Abstract
Purpose
To examine the changes in knee cartilage T2 values over 24 months in subjects with and without risk factors for knee osteoarthritis (OA) and their association with focal knee lesions at baseline.
Materials and Methods
Forty-one subjects without, and 101 subjects with OA risk factors (such as history of knee injury or surgery) were selected from the Osteoarthritis Initiative database (age: 45-55 years, no radiographic OA in the right knee). Baseline MR images of the right knee were assessed for prevalence and grade of focal knee lesions. Right knee cartilage T2 measurements were performed in five compartments (patella, medial/lateral femur/tibia) at baseline and 24 month follow-up.
Results
Compared to subjects without OA risk factors, those with OA risk factors showed no significant differences in baseline prevalence and grade of focal knee lesions (p>0.05), but had significantly higher T2 values in the medial femur compartment at both time points (p<0.05). T2 values averaged over all five compartments increased significantly over 24 months in both groups, but differences in T2 increase between the groups were not significant. Subjects with cartilage lesions showed significantly higher T2 values compared to subjects without cartilage lesions at both time points, but no accelerated T2 increase over 24 months (p>0.05).
Conclusion
Cartilage T2 values significantly increased over 24 months in subjects with and without OA risk factors, but neither the presence of OA risk factors nor the presence of cartilage lesions at baseline were associated with these T2 increases.
Keywords: Osteoarthritis, OAI, MRI, WORMS, T2 relaxation time, T2 laminar analysis
Introduction
Osteoarthritis (OA) is the most common form of arthritis and one of the leading causes of disability in the elderly. Nearly 27 million individuals in the United States have clinically symptomatic OA and radiographic evidence is seen in at least 70% of the population over the age of 65 years (1). OA is characterized by the progressive loss of hyaline articular cartilage and the most commonly affected joint is the knee. Magnetic resonance imaging (MRI) has been found advantageous in the monitoring of OA with typical imaging characteristics including meniscal and cartilage abnormalities as well as bone marrow edema pattern (BMEP) (2;3). The initial stages of OA include proteoglycan loss, increased water content and deterioration of the collagen network (4;5). Several studies have shown the potential of quantitative MRI to study the cartilage matrix given its sensitivity to tissue hydration and biochemical composition (6-12). One of these techniques is T2 relaxation time mapping, which may be used as a biomarker to non-invasively assess cartilage quality. However, there is little information available how T2 values change over time in subjects with and without risk factors for knee OA and on how the time course of T2 values is associated with focal morphological knee lesions anywhere in the joint at baseline. Risk factors for OA include female gender, overweight and obesity, knee injury, and repetitive use of joints (13). In this study we focused on non-obese subjects with the OA risk factors knee symptoms, history of knee injury, history of knee surgery, family history of total knee replacement or Heberden nodes. It is in particular important to study subjects with OA risk factors, since they may most benefit from treatment or behavioural interventions.
The NIH launched the Osteoarthritis Initiative (OAI), a longitudinal, observational multi-center study with 4,796 participants, to better understand the natural history of OA (http://www.oai.ucsf.edu/). The study population consists of subjects with symptomatic knee OA at baseline, those with no symptomatic knee OA, but with risk factors for OA at baseline, and subjects without knee OA, knee pain or OA risk factors at baseline. The OAI database contains clinical data, biological samples, radiographs and MR images including a T2 mapping sequence (14).
We used the OAI public database and image archive to identify knees without pain and without radiographic OA at baseline in order to: (i) compare T2 relaxation time measurements and the prevalence and grade of focal knee lesions between subjects with OA risk factors other than obesity and subjects without obesity or other OA risk factors, (ii) to assess the changes in cartilage T2 relaxation time over a period of 24 months in the two groups and (iii) to evaluate the association of the presence of OA risk factors and focal knee lesions at baseline with changes in cartilage T2 relaxation time over 24 months. We hypothesized that subjects with knee OA risk factors and focal knee lesions would have higher cartilage T2 relaxation times and greater increases in T2 over time, indicating decreased cartilage quality, compared to subjects without OA risk factors or focal knee lesions.
Materials and Methods
Subjects
Data used in the preparation of this article were obtained from the Osteoarthritis Initiative (OAI) database, which is available for public access at http://www.oai.ucsf.edu/. Specific OAI datasets used were baseline clinical dataset 0.2.2, baseline imaging datasets 0.E.1 and 0.C.2, 24 month follow-up clinical dataset 3.2.1, and 24 month follow-up imaging datasets 3.E.1 and 3.C.2.
We studied the right knees of 142 subjects selected from the OAI incidence and normal control subcohorts. Subjects in the normal control subcohort had no radiographic findings of OA (defined as a definite tibiofemoral osteophyte) in either knee at baseline and had no OA risk factors at baseline. Subjects in the incidence subcohort did not have symptomatic knee OA, defined as frequent symptoms and radiographic OA in the same knee, in either knee at baseline, but had at least one of the following OA risk factors at baseline: knee symptoms (“pain, aching, or stiffness in or around the knee” in the past 12 months), overweight or obesity, history of knee injury, history of knee surgery, family history of total knee replacement or Heberden nodes.
Specific inclusion criteria for the subjects from both subcohorts for this study were: 45-55 years of age, body mass index (BMI) of 19-27kg/m2, Western Ontario and McMaster University (WOMAC) pain score of zero in both knees at baseline, and Kellgren-Lawrence (KL) score ≤1 (based on an additional reading done for the present study) in the right knee at baseline. In addition, baseline and 24 month follow-up right knee MR images had to be available and useable. These specific inclusion criteria were applied to exclude obesity as an OA risk factor and to focus on younger, relatively asymptomatic subjects. Based on these criteria, 101 subjects with OA risk factors (50 males, 51 females) and 41 subjects without OA risk factors (15 males, 26 females) were eligible and included in the study.
