Abstract
Aims
Bladder compliance is one expression of the pressure and volume relationship as the bladder fills. In addition to passive elements, autonomous micromotional detrusor activity contributes to this relationship. In the mouse cystometric model, compliance pressure contributes to voiding expulsive pressure. During attempts to isolate the detrusor contractile component of this filling pressurization, we found that compliance reversibly diminishes under conditions which remove central control from the micturition cycle.
Methods
Ten mature female mice underwent constant infusion pressure/flow cystometry under urethane anesthesia, and five awake mature female mice underwent constant infusion pressure cystometry. Following baseline cystometry, all mice were anesthetized with isoflurane to abolish the micturition reflex, and cystometry conducted with manual emptying of the bladders. Animals were then allowed to recover from isoflurane to re-establish the micturition reflex, and cystometry again conducted. The urethane group was also studied immediately postmortem. Repeated measures comparisons of cystometric parameters were made across conditions.
Results
Compliance reversibly decreased in all mice with the abolishment of micturition responses by isoflurane anesthesia. A similar decrease was observed immediately postmortem in the urethaned mice. Bladder filling and voiding were not different between the intact micturition segments of the testing.
Conclusions
Enhanced compliance in mice with intact micturition responses suggests that autonomous micromotional activity is suppressed by central processes during normal filling. Since afferent activity during filling is also determined by the relationship between bladder pressure and volume, a feed-forward afferent signal conditioning mechanism may exist, creating novel therapeutic targets for urinary dysfunctions.
Keywords: Urinary Bladder, Urodynamics, Compliance
INTRODUCTION
Bladder compliance is a clinical term expressing the relationship of bladder volume to pressure prior to the onset of a voiding contraction. A highly compliant bladder, such as the normal human bladder, creates very little pressure in response to physiologic filling. Loss of compliance results in elevated filling pressures, is associated with pathologic conditions of the bladder or its neural control, and may result in renal damage. Unlike humans, in the mouse cystometric model bladder pressure gradually rises from baseline pressure until a voiding threshold pressure is achieved, followed by the voiding detrusor contraction The total pressure rises to its maximum value at which time flow commences, accompanied by a drop in total bladder pressure; at flow termination, a brief isovolumetric rise in bladder pressure is followed by resolution of the detrusor contraction (1). Rats and mice also generate an abdominal wall contraction during voiding(2), thus expulsive pressure during flow is created by the pre-existing compliance pressure, detrusor contraction, and abdominal wall contraction. Bladder pressure during filling, i.e. attributable to compliance, is the result of passive elements interacting with autonomous detrusor muscle contractile activity (3). Thus, the total contribution of detrusor muscle contraction to expulsive pressure is complex.
As a preliminary step towards using a mouse cystometric model to study the impact of aging and disease on detrusor contractile function, we sought to isolate the various contributors to bladder pressure during the mouse voiding cycle. We hypothesized that conditions eliminating the micturition reflex in vivo would diminish autonomous (micromotional) activity and therefore the pressure/volume slope during filling. We anticipated that if our hypothesis was refuted, it would be due to a failure to demonstrate any differences. However, we report the opposite finding, that the pressure/volume slope increases under conditions which abolish the micturition response, both reversibly and terminally.
MATERIALS AND METHODS
Fifteen mature female B6 mice were prepared for continuous infusion cystometry. Study protocols were approved by the institutional animal care committee. Ten mice were studied under urethane anesthesia to permit pressure/flow cystometry, and were prepared following the establishment of urethane anesthesia (1.2 gm/kg i.p.) using a previously reported protocol (1). Five mice underwent awake cystometry to confirm findings under urethane, and were prepared under isoflurane anesthesia. Via a low abdominal incision, a flared-tip 10 cm length of PE50 was placed into the bladder through the dome, and secured with 6-0 silk tie. In urethane animals, the catheter was exteriorized beneath the left forepaw, and in awake mice the catheter was tunneled over the left forepaw to exit via a small dorsal neck incision. Catheters were secured to the abdominal wall and skin to ensure the bladder was not under tension and the catheter did not lie against the bladder base, and the abdomen closed with running silk suture.
