature.
Keywords: Transforaminal epidural steroids, Radiculopathy, Sciatica, Steroids, Local anaesthetic
Introduction
Radicular nerve root pain is a common clinical problem of medical and economic significance [1]. The electrophysiological basis of sciatica has been characterised as ectopic firing of action potentials in nerve roots [2], most commonly caused by mechanical compression secondary to lumbar disc herniation or peripheral foraminal stenosis. These causes of nerve root compression may stimulate inflammatory processes [3, 4], forming a rationale for the use of epidural corticosteroids to inhibit inflammation and alleviate pain.
However, the efficacy of epidural corticosteroids for treatment of radicular pain remains controversial [5]. A major disadvantage of this technique is that the epidural infiltration of corticosteroids may not deliver an adequate concentration of corticosteroids to the locus of nerve root irritation [6] and may miss their target of delivery in up to 40% of cases [7]. The dorsal injection of epidural corticosteroids requires diffusion of active agents to the ventral target site, which may be prevented by the dorsal median epidural septum [8].
In contrast, the transforaminal (or periradicular) injection of corticosteroids under fluoroscopic guidance delivers high concentrations of therapeutic agents to the site of pathology [9]. But, the evidence for transforaminal injection of steroids is more contentious and there are only a few randomised controlled trials examining the efficacy of this treatment for alleviating radicular pain and disability. Thus, this meta-analysis was undertaken to review the available data for transforaminal injections into the lumbar spine with or without steroids.
Methods
An electronic search was performed using the Embase and PubMed databases from 1966 until 2009. The search terms ‘transforaminal’, “infiltration”, “periradicular”, “anesthetic” and MeSH headings “Glucocorticoids”[MeSH] and “Spinal Nerve Roots”[MeSH] were used in combination with the Boolean operators AND or OR. The reference lists of articles obtained were also searched to identify further relevant citations. Finally, the search included the Current Controlled Trials Register (http://www.controlled-trials.com) and the Cochrane Database of Controlled Trials.
Abstracts of the citations identified by the search were then scrutinised to determine eligibility for inclusion into the meta-analysis. Studies were included if they met each of the following criteria: randomised controlled trial, patients undergoing lumbar transforaminal or periradicular infiltration of glucocorticoids for radicular pain and randomisation to groups with or without radicular infiltration of steroids.
The primary outcome measures for the meta-analysis were the standardised mean difference in Visual Analogue Score (VAS) and Oswestry Disability Index (ODI) at specified time points following the injection. Data from the eligible trials were abstracted into a computerised spreadsheet for analysis. Between-study inconsistency was quantified by means of I squared = [(Q − df)/Q] × 100%, where Q is the chi-squared statistic and df is its degrees of freedom [10]. I squared defines the variability percentage in effect estimates that is due to heterogeneity rather than to sampling error or chance. A value >50% represents substantial heterogeneity. All analyses were performed with Revman analyses version 5.1.2 (2011, the Cochrane Collaboration). Forest plots were constructed to show standardised treatment effects.
Results
The initial search identified 126 papers. After screening, five randomised controlled trials were identified for analysis (Table 1, [11–15]). Of the five randomized trials evaluating lumbar transforaminal steroid injections, only three had followed-up patients systematically with VAS and ODI scores [12, 14, 15]. A total of 187 patients (‘treatment group’ receiving local anaesthetic/steroid transforaminal injection) were compared with 181 patients (‘control’ group, receiving local anaesthetic only [14, 15] or saline injection [12]). No trials reported significant between-group differences in baseline VAS or ODI before the transforaminal injections were administered.
Table 1.