The study protocol, amendments and informed consent documentation were reviewed and approved by the local institutional review boards.
WOMAC Questionnaire
The Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index was established to evaluate clinical symptoms of OA in the knee, including pain, stiffness, and physical function over the last seven days (15;16). A WOMAC pain score of zero was used to include only subjects without knee pain over the previous seven days in this study.
Imaging
Bilateral standing posterior-anterior fixed flexion knee radiographs were acquired at baseline. Knees were positioned in a plexiglas frame (SynaFlexer, CCBR-Synarc, San Francisco, CA, USA) with 20°–30° flexion and 10° internal rotation of the feet. In an additional reading performed for the present study, knee radiographs were graded by two radiologists (with 5, respectively 22 years of experience) in consensus by using the Kellgren-Lawrence (KL) scoring system (17).
All subjects underwent 3T MRI (Trio, Siemens, Erlangen, Germany) at baseline and 24 month follow-up. The following five sequences were obtained in right knees, as described in the OAI MRI protocol (14): (a) a coronal two-dimensional intermediate-weighted turbo spin-echo (TSE) sequence (TE / TR = 29 / 3850 ms, field of view (FOV) = 14 cm, slice thickness = 3 mm, in-plane spatial resolution = 0.365 × 0.456 mm2, flip angle = 180, bandwidth = 352 Hz / pixel), (b) a sagittal three-dimensional dual-echo steady-state (DESS) sequence with water excitation and coronal and axial reformations (TE / TR = 4.7 / 16.3 ms, field of view (FOV) = 14 cm, slice thickness = 0.7 mm, in-plane spatial resolution = 0.365 × 0.456 mm2, flip angle = 25, bandwidth = 185 Hz / pixel), (c) a sagittal two-dimensional intermediate-weighted turbo spin echo (TSE) sequence with fat suppression (TE / TR = 30 / 3200 ms, field of view (FOV) = 16 cm, slice thickness = 3 mm, in-plane spatial resolution = 0.357 × 0.511 mm2, flip angle = 180, bandwidth = 248 Hz / pixel), (d) a coronal three-dimensional T1-weighted fast low-angle shot (FLASH) sequence with water excitation (TE / TR = 7.57 / 20 ms, field of view (FOV) = 16 cm, slice thickness = 1.5 mm, in-plane spatial resolution = 0.313 × 0.313 mm2, flip angle = 12, bandwidth = 130 Hz / pixel), and (e) a sagittal two-dimensional multislice multiecho (MSME) spin echo sequence for T2 mapping (TR = 2700 ms, seven TEs = 10 ms, 20 ms, 30 ms, 40 ms, 50 ms, 60 ms, 70 ms, field of view (FOV) = 12 cm, slice thickness = 3 mm with 0.5mm gap, in-plane spatial resolution = 0.313 × 0.446 mm2, bandwidth = 250 Hz / pixel).
Image Analysis
Baseline MR images of the right knee were transferred to picture archiving communication system (PACS) workstations (Agfa, Ridgefield Park, NJ, USA) and assessed for the presence and grade of meniscal, cartilage lesions and bone marrow edema pattern (BMEP) using a modified whole organ MRI score (WORMS) as previously described (18-20). Assessment was performed by two radiologists (with 5, respectively 22 years of experience) independently. Consensus reading was performed in case of disagreement. Cartilage lesions and BMEP were not assessed by using the original 15 regions, but six condensed regions (patella, trochlea, medial / lateral femur and medial / lateral tibia) because of the small number of lesions expected in our asymptomatic study population. Cartilage lesions were graded using an 8-point scale: 0, normal thickness and signal intensity; 1, normal thickness or swelling with abnormal signal on fluid sensitive sequences; 2, partial-thickness focal defect <1 cm in greatest width; 2.5, full-thickness focal defect <1 cm in greatest width; 3, multiple areas of partial thickness (Grade 2) defects intermixed with areas of normal thickness, or a Grade 2 defect wider than 1 cm but <75% of the region; 4, diffuse (>75% of the region) partial-thickness loss; 5, multiple areas of full-thickness loss (Grade 2.5) or a Grade 2.5 lesion wider than 1 cm but <75% of the region; 6, diffuse (>75% of the region) full-thickness loss. Condensing our anatomical regions from 15 to 6 would have potentially affected the frequency of grade 4 and 6 lesions, however, grade 4 lesions are very rare and usually if there is >75% partial thickness cartilage loss, full thickness lesions are present and grade 6 lesions are not expected in this cohort. BMEP were defined as poorly marginated areas of increased T2 signal intensity and graded using a four-point scale: 0, none; 1, diameter of <5mm; 2, diameter of 5-20mm; 3, diameter of >20mm. Meniscal lesions were graded separately in 6 regions (medial / lateral and anterior / body / posterior) using the following 4-point scale: 0, normal; 1, intra-substance abnormal signal; 2, non-displaced tear; 3, displaced or complex tear; 4, complete destruction / maceration. Compared to the original WORMS system, grade 1 was added to better reflect presence of early degenerative meniscal disease.
Similar to previous studies (18-20), a WORMS maximum score (WORMS Max) was assigned to each knee by the greatest WORMS score in any compartment. WORMS Max >0 in any joint structure was taken as an indication of a lesion. A meniscal WORMS Max >1 indicated a non-displaced tear or worse, while a cartilage WORMS Max >1 identified subjects with at least a partial thickness defect. The cartilage WORMS Max >1 could be used to exclude lesions characterized only by signal abnormalities, i.e. Grade 1 lesions.
