Abstract
Spinal cord injury (SCI) induces a glial response in which astrocytes become activated and produce inflammatory mediators. The molecular basis for regulation of glial-innate immune responses remains poorly understood. Here, we examined the activation of retinoic acid inducible gene (RIG)-like receptors (RLRs) and their involvement in regulating inflammation following SCI. We show that astrocytes express two intracellular RLRs: RIG-I and melanoma differentiation-associated gene 5 (MDA5). SCI and stretch injury of cultured astrocytes stimulated RLR signaling as determined by phosphorylation of IRF3 leading to production of type I interferons (IFNs). RLR signaling stimulation with synthetic RNA resulted in RLR activation, phosphorylation of interferon regulatory factor 3 (IRF3), and increased expression of glial fibrillary acidic protein and vimentin, two hallmarks of reactive astrocytes. Moreover, mitochondrial E3 ubiquitin protein ligase 1 (MUL1), an RLR inhibitor, decreased production of glial fibrillary acidic protein (GFAP) and vimentin following RIG-I signaling stimulation. Our findings identify a role for RLR signaling and type I IFN in regulating astrocyte innate immune responses after SCI.
Keywords: Innate immunity, Neuroinflammation, Spinal Cord Injury, Astrocytes
INTRODUCTION
Spinal cord injury (SCI) triggers an inflammatory innate immune response (neuroinflammation) that is a key component of secondary injury pathomechanisms. Traditionally, inflammation in the central nervous system (CNS) is characterized by astrocyte/microglial activation and enhanced proinflammatory cytokine production, including type I interferons (IFNs). Previous findings from our laboratory and others have described a role of pattern recognition receptors such as Nucleotide Oligomerization Domain (NOD)-like receptors in the inflammasome (de Rivero Vaccari et al. 2008) and toll-like receptors (Kigerl et al. 2007) in regulating the inflammatory response after SCI. Type I IFN signaling has normally been associated with antiviral immune responses(Seo and Hahm 2010), but recent evidence suggests an involvement in the regulation of CNS inflammation following sterile injury (Khorooshi and Owens 2010). However, the precise signaling pathways and immune receptors and sensors regulating type I IFN after CNS injury remain poorly defined.
In the CNS, type I IFNs (IFN-α and IFN-β) are produced by astrocytes (Tedeschi et al. 1986) and are regulated by innate immune receptors termed retinoic acid-inducible gene-like receptors (RLRs) (Wilkins and Gale 2010). The RIG-like receptor family (RLR) is comprised of retinoic acid-inducible gene-1 (RIG-1), melanoma differentiation-associated gene 5 (MDA5) and laboratory of genetics and physiology 2 (LGP2) (Takeuchi and Akira 2009; Yoneyama and Fujita 2008). RLRs are localized in the cytoplasm and recognize genomic RNA of double stranded (ds) RNA viruses and dsRNA generated as replication intermediates of single stranded (ss) RNA viruses. The expression of RLRs is greatly enhanced in response to type I IFN stimulation or virus infection. RIG-I recognizes relatively short dsRNA (low molecular weight (LMW), up to 1 kb) whereas MDA5 detects long dsRNA (High Molecular Weight (HMW), more than 2 kb). The role of LGP2 in the innate immune response is not clearly understood, but it may function as a negative regulator of RIG-1 and MDA5 responses (Wilkins and Gale 2010). RLRs activate signaling cascades through interaction with the mitochondrial associated protein Virus Induced Signaling Adaptor (VISA) also known as mitochondrial antiviral signaling protein (MAVS), IFN-β-promoter stimulator 1 (IPS-1) or CARD adaptor inducing IFN-β (CARDIF) (Wilkins and Gale 2010). VISA then activates signaling cascades via phosphorylation of IRF3/7 leading to increased expression of IFN-stimulated genes (ISG), type I IFN and IL-1β. The role of type I IFN in SCI has not been reported. The aim of this study was to examine the involvement of RLR signaling in a clinically relevant model of cervical SCI and in astrocytes subjected to stretch injury or treatment with a synthetic RNA analog, Polyinosinic:polycytidylic acid repeats (poly(I:C)). We show that SCI induced expression of RLR signaling proteins, type I IFN production and Interferon Regulatory Factor 3 (IRF3) expression acutely after injury. Immunohistochemical staining and confocal microscopy localized RLR signaling intermediates to glial fibrillary acidic protein (GFAP) positive astrocytes in lesion-reactive spinal cords. Increased expression of RLR signaling components and type I IFNs were also induced in stretch injured astrocytes and astrocytes treated with short or long synthetic Ribonucleic Acid (RNA). These findings identify a critical role of astrocytes in RLR and type I IFN signaling governing reactive gliosis and the regulation of innate immune responses after SCI.