For cystometric study under urethane, animals were placed prone with the urethral meatus lying over a 1 cm opening, beneath which was positioned a 5 cm cup filled with tissue paper, suspended on a Grass FT01 force transducer. One gram of voided fluid was assumed to equal 1 ml. Awake cystometry was conducted with the animal freely mobile in a tall 15×20 cm Lucite box to avoid tension on the catheter; only bladder pressure was recorded in this group. Bladder catheters were connected to two infusion pumps in parallel, one programmed for a constant infusion rate of 1.5 ml/hour, and the other for withdrawal. A pressure transducer (BP1, World Precision Instruments) was connected in-line. Transducer outputs were digitized at 30Hz and acquired using WinDaq Pro+ (DataQ Instruments). Room temperature saline was used for all bladder infusions. Instruments were calibrated before each study.
All animals underwent sequential cystometric study under basal conditions (urethane or awake), then isoflurane inhalation anesthesia (nominally 1.5% in O2) to eliminated the voiding reflex, followed by return to basal condition allowing return of the voiding reflex. The urethane-anesthetized animals were then euthanized with CO2 and cystometry immediately repeated (typically completed within 10 minutes of death). Since isoflurane anesthesia (and euthanasia) abolished the micturition reflex, the bladder was actively emptied using the withdrawal pump under these conditions. Isoflurane concentration was adjusted slightly to use the smallest concentration resulting in elimination of the micturition reflex. Isoflurane was administered by hood to the urethane animals, and by closing and ventilating the box with the anesthetic mixture in the awake group. In the latter case, the box was flushed with oxygen and allowed to remain open following the isoflurane phase of the study.
In the initial basal state, following the recording of three filling/voiding cycles, mean base pressure (Pbase, lowest post-void bladder pressure), voiding contraction threshold pressure (Pthresh, bladder pressure at which the voiding contraction commenced), and filling pressure-time slope (ΔP/Δt between Pbase and Pthresh) were calculated. A hypothetical maximum compliance pressure (Pcomp) was calculated by taking the intercept of the slope at the moment flow started. Voiding flow rate (Q) was calculated for each urethane-anesthetized mouse. Under basal condition cystometry, the bladder was allowed to fill and empty according to the micturition response. Under conditions eliminating micturition, the bladder was filled to a pressure slightly above Pcomp, then emptied at the animal’s Q until pressure dropped somewhat below Pbase. This was done to allow bladder filling and emptying within the same compliance-associated pressure range of intact micturition, at the same rates of filling and emptying. In awake animals, the mean urethane Q, 0.26 ml/s, was used for emptying. For the post-euthanasia cystometry, the distal urethra was ligated prior to study.
Under each sequential condition, three or more filling/emptying cycles were recorded for analysis after a grossly consistent pattern was established in each condition. Following isoflurane anesthesia, typically ten minutes were required for reflex micturition to return. Until voiding was re-established, the bladder was emptied as above.
Analysis
For each cycle, an overall filling slope (Full Slope) was determined between the Pbase and Pthresh. For the non-voiding conditions (isoflurane, euthanasia), the pressure-time slope was taken between the Pbase and Pthresh values determined during the initial Urethane condition. Since the pressure/volume relationship of the bladder is not linear in the filling mouse bladder, the slope of the first 10% (10% Slope) and the last 10 (90% Slope) of the Pbase through Pthresh range were also determined. Mean values of body weight, voided volume and Q were calculated for voiding cycles in the urethane anesthesia animals, and slopes, Pbase and Pthresh for voiding cycles in urethane and awake cystometry. The distribution of mean values for the 15 animals was tested for normality using Kolmogorov-Smirnov test. Comparisons between the pre- and post-isoflurane voiding conditions in urethane and awake animals were made with paired t test. Slope data between conditions in each group were compared with repeated measures ANOVA with Bonferroni test or the nonparametric repeated measures Friedman test with Dunn’s multiple comparisons, as appropriate. Microsoft Excel and Prism 5 (GraphPad Software, LaJolla CA) were used for analysis and statistical calculations, p<0.05 regarded as statistically significant.
RESULTS
Mean animal weight was 30 +/− 1.3 gms. Figure 1 demonstrates representative tracings from one urethane-anesthetized animal under each sequential condition. Two mice in the urethane group leaked at pressures less than the contraction threshold pressure under urethane (23 vs. 41 cm.w, and 40 vs. 52 cm.w). These data were treated by regarding filling as occurring between Pbase and the leakage pressure (rather than Pthresh). A slope for the first 10% of filling was determined from this interval and used in the 10% slope results, but not in the overall or 90% results. In urethane and awake voiding groups, pressure, volume, and flow data did not significantly differ between pre- and post-isoflurane, and are presented in Table 1. Slopes under isoflurane and post-euthanasia in the urethane group did not differ.