The MSME spin echo sequences were transferred to a SUN workstation (Sun Microsystems, Mountain View, CA, USA) and T2 maps were calculated with custom-built software on a pixel-by-pixel basis skipping the first echo and using a noise-corrected exponential fitting. T2 measurements of the articular knee cartilage were performed in five compartments (patella, medial / lateral femur and medial / lateral tibia). The trochlea compartment was not evaluated due to flowing artifacts of the popliteal artery. Compartments were segmented with in-house software based on IDL (Interactive Data Language, Research Systems, Boulder, CO, USA) directly in the T2 maps. In order to exclude both fluid and chemical shift artifacts from the region of interest, a technique was used that allowed adjustment of the region of interest simultaneously in the T2 map and first echo of the multiecho sequence by opening separate image panels at the same time with synchronized cursor, slice number and zoom. This segmentation procedure has been applied in previous studies (18;20;21). T2 maps were segmented by one operator and supervised by a radiologist. Mean T2 values for each compartment were calculated after completed segmentation. In addition, we performed laminar analysis of the cartilage. As described previously (22;23), laminar analysis divided the segmented cartilage compartments in a superficial and a deep layer and corresponding mean T2 values were determined.
Statistical Analysis
The statistical analyses were performed with SPSS (SPSS Inc., Chicago, IL, USA) using a two-sided 0.05 level of significance. Differences in prevalence and severity of focal knee lesions as well as T2 values and changes in T2 values over time between subjects with and without OA risk factors were assessed by using multivariate linear and logistic regression models adjusting for age, gender, BMI and baseline KL-score. The T2 analysis was only performed using the values for the patella compartment, the medial femur compartment and for the mean of all five compartments to avoid multiple testing. The patella compartment was chosen to investigate cartilage degeneration in the patello-femoral joint, since this is a frequent site of degeneration. The medial femur was chosen, since it is a predominant weight bearing region and has a higher incidence of OA than the lateral side (24;25). Paired t-tests were used to determine differences between T2 values of the superficial and deep cartilage layer, respectively baseline and 24 month follow-up, in each group. T2 values and changes in T2 values over time of subjects with and without prevalent focal knee lesions anywhere in the joint were compared by using multivariate linear regression models adjusting for age, gender, BMI, baseline KL-score and subcohort (i.e. incidence or control subcohort).
We compared knees of subjects with OA risk factors versus those without OA risk factors. Given the differences in baseline KL-scores between the two groups we statistically adjusted for baseline KL-score. However, adjustment may be incorrectly performed when one group has only KL = 0 knees. Therefore we performed the statistical analysis not only in the whole sample size, but also repeated the statistical analysis in a restricted sample size with only KL = 0 knees. However, results were virtually identical when we restricted the analysis to only KL = 0 knees.
Reproducibility
To assess intra- and inter-reader reproducibility of the WORMS grading, 15 subjects were randomly selected and WORMS grading was performed two times by two readers independently. Intra-class correlation coefficients (ICC) were calculated to compare the exact WORMS score for meniscal and cartilage lesions and BMEP in each compartment (26).
An intra-reader (inter-reader) reproducibility for meniscal WORMS grading of 0.98 and 0.98 (0.95) was calculated, for cartilage WORMS grading of 0.96 and 0.97 (0.92) and for BMEP WORMS grading of 0.92 and 0.97 (0.93).
Intra-reader reproducibility for T2 measurements of each compartment and layer were determined in baseline T2 maps of 10 randomly selected subjects. T2 maps of each subject were segmented three times by one operator. Reproducibility errors for each compartment and layer were calculated as the root mean square error (ms) and as the root mean square error coefficient of variation (%) and are listed in Table 1 (27).
Table 1.
Intra-reader reproducibility errors for cartilage T2 measurements were calculated for each compartment and layer as the root mean square error (ms) and as the root mean square error coefficient of variation (%). LF: lateral femur, LT: lateral tibia, MF: medial femur, MT: medial tibia, and PAT: patella. The reproducibility errors averaged over all five compartments are printed in bold.
| whole compartment [%] |
whole compartment[ms] |
deep layer [%] |
deep layer [ms] |
superficial layer [%] |
superficial layer [ms] |
|
|---|---|---|---|---|---|---|
| LF | 1.52 | 0.53 | 2.13 | 0.72 | 2.05 | 0.75 |
| LT | 0.90 | 0.28 | 2.08 | 0.61 | 1.57 | 0.55 |
| MF | 0.90 | 0.35 | 1.48 | 0.56 | 1.52 | 0.60 |
| MT | 2.61 | 0.88 | 2.40 | 0.81 | 4.46 | 1.67 |
| PAT | 2.38 | 0.71 | 4.92 | 1.38 | 1.63 | 0.55 |
| mean | 1.66 | 0.55 | 2.60 | 0.82 | 2.24 | 0.82 |
Results
Baseline Subject Characteristics
Age, BMI, KL-score and frequency of OA risk factors of all subjects at baseline are listed in Table 2. Subjects with and without OA risk factors were not significantly different in age or BMI (p>0.05).
Table 2.