Summary of randomised trials of lumbar transforaminal epidural injections
| Study | Type of study | Outcome | p value |
|---|---|---|---|
| Riew et al. [11] | RCT, controlled, double blind C (1 ml 0.25% Bupivacaine alone, n = 27) T (1 ml (6 mg) betamethasone, n = 28) |
Mean f/u 23 months (13–28) C: 9/27 avoided operation T: 20/28 avoided operation |
p < 0.004 (favouring treatment group in avoiding need for operation) |
| Karppinen et al. [12] | RCT, double blinded (n = 160) C (Saline injection, n = 80) T (Bupivacaine/methylprednisolone, n = 80) Follow up at 2 weeks (n = 79) 4 weeks (n = 80) 3 months (n = 79) 6 months, 1 year (n = 158) |
Baseline C (n = 80): VAS 75.2 ± 19; ODI 43.5 ± 15 T (n = 80): VAS 71 ± 18; ODI 42.9 ± 16 (2 weeks leg pain better in steroid group (not shown, p = 0.02), but not at later f/u) |
|
|
3 months C (n = 79): VAS 34.3; ODI 22.6 T (n = 79): VAS 31.3; ODI 22.9 |
NS NS |
||
|
6 months C (n = 80): VAS 21.6; ODI 15.8 T (n = 78): VAS 30.7; ODI 18.9 |
p = 0.003 (favouring control group) | ||
|
12 months C (n = 80): VAS 24.2; ODI 16.3 T (n = 78): VAS 23.9; ODI 15.9 |
NS NS No additional benefit with steroids |
||
| Vad et al. [13] | RCT C (N/saline trigger point injection, n = 23) T [steroid (1.5 ml) betamethasone acetate (9 mg) and 2% xylocaine, n = 25] |
Baseline C (n = 23): VAS 9.4 ± 1.3 T (n = 25): VAS 8.8 ± 1.2 |
|
|
Average f/u [16 months (12–21)] C: VAS 3.6 (±1.1) T: VAS 1.6 ± 0.8 |
p < 0.05 (favouring treatment group) | ||
| Ng et al. [14] | RCT, double blind C (bupivacaine, n = 43) T (bupivacaine/steroid, n = 43) |
Baseline C: VAS 76.9 (60–82.5); ODI 48.4 (36–58) T: VAS 73 (60–80); ODI 47.8 (36–56) |
|
|
6 weeks C: VAS 55.9 (±4); ODI 35.5 (±3) T: VAS 51 (±4.2); ODI 40 (±2.8) |
VAS p = 0.85 ODI p = 0.21 |
||
|
3 months C: VAS 54.7 (±5.2); ODI 36.1 ± 3.2) T: VAS 50 (±5); ODI 37.8 (±3.4) |
VAS p = 0.94 ODI p = 0.68 No additional benefit with steroids |
||
| Tafazal et al. [15] | Prospective RCT, double blind C (2 ml 0.25% bupivacaine alone, n = 76) T (bupivacaine and 40 mg methylprednisolone, n = 74) |
Baseline C: VAS 76.4 (70–90); ODI 46.6 (34–58) T: VAS 72.7 (60–80); ODI 43.4 (32–54) |
|
|
6 weeks (n = 141) C: VAS 57.8 (±3.4); ODI 38.1 (±2.1) T: VAS 46.6 (±3.3); ODI 34.6 (±2.1) |
VAS p = 0.12 ODI p = 0.93 |
||
|
3 months (n = 124) C (n = 59): VAS 53.8 ± 4.1; ODI 35.9 (±2.6) T (n = 65): VAS 48.2 (3.6); ODI 34.1 (2.3) |
VAS p = 0.74 ODI p = 0.69 |
||
| 1 year subsequent surgery/injection (n = 129) | No additional benefit with steroids |
C control, T treatment, RCT randomised control trial, VAS Visual Analogue Score, ODI Oswestry Disability Score
The Forest plots for these studies are shown in Fig. 1 (VAS scores at 3 months) and Fig. 2 (ODI scores at 3 months) which display the cumulative meta-analysis comparison. The standardised mean difference in VAS (=0.2 in favour of steroid injection; 95%CI: −0.41 to 0.00, p = 0.05, I squared 0%) and ODI (=0; 95%CI: −0.21 to 0.20, p = 0.99, I squared 0%) between ‘treatment’ and ‘control’ groups was not significant. Additionally, the one study following patients for 12 months, did not find any significant difference in VAS and ODI between these groups [12].
