Abstract
The validity and reproducibility of echocardiographic methods used to quantify mitral regurgitation (MR) in children with congenital heart disease are unknown. We evaluated the usefulness of methods used to quantify MR in children enrolled in a multicenter trial of enalapril 6 months after surgical repair of an atrioventricular septal defect (AVSD). MR severity in this trial was assessed using body surface area (BSA)-adjusted vena contracta lateral (i-VCWlat) and anterior-posterior (i-VCWap) dimensions and cross-sectional area (i-VCA), regurgitant volume/BSA, regurgitant fraction, and qualitative MR grade. For each method, association with left ventricular end-diastolic volume (LVEDVz) and end-diastolic dimension (LVEDDz) z-scores and interobserver agreement were assessed. In 149 children (median age 1 year), i-VCWlat, i-VCWap, and i-VCA were best associated with LVEDVz (r2 = 0.54, r2 = 0.24, and r2 = 0.46, respectively; p < 0.001 for all) and showed the highest interobserver agreement (intraclass correlation coefficient = 0.62, 0.73, and 0.68, respectively). Qualitative MR grade was also associated with LVEDVz (r2 = 0.31, p < 0.001) and showed modest interobserver agreement (kappa 0.56). Regurgitant volume/BSA and regurgitant fraction were associated with LVEDVz (r2 = 0.45 and r2 = 0.45, p < 0.001 for both) but showed poor interobserver agreement [ICC = 0.28 (n = 91) and ICC = 0.17 (n = 76), respectively], and their values were negative in 75% of subjects. In conclusion, echocardiographic assessment of MR severity after AVSD remains challenging. Among the quantitative methods used in this trial, i-VCW and i-VCA performed the best but offered little advantage compared with qualitative MR grade. The utility of regurgitant volume and fraction was severely limited by poor interobserver agreement and frequently negative values.
Keywords: Mitral regurgitation, Quantification, Echocardiography, Children, Atrioventricular septal defect
Introduction
Assessment of the severity of mitral regurgitation (MR) is often performed using qualitative grading. Qualitative assessment may be confounded by several technical and hemodynamic factors, and quantitative methods have been sought that are less sensitive to these confounders [16]. Several echocardiographic methods for quantifying MR have been described and validated in adults with normal cardiac anatomy, and recommendations for their use have been published [20]. However, these techniques may have limitations in children, particularly those with congenital heart disease. Residual MR is an important complication after surgical repair of atrioventricular septal defect (AVSD) [3, 4, 15, 18]. Both qualitative and quantitative methods of evaluating the severity of MR in these children may be compromised by the presence of multiple jets, a noncircular cross-sectional jet profile, eccentric direction of flow into the left atrium with marked Coanda effect, and dynamic regurgitant orifice. However, a systematic comparison of these methods has not been previously performed in children with AVSD. In this study, we compared the usefulness of echocardiographic methods used to assess the severity of MR during a multicenter trial of enalapril in children after surgical repair of AVSD.
Methods
Subjects
To evaluate the effectiveness of enalapril for the treatment of MR, seven North American centers prospectively enrolled children after primary biventricular repair of an AVSD between June 2004 and February 2006. The study was funded by the National Heart Lung and Blood Institute. Children<17.5 years old and within 28 days of repair were eligible for entry into the trial. Inclusion and exclusion criteria for entry into the present study are listed in Table 1. Patients were diagnosed with AVSD and subtyped using guidelines from the Society of Thoracic Surgeons [9]. As part of the planned drug trial, consecutive children with parent or guardian consent were enrolled into an observation-only phase for 6 months after surgery to allow the heart to adapt to surgical intervention and to residual septal defects and/or valve regurgitation. Prospective echocardiographic data collected at the end of this 6 month period were used for the current analysis. The institutional review boards of each participating institution approved the trial, and written informed consent was obtained from each subject’s legal guardian. The conduct of the trial was monitored by an independent Data and Safety Monitoring Board appointed by the National Heart, Lung, and Blood Institute.
Table 1.
Inclusion/exclusion criteria for current analysis
| Inclusion criteria |
| Age < 17.5 year |
| AVSD (complete, partial or transitional) after biventricular repair |
| Echocardiographic data collected 6 months after repair |
| Exclusion criteria |
| AVSD with tetralogy of Fallot |
| Partially or totally anomalous pulmonary venous connection |
| Mitral stenosis (mean Doppler gradient > 10 mmHg) |
| Aortic regurgitation (vena contracta/annular diameter ratio > 0.2) |
| Left-ventricular outflow tract obstruction (peak Doppler gradient > 20 mmHg) |
| Residual atrial or ventricular septal defect (> 3 mm in diameter) |
| Associated hypertrophic obstructive cardiomyopathy |
Echocardiography
Two-dimensional and Doppler echocardiography was performed at participating centers using a standardized protocol developed by the core laboratory. Sedation was used at the discretion of the participating institution. Studies were recorded either digitally or on ½-inch VHS videotape. Study measurements were performed locally, as well as at a central core laboratory, by pediatric cardiologists specialized in echocardiography. At the core laboratory, all analog studies were converted into digital format, and measurements were made by a single observer using a microprocessor-based workstation custom programmed for electronic caliper overlay of captured digital images. Image quality for the entire echocardiographic examination was scored qualitatively at the core laboratory as unacceptable, fair, good, or excellent.