Figure 1.
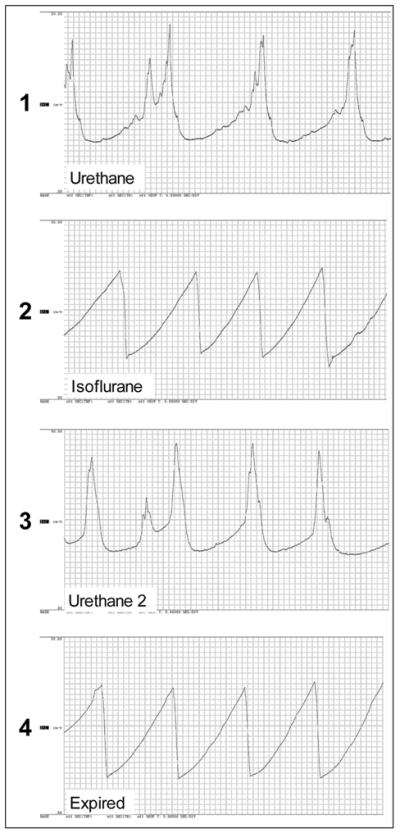
Tracings under each sequential condition for one animal, progression is top to bottom strip. Time scale 5 sec / division. Vertical scale 0 – 60 cm.w.
Table I.
Comparison of parameters for urethane and awake cystometry, before and after isoflurane suppression. Pbase: base pressure. Pthresh: voiding contraction threshold pressure. Comparisons by paired two-sided paired t-test.
| Urethane | Pre-Isoflurane | Post-Isoflurane | p | ||
|---|---|---|---|---|---|
| mean | s.e.m | mean | s.e.m | p | |
| per Void volume, ml | 0.14 | 0 .013 | 0.14 | 0.012 | 0.99 |
| Flow Rate, ml/s | 0.026 | 0.0031 | 0.020 | 0.0020 | 0.14 |
| Pbase, cm water | 9 | 1.1 | 9 | 1.2 | 0.66 |
| Pthresh, cm water | 29 | 3.8 | 22 | 3.9 | 0.06 |
|
| |||||
| Awake | Pre-Isoflurane | Post-Isoflurane | |||
|
| |||||
| mean | s.e.m | mean | s.e.m | p | |
|
| |||||
| Pbase, cm water | 11 | 1.9 | 10 | 1.9 | 0.74 |
| Pthresh, cm water | 23 | 3.5 | 25 | 5.5 | 0.36 |
Since bladder pressure is a function of filling volume, and filling always occurred at the same rate, analyzed slopes are expressed as change in pressure per change in time (compliance, as the term is generally used, is the inverse of this slope multiplied by the filling rate). In each of the 15 animals studied, the full slope during isoflurane was greater (to varying degrees) than the slope obtained during voiding cycles before and after isoflurane-induced suppression of voiding. With one exception, the post-euthanasia full slope was likewise greater than during the voiding cycles in the urethane-anesthetized animals; for one mouse, the post-euthanasia slope was greater than the first voiding cycle, but equal to the post-isoflurane voiding cycle. Figure 2 shows the change in slopes under sequential conditions of all 15 animals.
Figure 2.
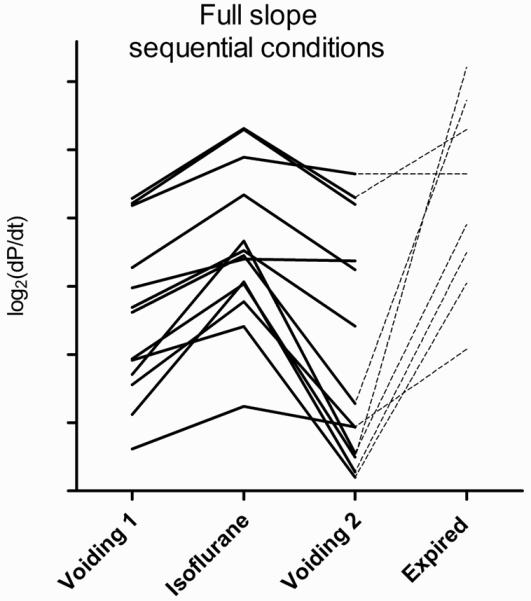
Sequential full slope (ΔP/Δt) values for each of 15 mice. Slopes expressed as log2(ΔP/Δt) used to clarify graphical representation. All isoflurane slopes are greater than pre- and post-isoflurane slopes. Dotted lines connect to post-euthanasia slopes, urethane group only.