MATERIALS AND METHODS
Astrocyte culture preparation and RLR stimulation
Primary astrocyte cells (Lonza) were plated and grown in culture for 7d prior to experimentation. Poly(I:C)Lyovec of low molecular weight (poly(I:C)LMW, Invivogen) was used to stimulate RIG-1 at different concentrations (3 and 6 μg). MDA5 was stimulated with poly(I:C)Lyovec of high molecular weight (Invivogen). Controls used were Lyovec vector alone (Invivogen) and no treatment. Stimulation with ligands was carried for 18 h according to manufacturer’s instructions. The RLR inhibitor mitochondrial E3 ubiquitin protein ligase 1 (MUL1, Novus Biologicals), also known as MULAN/FLJ12875/MAPL/RNF218, was used at a concentration of 1.0 μg/ml, 1 hr prior to poly(I:C)LMW delivery.
Stretch Injury
Primary astrocytes were grown on collagen I coated Flex Plates (Flexcell international Corporation) and subjected to stretch with the Cell Injury Controller II that regulates a pulse of compressed gas to transiently deform a SILASTIC membrane and adherent cells(Ellis et al. 1995). Astrocytes were injured using a 50 msec pulse and peak injury pressure of 3.8. Astrocytes were harvested at different time points after stretch (15, 30 min and 1h). Non-stretched wells were used as controls.
Immunoblotting
For detection of RLR proteins, spinal cord tissue and astrocyte cell cultures were harvested at different time points after SCI or stretch injury with extraction buffer (20 mM Tris–HCl, pH: 7.5, 150 mM NaCl, 1% Triton X-100; 1 mM ethylenediaminetetraacetic acid, 1 mM ethylene glycol tetraacetic acid, 2.5 mM pyrophosphate, 1 mM β-glycerophosphate) containing protease and phosphatase inhibitor cocktails (Sigma). Immunoblot analysis was carried with the Criterion system (Bio-Rad) as described in de Rivero Vaccari et al. 2008(de Rivero Vaccari et al. 2008) using antibodies (1:1000 dilution) to Rig1 (Anaspec), MDA5 (Abcam), VISA (Anaspec) and P-IRF3 (Novus Biologicals). For immunoblotting of the spinal cord a 10 mm of the spinal cord containing the area of the epicenter was used for analysis.
Animals
Adult female Fischer (180–200 g) rats were used in these studies. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Miami. Rats were anesthetized with ketamine (87 mg/kg) and xylazine (13 mg/kg). Adequate amounts of anesthesia were determined by monitoring toe touch. Using aseptic techniques, a midline incision was made in the neck in the skin and musculature to expose the C2–T1 vertebrae. A laminectomy was performed at vertebral level C5 exposing the cord. A moderate contusion injury (2000 kdyn of force) was induced by displacing the spinal cord 0.95 mm using the circular flap tip of the impactor of the Electromagnetic SCI Device (Ohio State University, Columbus, OH). Body and spinal cord temperature were maintained at 37.5°C with a feedback-controlled heating blanket, which was monitored by a rectal thermometer. After injury, the incision was closed with wound clips and sutures, and each rat was returned to its cage. Animals were killed at different times after SCI. Sham animals were used as controls.
Perfusion fixation
Animals were anesthetized with ketamine (87 mg/kg) and xylazine (13 mg/kg) and perfused with 500 ml of 4% paraformaldehyde. Spinal cords were removed and placed in 4% paraformaldehyde at 4°C for 20h. Then cords were transferred to 20% sucrose in 0.1 M PBS and stored at 4°C until sectioning.
Immunohistochemistry
Immunostained spinal cord sections of uninjured and injured rats at 6h were examined with a Zeiss (Jena, Germany) laser scanning confocal microscope (Zeiss). Rats were perfused with 4% paraformaldehyde as described and processed for cryostat sectioning (Leica SM 2000R Sliding Microtome). Sections (50 μm) were blocked by treatment with normal goat serum (Vector Laboratories, Burlingame, CA). Tissue sections were rinsed with 0.1 M PBS, pH 7.4, and incubated overnight at 4°C with primary antibodies. To determine the precise cellular distribution of RLR proteins, sections were double stained with cell-type-specific markers against astrocytes mouse anti-GFAP (Millipore Bioscience Research Reagents), anti-MAP2 (Chemicon), anti-APC-CC1 (CalBiochem) and anti-CD11b (Chemicon) and RLR proteins Rig1, MDA5 and VISA. Alexa Fluor secondary antibody conjugates (Invitrogen) were used as secondary antibodies. Sections were coverslipped with Vectashield mounting medium (Vector Laboratories) and analyzed with a Zeiss LSM510 laser scanning confocal microscope. Secondary antibodies alone were used as control for antibody specificity.
Statistical analysis
Data are expressed as ±SEM. Statistical comparisons between uninjured and injured groups were made using two-tailed Student’s t test and a one-way ANOVA followed by Tukey’s multiple comparison tests. p values of significance used were *p < 0.05 and #p < 0.10.