Assessment of MR
Two-dimensional and color Doppler flow mapping of the mitral valve were performed from the apical and parasternal long-axis views with activation of zoom mode to maximize spatial and temporal resolution. During this trial, MR severity was assessed by using the following four methods, which were thought to be best suitable to this patient population:
Method 1: Qualitative MR grade (none/trace, mild, moderate, severe) was assigned based on subjective assessment of the color Doppler appearance of the components of the MR jet from multiple views. No definite criteria for grading were prespecified, and the severity of MR was graded based on the reader’s overall subjective impression.
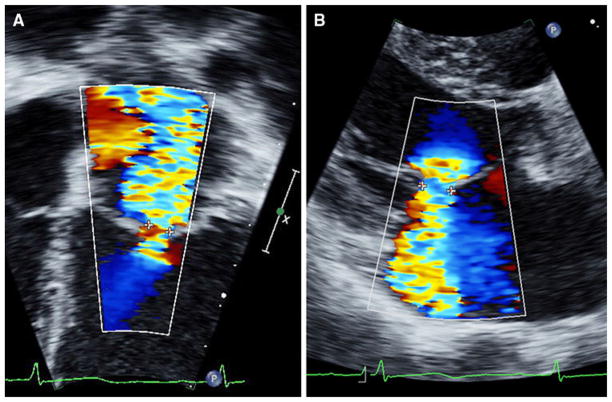
Method 2: Vena contracta width (VCW) in near-orthogonal [lateral (VCWlat) and anterior-posterior (VCWap)] planes were taken from apical and parasternal views (Fig. 1), respectively [8, 20]. To adjust for body size, indexed VCW (i-VCW) was calculated by dividing VCW by the square root of body surface area (BSA) [17]. Because the VCWs from multiple jets are not additive (flow across the regurgitant orifice relates to the square of the VCW of each orifice), subjects with >1 jet of regurgitation were excluded from this measurement.
-
Method 3: Vena contracta cross-sectional area (VCA) was calculated from orthogonal measurements of VCW using the formula for the area of an ellipse:
In case of multiple regurgitation jets, total VCA was calculated as the sum of the VCA for each jet. Total VCA was indexed (i-VCA) by dividing by BSA.
Method 4: For MR regurgitant volume and fraction, regurgitant volume was first calculated as the difference between left ventricular (LV) stroke volume from two-dimensional imaging and aortic stroke volume from Doppler echocardiography [6]. Regurgitant fraction was then calculated as regurgitant volume/LV stroke volume. Regurgitant volume was indexed (i-regurgitant volume) by dividing by BSA.
Fig. 1.
Measurement of VCW in near-orthogonal planes. a VCWlat was measured in the apical four-chamber view. b VCWap was measured in the parasternal long-axis view
Other methods for assessing the severity of MR, including the assessment of effective regurgitant orifice area, measurement of the jet area within the left atrium and mitral, and pulmonary venous inflow Doppler patterns, were not used in this study due to anticipated difficulties with their use in children with repaired AVSD. Left atrial volume can be a useful marker of MR severity, but this was not used because normative data in children were not available when we planned this study.
Assessment of LV Size and Function
Two-dimensional and M-mode echocardiography was used to obtain measures of LV size and systolic function. Ventricular dimensions were measured on M-mode echocardiography to obtain end-diastolic (LVEDD) and end-systolic LV dimensions. End-diastolic and end-systolic endocardial contours of the LV were traced on two-dimensional parasternal short-axis images, and LV length was measured on apical four-chamber view to obtain LV end-diastolic and end-systolic volumes. LV stroke volume and ejection fraction were calculated using an area-length method (volume = 5/6 * area * length) as recommended by the American Society of Echocardiography [13]. LV end-diastolic volume z-score (LVEDVz) and LV end-diastolic dimension z-score (LVEDDz) were calculated as the number of SDs from the normal population mean value relative to BSA [17].
Comparison of Methods for Assessing MR Severity
We compared the methods used to assess the severity of MR during this trial using the following criteria:
Criterion 1: Interobserver agreement between local and core laboratory observers.
Criterion 2: Degree of association with measures of LV dilation
Association with measures of LV dilation would support the validity of each method in these patients with stable MR where LV size is expected to increase with worsening regurgitation. With increasing regurgitant volume and no change in contractility, forward stroke volume (and hence cardiac output) are maintained by a gradual increase in LVEDV proportional to the severity of regurgitation [19].
