Abstract
Introduction
Current guidelines for management of critically ill stroke patients suggest that treatment in a neurocritical care unit (NCCU) and/or by a neurointensivist (NI) may be beneficial, but the contribution of each to outcome is unknown. The relative impact of a NCCU vs. NI on short- and long-term outcomes in patients with acute ischemic stroke (AIS), intracerebral hemorrhage (ICH) and aneurysmal subarachnoid hemorrhage (SAH) was assessed.
Methods
2,096 stroke patients admitted to a NCCU or non-neuro ICU at a tertiary stroke center were analyzed before the appointment of a NI, during the NI’s tenure, and after the NI departed and was not replaced. Data included admission ICU type, availability of a NI, age, NIHSS, ICH score and 3 and 12 month outcome.
Results
For AIS, compared to the time interval with a NI, departure of the NI predicted a worse rate of return to pre-stroke function at 3 months. For ICH, NCCU treatment predicted shorter ICU and hospital LOS but had no effect on short or long term outcomes. No effect of a NI was seen. For SAH, availability of an NI (but not an NCCU) predicted improved outcomes but longer ICU LOS. Disposition and in-hospital mortality improved when a NI was present, but continued improvement did not occur after the NI’s departure.
Conclusion
Presence of an NI was associated with improved clinical outcomes. This effect was more evident in patients with SAH. Patients with ICH tend to have poor outcomes regardless of the presence of a NCCU or a NI.
Keywords: ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, neurointensivist, neurocritical care, outcomes
Introduction
Over the past several decades, ICUs designed to focus on critically-ill neonatal, trauma, burn, cancer, neurologic, surgical and post-operative cardiac patients have become increasingly widespread. Staffed by physicians and nurses whose training emphasizes unique aspects of the disease process, some specialized ICUs have been shown to decrease mortality, reduce length of stay (LOS) and improve discharge disposition.1 However, building and staffing multiple small ICUs is costly, as the hospital must “forgo the economies of scale that would have been provided by a single large ICU”.1 Hospitals thus require solid evidence that specialized ICUs improve clinical outcomes and efficiently use healthcare resources.
In many hospitals, critically ill neurologic patients are now managed by neurointensivists (NIs). Since 2007, board certification in Neurocritical care has been offered by the United Council for Neurologic Subspecialties (UCNS). Currently, in order to become a board-certified NI two years of fellowship training in an UCNS-accredited program must follow residency. The NI is responsible for the interface between the brain and other organ systems in the setting of critical illness, managing both neurologic injury and any associated medical problems.
In critically ill stroke patients, the utility of a designated NCCU is unknown. Formal recommendations regarding NCCU use are lacking.2 For acute ischemic stroke (AIS), current guidelines strongly advise admission to specialized stroke units. The 2005 Brain Attack Coalition (BAC) consensus statement recommended that comprehensive stroke centers have an ICU available for AIS patients, but states that presence of a dedicated NCCU and/or a formally trained NI is “desirable but not required”.3 For deteriorating patients, the BAC recommends transfer to facilities with neurosurgical expertise, but does not mention preference for hospitals with a designated NCCU. For spontaneous intracerebral hemorrhage (ICH), the American Stroke Association guidelines call for admission to an ICU but do not advise for or against treatment by an NI or NCCU.4 For aneurysmal SAH patients, the American Heart Association/American Stroke Association guidelines suggest early referral to high-volume centers that have experienced cerebrovascular surgeons and endovascular specialists, but again do not mention ICU preference or advocate for specialized NI care.5
Following the BAC consensus, three retrospective studies have provided evidence that NIs and NCCUs improve outcome in critically ill stroke patients.6,7,8 All reported a positive association between NCCU/NI and decreased hospital LOS, and two reported improved discharge disposition as well. However, both short- and long-term mortality rates remained unchanged in all three studies, and post-discharge functional outcomes were not assessed. These findings contradict an earlier, larger retrospective study which found that ICH patients treated in an NCCU had a lower in-hospital mortality rate but a longer length of stay compared to those treated in a non-neuro ICU.1
Existing research has been limited by lack of long-term follow up and confounders such as general advances in clinical medicine during the time after which NI care was introduced. Current studies also fail to distinguish whether active management by a NI or simply the existence of an NCCU are responsible for the improved outcomes. The popular but mostly untested hypothesis is that NIs and specialized support staff provide superior prevention and management of post-stroke complications; institution of potentially neuroprotective protocols for normothermia, glycemic control and blood pressure management; placement and interpretation of advanced neuromonitoring techniques and early identification and subsequent expert management of cerebral edema and brain herniation.6,8,9
The goal of this study was to evaluate the effect of the arrival of a NI on AIS, ICH and SAH outcomes in a semi-closed NCCU at a tertiary university affiliated hospital. To clarify whether the presence of a NI or the NCCU setting in general that potentially improves stroke outcome, we assessed stroke outcomes in the NCCU vs. other ICUs (MICU/SICU/CCU). To control for general advances in medicine over time, we compared stroke outcomes before the arrival of a NI, during the presence of an NI, and after his departure from the hospital (he has not yet been replaced).