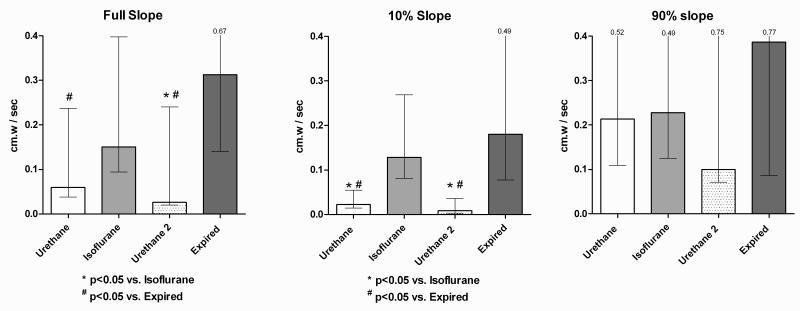
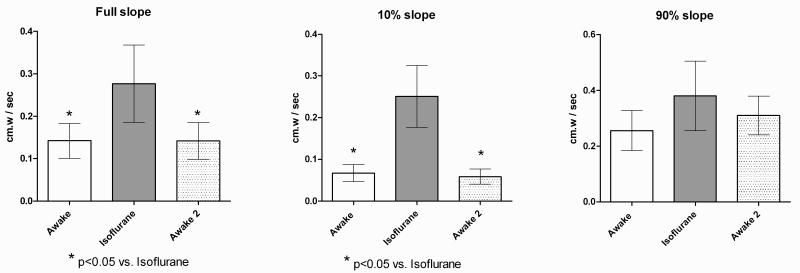
In the urethane group, the full slope (n=8) of euthanized animals was significantly greater than both in the pre- and post-isoflurane basal voiding conditions, and the slope during isoflurane was significantly greater than in the post-isoflurane phase. In the awake group, the full slope during isoflurane cystometry was significantly greater than the intact voiding cycles before and after isoflurane. For both urethane and awake groups, comparison of the first 10% Slopes demonstrated significant differences between the voiding conditions vs. the two non-voiding conditions, however no significant differences between conditions were found for the 90% Slope. Figures 3 and 4 show the comparisons of mean slope values between sequential conditions for urethane and awake cystometry groups.
Figure 3.
Pressure – time slopes, sequential urethane / isoflurane / urethane / expired conditions. Constant filling rate 1.5 ml/hr. Slopes taken between basal and voiding contraction threshold pressure. Isoflurane and Expired slopes taken between mean base and threshold pressures. Comparisons by nonparametric repeated measures Friedman test with Dunn’s multiple comparisons. Bars show medians with interquartile range.
Figure 4.
Pressure – time slopes, awake / isoflurane conditions / awake conditions. Constant filling rate 1.5 ml/hr. Slopes taken between base and voiding contraction threshold pressure. Isoflurane slopes taken between mean base and threshold pressures. Comparisons by repeated measures ANOVA with Bonferroni post-test. Means with error bars showing s.e.m..
DISCUSSION
We have demonstrated a clear and consistent increase in pressure-volume slope (and thus decreased compliance) under conditions eliminating central control of the micturition response in the mature female mouse cystometric model. The pressure-volume slope expresses bladder pressure as a function of volume, reflecting that pressure responses to increasing volume are a function of bladder wall characteristics. This relationship is dependent on the passive elastic and viscoelastic elements (collagen, elastin, and the passive mechanical characteristics of smooth muscle cells), the autonomous “micromotional” activity of the smooth muscle cells (the active element), filling rate, and the bladder’s geometry (3). The sequential methodology of this study allows the assumption that the last two features are constant across conditions for each animal. Furthermore, the passive elements are unlikely to undergo significant change during the time course of our methodology. Use of a consistent filling and emptying rate in all conditions minimizes the potential impact of viscoelasticity due to filling at supraphysiologic cystometric rates. Therefore, the observed changes in pressure-volume slope may be attributed to altered micromotional activity of the detrusor during filling. The increased slope observed during suppression or elimination of the micturition response thus reflects increased detrusor tone via enhanced micromotional activity.