Statistical Analyses
Agreement between local and core laboratory observers was evaluated using the intraclass correlation coefficient (ICC) for continuous measures and a weighted kappa statistic for qualitative MR grade. Linear regression modeling was used to evaluate the relationship between measures of MR and LVEDV and LVEDD z-scores and to test for the presence of interactions between predictor and three subgroup variables (type of AVSD, single vs. multiple jets, and sedation status). Quadratic terms for predictors of LVEDDz and LVEDVz were employed, where appropriate, to address nonlinear associations. Analyses examining sedation effects were restricted to subjects <2 years old because older subjects were not likely to need sedation for the echocardiogram. Analyses examining effect of single versus multiple jets were not conducted for VCWlat, and VCWap because these measures were calculated only in the presence of a single jet.
Results
Of the 181 children enrolled in the main trial, 149 [median age 1 year (range 0.5–17)] satisfied criteria for inclusion into the present study. Among these, 91 (61%) had undergone repair of a complete AVSD or an atrioventricular canal-type ventricular septal defect, whereas 58 (39%) had undergone repair of a partial or transitional AVSD.
Comparison of Methods for Assessing MR Severity
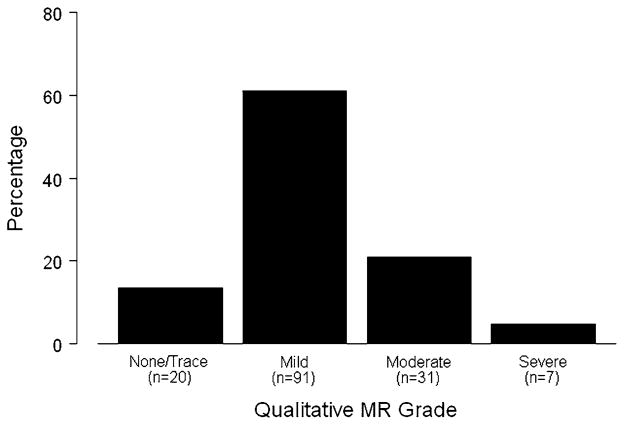
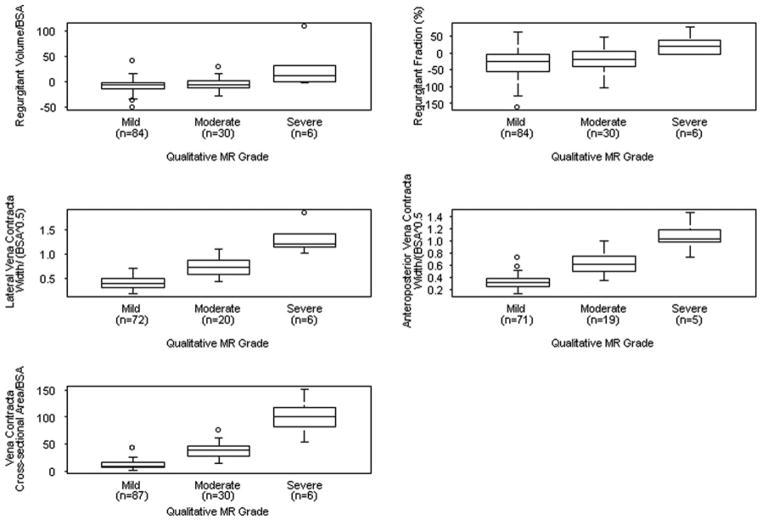
The distribution of qualitative MR grade is summarized in Fig. 2, and results of quantitative measures of MR are listed in Table 2. Qualitative MR grade was equal to or greater than moderate in 38 of 149 (26%) children. Relations between each of the quantitative measures and qualitative MR grade are shown in Fig. 3. Values for i-regurgitant volume and regurgitant fraction were often negative (in 75% of subjects); however, these measures nevertheless correlated with qualitative MR grade. Other quantitative measures, including i-VCWap, i-VCWlat, and i-VCA, also correlated with qualitative MR grade.
Fig. 2.
Distribution of patients by qualitative MR grade
Table 2.
Measures of MR and LV size and function
| Median (range) or mean ± SD | |
|---|---|
| i-VCWlat (cm/m) | 0.44 (0.2, 1.8) |
| i-VCWap (cm/m) | 0.35 (0.1, 1.5) |
| i-VCA (mm2/m2) | 16.0 (2.2, 150.7) |
| Regurgitant fraction (%) | −20 (−161.5, 78.9) |
| i-Regurgitant volume (ml/m2) | −6.4 (−50.7, 109.4) |
| LVEDD z-score | −0.49 ± 1.7 |
| LVEDV z-score | −0.005 ± 1.8 |
Fig. 3.