Materials and Methods
Data were extracted from an IRB-approved registry maintained by the Stroke Center at Hartford Hospital. The registry was begun in May 2001 for patients with acute ischemic stroke but was expanded to include hemorrhagic strokes in January 2003. The current study included patients from January 2003 through January 2011. Clinical data for the registry was abstracted from patient charts and discharge documentation and entered into a Microsoft Access database during or shortly after the hospital stay by a trained nurse and data analyst. Pre-morbid and admission functional status data were collected prospectively from patients and/or their relative proxies during their stay and follow-up data for functional outcomes were collected by telephone interview by trained staff at 3 and 12 months. Data were extracted and analyzed using SPSS version 14.
Study Population
Applying inclusion criteria of primary diagnosis of AIS, ICH or SAH with treatment in the ICU resulted in a study sample of 2,096. Excluded were all patients with a diagnosis of transient ischemic attack (TIA) or stroke rule-out or who were not admitted to an ICU. For patients with more than one qualifying admission, the study sample included only their first admission in order to avoid within-subjects comparisons.
Structure of the NCCU during the Study Period
The study facility was a tertiary regional teaching hospital and primary stroke center certified by JACHO with a Level One Trauma Center. The catchment area included central Connecticut, eastern New York and western Massachusetts. The NCCU was established in 1979, and consists of an 18-bed unit that houses most neurology and neurosurgical patients requiring critical care. The NCCU follows a ‘semiclosed’ model, with a primary team consisting of Advanced Practice Practioners (physician assistants and acute care nurse practitioners responsible for 24-hour patient care), nurses and respiratory technicians. A critical care physician serves as the attending physician during daytime hours, with different critical care physicians rotating through on a weekly basis. Until the NI was hired, all of the attending physicians were board-certified surgical critical care specialists. The primary admitting services – neurology, neurosurgery, trauma and surgery – round on their patients daily and coordinate with the critical care team that carries out daily care and management. In 2003, two interventional neuroradiologists joined the staff, allowing for the performance of procedures including intra-arterial tPA, aneurysm coiling for patients with SAH, and mechanical thrombolysis.
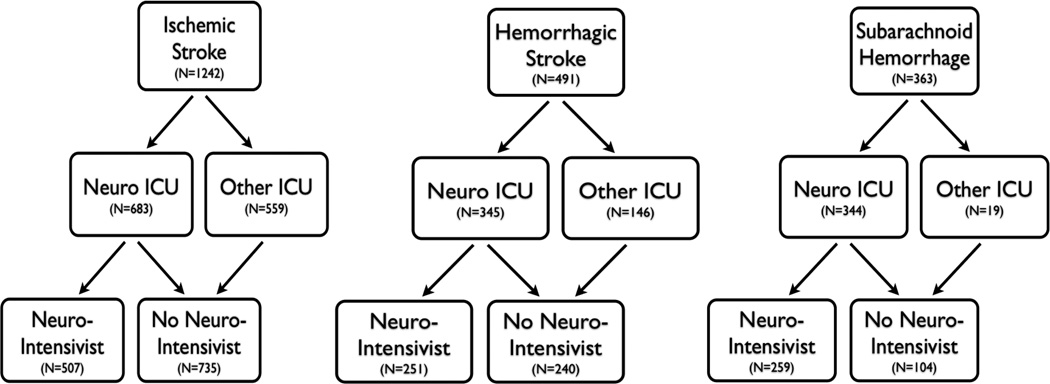
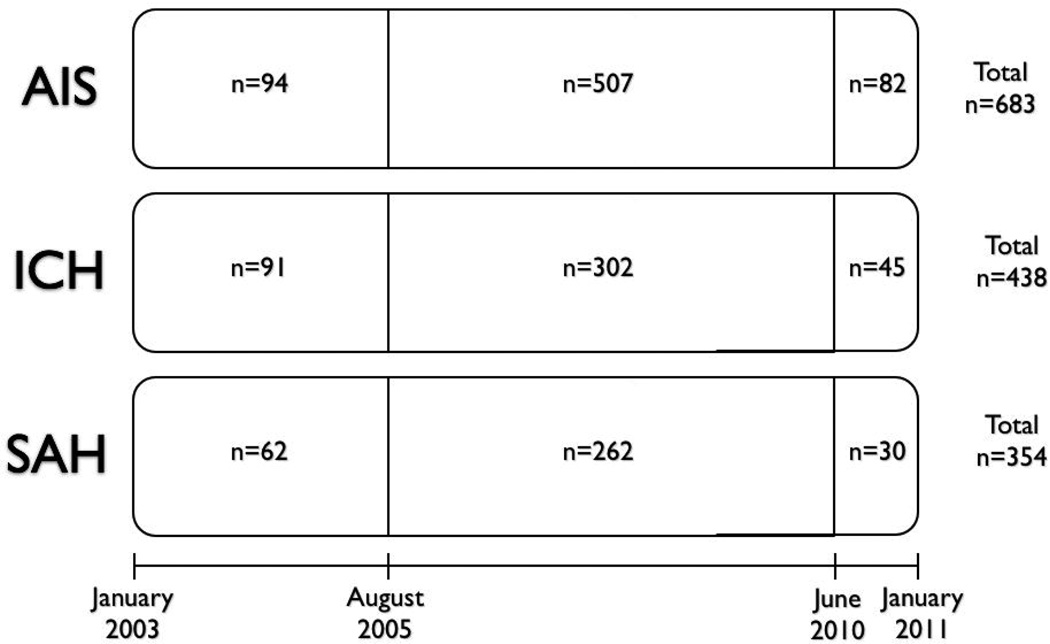
In August 2005, a NI was recruited who spent 20 weeks per year on service full-time in the NCCU. Numerous changes occurred in the NCCU during the NI’s tenure. An intensive educational program for nurse practitioners regarding specific neurocritical care issues was established. For the management of increased ICP, a step-wise protocol that included hypertonic saline as an option was introduced. Protocolized guidelines (e.g. DVT prophylaxis, death by neurologic criteria) were adapted to the unique issues of the NCCU – for example, timing of heparin initiation after ICH – and were rigorously adhered to by NCCU staff. Guidelines for the management of aneurysmal SAH and vasospasm were also followed closely. Advanced neuromonitoring (i.e. cerebral oxymetry) was introduced shortly after his arrival. Previously-established goal sheets (i.e. bundles) for reduction in ventilator-associated pneumonia and central line associated bloodstream infections were also adapted to the NCCU and monitored daily for compliance. In June 2010, the NI departed and has not yet been replaced by a trained NI. The arrival and departure of the NI, thus, creates three time periods in the treatment of patients: pre-NI (2003–2005), NI (2005–2010), and post-NI (2010–2011). Patients were also categorized as having been treated in the NCCU or a non-neuro ICU (specifically medical, surgical, and cardiac ICU). These events and locations are summarized in Figures 1 and 2, which also provide the number of patients for each type of stroke, treated in each ICU, during the three time periods.