Several factors suggest these changes are due to the suppression or elimination of the micturition response, rather than to other effects of the anesthetic agents. Findings from the complete pressure-flow model under urethane anesthesia were confirmed by awake cystometry, eliminating potential influence by urethane. The effect is reversible upon re-establishment of central control and therefore cannot be ascribed to iatrogenic tissue damage. Euthanasia by definition removes central control as well as the ongoing delivery of pharmacologic agents, and spontaneous detrusor muscle activity is noted in in-vitro strip studies (4, 5), suggesting an uninhibited active detrusor component to the postmortem pressure-flow relationship . Therefore, we interpret our findings as indicative of central suppression of detrusor micromotional activity during normal filling, thus increasing bladder compliance (decreased pressure/time slope) to allow low-pressure filling
While the anesthetic agents employed potentially affected the magnitude of responses, the direction of the responses cannot be attributed directly to anesthetic pharmacology. Despite its inhibitory effect on several signaling pathways and toxicity concerns, urethane is an accepted anesthetic agent for continuous filling cystometry in rodent models due to its preservation of the micturition reflex (6-8). Urethane and isoflurane are both associated with changes in suppression of central activity and modulation of relevant ion channel receptor activity. including potentiated inhibitory GABAA, glycine, and nicotinic receptor activity (9, 10). Urethane also inhibits stimulatory ionotropic glutamate receptor (NMDA, AMPA) activity (9). The effects of both drugs are therefore primarily suppressive of central processes pertaining to afferent processing and motor control (and therefore the micturition reflex to volume) rather than a direct effect on the detrusor muscle. Local effects are likely to be relaxatory, contrary to our findings of increased slope under the influence of isoflurane. Isoflurane inhibits M3 receptor activity in vitro (11), and is associated with relaxation of cardiovascular smooth muscle (12). Furthermore, while at higher concentrations isoflurane abolishes the micturition response (13) (ca. 1.5% as we observed), at intermediate concentrations isoflurane increases the interval between voiding contractions without affecting baseline or threshold pressures, therefore decreasing the pressure-volume slope (14).
Micromotional activity has been postulated to be necessary to allow individual muscle bundles to adjust the length in response to filling, thus allowing the bladder to minimize surface area in preparation for the generation of expulsive pressure with voiding (15). This is balanced by an unidentified relaxing factor (16), allowing minimal resistive pressure to renal drainage into the bladder. A similar loss of compliance following sedation vs. the fully awake state was demonstrated in a pig model, and was found to be sensitive to atropine(17). A tonic low-level parasympathetic discharge was therefore hypothesized to be responsible for loss of compliance during sedation. Investigations using feline decentralized bladder suggested that early filling bladder wall stiffness is modulated downwards by sympathetic activity (18). Thus, in this context our data suggest that rather than an active increase in pro-contractile neurotransmitter in the decentralized bladder, detrusor activity is actively suppressed when central control is intact. Bladder filling characteristics in the awake animal could thus be viewed as an active sympathetic/parasympathetic balance.
Interpreting the pressure-volume relationships of bladder filling over large intervals risks mischaracterizing differences in pressure responses to gradual increases in volume, in part because the pressure/volume relationship demonstrates exponential, rather than linear, behavior (3). In our study, we observed grossly larger slope differences in the first 10% of filling when compared to the total filling interval, and no significant differences in the final 10% of filling, suggesting that most of the difference in pressure-volume response between conditions occurs early in filling. This suggests that over the physiologic range of bladder volumes, filling bladder compliance is primarily determined by detrusor muscle tone at very low volumes, and with progressive filling the pressure/volume relationship becomes increasingly dependent upon the interaction between the active and passive elements of the bladder wall. The importance of these interactions is the potential for alterations in the sensory transduction of both volume and rate of volume change information, associated with age and disease-induced changes in bladder structure and detrusor tone regulation.