Baseline characteristics of subjects with OA risk factors and those without OA risk factors. KL-score and frequency of OA risk factors are given as n (%).
| Subjects with OA risk factors (n=101) |
Subjects without OA risk factors (n=41) |
|
|---|---|---|
| age [years] | 50.8±2.9 | 50.6±3.1 |
| BMI [kg/m2] | 24.0±1.8 | 23.4±2.0 |
| right KL-score = 0 | 75 (74.3%) | 41 (100%) |
| right KL-score = 1 | 26 (25.7%) | 0 (0%) |
| left KL-score = 0 | 77 (76.2%) | 41 (100%) |
| left KL-score = 1 | 21 (20.8%) | 0 (0%) |
| left KL-score = 2 | 3 (3.0%) | 0 (0%) |
| OA risk factors: | ||
| knee symptoms in the past 12 months | 88 (87.1%) | 0 (0%) |
| history of knee injury | 54 (53.5%) | 0 (0%) |
| history of knee surgery | 24 (23.8%) | 0 (0%) |
| family history of knee replacement surgery | 23 (22.8%) | 0 (0%) |
| Heberden nodes | 17 (16.8%) | 0 (0%) |
Focal Knee Lesions
Table 3 shows the prevalence of focal knee lesions by compartment. The greatest prevalence of meniscal lesions (WORMS >0) was seen in the medial posterior horn in subjects with and without OA risk factors, while the greatest prevalence of cartilage lesions and BMEP (WORMS >0) was observed in the patella in subjects with and without OA risk factors.
Table 3.
Prevalence of meniscal and cartilage lesions and BMEP (WORMS >0) in subjects with and without OA risk factors given as n (%).
| Subjects with OA risk factors (n=101) |
Subjects without OA risk factors (n=41) |
|
|---|---|---|
| Meniscus: | ||
| Medial Anterior WORMS >0 | 3 (3.0%) | 0 (0.0%) |
| Medial Body WORMS >0 | 21 (20.8%) | 9 (22.0%) |
| Medial Posterior WORMS >0 | 46 (45.5%) | 13 (31.7%) |
| Lateral Anterior WORMS >0 | 10 (9.9%) | 1 (2.4%) |
| Lateral Body WORMS >0 | 8 (7.9%) | 0 (0.0%) |
| Lateral Posterior WORMS >0 | 13 (12.9%) | 4 (9.8%) |
| Cartilage: | ||
| Patella WORMS >0 | 62 (61.4%) | 23 (56.1%) |
| Trochlea WORMS >0 | 24 (23.8%) | 8 (19.5%) |
| Medial Femur WORMS >0 | 18 (17.8%) | 4 (9.8%) |
| Lateral Femur WORMS >0 | 12 (11.9%) | 1 (2.4%) |
| Medial Tibia WORMS >0 | 10 (9.9%) | 1 (2.4%) |
| Lateral Tibia WORMS >0 | 23 (22.8%) | 8 (19.5%) |
| BMEP: | ||
| Patella WORMS >0 | 27 (26.7%) | 11(26.8%) |
| Trochlea WORMS >0 | 10 (9.9%) | 4 (9.8%) |
| Medial Femur WORMS >0 | 3 (3.0%) | 2 (4.9%) |
| Lateral Femur WORMS >0 | 3 (3.0%) | 1 (2.4%) |
| Medial Tibia WORMS >0 | 4 (4.0%) | 0 (0.0%) |
| Lateral Tibia WORMS >0 | 7 (6.9%) | 2 (4.9%) |
Prevalence of meniscal lesions (WORMS Max >0) and tears (WORMS Max >1) were not significantly different between subjects with and without OA risk factors after adjustment for age, gender, BMI and KL-score (p>0.05; Table 4). These groups also showed no significant differences in the grade of meniscal lesions (WORMS Max; p>0.05; Table 4). Prevalence of cartilage lesions (WORMS Max >0) and grade 2 or higher cartilage lesions (WORMS Max >1) were not significantly different between the two groups (p>0.05; Table 4). Similarly, differences in the grade of cartilage lesions (WORMS Max) were not significant between the two groups (p>0.05; Table 4). Subjects with and without OA risk factors also showed no significant differences in the prevalence of BMEP (WORMS Max >0) as presented in Table 4 (p>0.05).
Table 4.
Focal knee lesions of subjects with and without OA risk factors. WORMS Max >0 expresses prevalence of both, meniscal intra-substance degeneration and tear (respectively of both, cartilage signal abnormality and more severe lesion), whereas WORMS Max >1 specifies prevalence of meniscal tear (respectively of grade 2 or higher cartilage lesion). WORMS Max express grade of meniscal, respectively cartilage lesions.
| Subjects with OA risk factors (n=101) |
Subjects without OA risk factors (n=41) |
p-value* | |
|---|---|---|---|
| meniscal WORMS Max >0 | 52 (51.5%) | 15 (36.6%) | 0.560 |
| meniscal WORMS Max >1 | 33 (32.7%) | 6 (14.6%) | 0.347 |
| meniscal WORMS Max | 1.04±1.24 | 0.56±0.87 | 0.362 |
| cartilage WORMS Max >0 | 73 (72.3%) | 29 (70.7%) | 0.612 |
| cartilage WORMS Max >1 | 55 (54.5%) | 15 (36.6%) | 0.183 |
| cartilage WORMS Max | 1.92±1.66 | 1.29±1.18 | 0.094 |
| BMEP WORMS Max >0 | 39 (38.6%) | 16 (39.0%) | 0.472 |
Adjusted for age, gender, BMI and baseline KL-score.
Baseline T2 Measurements
Averaged over all five compartments, T2 values at baseline were higher in subjects with OA risk factors than in those without OA risk factors as shown in Table 5. However, differences were not significant (p>0.05). Compared to subjects without OA risk factors, subjects with OA risk factors had significantly higher T2 values in the medial femur compartment (whole compartment: 37.9±2.3ms vs. 36.9±2.3ms; p=0.044; deep cartilage layer: 35.7±2.3 ms vs. 34.6±2.3 ms; p=0.016), adjusted for age, gender, BMI and KL-score). T2 values of the patella compartment were similar between subjects with and without OA risk factors (p>0.05; Table 5).