RESULTS
Astrogliosis is induced by short and long dsRNAs and inhibited by MUL1
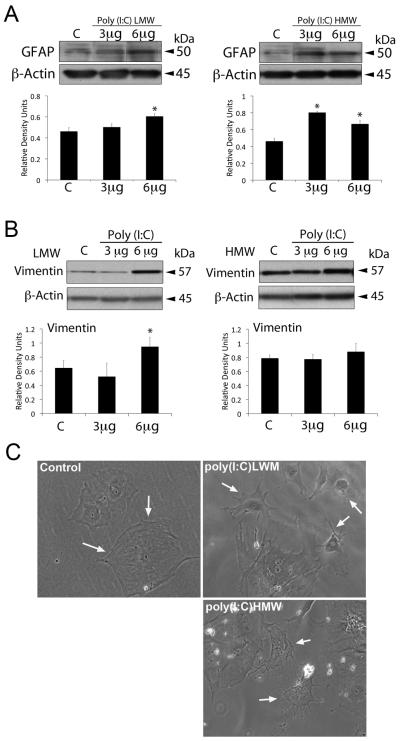
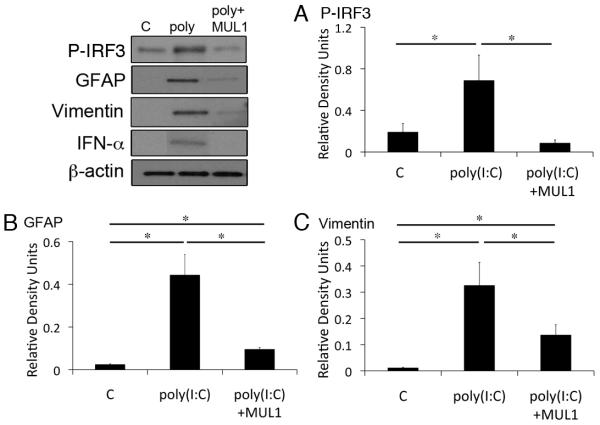
To examine whether astrocytes detect short and long dsRNAs, we treated primary astrocytes with different size lengths of synthetic RNA poly(I:C). As shown in Figure 1 treatment of astrocytes with short poly(I:C) LMW activated RIG-I signaling (Figure 1A), whereas poly(I:C) HMW treatment activated MDA-5 signaling (Figure 1B). RLR signaling activation resulted in phosphorylation of IFN-regulatory factor 3 (p-IRF3) (de Veer et al. 2001; Wilkins and Gale 2010). Both size classes of poly(I:C) increased levels of p-IRF3 (Figure 1A and 1B). RIG-1 protein expression was elevated by the treatment of astrocytes with 6 μg of poly(I:C)HMW (not shown). We next investigated whether treatment of astrocytes with synthetic RNA induced biochemical alterations of reactive gliosis as characterized by increased production of intermediate filament proteins GFAP (Figure 2A) and vimentin (Figure 2B), and morphological characteristics of reactive gliosis such as extension of the processes (Figure 2C). Significant increases in levels of GFAP were induced in astrocytes treated with both short and long poly(I:C) (Figure 2A), whereas increased levels of vimentin were only detected in cultures treated with short synthetic RNA (Figure 2B). In addition, astrocytes treated with dsRNAs showed extended processes unlike untreated astrocytes. Next, we delivered the VISA inhibitor mitochondrial E3 ubiquitin protein ligase (MUL1) protein 1 hr prior to poly(I:C) LMW delivery (6 μg/ml). As shown in Figure 3 treatment with the RLR signaling inhibitor MUL1 resulted in decreased phosphorylation of IRF3 and decreased expression of both GFAP and vimentin when compared to poly(I:C) LMW alone. Thus, RIG-1 and MDA-5 signaling pathways are induced in astrocytes by treatment with synthetic RNA leading to increased GFAP and vimentin expression.
Fig. 1.
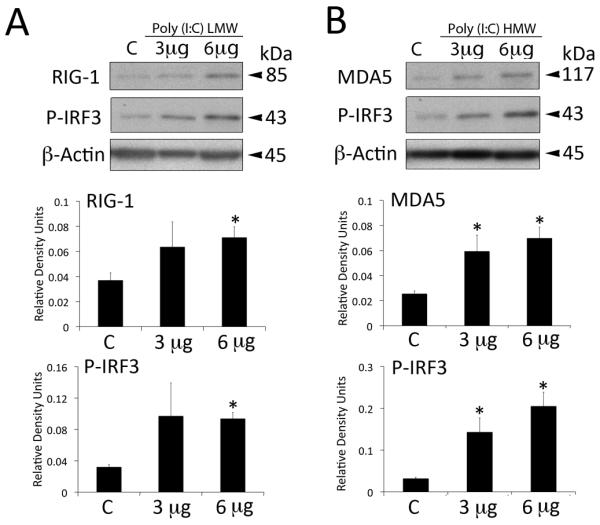
Synthetic dsRNA alters RLR protein expression in astrocytes. Representative immunoblot analysis of astrocyte lysates of cells stimulated with 3 or 6 μg of poly(I:C) LMW (Rig-I ligand) or poly(I:C)HMW (MDA5 ligand) for 18h. Non-stimulated cells were used as a control (C). Poly(I:C) vector alone showed similar result (C, control). Cell lysates were immunoblotted with antibodies against (A) Rig-I, (B) MDA5. β-actin was used as internal standard and control for protein loading. Data presented as mean +/− SEM. *p < 0.05. n=6.