Box plots showing the relationship between quantitative measures of MR and qualitative MR grade. The middle line in the box represents the median. The lower and upper edges of the box represent the 25th and 75th percentile, respectively. All measures of MR correlated with qualitative MR grade. Values for regurgitant volume and fraction were frequently negative
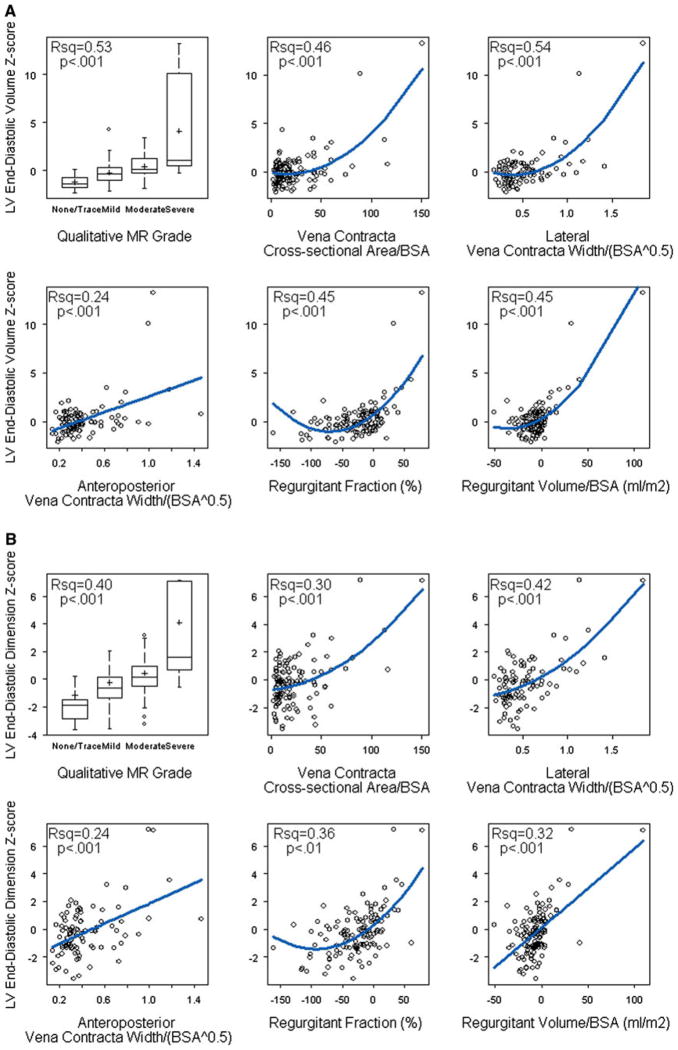
MR Severity and LV Size
Figure 4 shows the relationship between measures of MR and LV size (i.e., LVEDVz and LVEDDz). Note that to allow for stabilization of both MR severity and LV size, both were evaluated on the same echocardiogram performed 6 months after surgical repair. All methods, including i-regurgitant volume, regurgitant fraction, i-VCWlat, i-VCWap, and i-VCA and qualitative MR grade, were associated with LV size, and most of the relationships were nonlinear. With the exception of i-VCWap, each of these measures accounted for 46–54% of the variation in LVEDVz. As previously noted, values for i-regurgitant volume and regurgitant fraction were frequently negative. In multivariable modeling that allowed for nonlinear associations, LVEDVz was independently associated with qualitative MR grade, i-regurgitant volume, and regurgitant fraction but not with i-VCA, with the model explaining 77% of variability in LVEDVz.
Fig. 4.
Relationship between measures of MR and a LVEDVz and b LVEDDz. Blue lines denote the predicted regression values. All methods were associated with LV size, and most of the relationships were nonlinear. As previously noted, values for i-regurgitant volume (regurgitant volume/BSA) and regurgitant fraction were frequently negative
We then investigated whether the strength of association between each measure of MR and LV size was affected by either of the following factors: presence of>1 regurgitation jet and type of AVSD. Among the patients with MR, 28 had >1 jet of regurgitation. The presence of >1 regurgitation jet had a significant impact on the association between LVEDVz and qualitative MR grade (interaction p = 0.01). Qualitative MR grade was significantly associated with LVEDVz only in subjects with a single jet of regurgitation. This interaction was not seen with the other measures of MR.
The type of AVSD had a significant impact on the correlation between LVEDVz and severity of MR as assessed by i-VCWap, i-VCA, and regurgitant fraction (interaction p < 0.001 for all three measures). For each measure, the correlation with LVEDVz was stronger in patients with a partial AVSD. This interaction was not seen with i-regurgitant volume, i-VCWlat, or qualitative MR grade.
Interobserver Agreement
Results of tests of agreement between local and core laboratory observers for the various methods used to assess MR are listed in Table 3. Agreement between observers was modest for i-VCWap, i-VCWlat, and i-VCA and was marginally lower for qualitative MR grade. Agreement was poor for i-regurgitant volume and regurgitant fraction. The level of agreement was not impacted by either the number of regurgitation jets or the type of AVSD for any of the measures of MR.
Table 3.