Figure 1.
Study Design
Figure 2.
Time Period Analysis Study Design
Study Design and Data Collection
These naturally occurring events allowed for several approaches to the analysis of process and outcomes. The initial analysis compared patients first treated in NCCU with those first treated in the non-neuro ICUs. Second, the role of the NI was highlighted by comparing patients treated in the NCCU during the tenure of the NI to patients treated without an NI, in the NCCU before and after his tenure and in the non-neuro ICUs during the entire time period. A comparison of the three time periods within the NCCU provided the opportunity to explore whether any changes that occurred during the time period with a NI were sustained after his departure. Finally, a look at changes over these time periods in both the neuro and non-neuro ICU, where no similar personnel changes occurred, attempted to control for general improvements in stroke care during this eight year time period. These univariate analyses were supplemented by multivariate approaches looking simultaneously at type of unit, presence of NI, etc. All analyses were done independently for the three stroke types.
For all included subjects, the following predictor variables were extracted: demographics (age, gender, and race), stroke type, source of admission, stroke severity on admission (measured via NIHSS, ICH score and Hunt/Hess score for AIS, ICH and SAH respectively), presence/absence of intraventricular hemorrhage (IVH), receipt of tPA (in AIS), modified Barthel Index (a measure of patient’s functional status as defined by ability to perform activities of daily living10) pre-admission and on admission, the type of ICU, and whether or not they were managed by a NI. Outcome variables included length of stay (LOS) in the ICU, LOS in the hospital, mortality in-hospital and at 3- and 12- month follow up, location of discharge (home with/without services, acute/sub-acute rehabilitation, skilled nursing facility, hospice or death), medical complications during hospitalization, NIHSS on discharge (for ischemic strokes) and modified Barthel Index (MBI) at 3- and 12-month follow up. Complications examined were selected based on prior research identifying the most common complications in ICU patients.11
Statistical methods
For the univariate analyses of type of ICU and presence of NI, categorical variables such as gender, race and pre-stroke living situation, mortality and discharge destination, were analyzed with chi-square tests of proportions; continuous variables, such as age, hospital and ICU length of stay (LOS), and functional outcome scales were analyzed with independent group t-tests. For the comparison of the time points within the NCCU, a one-way analysis of variance (ANOVA) was conducted for all continuous variables; chi-square tests of proportion were still used for the categorical variables. A factorial two way ANOVA included the non neuro ICU patients and focused on the interaction effects of time period and ICU to again highlight the presence of the NI for study. Multivariate analyses included linear regression for continuous outcomes and logistic regression for all dichotomous variables. To correct for a strong positive skew and better meet the assumptions for parametric analysis, LOS data were transformed to their logarithmic value prior to analysis. In addition, all LOS analyses were repeated excluding the subpopulation of patients who died during hospitalization or were discharged to hospice, in order to avoid mislabeling early withdrawal of care as a short hospital stay.