Fig. 2.
RLR ligands alter astrocytes protein expression. Representative immunoblot analysis of astrocyte lysates of cells stimulated with 3 or 6 μg of poly(I:C) LMW (Rig-I ligand) or poly(I:C)HMW (MDA5 ligand) for 18h. Non-stimulated cells were used as a control (C). (A) GFAP and (B) Vimentin. β-actin was used as internal standard and control for protein loading. Data presented as mean +/− SEM. *p < 0.05. n=6. (C) Light microscopy image showing changes in morphology associated with delivery of poly(I:C) to astrocytes in culture (arrows). Magnification 40X.
Fig. 3.
MUL1 inhibits reactive astrogliosis in culture. Representative immunoblot analysis of astrocyte lysates of cells stimulated with 3 or 6 μg of poly(I:C) LMW (Rig-I ligand) for 18h and pretreated with MUL1 for 1 hr. Non-stimulated cells were used as a control (C). (A) P-IRF3, (B) GFAP and (C) Vimentin. β-actin was used as internal standard and control for protein loading. Data presented as mean +/− SEM. *p < 0.05. n=5.
Stretch injury induces RIG-I signaling components in astrocytes
Tissue strain is an important component of in vivo CNS injury and causes inflammation. In order to establish whether injury induces RLR signaling in astrocytes, we employed the well-characterized in vitro model of stretch injury (Ellis et al. 1995) that produces many of the post-traumatic responses in vivo. Primary astrocytes were grown on deformable SILASTIC membranes and subjected to stretch injury. Uninjured cells in a well of the Flex Plate served as controls. Cultures were returned to the incubator, and at 15, 30 min and 1 hr after injury cells were assessed for RLR signaling components. As shown in Figure 4, marked increases in RIG-1, MDA5, VISA and P-IRF3 were observed at 15 min post-injury. These results indicate that RLR signaling involving Rig-I and/or MDA5 is activated in astrocytes after stretch injury.
Fig. 4.
Stretch injury activates RLR signaling in primary astrocytes in culture. Representative immunoblot analysis of astrocyte lysates of non-stretch (C, control) and stretch cells harvested at 15 min, 30 min and 1h after stretch injury. Cell lysates were immunoblotted with antibodies against Rig-I, MDA5, VISA and phosphorylated IRF3. β-actin was used as internal standard and control for protein loading. Data presented as mean +/− SEM. *p < 0.05. n=6.
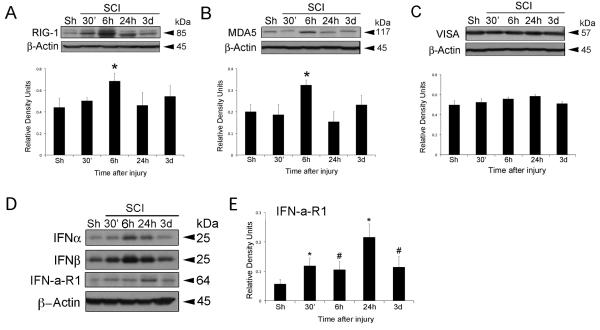
SCI increases protein expression of RIG-I, MDA5 and Type I IFN
Since excessive levels of type I IFNs are associated with secondary damage after SCI, we next examined the involvement of RIG-I, MDA5 and type I IFN signaling induced by SCI. Spinal cord lysates from injured and sham-operated animals were analyzed for RLR signaling proteins (Figure 5). SCI resulted in a significant increase in the levels of RIG-I (Figure 5A) and MDA5 (Figure 5B) at 6h, whereas the levels of the mitochondrial-associated protein VISA did not change significantly at any of the time points tested (Figure 5C). Lastly, an increase in IFN-α and IFN-β was detected at 6h after SCI (Figure 5D), and is consistent with increased levels of RIG-I and MDA5 at a similar time point (Figure 5A and B). Since these cytokines are secreted, we quantified levels of the IFN-a-R1, a receptor that binds to type I IFNs to better determine the signaling contribution of these cytokines after trauma. Significantly higher levels of IFN-a-R1 were detected in injured lysates as early as 30 min after trauma and peak levels were observed at 1d after injury (Figure 5D).
Fig. 5.
RLR signaling is activated after SCI. Representative immunoblot analysis of spinal cord lysates of sham (Sh) and injured rat cords at 30 min, 6h, 24h and 3d after trauma. Spinal cord lysates were immunoblotted with antibodies against (A) Rig-I, (B) MDA5 and (C) VISA, (D) IFNα, IFNβ and the receptor IFN-a-R1. (E) Densitometric analysis of IFN-a-R1 at different time points after SCI. β-actin was used as internal standard and control for protein loading. Data presented as mean +/− SEM. *p < 0.05 and #p < 0.10. n=5.