Fig. 1.
Forest plot showing the standardised mean difference in VAS between ‘treatment’ and ‘control’ groups at 3 months
Fig. 2.
Forest plot showing the standardised mean difference in ODI between ‘treatment’ and ‘control’ groups at 3 months
Study descriptions
Riew et al. [11] studied the effect of nerve root injections in avoiding operative treatment for lumbar radiculopathy. Fifty-five patients who were deemed surgical candidates were treated and randomized to receive either a transforaminal epidural injection of betamethasone with bupivacaine or bupivacaine alone. The mean follow up of patients was 23 months (range 13–28 months). Unfortunately, there was no set follow-up evaluation and at the end of the study, 9/27 (33%) patients receiving bupivacaine only had avoided an operation compared with 20/28 (71%) patients receiving the combination of betamethasone and bupivacaine. Although these results were significant, in their follow up study, they went on to show positive long-term results with or without the use of steroids [16].
Karppinen et al. [12], evaluated transforaminal epidural steroid injections in patients with sciatica. Eighty patients received transforaminal epidural injections of methylprednisolone and bupivacaine and another eighty patients received saline injections via a transforaminal route. Both groups showed improvement in VAS leg pain and ODI scores, with the steroid group doing better than the saline at 2 weeks and the saline group doing better at 3 and 6 months (rebound phenomenon observed with steroid/local anaesthetic group). At 1-year follow up, there was no statistical difference between the groups. Interestingly, the same authors performed a further cost analysis [17], and found that at the 4-week follow-up period the patients who had received steroid/local anaesthetic injection had utilized fewer therapy visits and less drugs resulting in significantly lower costs. At all other times there was no significant cost difference in the groups. To our knowledge, no other study has performed any kind of cost analysis.
Vad et al. [13] evaluated the effect of transforaminal epidural injection of betamethasone with lidocaine in 25 patients against 23 patients who received a lumbar paraspinal muscle trigger point injection of saline. Outcomes included pain score, patient satisfaction, and other measures of function. After an average follow up of 16 months (range 12–21 months), both groups improved but the transforaminal group did significantly better with a much lower pain score at the end and a larger percentage of patients (84 vs. 48%) achieving a successful outcome in a shorter period of time than the trigger point group (6 vs. 12 weeks).
Ng et al. [14] randomised patients to bupivaciane versus bupivacaine and methylprednisolone with 43 patients in each treatment arm. They found no significant difference in VAS or ODI scores at 6 weeks or 3 months. In a study from the same unit, Tafazal et al. [15] found similar results with no additional benefit of adding steroids to the injection. These same authors also found that the rate of surgery at 1 year was not significantly different between these groups, which is in contrast to Riew’s earlier study [11].
Discussion
The current meta-analysis shows that transforaminal epidural steroid injections, when appropriately performed, result in improvement in pain (standardised mean difference in VAS 0.2 in favour of steroid injection), but not disability (standardised mean difference in ODI 0). However, the three RCTs that followed patients to 3 months, have found no benefit by the addition of steroids [12, 14, 15] and this effect was maintained at 12 months [12].
The mechanism of action of steroid and local anaesthetic epidural injection is thought to be neural blockade which alters or interrupts nociceptive input, the reflex mechanism of the afferent fibres, self-sustaining activity of the neurons, and the pattern of central neuronal activities [18, 19]. Corticosteroids have also been shown to reduce inflammation by inhibiting either the synthesis or release of a number of pro-inflammatory mediators and by causing a reversible local anaesthetic effect [19, 20]. However, further evidence has shown that the long-lasting effect may be obtained with local anaesthetic with or without steroids [21–28]. For example, Tachihara et al. [20], in rat experiments of nerve root infiltration, showed that mechanical allodynia was prevented by local anaesthetic with or without steroid, and no additional benefit from using corticosteroid was found. Thus, it is suggested that corticosteroids may be unnecessary for nerve root blocks. This concept has been reinforced by numerous randomized and observational studies [24–38].