Results
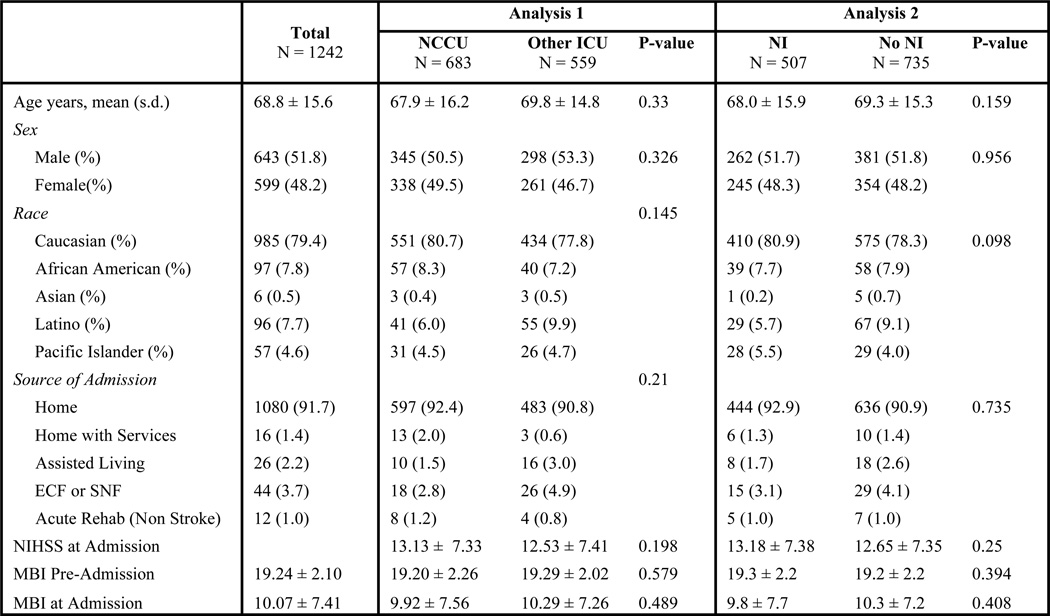
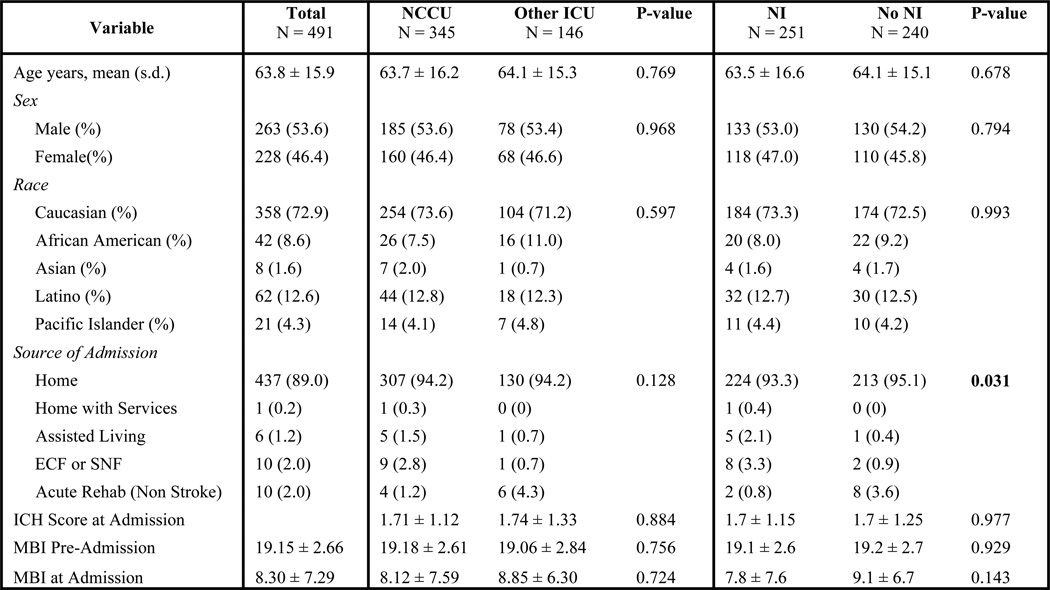
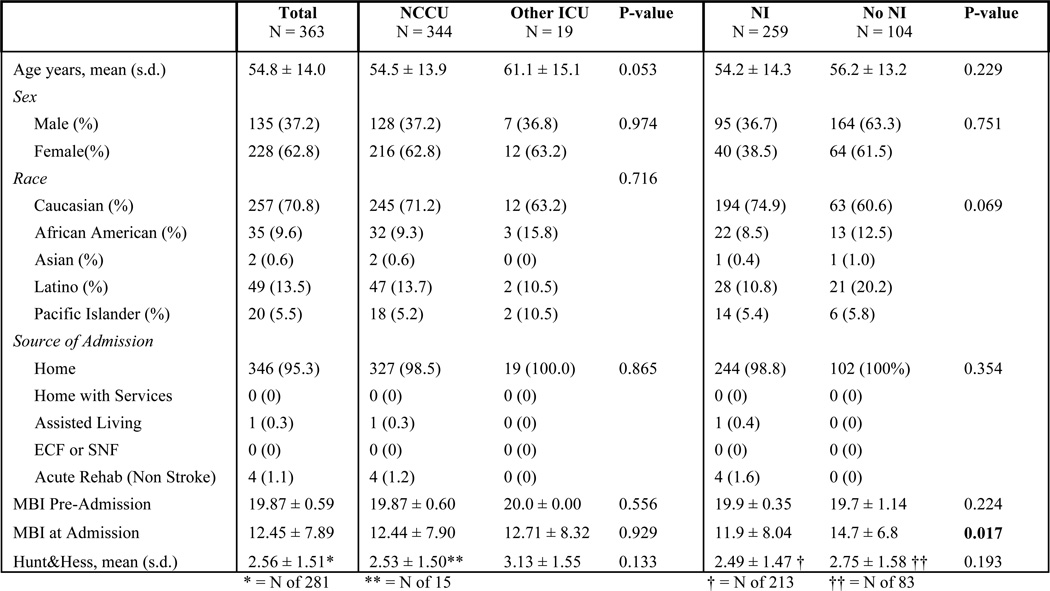
Of the 2,096 stroke patients who met eligibility criteria, there were 1242 AIS patients, 491 ICH patients and 363 SAH patients. Figures 3, 4 and 5 show patient demographics for AIS, ICH and SAH respectively. The mean age was 68.8 ± 15.6 for AIS, 63.8 ± 15.9 for ICH and 54.8 ± 14.0 for SAH. AIS patients did not differ demographically across groups. For ICH, only source of admission differed significantly. However, because these two groups had no difference in pre-admission MBI (a better proxy for pre-morbid functional status), differing admission source was not considered a confounder. For SAH, the only demographic difference was that NI-treated patients had a significantly lower MBI on admission. However, Hunt and Hess scores did not differ, indicating that the patient groups were equally impaired at time of admission.
Figure 3.
Ischemic Stroke Demographics
Figure 4.
Intracerebral Hemorrhage Demographics
Figure 5.
Subarachnoid Hemorrhage Demographics
Ischemic Stroke
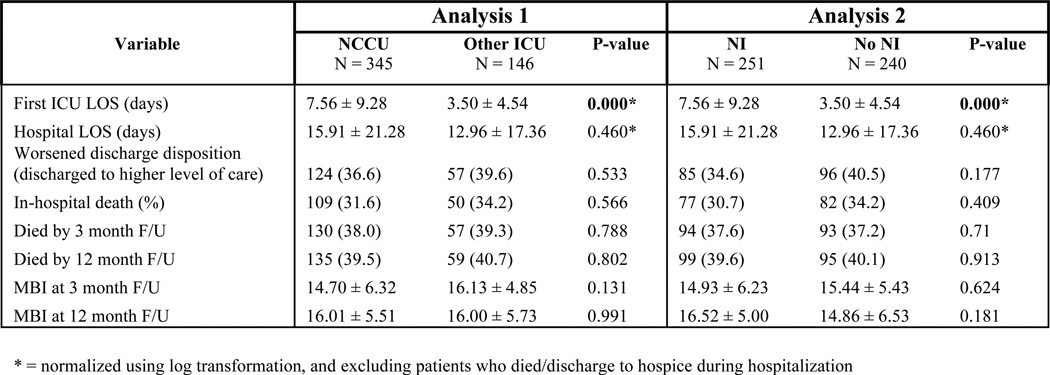
Figure 6 shows outcomes of the univariate analysis for AIS patients. Patients treated in the NCCU or by an NI had lower in-hospital mortality, better discharge disposition, and shorter hospital LOS compared to controls. When LOS data was re-analyzed excluding the subpopulation who died during hospitalization or were discharged