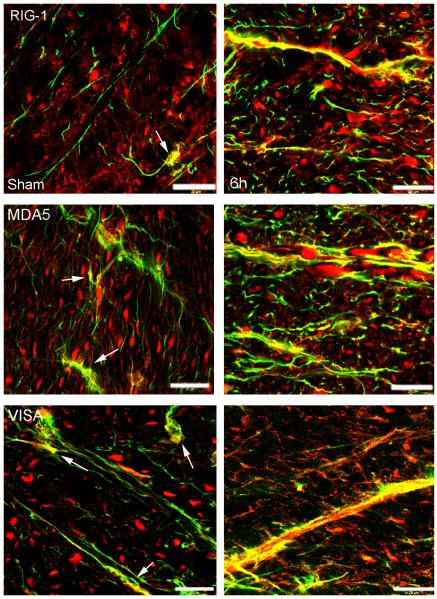
RLR signaling proteins are present in spinal cord astrocytes, and SCI induces alteration in protein expression pattern
Figure 6 shows confocal images of the cell type expression and regional distribution of RLR signaling proteins in astrocytes in the ventral horn of sham and injured spinal cords at 6h after injury. Sections were stained for RIG-I, MDA5 and VISA (red) and the astrocytic marker GFAP (green). RIG-I, MDA5 and VISA immunoreactivity was present mainly in the cytoplasm of cells. At 6h after injury, the immunoreactivity of RLR signaling proteins increased in GFAP positive cells. In addition to astrocytes, other cells in the CNS express RLR signaling proteins. In motor neurons of the uninjured ventral horn (Supporting Information Figure 1), Rig1 was present mainly in the cytoplasm, whereas MDA5 was seen in the nucleus and VISA was expressed in the cytoplasm and nucleus. Six hrs after SCI, there was protein redistribution, with Rig1 located in the nucleus, MDA5 in the cytoplasm and VISA in the cell processes. These findings are consistent with previous reports showing a role of VISA in RLR signaling upon VISA redistribution within the cell (Onoguchi et al. 2010). Moreover, oligodendrocytes (Supporting Information Figure 2), and microglia/macrophages (Supporting Information Figure 3), in the spinal cord also express RLR proteins. The contribution of these cells to the innate immune response after SCI is currently under investigation.
Fig. 6.
Rig-I, MDA5 and VISA are present in astrocytes of the spinal cord, and SCI induces alterations in protein expression patterns. Confocal images show astrocytes in the ventral horn of sham (left) and 6h (right) injured spinal cords. Sections were stained for Rig-I, MDA5 and VISA (red) and GFAP (green). Arrows point to Rig-I, MDA5 and VISA in sham animals in the processes of GFAP positive cells. Scale bar = 20 μm.
DISCUSSION
In this study, we show for the first time the time course of expression of RLR proteins and type I IFN signaling proteins after SCI. Although RLR and type I IFN signaling in the CNS has been normally associated with antiviral immune responses (Wilkins and Gale 2010), our data demonstrate that injury to the spinal cord and stretch injury to astrocytes in culture elevates the protein levels of RLR signaling intermediates, phosphorylated IRF3 and the type I IFNs, IFN α and β. In support of our findings is the recent observation that sterile axonal lesion induces IRF7 and IRF9 gene expression (Khorooshi and Owens 2010). Thus, RLR and type I IFN signaling appears to have a significant role in CNS injury-induced inflammatory responses supporting the idea that RLR signaling contributes to the wide variety of effects on inflammatory processes after injury and in CNS diseases (Paul et al. 2007).
RLRs are a family of intracellular innate immune receptors also known as pattern recognition receptors (PRRs) that recognize structures conserved among microbial or viral species called pathogen-associated molecular patterns (PAMPs), such as flagellin, lipopolysaccharide or muramyl dipeptide. PRRs are also responsible for recognizing endogenous molecules released from damaged cells, termed damage-associated molecular patterns (DAMPs), such as Adenosine Triphosphate (ATP), Deoxyribonucleic Acid (DNA), RNA, Heat Shock Proteins or High Mobility Group Box1 (HMGB1). The cell-type, organ-, and species-specific expression of surface IFN receptors are well documented (Auerbuch et al. 2004), but little is known about the respective cell expression profiles of cytosolic PRR molecules in CNS cells. Our data show that upregulation of RIG-I, MDA5 and VISA in GFAP+ astrocytes leads to production of type I IFNs. These findings are in agreement with our earlier work demonstrating IFN-α/β production by astrocytes in the regulation of MHC antigen expression (Tedeschi et al. 1986). However, others have reported that neurons produce IFN-α in response to infection (Delhaye et al. 2006) and macrophages/microglia produce IFN-β (Khorooshi and Owens 2010; Teige et al. 2003) and IFN-α (Khorooshi and Owens 2010) in the experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis. However, it is yet to be determined whether neurons or macrophages/microglia express RLR signaling intermediates that activate IFN production after CNS trauma.