Interobserver agreement in assessing MR
| ICC | Kappa statistic | |
|---|---|---|
| i-VCWlat (n = 75) | 0.62 | – |
| i-VCWap (n = 73) | 0.73 | – |
| i-VCA (n = 111) | 0.68 | – |
| Regurgitant fraction (n = 76) | 0.167 | – |
| i-Regurgitant volume (n = 91) | 0.281 | – |
| Qualitative MR grade (n = 149) | – | 0.56 |
Effect of Sedation
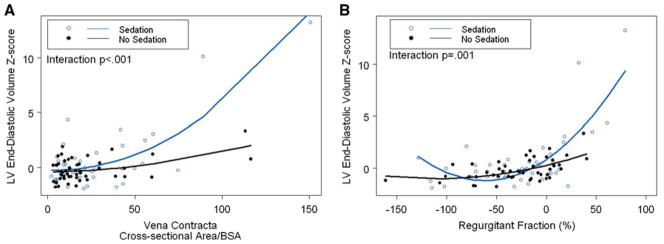
Sedation was administered based on each institution’s policy. Of the 117 echocardiograms performed in subjects <2 years of age, 54 (46%) were performed using sedation. Sedated echocardiograms yielded greater image-quality scores (Table 4). Administration of sedation also had a significant impact on the association between LVEDVz and severity of MR as assessed by most measures, including i-VCWap, i-VCA, regurgitant fraction, and qualitative MR grade (all interaction p values ≤0.001). Compared with unsedated children, children who received sedation had stronger associations (Fig. 5). In contrast, administration of sedation did not have a significant impact on interobserver agreement for any of the measures of MR.
Table 4.
Effect of sedation on image quality (in children <2 year old)
| Image-quality grade | n | % Sedateda |
|---|---|---|
| Excellent | 7 | 100 |
| Good | 94 | 46 |
| Fair | 15 | 27 |
| Unacceptable | 1 | 0 |
Mantel-Haenszel test for linear trend p = 0.002
Fig. 5.
Effect of sedation (in patients<2 years of age) on relationship between i-VCA and regurgitant fraction and LVEDVz. Administration of sedation had a significant impact on the association between LVEDVz and the severity of MR as assessed by most measures (all interaction p values ≤ 0.001). Compared with unsedated children, the associations were stronger in children <2 years of age who received sedation
Challenges in Calculating i-Regurgitant Volume and Fraction
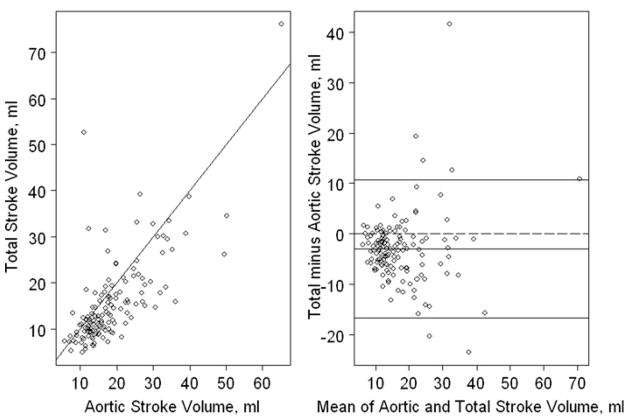
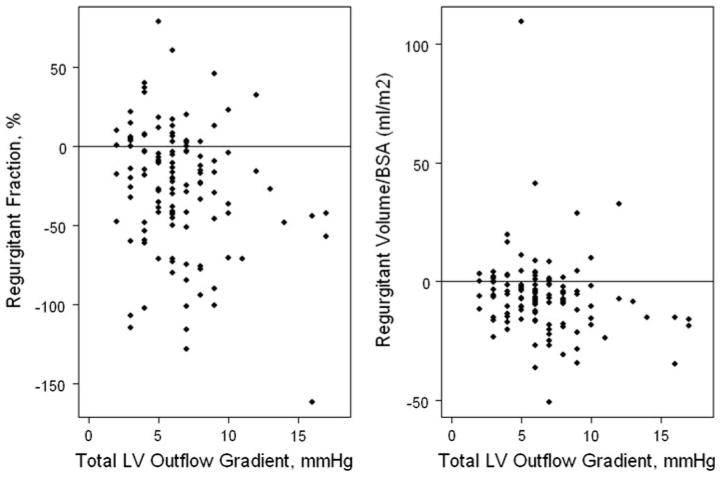
We explored possible reasons for the negative values obtained for i-regurgitant volume and fraction in the majority (75%) of subjects. Because regurgitant volume was calculated as the difference between total LV stroke volume and aortic stroke volume, underestimation could have occurred due to either an overestimation of aortic or underestimation of LV stroke volume. Figure 6 shows that in most subjects, aortic was greater than LV stroke volume, resulting in a negative value for regurgitant volume and fraction. As shown in Fig. 7, greater LV outflow tract gradient was associated weakly in an inverse fashion with i-regurgitant volume (r = −0.18, p = 0.04) and regurgitant fraction (r = −0.2, p = 0.03), suggesting that greater aortic outflow velocities may have contributed to overestimation of aortic stroke volume. Furthermore, the mean LVEDVz in subjects with none/trace MR by qualitative grading was −0.18, and the cardiac index calculated using LV measurements (2.8 ± 0.79 L/min/m2) was lower than the cardiac index calculated using aortic Doppler measurements (4.24 ± 1.01 L/min/m2, p < 0.001). These data suggest that a systematic underestimation of LVEDVz may have contributed to an underestimation of left-ventricular stroke volume.
Fig. 6.