to hospice, this finding remained significant. However, neither the presence of an NI nor an NCCU significantly improved NIHSS on discharge (a surrogate marker of functional status). In contrast, treatment in the NCCU (but not by an NI) was associated with better long-term outcomes compared to controls: cumulative mortality was lower for NCCU patients at 3 and 12 month follow-up, and their functional status (MBI) at 3 months was also significantly better.
Figure 6.
Ischemic Stroke: Univariate Analysis Outcomes
In subsequent multivariate analysis, after adjusting for covariates (age, NIHSS on admission, and receipt of tPA), the short-term outcome improvements on LOS and in-hospital mortality disappeared. However, treatment with a NI predicted good outcome at 3 month follow-up. This is specific to NI care, as treatment in any ICU (including the NCCU) in the post-NI period predicted significantly lower rates of return to pre-stroke MBI (OR 0.165, 95% CI 0.032 – 0.850, P = 0.031) compared to AIS patients treated by a NI.
For the time period analysis of AIS outcomes in the NCCU before, during and after an NI, race was a confounder (P = 0.038). For ICU LOS, there was a trend toward shorter ICU stay in the NI period compared to the pre-NI period (P = 0.064) but there was no change in LOS after the NI departure suggesting this measure is not influenced by specific NI care. No differences over the time periods were seen in any other short- or long-term outcomes.