The sensing of PAMPs and DAMPs by PRRs upregulates the transcription of genes involved in inflammatory responses. Foreign nucleic acids, the signature of invading viruses and certain bacteria, are sensed intracellularly by RIG-I and MDA5 (Wilkins and Gale 2010). However, IFN production may be activated by small self-RNA cleavage products generated by RNase L that stimulate signaling of RIG-I, MDA5 and VISA(Malathi et al. 2007). Since damaged CNS cells release small self-nucleic acids after SCI, these DAMPs appear to have an important role in the initiation of the innate immune response after CNS injury (Rainer and Lam 2006). However, it is possible that other yet unidentified ligands are involved in the activation of RLR signaling following CNS injury and disease. CNS injury induces an innate-glial response in which astrocytes become activated. This response is essential for the induction of cytokines and chemokines, which initiate a variety of cellular responses.
The molecular basis for glial activation is poorly understood. Following SCI, one of the major obstacles to neuronal regeneration is glial scarring that occurs in part as a result of reactive astrogliosis (Wilhelmsson et al. 2004; Wu et al. 1998; Wu and Schwartz 1998). Reactive astrogliosis involves a spectrum of astrocytic changes that vary with the severity of the insult. Alterations in molecular expression of intermediate filament proteins GFAP and vimentin (Pekny and Pekna 2004) are characteristic features of reactive astrocytes as well as cellular hypertrophy, and in severe cases, scar formation (Sofroniew 2009). Thus, reactive astrogliosis may be responsible for isolation of injured tissue from healthy tissue to prevent the spread of damage at the expense of blocking neuronal regeneration (Myer et al. 2006; Sofroniew 2005). In contrast, reactive astrogliosis has been reported to be beneficial in that it: 1) aids in the repair of the blood brain barrier; 2) prevents of the death of neurons and oligodendrocytes (Bush et al. 1999; Faulkner et al. 2004); 3) regulates blood flow and extracellular ion balance; 4) participates in synaptic function and plasticity and 5) provides energy metabolites to neurons (Barres 2008; Nedergaard et al. 2003; Pellerin et al. 2007; Seifert et al. 2006). Moreover, reactive astrogliosis has been suggested to be involved in neurotoxicity, inflammation and chronic pain (Sofroniew 2009). Reactive astrogliosis plays a role in inflammatory events. Reactive astrocytes prevent the spread of inflammatory cells and the spread of infections after SCI (Bush et al. 1999; Faulkner et al. 2004; Herrmann et al. 2008; Okada et al. 2006). Nevertheless, deletion of certain proteins in astrocytes reduces the deleterious inflammatory response after SCI (Brambilla et al. 2005). Recently, Toyooka et al. downregulated GFAP and vimentin expression with RNAi, resulting in improved acute dysfunction in a rodent model of SCI (Pekny and Pekna 2004). In addition, stimulation of reactive astrocytes exacerbates the inflammatory response via cytokine production and formation of reactive oxygen species (ROS) (Hamby et al. 2006; Swanson et al. 2004).
Our findings show injured spinal cords and astrocyte cultures induce type I IFN production and a common IFN cell surface receptor, IFN-a-R1. IFN-a-R1 stimulates type I IFN-dependent gene transcription with diverse functions, including host defense, regulation of growth and apoptosis (de Veer et al. 2001). Although there were no significant changes in VISA protein expression after SCI, immunohistochemical analysis revealed that VISA expression within cells changed after injury. This alteration in VISA protein expression pattern after SCI is consistent with previous reports showing a role of VISA in RLR signaling upon VISA redistribution within the cell (Onoguchi et al. 2010)The upregulation of IRF3 in response to CNS injury suggests an involvement in noninfectious responses as well. Immunoblot analysis showed that levels of IRF3 were higher in astrocytes subjected to stretch injury and spinal cords subjected to moderate contusive injury, suggesting that type I IFNs play a critical role in glial activation after CNS injury. We tested this idea by treating astrocyte cultures with poly(I:C) of approximately 300 bp to stimulate RLR signaling or treatment with longer segments of poly(I:C) to stimulate MDA5 signaling. Both long and short synthetic RNA induced significantly higher levels of GFAP and vimentin in astrocytes, two proteins characteristic of reactive astrocytes. Thus, the initial sensing of DAMPs by RIG-I and MDA5 may trigger inflammatory mediators that coordinate the activation of astrocytes in response to injury. Although poly(I:C) has been shown to stimulate TLR3 signaling (Yu et al. 2011) and it remains controversial whether DNA viruses also activate RLR signaling(Yoneyama and Fujita 2007a; Yoneyama and Fujita 2007b), it does not appear that the increases in GFAP and vimentin observed after poly(I:C) treatment are mediated by TLR3 because treatment of stimulated astrocytes with MUL1 a specific inhibitor RIG-1 and MDA5 decreased the levels of GFAP and vimentin in cultured astrocytes thus supporting the idea that RLR signaling is involved in reactive gliosis. Although the exact mechanism of how RLR signaling triggers increased expression of GFAP and vimentin remains unknown, it is possible that cytokine production such as IL-6 or other IFN stimulated genes may result in the stimulation of GFAP for it has been previously shown that cytokines are involved in astrocytes activation(Wu et al. 1998).