A recent systematic review has found that there was no significant difference between transforaminal and caudal epidural injection [39]. Transforaminal injection of steroids can be hazardous [40–42], and especially if carelessly performed. Guidelines for the safe conduct of transforaminal injections have been published by the International Spinal Intervention Society [43], emphasizing particularly the need to be vigilant for unintended, intra-arterial injection of particulate steroids. However, the risks are low and considering the less expensive nature of the procedure compared with surgical interventions, transforaminal epidural injections with or without steroids appear to be cost effective [17, 31]. Furthermore, transforaminal epidural injections are always performed under fluoroscopy with (out) contrast injection [44–48], and thus chances of inaccurate needle placement minimised.
The results of this meta-analysis have significant implications for clinical practice. Transforaminal epidural injections show a significant reduction of pain scores in patients with lumbar radiculopathy when compared with doing nothing, conservative management without injection therapy, and probably lumbar interlaminar epidural injections [49]. In this meta-analysis, no additional benefit has been found by the addition of steroids to local anaesthetic [12, 14, 15] and this effect was maintained at 12 months [12].
The limitations of this study include that we were able to find only five appropriately performed studies which met the inclusion criteria and were clinically relevant. Further, only three studies actually followed-up patients in a systematic way with pain and disability scores. Finally, it is worth bearing in mind that this meta-analysis was performed based on RCTs reporting their mean differences in VAS and ODI scores enabling this paper to compare the standardised treatment effects. But, when using these mean values of a group response, treatments emerge as significantly more effective only if all patients consistently benefit to some degree, or if a substantial majority of patients benefit to at least a moderate degree. Thus, group data can indeed camouflage good responses when they occur in a subgroup of patients as the poor outcomes of the other patients statistically cancel the good responses of the subgroup. It can be argued that such an analysis is not appropriate as the data are not normally distributed and outcome distribution may differ. The future implications for research should include a clear case definition with consistent inclusion and exclusion criteria, clear outcome measures, appropriate design, and reporting of randomized trials [50].
Acknowledgments
I would very much like to acknowledge and thank the help of Dr Graham Warren, Research Fellow in Medical Statistics, The NIHR Research Design Service for the East Midlands, Queen’s Medical Centre, Nottingham, UK.
Conflict of interest None.
References
- 1.Frymoyer JW. Back pain and sciatica. N Engl J Med. 1988;318:291–300. doi: 10.1056/NEJM198802043180506. [DOI] [PubMed] [Google Scholar]
- 2.Muramoto T, Atsuta Y, Iwahara T, et al. The action of prostaglandin E2 and triamcinolone acetonide on the firing activity of lumbar nerve roots. Int Orthop. 1997;21:172–175. doi: 10.1007/s002640050144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saal JS. The role of inflammation in lumbar pain. Spine. 1995;20:1821–1827. doi: 10.1097/00007632-199508150-00013. [DOI] [PubMed] [Google Scholar]
- 4.Olmarker K, Størkson R, Berge OG. Pathogenesis of sciatic pain: a study of spontaneous behaviour in rats exposed to experimental disc herniation. Spine. 2002;27:1312–1317. doi: 10.1097/00007632-200206150-00013. [DOI] [PubMed] [Google Scholar]