Bladder afferent activity is a function of bladder wall stress (19) and therefore the pressure-volume relationship of the bladder wall during filling. Therefore, by modulating the active (micromotional) contribution to bladder wall compliance, afferent activity as a function of volume may be modulated in real time. This leads to the interpretation that under normal circumstances, bladder wall stiffness during filling may be actively adjusted by a feed-forward mechanism in order that received afferent signals are consistent with cognitive/executive expectations of urine storage. Analogous feed-forward afferent signal conditioning is thought to exist in other sensory systems such as vision (20, 21) and hearing (22). Indeed, disease processes associated with impaired brain processes, such as white matter hyperintensity disease, Lewy-body dementia, and autonomic failure have been associated with diminished bladder compliance (23-25). This concept may allow the brain to adjust for variability in the relatively fixed aspects of end-organ mechanotransduction process (within limits). An inability to adjust for end-organ dysfunction and/or malfunctions in this central regulation of afferent activity generation could thus contribute to urinary dysfunction and symptoms. Enhanced or diminished volume afferent responses would occur if detrusor filling suppression is inappropriately decreased or increased. This is consistent with previous suggestions that urodynamic pathologies such as volume hypersensitivity (“sensory overactivity”), detrusor overactivity and detrusor underactivity may be understood as reflecting dysfunctional control of micromotional activity during filling (26).
CONCLUSIONS
Bladder compliance during urine storage, primarily in the early filling phases, reversibly decreased upon abolition of micturition responses. Based on our findings, we now hypothesize a feed-forward mechanism by which central processes regulate the translation of bladder volume to afferent activity as the bladder fills, in order to condition sensory signaling and processing. Aberrations in this regulation would logically affect volume sensory transduction and therefore may lead to abnormal sensations (perceived as urinary symptoms) and further dysregulation. Confirmation of this effect in humans, the impact of aging and disease on the existence and magnitude of the effect, and further investigation into the involved neuropharmacologic pathways will be required as this potential therapeutic target is elucidated.
Acknowledgements
This research funded by: Dennis A. Jahnigen Scholars Career Development Award, American Geriatrics Society, PPS, PI NIH R01AG028657, GAK PI
REFERENCES
- 1.Smith PP, Kuchel GA. Continuous uroflow cystometry in the urethane-anesthetized mouse. Neurourol Urodyn. 2010 Feb 1; doi: 10.1002/nau.20850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cruz Y, Downie JW. Abdominal muscle activity during voiding in female rats with normal or irritated bladder. Am J Physiol Regul Integr Comp Physiol. 2006 May;290(5):R1436–45. doi: 10.1152/ajpregu.00556.2005. [DOI] [PubMed] [Google Scholar]
- 3.Coolsaet BL. Bladder Compliance and Detrusor Activity During the Collection Phase. Neurourol Urodyn. 1985;4:263–73. 1985. [Google Scholar]
- 4.Meng E, Young JS, Brading AF. Spontaneous activity of mouse detrusor smooth muscle and the effects of the urothelium. Neurourology and urodynamics. 2008;27(1):79–87. doi: 10.1002/nau.20456. [DOI] [PubMed] [Google Scholar]
- 5.Sibley GN. A comparison of spontaneous and nerve-mediated activity in bladder muscle from man, pig and rabbit. The Journal of physiology. 1984 Sep;354:431–43. doi: 10.1113/jphysiol.1984.sp015386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maggi CA, Meli A. Suitability of urethane anesthesia for physiopharmacological investigations. Part 3: Other systems and conclusions. Experientia. 1986 May 15;42(5):531–7. doi: 10.1007/BF01946692. [DOI] [PubMed] [Google Scholar]
- 7.Matsuura S, Downie JW. Effect of anesthetics on reflex micturition in the chronic cannula-implanted rat. Neurourol Urodyn. 2000;19(1):87–99. doi: 10.1002/(sici)1520-6777(2000)19:1<87::aid-nau9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 8.Cannon TW, Damaser MS. Effects of anesthesia on cystometry and leak point pressure of the female rat. Life Sci. 2001 Jul 27;69(10):1193–202. doi: 10.1016/s0024-3205(01)01182-1. [DOI] [PubMed] [Google Scholar]