Intracerebral Hemorrhage
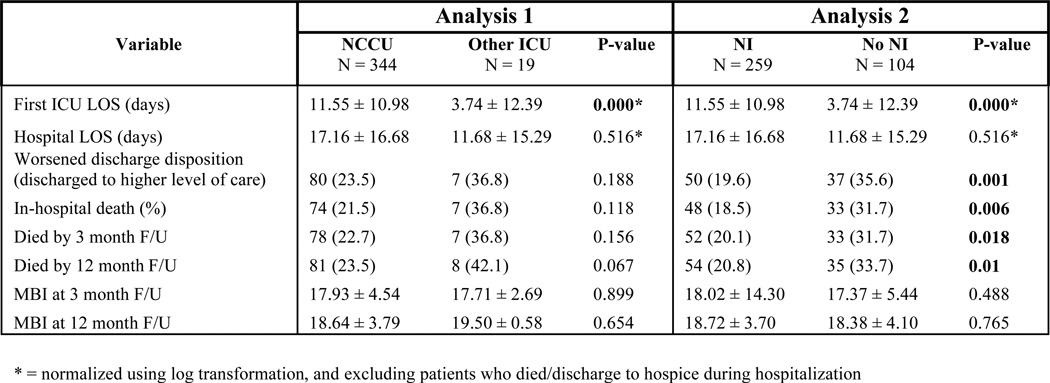
Figure 7 shows outcomes of the univariate analysis for ICH patients. For patients treated in the NCCU or by an NI, ICU LOS was significantly longer compared to controls, a difference that remained when excluding the subpopulation who died or went to hospice. There were no differences in any other outcome. In subsequent multivariate analysis, after adjusting for covariates (age, ICH score on admission, and presence of IVH), treatment in the NCCU independently predicted shorter ICU and hospital LOS (OR 0.625, 95% CI 0.427 – 0.915, P = 0.016 and OR 0.649, 95% CI 0.444 – 0.947, P = 0.025). Thus, the prolonged LOS seen in initial univariate analysis was likely secondary to NCCU patients being older and sicker – and thus requiring more prolonged intensive care – than non-NCCU patients. For the time period analysis for the presence or absence of a NI for ICH patients, no significant differences in any outcome measure was seen.
Figure 7.
Intracerebral Hemorrhage: Univariate Analysis Outcomes
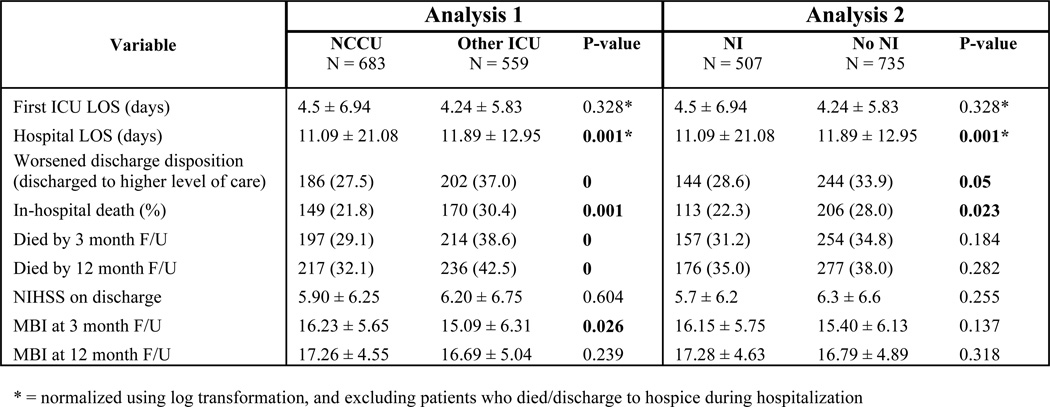
Subarachnoid Hemorrhage
Figure 8 shows outcomes of the univariate analysis for SAH patients. There was a longer ICU LOS in both the NCCU and NI groups compared to controls, a difference which remained when excluding the subpopulation who died or went to hospice. Most notably, treatment by an NI conferred specific benefits that were not seen in treatment in an NCCU: these included improved discharge disposition, lower in-hospital mortality or withdrawal of care, and lower mortality at 3 and 12 month follow-up. On multivariate analysis, after adjusting for covariates (age, Hunt & Hess score, and presence of IVH), several outcome variables showed a main effect of time. Compared to the during-NI period, treatment in any ICU in the post-NI period predicted a greater rate of in-hospital mortality or discharge to hospice (OR 2.206, 95% CI 1.062 – 4.584, P = 0.034), worsened discharge disposition (OR 2.455, 95% CI 1.165 – 5.177, P = 0.018), and shorter ICU LOS (OR 0.459, 95% CI 0.229 – 0.917, P = 0.028).
Figure 8.
Subarachnoid Hemorrhage: Univariate Analysis Outcomes
Analysis of SAH outcomes in the NCCU in the pre-, during- and post-NI time periods yielded several notable results. MBI (pre-admission and at admission) was a confounder: patients in the “during-NI” period were more functionally impaired than those in the pre-NI period. Hospital LOS significantly shortened after departure of the NI (P =0.012). Discharge disposition improved over time (P = 0.002), most dramatically throughout the during-NI period compared to the pre-NI period. In-hospital mortality also improved over time (P = 0.003), with the greatest improvement occurring in the during-NI time period compared to the pre-NI time period.