- 5.Koes BW, Scholten RJ, Mens JM, et al. Efficacy of epidural steroid injections for low-back pain and sciatica: a systematic review of randomized clinical trials. Pain. 1995;63:279–288. doi: 10.1016/0304-3959(95)00124-7. [DOI] [PubMed] [Google Scholar]
- 6.Bogduk N. Epidural steroids. Spine. 1995;20:845–848. doi: 10.1097/00007632-199504000-00021. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein SM, Herring SA, Derby R. Contemporary concepts in spine care: epidural steroid injections. Spine. 1995;20:1842–1846. doi: 10.1097/00007632-199508150-00018. [DOI] [PubMed] [Google Scholar]
- 8.O’Neill C, Derby R, Knederes L. Precision injection techniques for the diagnosis and treatment of lumbar disc disease. Semin Spine Surg. 1999;11:104–118. [Google Scholar]
- 9.Derby R, Kine G, Saal JA, et al. Response to steroid and duration of radicular pain as predictors of surgical outcome. Spine. 1992;17:S176–S183. doi: 10.1097/00007632-199206001-00020. [DOI] [PubMed] [Google Scholar]
- 10.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riew KD, Yin Y, Gilula L, Bridwell KH, Lenke LG, Lauryssen C, Goette K. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain. A prospective, randomized, controlled, double-blind study. J Bone Jt Surg Am. 2000;82-A(11):1589–1593. doi: 10.2106/00004623-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Karppinen J, Malmivaara A, Kurunlahti M, Kyllönen E, Pienimäki T, Nieminen P, Ohinmaa A, Tervonen O, Vanharanta H. Periradicular infiltration for sciatica: a randomized controlled trial. Spine. 2001;26(9):1059–1067. doi: 10.1097/00007632-200105010-00015. [DOI] [PubMed] [Google Scholar]
- 13.Vad VB, Bhat AL, Lutz GE, Cammisa F. Transforaminal epidural steroid injections in lumbosacral radiculopathy: a prospective randomized study. Spine. 2002;27(1):11–16. doi: 10.1097/00007632-200201010-00005. [DOI] [PubMed] [Google Scholar]
- 14.Ng L, Chaudhary N, Sell P. The efficacy of corticosteroids in periradicular infiltration for chronic radicular pain: a randomized, double-blind, controlled trial. Spine. 2005;30(8):857–862. doi: 10.1097/01.brs.0000158878.93445.a0. [DOI] [PubMed] [Google Scholar]
- 15.Tafazal S, Ng L, Chaudhary N, Sell P. Corticosteroids in periradicular infiltration for radicular pain: a randomised double blind controlled trial. One year results and subgroup analysis. Eur Spine J. 2009;8:1220–1225. doi: 10.1007/s00586-009-1000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riew KD, Park JB, Cho YS, Gilula L, Patel A, Lenke LG, Bridwell KH. Nerve root blocks in the treatment of lumbar radicular pain. A minimum five-year follow-up. J Bone Jt Surg Am. 2006;88:1722–1725. doi: 10.2106/JBJS.E.00278. [DOI] [PubMed] [Google Scholar]
- 17.Karppinen J, Ohinmaa A, Malmivaara A, Kurunlahti M, Kyllönen E, Pienimäki T, Nieminen P, Tervonen O, Vanharanta H. Cost effectiveness of periradicular infiltration for sciatica: subgroup analysis of a randomized controlled trial. Spine. 2001;26:2587–2595. doi: 10.1097/00007632-200112010-00013. [DOI] [PubMed] [Google Scholar]
- 18.Manchikanti L. Role of neuraxial steroids in interventional pain management. Pain Physician. 2002;5:182–199. [PubMed] [Google Scholar]
- 19.Dietrich CL, Smith CE. Epidural granuloma and intracranial hypotension resulting from cervical epidural steroid injection. Anesthesiology. 2004;100:445–447. doi: 10.1097/00000542-200402000-00039. [DOI] [PubMed] [Google Scholar]
- 20.Tachihara H, Sekiguchi M, Kikuchi S, Konno S. Do corticosteroids produce additional benefit in nerve root infiltration for lumbar disc herniation. Spine. 2008;33:743–747. doi: 10.1097/BRS.0b013e3181696132. [DOI] [PubMed] [Google Scholar]