Our previous studies have shown that inflammasomes in neurons play a critical role in the innate immune response following SCI (de Rivero Vaccari et al. 2008), traumatic brain injury (de Rivero Vaccari et al. 2009) and stroke (Abulafia et al. 2009). Here we showed that short nucleic acids, a signature of damaged cells, are sensed intracellularly by RIG-I and MDA5 in astrocytes. The nucleic acids trigger RLR signaling leading to type I IFN production and astrogliosis. Thus, RLR innate immune signaling pathways in the CNS offer novel therapeutic targets in CNS injury and neuroinflammatory diseases.
Supplementary Material
Supporting Information Fig. 1. Rig-I, MDA5 and VISA are present in neurons of the spinal cord, and SCI induces alterations in protein expression patterns. Confocal images show neurons in the ventral horn of sham (left) and 6h (right) injured spinal cords. Sections were stained for Rig-I, MDA5 and VISA (red) and MAP2 (green). Scale bar = 20 μm.
Supporting Information Fig. 2. Rig-I, MDA5 and VISA are present in oligodendrocytes of the spinal cord, and SCI induces alterations in protein expression patterns. Confocal images show oligodendrocytes in the ventral horn of sham (left) and 6h (right) injured spinal cords. Sections were stained for Rig-I, MDA5 and VISA (red) and APC-CC1 (green). Scale bar = 20 μm.
Supporting Information Fig. 3. Rig-I, MDA5 and VISA are present in microglia/macrophages of the spinal cord, and SCI induces alterations in protein expression patterns. Confocal images show microglia/macrophages in the ventral horn of sham (left) and 6h (right) injured spinal cords. Sections were stained for Rig-I, MDA5 and VISA (red) and CD11b (green). Scale bar = 20 μm.
ACKNOWLEDGMENTS
We thank Frank Brand for assistance. This work was supported in part by The Miami Project to Cure Paralysis, and an NIH grant to RWK (NS059836).
REFERENCES
- Abulafia DP, de Rivero Vaccari JP, Lozano JD, Lotocki G, Keane RW, Dietrich WD. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J Cereb Blood Flow Metab. 2009;29(3):534–44. doi: 10.1038/jcbfm.2008.143. [DOI] [PubMed] [Google Scholar]
- Auerbuch V, Brockstedt DG, Meyer-Morse N, O’Riordan M, Portnoy DA. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J Exp Med. 2004;200(4):527–33. doi: 10.1084/jem.20040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60(3):430–40. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202(1):145–56. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23(2):297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Lotocki G, Alonso OF, Bramlett HM, Dietrich WD, Keane RW. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab. 2009;29(7):1251–61. doi: 10.1038/jcbfm.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Lotocki G, Marcillo AE, Dietrich WD, Keane RW. A molecular platform in neurons regulates inflammation after spinal cord injury. J Neurosci. 2008;28(13):3404–14. doi: 10.1523/JNEUROSCI.0157-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Veer MJ, Holko M, Frevel M, Walker E, Der S, Paranjape JM, Silverman RH, Williams BR. Functional classification of interferon-stimulated genes identified using microarrays. J Leukoc Biol. 2001;69(6):912–20. [PubMed] [Google Scholar]
- Delhaye S, Paul S, Blakqori G, Minet M, Weber F, Staeheli P, Michiels T. Neurons produce type I interferon during viral encephalitis. Proc Natl Acad Sci U S A. 2006;103(20):7835–40. doi: 10.1073/pnas.0602460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis EF, McKinney JS, Willoughby KA, Liang S, Povlishock JT. A new model for rapid stretch-induced injury of cells in culture: characterization of the model using astrocytes. J Neurotrauma. 1995;12(3):325–39. doi: 10.1089/neu.1995.12.325. [DOI] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24(9):2143–55. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamby ME, Hewett JA, Hewett SJ. TGF-beta1 potentiates astrocytic nitric oxide production by expanding the population of astrocytes that express NOS-2. Glia. 2006;54(6):566–77. doi: 10.1002/glia.20411. [DOI] [PubMed] [Google Scholar]
- Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28(28):7231–43. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorooshi R, Owens T. Injury-induced type I IFN signaling regulates inflammatory responses in the central nervous system. J Immunol. 2010;185(2):1258–64. doi: 10.4049/jimmunol.0901753. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Lai W, Rivest S, Hart RP, Satoskar AR, Popovich PG. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J Neurochem. 2007;102(1):37–50. doi: 10.1111/j.1471-4159.2007.04524.x. [DOI] [PubMed] [Google Scholar]
- Malathi K, Dong B, Gale M, Jr., Silverman RH. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature. 2007;448(7155):816–9. doi: 10.1038/nature06042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myer DJ, Gurkoff GG, Lee SM, Hovda DA, Sofroniew MV. Essential protective roles of reactive astrocytes in traumatic brain injury. Brain. 2006;129(Pt 10):2761–72. doi: 10.1093/brain/awl165. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26(10):523–30. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12(7):829–34. doi: 10.1038/nm1425. others. [DOI] [PubMed] [Google Scholar]