Discussion
The primary aim of this study was to explore the relationship between the presence of a NI and outcomes of AIS, ICH and SAH. We sought to determine whether outcomes were affected by the physical presence of a NCCU and/or the availability of a NI. This is the largest study of stroke outcomes from NCCU patients to date. It is also the first study that tracked clinical outcomes in critically ill stroke patients out to 12 months of follow-up, and the only study to assess the relative influence of improved medical care over time (as opposed to NI/NCCU care) on patient outcomes. Overall, this study suggests that presence of a NI is associated with improved outcomes in stroke patients, but the advantages of NCCU or NI care differ according to stroke type. Patients with SAH were the most likely to benefit when their care was coordinated by a neurointensivist.
For SAH patients, treatment during the time period when an NI was on staff resulted in significantly improved outcomes (mortality rate and discharge disposition), which was still present after controlling for possible confounders (age and Hunt/Hess score). Surprisingly, treatment in a dedicated NCCU did not confer these same benefits. Moreover, disposition and in-hospital mortality ‘leveled off’ after the NI departed, suggesting that this effect was independent of general advances in medical care over time and NCCU-specific care. However, outcomes did not worsen in the post-NI period, which is reassuring. This could be due to the persistence of systemic changes introduced by the NI, rather than his physical presence/personal expertise. Our ICU model – a community hospital setting where the nurse practitioners and physician assistants responsible for primary bedside care were initially trained by an NI and effectively maintained better outcomes after his departure – could represent a more cost-effective way of bringing the benefits of subspecialist care to a larger segment of society. However, this data will have to be followed closely, as our results could simply be an effect of the small sample size and low power in the post-NI cohort. As more SAH patients are treated without a NI, but with otherwise similar services (NCCU, neurosurgery, interventional neuroradiology, with equivalent SAH volume), if outcomes worsen, this would have important implications for the development of recommendations for NI expertise at all sites treating SAH patients. A follow up study is planned for next year that will include a minimum of 18 months of post-NI data, and perhaps longer depending on recruitment of a new NI.
Although SAH patients enjoyed improved outcomes with an NI, they also required greater resource utilization: departure of the NI resulted in significantly shorter ICU LOS in the multivariate analysis, and shorter hospital LOS in the time cohort analysis. This contradicts an earlier study, which found that NI co-management of an NCCU with neurosurgery decreased LOS in SAH patients.8 This may be partially attributable to our inability to control for functional status pre-stroke and on admission: although NI-treated patients had worse MBI on admission compared to controls, missing data made it too difficult to use MBI as a covariate in the multivariate analysis.
In contrast to SAH, availability of an NCCU or NI conferred only small benefits to AIS patients, and no benefit at all to ICH patients. For AIS, patient characteristics (age, NIHSS on admission and receipt of tPA) were the only predictors of stroke outcomes, with one exception: return to pre-stroke MBI at 3 month follow-up was significantly worse in the post-NI period. However, the latter finding must be approached with caution, as many patients were lost to follow-up and a survival bias could skew these results. For ICH, outcomes were also largely unaffected by type of ICU or specialty of the treating physician, and were the poorest of the three cohorts, consistent with previous studies14, 15.
It is not surprising that SAH patients benefited from availability of a NI, while AIS and ICH patients generally did less so or not at all. SAH represents a disease at the intersection of neurologic and neurosurgical illness, where coordination and collaboration of healthcare teams may be most crucial. Because NIs are often trained in bedside procedures such as insertion of ICP monitors and EVDs, they can provide close monitoring and early intervention for cerebral edema and hydrocephalus that often arise in SAH patients without requiring immediate access to a neurosurgeon. Future analyses should explore this hypothesis, asking: what specific qualities of a NI are responsible for better SAH outcomes? As mentioned, one theory is that NIs improve rates of post-stroke complications. Complications were initially collected in this study, but ultimately excluded from analysis due to lack of inter-rater reliability (there were no clear criteria for what qualified as a complication worthy of entry into the database). This is a good topic for future exploration. Future analyses should also examine the role of specific NI-introduced procedures and protocols on SAH outcomes. Did bedside procedures – e.g., ICP monitor use, extraventricular drain (EVD insertion), transcranial doppler, and continuous EEG – increase with arrival of a NI? Did they decrease after his departure, or were they continued by the practitioners that he trained? Most importantly, did these procedures contribute to better SAH outcome?