Relationship between LV stroke and aortic stroke volume (a) scatterplot. The solid line represents y = x. b Bland-Altman plot. The middle solid line represents the mean difference between LV stroke volume and aortic stroke volume. The lower and upper solid lines represent the 95% CIs for the difference. In most subjects, aortic stroke volume was greater than LV stroke volume, resulting in a negative value for regurgitant volume and fraction
Fig. 7.
Relationship between Doppler-derived maximum instantaneous LV outflow tract pressure gradient and a regurgitant fraction and b regurgitant volume/BSA. A greater LV outflow tract pressure gradient was associated weakly in an inverse fashion with BSA-indexed regurgitant volume (r = −0.18, p = 0.04) and regurgitant fraction (r = −0.2, p = 0.03), suggesting that greater aortic outflow velocities may have contributed to overestimation of aortic stroke volume. This may have contributed to negatives values frequently observed for regurgitant volume and fraction
Discussion
In this multicenter, prospective trial of enalapril in children after repair of AVSD, several echocardiographic methods were used to assess the severity of MR before drug treatment. Among the techniques used, quantitative measures of MR based on measurement of the vena contracta, including i-VCWlat, i-VCWap, and i-VCA, demonstrated the highest interobserver agreement and highest association with measures of LV dilation, but these techniques were not significantly superior to qualitative MR grade. Regurgitant fraction and i-regurgitant volume were also associated with measures of LV dilation, but their usefulness was significantly limited by frequently negative values and poor agreement between local and core laboratory observers. Assessment of MR was easiest in the presence of a single jet of regurgitation, in patients with partial AVSD type and when echocardiography in children <2 years of age was performed with the child under sedation.
Accurate and reproducible quantification of the severity of residual MR is useful in guiding clinical management after repair of AVSD, and, in theory, quantitative methods may overcome some of the limitations of qualitative assessment. However, this theoretical advantage has proven difficult to translate into practice. Biner et al., in a recent study, evaluated the reproducibility of proximal isovelocity surface area, VCW, and regurgitant jet area for assessing MR severity in adults and found them to be only modestly reliable and associated with suboptimal interobserver agreement [2]. They also found that presence of an eccentric regurgitant jet and difficulty in visualizing the regurgitant orifice were predictors of poor interobserver agreement. Suboptimal interobserver agreement in evaluating MR by both qualitative and quantitative methods has also been reported by other investigators [1]. In contrast to these discouraging results, Foster et al. found adequate agreement between observers in assessing MR using qualitative grading, VCW, and regurgitant fraction and volume [7]. Importantly, none of these previous studies included children with congenital heart disease. This study was the first to examine this question in repaired AVSD, and although we did not have a reference technique for comparison, our results nevertheless highlight the practical difficulties with several quantitative methods that seem to offer little advantage compared with qualitative assessment.
Assessment of MR severity by both qualitative and quantitative methods in children with repaired AVSD may be complicated by several factors. In addition to difficulties associated with eccentric jet orientation and adequate visualization of the regurgitant orifice, other factors unique to this population may play an important role. Multiple jets of MR are common after repair of AVSD, and this can make the assessment of overall severity difficult, even with quantitative techniques, such as i-VCA [20]. Our finding that qualitative MR grade correlates with LV size only in the presence of a single jet of MR is in agreement with in vitro work performed by Lin et al. [11]. Adequate imaging in young children often requires sedation, and image quality may be comprised without its use. Our data confirm that image quality is improved in the sedated group, and as a result, quantitative measures of MR had a stronger association with LV size in the younger patients. A combination of these factors was likely responsible for the difficulties seen with both qualitative and quantitative assessment of MR in this study. Recently, three-dimensional (3-D) echocardiographic techniques for assessing the severity of MR have been proposed, and in the future these may help overcome some of the limitations of 2-D echocardiography [12].
Values for regurgitant fraction and i-regurgitant volume were frequently negative in our study. These parameters have been shown to be reliable indicators of the severity of MR in adult patients who do not have congenital heart disease and are not confounded by factors, such as eccentricity of the regurgitation jet, presence of multiple jets, difficulty in visualizing the regurgitation orifice, and technical settings, such as Doppler gain, Nyquist limit, and sampling frequency [5, 7, 10]. Due to these advantages, these techniques seemed potentially attractive in children after AVSD repair and were used to assess MR severity in this trial. However, several other factors likely compromised their reliability in practice. Previous studies have shown that 2-D echocardiographic techniques systematically underestimate LV volume by approximately 25% compared with cardiac magnetic resonance imaging (MRI) [14]. In our dataset, both mean LVEDV z-score and LV cardiac index were lower than expected in children with none/trace MR consistent with a systematic underestimation of LV volumes. Additionally, due to an elongated left-ventricular outflow tract, which is typical of AVSD, increased flow velocity at the aortic valve may lead to overestimation of aortic stroke volume and thus further underestimation of the regurgitant volume or fraction and negative values in children with trace or mild regurgitation. Despite the negative values, both regurgitant fraction and i-regurgitant volume were associated with measures of LV dilation, implying that although their values cannot be directly applied to the clinical situation, they do correlate with the severity of regurgitation. In future studies, it may be worth investigating whether these measurements could be included in an equation predicting regurgitation measured using a reference technique, such as MRI. However, their poor interobserver agreement will likely continue to limit their clinical usefulness. High variability between observers may be related to the compounded variance of the several independent 2-D and Doppler measurements required to calculate these parameters.