Table 5.
Cartilage T2 values (ms) of subjects with and without OA risk factors for the deep and superficial cartilage layer as well as the whole compartment. Values printed in bold indicate statistically significant differences between subject groups (p<0.05).
| Subjects with OA risk factors (n=101) |
Subjects without OA risk factors (n=41) |
p-value* | |
|---|---|---|---|
| baseline T2 patella (deep layer) | 29.9±3.7+ | 29.4±3.3~ | 0.304 |
| 24 month T2 patella (deep layer) | 31.0±3.2+ | 30.9±3.2~ | 0.836 |
| baseline T2 patella (superficial layer) | 33.6±2.9+ | 33.9±3.8~ | 0.613 |
| 24 month T2 patella (superficial layer) | 35.4±3.7+ | 35.7±3.0~ | 0.557 |
| baseline T2 patella (whole compartment) | 31.7±2.8+ | 31.7±3.3~ | 0.744 |
| 24 month T2 patella (whole compartment) | 33.2±3.1+ | 33.3±2.5~ | 0.811 |
| delta T2 patella (whole compartment) | 1.3±2.7 | 1.7±2.6 | 0.449 |
| baseline T2 medial femur (deep layer) | 35.7±2.3 + | 34.6±2.3 # | 0.016 |
| 24 month T2 medial femur (deep layer) | 36.3±2.9 + | 35.0±2.1 # | 0.027 |
| baseline T2 medial femur (superficial layer) | 40.0±2.7- | 39.2±2.6# | 0.196 |
| 24 month T2 medial femur (superficial layer) | 40.1±2.9 | 38.6±2.4 # | 0.016 |
| baseline T2 medial femur (whole compartment) | 37.9±2.3 - | 36.9±2.3 # | 0.044 |
| 24 month T2 medial femur (whole compartment) | 38.2±2.7 - | 36.8±2.1 # | 0.013 |
| delta T2 medial femur (whole compartment) | 0.4±2.5 | −0.1±1.4 | 0.731 |
| baseline T2 averaged over all five compartments (deep layer) |
30.7±2.1+ | 30.1±1.6~ | 0.059 |
| 24 month T2 averaged over all five compartments (deep layer) |
31.6±2.9+ | 30.9±1.4~ | 0.276 |
| baseline T2 averaged over all five compartments (superficial layer) |
34.5±2.0+ | 34.1±1.7~ | 0.267 |
| 24 month T2 averaged over all five compartments (superficial layer) |
37.0±2.5+ | 36.1±1.5~ | 0.138 |
| baseline T2 averaged over all five compartments (whole compartment) |
32.7±1.8+ | 32.1±1.5~ | 0.099 |
| 24 month T2 averaged over all five compartments (whole compartment) |
34.3±2.6+ | 33.5±1.4~ | 0.175 |
| delta T2 averaged over all five compartments (whole compartment) |
1.7±2.5 | 1.4±1.0 | 0.983 |
Adjusted for age, gender, BMI and baseline KL-score.
p<0.05 for paired t-test for change in T2 over 24 months in subjects with OA risk factors
p>0.05 for paired t-test for change in T2 over 24 months in subjects with OA risk factors
p<0.05 for paired t-test for change in T2 over 24 months in subjects without OA risk factors
p>0.05 for paired t-test for change in T2 over 24 months in subjects without OA risk factors
The superficial cartilage layer had significantly higher T2 values than the deep cartilage layer in the patella, medial femur and averaged over all five compartments in both subject groups at baseline (p<0.05; Table 5).
24 Month Follow-up T2 Measurements
Averaged over all five compartments, T2 values at 24 month follow-up were higher in subjects with OA risk factors than in those without OA risk factors (Table 5). While these differences were not significant (p>0.05), T2 differences between the two groups in the weight-bearing medial femur compartment were significant in the superficial and deep cartilage layer as well as the whole compartment (p<0.05; Table 5). T2 values of the patella compartment were similar between subjects with and without OA risk factors (p>0.05; Table 5).
Similar to baseline, the superficial cartilage layer had significantly higher T2 values than the deep cartilage layer in the patella, medial femur and averaged over all compartments in both subject groups at 24 month follow-up (p<0.05; Table 5).
T2 values of the patella and averaged over all five compartments increased significantly over 24 months in both groups (p<0.05; Table 5), but medial femur T2 values did not increase significantly (p>0.05; Table 3). ΔT2 (24 month T2 – baseline T2) was not significantly different in subjects with and without OA risk factors in the patella, medial femur and averaged over all five compartments (Table 5; p>0.05).
Association of Focal Knee Lesions and T2 Measurements
T2 values averaged over all five compartments were significantly higher in knees with cartilage lesion anywhere in the joint (n=102) compared to knees without cartilage lesion (n=40) at baseline (32.7±1.8ms vs. 31.9±1.5ms; p=0.030) and 24 month follow-up (34.4±2.6ms vs. 33.4±1.4ms; p=0.025) as shown in Figure 1 and 2. Similar results were observed for the superficial and deep cartilage layer averaged over all five compartments (Figure 1). However, ΔT2 (24 month T2 – baseline T2) was not significantly different in knees with and without cartilage lesion (1.7±2.4ms vs. 1.4±1.2ms; p>0.05). T2 values in the medial femur compartment were significantly different between knees with and without cartilage lesion at baseline (38.1±2.2ms vs. 36.3±2.0ms; p<0.001) and 24 month follow-up (38.3±2.8ms vs. 36.6±1.8ms; p=0.001), while differences in ΔT2 were not statistically significant (0.2±2.4ms vs. 0.3±1.7ms; p>0.05).