- 9.Hara K, Harris RA. The anesthetic mechanism of urethane: the effects on neurotransmitter-gated ion channels. Anesth Analg. 2002 Feb;94(2):313–8. doi: 10.1097/00000539-200202000-00015. table of contents. [DOI] [PubMed] [Google Scholar]
- 10.Yamakura T, Harris RA. Effects of gaseous anesthetics nitrous oxide and xenon on ligand-gated ion channels. Comparison with isoflurane and ethanol. Anesthesiology. 2000 Oct;93(4):1095–101. doi: 10.1097/00000542-200010000-00034. [DOI] [PubMed] [Google Scholar]
- 11.Nietgen GW, Honemann CW, Chan CK, Kamatchi GL, Durieux ME. Volatile anaesthetics have differential effects on recombinant m1 and m3 muscarinic acetylcholine receptor function. British journal of anaesthesia. 1998 Oct;81(4):569–77. doi: 10.1093/bja/81.4.569. [DOI] [PubMed] [Google Scholar]
- 12.Torri G. Inhalation anesthetics: a review. Minerva Anestesiol. 2010 Mar;76(3):215–28. [PubMed] [Google Scholar]
- 13.Yaksh TL, Durant PA, Brent CR. Micturition in rats: a chronic model for study of bladder function and effect of anesthetics. Am J Physiol. 1986 Dec;251(6 Pt 2):R1177–85. doi: 10.1152/ajpregu.1986.251.6.R1177. [DOI] [PubMed] [Google Scholar]
- 14.Chang HY, Havton LA. Differential effects of urethane and isoflurane on external urethral sphincter electromyography and cystometry in rats. Am J Physiol Renal Physiol. 2008 Oct;295(4):F1248–53. doi: 10.1152/ajprenal.90259.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brading AF. Spontaneous activity of lower urinary tract smooth muscles: correlation between ion channels and tissue function. 2006. pp. 13–22. [DOI] [PMC free article] [PubMed]
- 16.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev. 2004 Jul;84(3):935–86. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- 17.Mills IW, Drake MJ, Greenland JE, Noble JG, Brading AF. The contribution of cholinergic detrusor excitation in a pig model of bladder hypocompliance. BJU Int. 2000 Sep;86(4):538–43. doi: 10.1046/j.1464-410x.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- 18.Skehan AM, Downie JW, Awad SA. Control of detrusor stiffness in the chronic decentralized feline bladder. J Urol. 1993 May;149(5):1165–73. doi: 10.1016/s0022-5347(17)36340-1. [DOI] [PubMed] [Google Scholar]
- 19.le Feber J, van Asselt E, van Mastrigt R. Afferent bladder nerve activity in the rat: a mechanism for starting and stopping voiding contractions. Urological Research. 2004;32(6):395–405. doi: 10.1007/s00240-004-0416-8. [DOI] [PubMed] [Google Scholar]
- 20.Theeuwes J. Top-down and bottom-up control of visual selection. Acta Psychol (Amst) 2010 Oct;135(2):77–99. doi: 10.1016/j.actpsy.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Mihalas S, Dong Y, von der Heydt R, Niebur E. Mechanisms of perceptual organization provide auto-zoom and auto-localization for attention to objects. Proc Natl Acad Sci U S A. 2011 Apr 18; doi: 10.1073/pnas.1014655108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson D. Centrifugal control in mammalian hearing. Clin Exp Pharmacol Physiol. 2009 Jul;36(7):603–11. doi: 10.1111/j.1440-1681.2009.05185.x. [DOI] [PubMed] [Google Scholar]
- 23.Sakakibara R, Hattori T, Uchiyama T, Yamanishi T. Urinary function in elderly people with and without leukoaraiosis: relation to cognitive and gait function. J Neurol Neurosurg Psychiatry. 1999 Nov;67(5):658–60. doi: 10.1136/jnnp.67.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakakibara R, Ito T, Uchiyama T, Asahina M, Liu Z, Yamamoto T, et al. Lower urinary tract function in dementia of Lewy body type. J Neurol Neurosurg Psychiatry. 2005 May;76(5):729–32. doi: 10.1136/jnnp.2004.046243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakakibara R, Uchiyama T, Asahina M, Suzuki A, Yamanishi T, Hattori T. Micturition disturbance in acute idiopathic autonomic neuropathy. J Neurol Neurosurg Psychiatry. 2004 Feb;75(2):287–91. [PMC free article] [PubMed] [Google Scholar]
- 26.Gillespie JI. The autonomous bladder: a view of the origin of bladder overactivity and sensory urge. BJU International. 2004;93(4):478–83. doi: 10.1111/j.1464-410x.2003.04667.x. [DOI] [PubMed] [Google Scholar]