This study had several important limitations. First, we could not directly assess the accuracy of each method because no reference technique for quantifying MR was available. Although direct comparison with cardiac MRI would be useful, the feasibility of a side-by-side comparison may be limited by the requirement for additional sedation or anesthesia for MRI imaging. In the absence of a reference technique, we used the degree of association of each method with measures of LV dilation as a surrogate for validity. Second, although we had a sufficient number of patients with moderate or greater MR, the proportion of study subjects with severe MR was relatively small. Third, guidelines for administration of sedation for echocardiography varied among participating institutions, which likely led to nonuniform image quality. Fourth, although we present data on interobserver variability in assessing MR severity, intraobserver variability was not assessed. Finally, results from this population of children with AVSD may not apply to children or adults with MR due to other etiologies.
Conclusion
In conclusion, our study takes the first step in systematically comparing echocardiographic techniques for assessing the severity of MR in children after repair of AVSD by providing data on interobserver agreement and relationship with measures of LV dilation. Using these criteria, we found that reliable assessment of MR severity in this population remains challenging. Quantitative methods that used assessment of the vena contracta were only marginally superior to qualitative MR grade. Other quantitative methods, such as regurgitant fraction and volume, frequently yielded negative values with poor interobserver agreement, thus severely limiting their usefulness. Further work is needed to improve echocardiographic assessment of the severity of MR in children with congenital heart disease. In the future, comparison of a battery of 2-D with 3-D echocardiographic methods with a reference technique, such as cardiac MRI, may provide useful data.
Acknowledgments
This work was supported by U01 grants from the National Heart, Lung, and Blood Institute (Grants no. HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, and HL068288).
Footnotes
This study is conducted for the Pediatric Heart Network Investigators.
Contributor Information
Ashwin Prakash, Email: ashwin.prakash@cardio.chboston.org, Department of Cardiology, Children’s Hospital Boston, 300 Longwood Avenue, Boston, MA 02115, USA. Columbia University College of Physicians and Surgeons, New York, NY, USA.
Ronald V. Lacro, Department of Cardiology, Children’s Hospital Boston, 300 Longwood Avenue, Boston, MA 02115, USA
Lynn A. Sleeper, New England Research Institutes, Watertown, MA, USA
L. LuAnn Minich, University of Utah, Salt Lake City, UT, USA.
Steven D. Colan, Department of Cardiology, Children’s Hospital Boston, 300 Longwood Avenue, Boston, MA 02115, USA. New England Research Institutes, Watertown, MA, USA
Brian McCrindle, The Hospital for Sick Children, Toronto, ON, Canada.
Wesley Covitz, Wake Forest University Health Sciences, Winston Salem, NC, USA.
Fraser Golding, The Hospital for Sick Children, Toronto, ON, Canada.
Anthony M. Hlavacek, Medical University of South Carolina, Charleston, SC, USA
Jami C. Levine, Department of Cardiology, Children’s Hospital Boston, 300 Longwood Avenue, Boston, MA 02115, USA
Meryl S. Cohen, Children’s Hospital of Philadelphia, Philadelphia, PA, USA
References
- 1.Acker MA, Bolling S, Shemin R, Kirklin J, Oh JK, Mann DL, Jessup M, Sabbah HN, Starling RC, Kubo SH. Mitral valve surgery in heart failure: Insights from the Acorn Clinical Trial. J Thorac Cardiovasc Surg. 2006;132:568–577. doi: 10.1016/j.jtcvs.2006.02.062. [DOI] [PubMed] [Google Scholar]
- 2.Biner S, Rafique A, Rafii F, Tolstrup K, Noorani O, Shiota T, Gurudevan S, Siegel RJ. Reproducibility of proximal isovelocity surface area, vena contracta, and regurgitant jet area for assessment of mitral regurgitation severity. J Am Coll Cardiol Imag. 2010;3:235–243. doi: 10.1016/j.jcmg.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 3.Chowdhury UK, Airan B, Malhotra A, Bisoi AK, Kalaivani M, Govindappa RM, Venugopal P. Specific issues after surgical repair of partial atrioventricular septal defect: actuarial survival, freedom from reoperation, fate of the left atrioventricular valve, prevalence of left ventricular outflow tract obstruction, and other events. J Thorac Cardiovasc Surg. 2009;137:548–555. e542. doi: 10.1016/j.jtcvs.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 4.Dragulescu A, Fouilloux V, Ghez O, Fraisse A, Kreitmann B, Metras D. Complete atrioventricular canal repair under 1 year: Rastelli one-patch procedure yields excellent long-term results. Ann Thorac Surg. 2008;86:1599–1604. doi: 10.1016/j.athoracsur.2008.07.002. discussion 1604–1596. [DOI] [PubMed] [Google Scholar]