Figure 1.
The box plots show T2 values averaged over all five compartments for (A) the superficial cartilage layer, (B) the deep cartilage layer, and (C) the whole cartilage at baseline and 24 month follow-up. Subjects were stratified by prevalence of cartilage lesion at baseline. Elevated T2 values were observed in subjects with cartilage lesion, compared to subjects without cartilage lesion (p<0.05). The p-values are adjusted for age, gender, BMI, baseline KL-score and subcohort.
Figure 2.
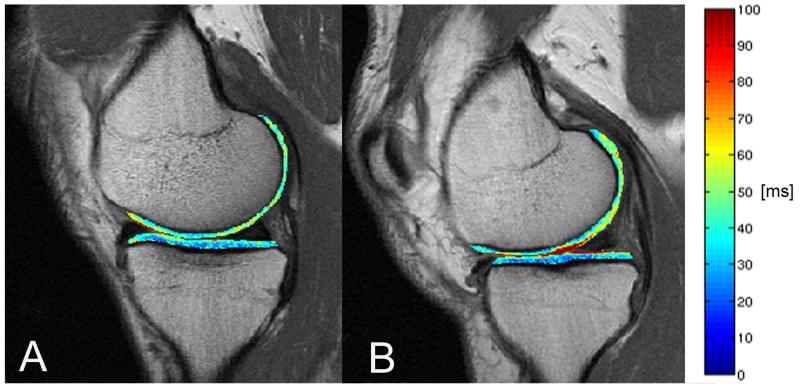
T2 color maps of the medial femur and medial tibia compartments of the right knee overlaid with the first-echo images of MSME sequence. (A) Representative subject without cartilage lesion and (B) representative subject with cartilage lesion. Blue color indicates low, red color high cartilage T2 values. High cartilage T2 values are associated with increased water content and deterioration of the collagen network. The subject with cartilage lesion showed elevated T2 values compared to the subject without cartilage lesion.
T2 values of the patella compartment were higher in subjects with cartilage lesion, compared to subjects without cartilage lesion at baseline (32.0±3.0ms vs. 30.9±2.5ms) and 24 month follow-up (33.4±3.1ms vs. 32.6±2.4ms). However, differences were not statistically significant (p=0.077, respectively p=0.133). Differences in ΔT2 of the patella compartment between subjects with and without cartilage lesion were also not statistically significant (1.3±2.9ms vs. 1.6±1.7ms; p>0.05). No significant differences were found for T2 values at baseline, at 24 months follow-up, or changes in T2 values over 24 months between knees with and without meniscal lesion or knees with and without BMEP (p>0.05; adjusted for age, gender, BMI, KL-score and subcohort).
Discussion
This study demonstrated that prevalence and grade of focal knee lesions were not significantly different between subjects with and without OA risk factors, whereas cartilage T2 values were significantly elevated in the weight-bearing medial femur compartment of subjects with OA risk factors. Significant T2 increases over 24 months were found in both subject groups. However, the increase in T2 over 24 months was not significantly different between subjects with and without OA risk factors. Cartilage lesions at baseline anywhere in the joint were associated with higher T2 values at baseline and 24 month follow-up, but not with a greater T2 increase over 24 months.
This study focused on younger subjects without radiographic OA and without pain, since they may most benefit from treatment or behavioural interventions. While previous studies compared subjects with radiographic OA with normal controls and reported more and more severe meniscal and cartilage lesions in OA subjects (6;28;29), we found no statistically significant differences in prevalence and grade of meniscal and cartilage lesions and BMEP between subjects with and without OA risk factors in this study. We measured patellar cartilage T2 relaxation times to investigate early cartilage degeneration in the patello-femoral joint in subjects with OA risk factors. However, we observed no differences in patellar T2 values between subjects with versus without OA risk factors. Interestingly, T2 values in the weight-bearing medial femur compartment were significantly higher in subjects with OA risk factors compared to those without OA risk factors at baseline and after 24 months in this study. Laminar analysis demonstrated that elevated T2 values in subjects with OA risk factors were found in the deep as well as the superficial cartilage layer compared to subjects without OA risk factors. Since elevated T2 values indicate increased water content and deterioration of the collagen network (4;5), T2 measurements may be a more sensitive biomarker for cartilage matrix degeneration in the initial phase of OA than the semi-quantitative morphologic assessment of focal knee lesions provided by the WORMS grading.
ΔT2 values were not significantly different between subjects with and without OA risk factors. Therefore T2 increase over 24 months in subjects with OA risk factors is not accelerated compared to subjects without OA risk factors, but aging in general may lead to a T2 increase over 24 months. Previous studies reported an age dependency of T2 relaxation times (9;30), which may explain our finding of a significant T2 increase over 24 months.
Knees with cartilage lesions showed significantly higher T2 values than knees without cartilage lesions at baseline and 24 months of follow-up, suggesting a close interrelationship between prevalence of cartilage lesions anywhere in the joint and accompanying proteoglycan loss, increased water content and deterioration of the collagen network in the whole articular cartilage. Interestingly, knees with cartilage lesions showed no significantly higher ΔT2, compared to those without cartilage lesions, which may be related to the relatively short follow-up interval of 24 months. Further studies with longer follow-up time intervals are clearly needed to investigate this finding. In contrast to previous studies (7;29;31), we observed no association between cartilage T2 values and meniscal lesions, respectively BMEP. Our subjects were all asymptomatic (WOMAC pain score of zero), while the previous studies included subjects based on clinically prevalent symptoms of OA. This might explain why no associations were found in our study.