- Onoguchi K, Onomoto K, Takamatsu S, Jogi M, Takemura A, Morimoto S, Julkunen I, Namiki H, Yoneyama M, Fujita T. Virus-infection or 5’ppp-RNA activates antiviral signal through redistribution of IPS-1 mediated by MFN1. PLoS Pathog. 2010;6(7):e1001012. doi: 10.1371/journal.ppat.1001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Ricour C, Sommereyns C, Sorgeloos F, Michiels T. Type I interferon response in the central nervous system. Biochimie. 2007;89(6-7):770–8. doi: 10.1016/j.biochi.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Pekny M, Pekna M. Astrocyte intermediate filaments in CNS pathologies and regeneration. J Pathol. 2004;204(4):428–37. doi: 10.1002/path.1645. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia. 2007;55(12):1251–62. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Rainer TH, Lam NY. Circulating nucleic acids and critical illness. Ann N Y Acad Sci. 2006;1075:271–7. doi: 10.1196/annals.1368.035. [DOI] [PubMed] [Google Scholar]
- Seifert G, Schilling K, Steinhauser C. Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci. 2006;7(3):194–206. doi: 10.1038/nrn1870. [DOI] [PubMed] [Google Scholar]
- Seo YJ, Hahm B. Type I interferon modulates the battle of host immune system against viruses. Adv Appl Microbiol. 2010;73:83–101. doi: 10.1016/S0065-2164(10)73004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11(5):400–7. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32(12):638–47. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Ying W, Kauppinen TM. Astrocyte influences on ischemic neuronal death. Curr Mol Med. 2004;4(2):193–205. doi: 10.2174/1566524043479185. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. Innate immunity to virus infection. Immunol Rev. 2009;227(1):75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedeschi B, Barrett JN, Keane RW. Astrocytes produce interferon that enhances the expression of H-2 antigens on a subpopulation of brain cells. J Cell Biol. 1986;102(6):2244–53. doi: 10.1083/jcb.102.6.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teige I, Treschow A, Teige A, Mattsson R, Navikas V, Leanderson T, Holmdahl R, Issazadeh-Navikas S. IFN-beta gene deletion leads to augmented and chronic demyelinating experimental autoimmune encephalomyelitis. J Immunol. 2003;170(9):4776–84. doi: 10.4049/jimmunol.170.9.4776. [DOI] [PubMed] [Google Scholar]
- Wilhelmsson U, Li L, Pekna M, Berthold CH, Blom S, Eliasson C, Renner O, Bushong E, Ellisman M, Morgan TE. Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J Neurosci. 2004;24(21):5016–21. doi: 10.1523/JNEUROSCI.0820-04.2004. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins C, Gale M., Jr. Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol. 2010;22(1):41–7. doi: 10.1016/j.coi.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu VW, Nishiyama N, Schwartz JP. A culture model of reactive astrocytes: increased nerve growth factor synthesis and reexpression of cytokine responsiveness. J Neurochem. 1998;71(2):749–56. doi: 10.1046/j.1471-4159.1998.71020749.x. [DOI] [PubMed] [Google Scholar]
- Wu VW, Schwartz JP. Cell culture models for reactive gliosis: new perspectives. J Neurosci Res. 1998;51(6):675–81. doi: 10.1002/(SICI)1097-4547(19980315)51:6<675::AID-JNR2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Fujita T. Cytoplasmic double-stranded DNA sensor. Nat Immunol. 2007a;8(9):907–8. doi: 10.1038/ni0907-907. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Fujita T. Function of RIG-I-like receptors in antiviral innate immunity. J Biol Chem. 2007b;282(21):15315–8. doi: 10.1074/jbc.R700007200. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Fujita T. Structural mechanism of RNA recognition by the RIG-I-like receptors. Immunity. 2008;29(2):178–81. doi: 10.1016/j.immuni.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Yu N, Zhang S, Sun T, Kang K, Guan M, Xiang L. Double-stranded RNA induces melanocyte death via activation of Toll-like receptor 3. Exp Dermatol. 2011;20(2):134–9. doi: 10.1111/j.1600-0625.2010.01208.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information Fig. 1. Rig-I, MDA5 and VISA are present in neurons of the spinal cord, and SCI induces alterations in protein expression patterns. Confocal images show neurons in the ventral horn of sham (left) and 6h (right) injured spinal cords. Sections were stained for Rig-I, MDA5 and VISA (red) and MAP2 (green). Scale bar = 20 μm.
Supporting Information Fig. 2. Rig-I, MDA5 and VISA are present in oligodendrocytes of the spinal cord, and SCI induces alterations in protein expression patterns. Confocal images show oligodendrocytes in the ventral horn of sham (left) and 6h (right) injured spinal cords. Sections were stained for Rig-I, MDA5 and VISA (red) and APC-CC1 (green). Scale bar = 20 μm.
Supporting Information Fig. 3. Rig-I, MDA5 and VISA are present in microglia/macrophages of the spinal cord, and SCI induces alterations in protein expression patterns. Confocal images show microglia/macrophages in the ventral horn of sham (left) and 6h (right) injured spinal cords. Sections were stained for Rig-I, MDA5 and VISA (red) and CD11b (green). Scale bar = 20 μm.