- 5.Dujardin KS, Enriquez-Sarano M, Bailey KR, Nishimura RA, Seward JB, Tajik AJ. Grading of mitral regurgitation by quantitative Doppler echocardiography: calibration by left ventricular angiography in routine clinical practice. Circulation. 1997;96:3409–3415. doi: 10.1161/01.cir.96.10.3409. [DOI] [PubMed] [Google Scholar]
- 6.Enriquez-Sarano M, Bailey KR, Seward JB, Tajik AJ, Krohn MJ, Mays JM. Quantitative Doppler assessment of valvular regurgitation. Circulation. 1993;87:841–848. doi: 10.1161/01.cir.87.3.841. [DOI] [PubMed] [Google Scholar]
- 7.Foster E, Wasserman HS, Gray W, Homma S, Di Tullio MR, Rodriguez L, Stewart WJ, Whitlow P, Block P, Martin R, Merlino J, Herrmann HC, Wiegers SE, Silvestry FE, Hamilton A, Zunamon A, Kraybill K, Gerber IL, Weeks SG, Zhang Y, Feldman T. Quantitative assessment of severity of mitral regurgitation by serial echocardiography in a multicenter clinical trial of percutaneous mitral valve repair. Am J Cardiol. 2007;100:1577–1583. doi: 10.1016/j.amjcard.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 8.Irvine T, Li XK, Sahn DJ, Kenny A. Assessment of mitral regurgitation. Heart. 2002;88(Suppl 4):iv11–iv19. doi: 10.1136/heart.88.suppl_4.iv11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs JP, Burke RP, Quintessenza JA, Mavroudis C. Congenital Heart Surgery Nomenclature and Database Project: atrioventricular canal defect. Ann Thorac Surg. 2000;69:S36–S43. doi: 10.1016/s0003-4975(99)01235-7. [DOI] [PubMed] [Google Scholar]
- 10.Kizilbash AM, Hundley WG, Willett DL, Franco F, Peshock RM, Grayburn PA. Comparison of quantitative Doppler with magnetic resonance imaging for assessment of the severity of mitral regurgitation. Am J Cardiol. 1998;81:792–795. doi: 10.1016/s0002-9149(97)01024-2. [DOI] [PubMed] [Google Scholar]
- 11.Lin BA, Forouhar AS, Pahlevan NM, Anastassiou CA, Grayburn PA, Thomas JD, Gharib M. Color Doppler jet area overestimates regurgitant volume when multiple jets are present. J Am Soc Echocardiogr. 2010;23:993–1000. doi: 10.1016/j.echo.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Little SH, Pirat B, Kumar R, Igo SR, McCulloch M, Hartley CJ, Xu J, Zoghbi WA. Three-dimensional color Doppler echocardiography for direct measurement of vena contracta area in mitral regurgitation: in vitro validation and clinical experience. JACC Cardiovasc Imaging. 2008;1:695–704. doi: 10.1016/j.jcmg.2008. 05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, Lai WW, Geva T. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23:465–495. doi: 10.1016/j.echo.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Margossian R, Schwartz ML, Prakash A, Wruck L, Colan SD, Atz AM, Bradley TJ, Fogel MA, Hurwitz LM, Marcus E, Powell AJ, Printz BF, Puchalski MD, Rychik J, Shirali G, Williams R, Yoo SJ, Geva T. Comparison of echocardiographic and cardiac magnetic resonance imaging measurements of functional single ventricular volumes, mass, and ejection fraction (from the Pediatric Heart Network Fontan Cross-Sectional Study) Am J Cardiol. 2009;104:419–428. doi: 10.1016/j.amjcard.2009.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murashita T, Kubota T, Oba J, Aoki T, Matano J, Yasuda K. Left atrioventricular valve regurgitation after repair of incomplete atrioventricular septal defect. Ann Thorac Surg. 2004;77:2157–2162. doi: 10.1016/j.athoracsur.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Sahn DJ. Instrumentation and physical factors related to visualization of stenotic and regurgitant jets by Doppler color flow mapping. J Am Coll Cardiol. 1988;12:1354–1365. doi: 10.1016/0735-1097(88)92621-6. [DOI] [PubMed] [Google Scholar]
- 17.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–457. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 18.Ten Harkel AD, Cromme-Dijkhuis AH, Heinerman BC, Hop WC, Bogers AJ. Development of left atrioventricular valve regurgitation after correction of atrioventricular septal defect. Ann Thorac Surg. 2005;79:607–612. doi: 10.1016/j.athoracsur.2004.07. 010. [DOI] [PubMed] [Google Scholar]
- 19.Uretsky S, Supariwala A, Nidadovolu P, Khokhar SS, Comeau C, Shubayev O, Campanile F, Wolff SD. Quantification of left ventricular remodeling in response to isolated aortic or mitral regurgitation. J Cardiovasc Magn Reson. 2010;12:32. doi: 10.1186/1532-429X-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, Stewart WJ, Waggoner A, Weissman NJ. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S08 94-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]