This study has several limitations. First of all, WORMS grading of focal knee lesions was only performed for the baseline images. However, our study focused on the time course of T2 measurements and we were interested in the prospective effect of baseline morphological changes on T2 measurements. The second limitation is the exclusion of obese subjects. Obese subjects may be more sensitive to changes over time than the selected study population. Therefore the impact of obesity on cartilage degeneration measured by T2 mapping has to be evaluated in future studies. Thirdly, reproducibility of cartilage segmentation is crucial. The mean T2 reproducibility error amounted 0.55ms for whole compartments. Higher T2 reproducibility errors were observed for laminar analysis (mean: 0.82ms). However, statistically significant differences between subjects with versus without OA risk factors, respectively subjects with versus without cartilage lesion, were demonstrated and were clearly higher than the reproducibility errors. Lastly, the comparison of baseline and 24 month follow-up T2 measurements requires reliable and accurate MR imaging, which is challenging. However, in the OAI great care was taken to achieve high reproducibility with rigorous quality assurance methods that were established to allow a high quality of cartilage T2 measurements over 24 months (32).
In conclusion, the results of this study demonstrate that subjects with OA risk factors showed significantly elevated T2 values in the weight-bearing medial femur compartment compared to subjects without OA risk factors, but no significant difference in prevalence and grade of focal knee lesions. Therefore T2 measurements may be a more sensitive biomarker for cartilage degeneration in the initial phase of OA. Also T2 measurements showed a significant increase over a period of 24 months suggesting their sensitivity to change, which strengthens this hypothesis. In addition, cartilage lesions at baseline were associated with increased T2 values at baseline and 24 month follow-up, but not with an accelerated T2 increase over 24 months.
Acknowledgement
The OAI is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Pfizer, Inc.; Novartis Pharmaceuticals Corporation; Merck Research Laboratories; and GlaxoSmithKline. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health.
This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation.
This study was supported by NIH U01AR059507-01 (T.M.L.) and NIH F32AR059478-01 (G.B.J.).
Grant support: This study was supported by NIH U01AR059507-01 and NIH F32AR059478-01. The Osteoarthritis Initiative is a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Pfizer, Inc.; Novartis Pharmaceuticals Corporation; Merck Research Laboratories; and GlaxoSmithKline. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health.
Reference List
- 1.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the Prevalence of Arthritis and Other Rheumatic Conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burstein D, Gray M, Mosher T, Dardzinski B. Measures of Molecular Composition and Structure in Osteoarthritis. Radiol.Clin.North Am. 2009;47(4):675–686. doi: 10.1016/j.rcl.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Link TM, Steinbach LS, Ghosh S, et al. Osteoarthritis: MR Imaging Findings in Different Stages of Disease and Correlation With Clinical Findings. Radiology. 2003;226(2):373–381. doi: 10.1148/radiol.2262012190. [DOI] [PubMed] [Google Scholar]
- 4.Blumenkrantz G, Majumdar S. Quantitative Magnetic Resonance Imaging of Articular Cartilage in Osteoarthritis. Eur.Cell Mater. 2007;13:76–86. doi: 10.22203/ecm.v013a08. [DOI] [PubMed] [Google Scholar]
- 5.Liess C, Lusse S, Karger N, Heller M, Gluer CC. Detection of Changes in Cartilage Water Content Using MRI T2-Mapping in Vivo. Osteoarthritis.Cartilage. 2002;10(12):907–913. doi: 10.1053/joca.2002.0847. [DOI] [PubMed] [Google Scholar]
- 6.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 Relaxation Time of Cartilage at MR Imaging: Comparison With Severity of Knee Osteoarthritis. Radiology. 2004;232(2):592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedrich KM, Shepard T, de Oliveira VS, et al. T2 Measurements of Cartilage in Osteoarthritis Patients With Meniscal Tears. AJR Am.J.Roentgenol. 2009;193(5):W411–W415. doi: 10.2214/AJR.08.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li X, Pai A, Blumenkrantz G, et al. Spatial Distribution and Relationship of T1rho and T2 Relaxation Times in Knee Cartilage With Osteoarthritis. Magn Reson.Med. 2009;61(6):1310–1318. doi: 10.1002/mrm.21877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosher TJ, Liu Y, Yang QX, et al. Age Dependency of Cartilage Magnetic Resonance Imaging T2 Relaxation Times in Asymptomatic Women. Arthritis Rheum. 2004;50(9):2820–2828. doi: 10.1002/art.20473. [DOI] [PubMed] [Google Scholar]
- 10.Mosher TJ, Liu Y, Torok CM. Functional Cartilage MRI T2 Mapping: Evaluating the Effect of Age and Training on Knee Cartilage Response to Running. Osteoarthritis.Cartilage. 2010;18(3):358–364. doi: 10.1016/j.joca.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishii T, Kuroda K, Matsuoka Y, Sahara T, Yoshikawa H. Change in Knee Cartilage T2 in Response to Mechanical Loading. J.Magn Reson.Imaging. 2008;28(1):175–180. doi: 10.1002/jmri.21418. [DOI] [PubMed] [Google Scholar]