Our study has several limitations and the data should be interpreted with these in mind. This was a single-center retrospective study, so causal relationships between NCCU/NI presence and improved stroke outcome cannot be definitively proven. Additionally, as only 6 months of data were available in the post-NI cohort, the small number of patients precludes detailed analysis of outcomes after SAH in this cohort. Continued data collection will improve the power of future studies. It is also likely that a “lag phase” exists for any improved outcomes that were secondary to the NI’s arrival, which may have minimized the impact of the NI in AIS and ICH. Indeed there may also be a “lag phase” from his departure, and outcomes could conceivably worsen over the next year, again stressing the importance of performing a follow up study in the future. Possible changes related to outcomes that occurred during the NI’s “off-service weeks” could also not be determined, due to the complexity of determining the patient sample (as some patients were in the NCCU for several weeks, some with the NI and some without the NI as the primary attending). As the NI also performed consults and led the stroke team on off service weeks, it is likely that his physical presence encouraged compliance with protocols, but this cannot be definitively shown in this study. Retrospective analyses may also be subject to inherent bias. However, much of the information, including in-hospital and long-term mortality, LOS, stroke severity measures (NIHSS, ICH score and Hunt/Hess score), and functional status (MBI) at admission and follow-up, was obtained from a prospectively-collected database and is consistent with previous literature.1,6–8,12,13. In addition, factors that influence outcome after stroke (i.e., NIHSS or ICH score, age, sex, time to treatment, and medical complications) were included in our multivariate analysis. Using the three time periods and comparing between ICU types allowed us to rule out improvements in general medical care over time, which has been impossible in earlier studies.
It is clear from this study that factors that influence outcomes in critically ill stroke patients are complex and multifactorial, and are often more related to patient factors than the specifics of their ICU care. This work suggests that the NCCU improves outcomes for AIS patients, and the presence of a NI improves outcomes specifically in SAH patients. As evidence that the availability of a trained NI improves outcome in critically ill stroke patients need for this specialized physician will continue to rise.
References
- 1.Diringer MN, Edwards DF. Admission to a neurologic/neurosurgical intensive care unit is associated with reduced mortality rate after intracerebral hemorrhage. Crit Care Med. 2001;29:635–640. doi: 10.1097/00003246-200103000-00031. [DOI] [PubMed] [Google Scholar]
- 2.Hemphill JC, III, Bleck T, Carhuapoma R, et al. Is neurointensive care really optional for comprehensive stroke care? Stroke. 2005;36:2344–2345. doi: 10.1161/01.STR.0000185667.61420.94. [DOI] [PubMed] [Google Scholar]
- 3.Alberts MJ, Latchaw RE, Selman WR, et al. Recommendations for comprehensive stroke centers: a consensus statement from the brain attack coalition. Stroke. 2005;36:1597–1616. doi: 10.1161/01.STR.0000170622.07210.b4. [DOI] [PubMed] [Google Scholar]
- 4.Broderick J, Connolly S, Feldmann E, et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update: a guideline from the American Heart Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38:2001–2023. doi: 10.1161/STROKEAHA.107.183689. [DOI] [PubMed] [Google Scholar]
- 5.Bederson JB, Sander Connolly E, Batjer H, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- 6.Bershad EM, Feen ES, Hernandez OH, Fareed M, Suri K, Saurez JI. Impact of a specialized neurointensive care team on outcomes of critically ill acute ischemic stroke patients. Neurocrit Care. 2008;9:287–292. doi: 10.1007/s12028-008-9051-5. [DOI] [PubMed] [Google Scholar]
- 7.Varelas PN, Schultz L, Conti M, Spanaki M, Genarrelli T, Hacein-Bey L. The impact of a neuro-intensivist on patients with stroke admitted to a neurosciences intensive care unit. Neurocrit Care. 2008;9:293–299. doi: 10.1007/s12028-008-9050-6. [DOI] [PubMed] [Google Scholar]
- 8.Josephson SA, Douglas VC, Lawton MT, English JD, Smith WS, Ko NU. Improvement in intensive care unit outcomes in patients with subarachnoid hemorrhage after initiation of neurointensivist co-management. J Neurosurg. 2010;112:626–630. doi: 10.3171/2009.8.JNS09441. [DOI] [PubMed] [Google Scholar]
- 9.Kraus JJ, Metzler MD, Coplin WM. Critical care issues in stroke and subarachnoid hemorrhage. Neurol Res. 2002;24 Suppl 1:S47–S57. doi: 10.1179/016164102101200032. [DOI] [PubMed] [Google Scholar]
- 10.Shah S. Modified Barthel Index or Barthel Index (Expanded) In: Salek S, editor. Compendium of quality of life instruments Part II. Chichester: Wiley & Sons; 1998. [Google Scholar]
- 11.de Vos M, Graafmans W, Keesman E, Westert G, van der Voort PHJ. Quality measurement at intensive care units: which indicators should we use? J Crit Care. 2007;22:267–274. doi: 10.1016/j.jcrc.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Suarez JI, Zaidat OO, Suri MF, et al. Length of stay and mortality in neurocritically ill patients: impact of a specialized neurocritical care team. Crit Care Med. 2004;32:2311–2317. doi: 10.1097/01.ccm.0000146132.29042.4c. [DOI] [PubMed] [Google Scholar]
- 13.Varelas PN, Conti MM, Spanaki MV, et al. The impact of a neurointensivist-led team on a semiclosed neurosciences intensive care unit. Crit Care Med. 2004;32:2191–2198. doi: 10.1097/01.ccm.0000146131.03578.21. [DOI] [PubMed] [Google Scholar]
- 14.van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 15.Flaherty ML, Haverbusch M, Sekar P, et al. Long-term mortality after intracerebral hemorrhage. Neurology. 2006;66:1182–1186. doi: 10.1212/01.wnl.0000208400.08722.7c. [DOI] [PubMed] [Google Scholar]