- 21.Katz WA, Rothenberg R. The nature of pain: pathophysiology. J Clin Rheumatol. 2005;11(Suppl 2):S11–S15. doi: 10.1097/01.rhu.0000158686.43637.af. [DOI] [PubMed] [Google Scholar]
- 22.Mao J, Chen LL. Systemic lidocaine for neuropathic pain relief. Pain. 2000;87:7–17. doi: 10.1016/S0304-3959(00)00229-3. [DOI] [PubMed] [Google Scholar]
- 23.Arner S, Lindblom U, Meyerson BA, Molander C. Prolonged relief of neuralgia after regional anesthetic block. A call for further experimental and systematic clinical studies. Pain. 1990;43:287–297. doi: 10.1016/0304-3959(90)90026-A. [DOI] [PubMed] [Google Scholar]
- 24.Manchikanti L, Singh V, Rivera JJ, Pampati V, Beyer CD, Damron KS, Barnhill RC. Effectiveness of caudal epidural injections in discogram positive and negative chronic low back pain. Pain Physician. 2002;5:18–29. [PubMed] [Google Scholar]
- 25.Manchikanti L, Singh V, Falco FJ, Cash KA, Pampati V. Lumbar facet joint nerve blocks in managing chronic facet joint pain: one-year follow-up of a randomized, double-blind controlled trial: clinical trial NCT00355914. Pain Physician. 2008;11:121–132. [PubMed] [Google Scholar]
- 26.Manchikanti L, Singh V, Falco FJ, Cash KA, Fellows B. Cervical medial branch blocks for chronic cervical facet joint pain: a randomized double-blind, controlled trial with one-year follow-up. Spine. 2008;33:1813–1820. doi: 10.1097/BRS.0b013e31817b8f88. [DOI] [PubMed] [Google Scholar]
- 27.Manchikanti L, Singh V, Falco FJE, Cash KA, Pampati V. Effectiveness of thoracic medial branch blocks in managing chronic pain: a preliminary report of a randomized, double-blind controlled trial; clinical trial NCT00355706. Pain Physician. 2008;11:491–504. [PubMed] [Google Scholar]
- 28.Kawakami M, Weinstein JN, Chatani K, Spratt KF, Meller ST, Gebhart GF. Experimental lumbar radiculopathy. Behavioral and histologic changes in a model of radicular pain after spinal nerve root irritation with chromic gut ligatures in the rat. Spine. 1994;19:1795–1802. doi: 10.1097/00007632-199408150-00002. [DOI] [PubMed] [Google Scholar]
- 29.Manchikanti L. Transforaminal lumbar epidural steroid injections. Pain Physician. 2000;3:374–398. [PubMed] [Google Scholar]
- 30.Manchikanti L, Cash KA, McManus CD, Pampati V, Smith HS. Preliminary results of randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: Part 1. Discogenic pain without disc herniation or radiculitis. Pain Physician. 2008;11:785–800. [PubMed] [Google Scholar]
- 31.Huntoon MC, Burgher AH. Back to the future: the end of the steroid century. Pain Physician. 2008;11:713–716. [PubMed] [Google Scholar]
- 32.Manchikanti L, Singh V, Cash KA, Pampati V, Datta S. Preliminary results of randomized, equivalence trial of fluoroscopic caudal epidural injections in managing chronic low back pain: Part 3. Post surgery syndrome. Pain Physician. 2008;11:817–831. [PubMed] [Google Scholar]
- 33.Manchikanti L, Cash KA, McManus CD, Pampati V, Abdi S. Preliminary results of randomized, equivalence trial of flu oroscopic caudal epidural injections in managing chronic low back pain: Part 4. Spinal stenosis. Pain Physician. 2008;11:833–848. [PubMed] [Google Scholar]
- 34.Manchikanti L, Pampati V, Rivera JJ, Beyer CD, Damron KS, Barnhill RC. Caudal epidural injections with Sarapin steroids in chronic low back pain. Pain Physician. 2001;4:322–335. [PubMed] [Google Scholar]
- 35.Manchikanti KN, Pampati V, Damron KS, McManus CD. A double-blind, controlled evaluation of the value of Sarapin in neural blockade. Pain Physician. 2004;7:59–62. [PubMed] [Google Scholar]