- 12.Souza RB, Stehling C, Wyman BT, et al. The Effects of Acute Loading on T1rho and T2 Relaxation Times of Tibiofemoral Articular Cartilage. Osteoarthritis.Cartilage. 2010;18(12):1557–1563. doi: 10.1016/j.joca.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Jordan JM. Epidemiology of Osteoarthritis. Clin.Geriatr.Med. 2010;26(3):355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peterfy CG, Schneider E, Nevitt M. The Osteoarthritis Initiative: Report on the Design Rationale for the Magnetic Resonance Imaging Protocol for the Knee. Osteoarthritis.Cartilage. 2008;16(12):1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation Study of WOMAC: a Health Status Instrument for Measuring Clinically Important Patient Relevant Outcomes to Antirheumatic Drug Therapy in Patients With Osteoarthritis of the Hip or Knee. J.Rheumatol. 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 16.Bellamy N. The WOMAC Knee and Hip Osteoarthritis Indices: Development, Validation, Globalization and Influence on the Development of the AUSCAN Hand Osteoarthritis Indices. Clin.Exp.Rheumatol. 2005;23(5 Suppl 39):S148–S153. [PubMed] [Google Scholar]
- 17.Kellgren J, Lawrence J. Radiological Assessment of Osteo-Arthrosis. Ann.Rheum.Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan J, Stehling C, Muller-Hocker C, et al. Vastus Lateralis/Vastus Medialis Cross-Sectional Area Ratio Impacts Presence and Degree of Knee Joint Abnormalities and Cartilage T2 Determined With 3T. Osteoarthritis.Cartilage. 2011;19(1):65–73. doi: 10.1016/j.joca.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stehling C, Lane NE, Nevitt MC, Lynch J, McCulloch CE, Link TM. Subjects With Higher Physical Activity Levels Have More Severe Focal Knee Lesions Diagnosed With 3T MRI: Analysis of a Non-Symptomatic Cohort of the Osteoarthritis Initiative. Osteoarthritis.Cartilage. 2010;18(6):776–786. doi: 10.1016/j.joca.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stehling C, Liebl H, Krug R, et al. Patellar Cartilage: T2 Values and Morphologic Abnormalities at 3.0-T MR Imaging in Relation to Physical Activity in Asymptomatic Subjects From the Osteoarthritis Initiative. Radiology. 2010;254(2):509–520. doi: 10.1148/radiol.09090596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stehling C, Baum T, Mueller-Hoecker C, et al. A Novel Fast Knee Cartilage Segmentation Technique for T(2) Measurements at MR Imaging - Data From the Osteoarthritis Initiative. Osteoarthritis.Cartilage. 2011;19(8):984–989. doi: 10.1016/j.joca.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carballido-Gamio J, Stahl R, Blumenkrantz G, Romero A, Majumdar S, Link TM. Spatial Analysis of Magnetic Resonance T1rho and T2 Relaxation Times Improves Classification Between Subjects With and Without Osteoarthritis. Med.Phys. 2009;36(9):4059–4067. doi: 10.1118/1.3187228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carballido-Gamio J, Blumenkrantz G, Lynch JA, Link TM, Majumdar S. Longitudinal Analysis of MRI T(2) Knee Cartilage Laminar Organization in a Subset of Patients From the Osteoarthritis Initiative. Magn Reson.Med. 2010;63(2):465–472. doi: 10.1002/mrm.22201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schipplein OD, Andriacchi TP. Interaction Between Active and Passive Knee Stabilizers During Level Walking. J.Orthop.Res. 1991;9(1):113–119. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- 25.Winby CR, Lloyd DG, Besier TF, Kirk TB. Muscle and External Load Contribution to Knee Joint Contact Loads During Normal Gait. J.Biomech. 2009 Oct 16;42(14):2294–2300. doi: 10.1016/j.jbiomech.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Shrout PE, Fleiss JL. Intraclass Correlations: Uses in Assessing Rater Reliability. Psychol.Bull. 1979;86(2):420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 27.Gluer CC, Blake G, Lu Y, Blunt BA, Jergas M, Genant HK. Accurate Assessment of Precision Errors: How to Measure the Reproducibility of Bone Densitometry Techniques. Osteoporos.Int. 1995;5(4):262–270. doi: 10.1007/BF01774016. [DOI] [PubMed] [Google Scholar]
- 28.Reichenbach S, Yang M, Eckstein F, et al. Does Cartilage Volume or Thickness Distinguish Knees With and Without Mild Radiographic Osteoarthritis? The Framingham Study. Ann.Rheum.Dis. 2010;69(1):143–149. doi: 10.1136/ard.2008.099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zarins ZA, Bolbos RI, Pialat JB, et al. Cartilage and Meniscus Assessment Using T1rho and T2 Measurements in Healthy Subjects and Patients With Osteoarthritis. Osteoarthritis.Cartilage. 2010;18(11):1408–1416. doi: 10.1016/j.joca.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mosher TJ, Dardzinski BJ, Smith MB. Human Articular Cartilage: Influence of Aging and Early Symptomatic Degeneration on the Spatial Variation of T2--Preliminary Findings at 3 T. Radiology. 2000;214(1):259–266. doi: 10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- 31.Bining HJ, Santos R, Andrews G, Forster BB. Can T2 Relaxation Values and Color Maps Be Used to Detect Chondral Damage Utilizing Subchondral Bone Marrow Edema As a Marker? Skeletal Radiol. 2009;38(5):459–465. doi: 10.1007/s00256-008-0629-y. [DOI] [PubMed] [Google Scholar]
- 32.Schneider E, NessAiver M, White D, et al. The Osteoarthritis Initiative (OAI) Magnetic Resonance Imaging Quality Assurance Methods and Results. Osteoarthritis.Cartilage. 2008;16(9):994–1004. doi: 10.1016/j.joca.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]