- 36.Manchikanti L, Damron KS, Cash KA, Manchukonda R, Pampati V. Therapeutic medial branch blocks in managing chronic neck pain: a preliminary report of a randomized, double-blind, controlled trial: clinical trial CT0033272. Pain Physician. 2006;9:333–346. [PubMed] [Google Scholar]
- 37.Manchikanti L, Manchikanti KN, Manchukonda R, Pampati V, Cash KA. Evaluation of therapeutic thoracic medial branch block effectiveness in chronic thoracic pain: a prospective outcome study with minimum 1-year follow up. Pain Physician. 2006;9:97–105. [PubMed] [Google Scholar]
- 38.Manchikanti L, Manchikanti K, Manchukonda R, Cash KA, Damron KS, Pampati V, McManus CD. Evaluation of lumbar facet joint nerve blocks in the management of chronic low back pain: a preliminary report of a randomized, double-blind controlled trial: clinical trial NCT000355914. Pain Physician. 2007;10:425–440. [PubMed] [Google Scholar]
- 39.Conn A, Buenaventura R, Datta S, Abdi S, Diwan S. Systematic review of caudal epidurals injections in the management of chronic low back pain. Pain Physician. 2009;12:109–135. [PubMed] [Google Scholar]
- 40.Bogduk N. Complications associated with transforaminal injections. In: Neal JM, Rathmell JP, editors. Complications in regional anesthesia and pain medicine. PA: Saunders Elsevier; 2007. pp. 259–265. [Google Scholar]
- 41.Bogduk N, Dreyfuss P, Baker R, et al. Complications of spinal diagnostic and treatment procedures. Pain Med. 2008;6:S11–S34. doi: 10.1111/j.1526-4637.2008.00437.x. [DOI] [Google Scholar]
- 42.Kennedy DJ, Dreyfuss P, Aprill CN, Bogduk N. Paraplegia following image-guided transforaminal lumbar spine epidural steroid injection: two case reports. Pain Med. 2009;10:1389–1394. doi: 10.1111/j.1526-4637.2009.00728.x. [DOI] [PubMed] [Google Scholar]
- 43.Society International Spinal Intervention. Lumbar transforaminal injections. In: Bogduk N, editor. Practice guidelines for spinal diagnostic and treatment procedures. San Francisco, CA: International Spinal Intervention Society; 2004. pp. 163–187. [Google Scholar]
- 44.Fredman B, Nun MB, Zohar E, Iraqi G, Shapiro M, Gepstein R, Jedeikin R. Epidural steroids for treating “failed back surgery syndrome”: is fluoroscopy really necessary? Anesth Analg. 1999;88:367–372. [PubMed] [Google Scholar]
- 45.Mehta M, Salmon N. Extradural block. Confirmation of the injection site by X-ray monitoring. Anaesthesia. 1985;40:1009–1012. doi: 10.1111/j.1365-2044.1985.tb10558.x. [DOI] [PubMed] [Google Scholar]
- 46.Bartynski WS, Grahovac SZ, Rothfus WE. Incorrect needle position during lumbar epidural steroid administration: Inaccuracy of loss of air pressure resistance and requirement of fluoroscopy and epidurography during needle insertion. Am J Neuroradiol. 2005;26:502–505. [PMC free article] [PubMed] [Google Scholar]
- 47.Botwin KP, Natalicchio J, Hanna A. Fluoroscopic guided lumbar interlaminar epidural injections: a prospective evaluation of epidurography contrast patterns and anatomical review of the epidural space. Pain Physician. 2004;7:77–80. [PubMed] [Google Scholar]
- 48.Weil L, Frauwirth NH, Amirdelfan K, Grant D, Rosenberg JA. Fluoroscopic analysis of lumbar epidural contrast spread after lumbar interlaminar injection. Arch Phys Med Rehabil. 2008;89:413–416. doi: 10.1016/j.apmr.2007.08.161. [DOI] [PubMed] [Google Scholar]
- 49.Parr AT, Diwan S, Abdi S. Lumbar interlaminar epidural injections in managing chronic low back and lower extremity pain: a systematic review. Pain Physician. 2009;12:163–188. [PubMed] [Google Scholar]
- 50.Buenaventura RM, Datta S, Abdi S, Smith HS. Systematic review of therapeutic lumbar transforaminal epidural steroid injections. Pain Physician. 2009;12:233–251. [PubMed] [Google Scholar]