SUMMARY
The intestine is host to a diverse bacterial community whose structure, at the phylum level, is maintained through unknown mechanisms. Acute inflammation triggered by enteric pathogens, such as Salmonella enterica serotype Typhimurium (S. Typhimurium), is accompanied by changes in the bacterial community structure marked by an outgrowth of the pathogen. Recent studies show that S. Typhimurium can harness benefit from the host response to edge out the beneficial bacterial species that dominate in the healthy gut. The elucidation of how S. Typhimurium alters the bacterial community structure during gastroenteritis is beginning to provide insights into mechanisms that dictate the balance between the host and its microbiota.
INTRODUCTION
Salmonella enterica serotype Typhimurium (S. Typhimurium) is an important food-borne pathogen that in humans causes a self-limited gastroenteritis, characterized by fever, acute intestinal inflammation, diarrhea, and the presence of neutrophils in stool samples [1]. In addition, S. Typhimurium is a model organism for studying bacterial genetics and microbial pathogenesis. As the frontier in bacterial pathogenesis research is moving towards understanding the complexity of host-pathogen interaction at the tissue level, studies on the pathogenesis of S. Typhimurium gastroenteritis using animal models have helped establish important new concepts that exert a strong influence on the research field. Recent studies on S. Typhimurium pathogenesis reveal how tissue-specific host factors and the presence of other bacterial species shape the outcome of host-pathogen interaction in the intestinal lumen. Here, we will review these new paradigms for the interplay between the pathogen, the host and its resident microbial community.
VIRULENCE MECHANISMS
Upon ingestion, S. Typhimurium colonizes the terminal ileum and colon, commonly eliciting symptoms of gastroenteritis within less than 24 hours. The signs of disease and the pathological changes in the human terminal ileum and colon can be reproduced in a calf model [2]. Studies in this animal model identified motility and two type III secretion systems as the main S. Typhimurium virulence factors important for triggering intestinal inflammation [3,4]. Motility and the invasion-associated type III secretion system (T3SS-1) work in concert to enable a fraction of the S. Typhimurium population to invade intestinal epithelial cells [5,6]. Acting as a molecular syringe, the T3SS-1 injects proteins, termed effectors, into host cells [7]. Five T3SS-1 effectors, named SipA, SopA, SopB (SigD), SopD and SopE2, act in concert to trigger alterations in the actin cytoskeleton of host cells, thereby promoting epithelial invasion [8] and intestinal inflammation [9]. Once S. Typhimurium has crossed the epithelial lining, a second type III secretion (T3SS-2) system enables the pathogen to survive within tissue mononuclear cells (macrophages and dentritic cells) [10]. Finally, S. Typhimurium invasion of host tissue is detected by the innate immune surveillance (Figure 1) [11], resulting in the rapid induction of intestinal inflammation, which is largely responsible for the signs of disease [12]. Mechanisms by which S. Typhimurium first induces and then benefits from the host inflammatory response have been elucidated using a mouse colitis model [2].
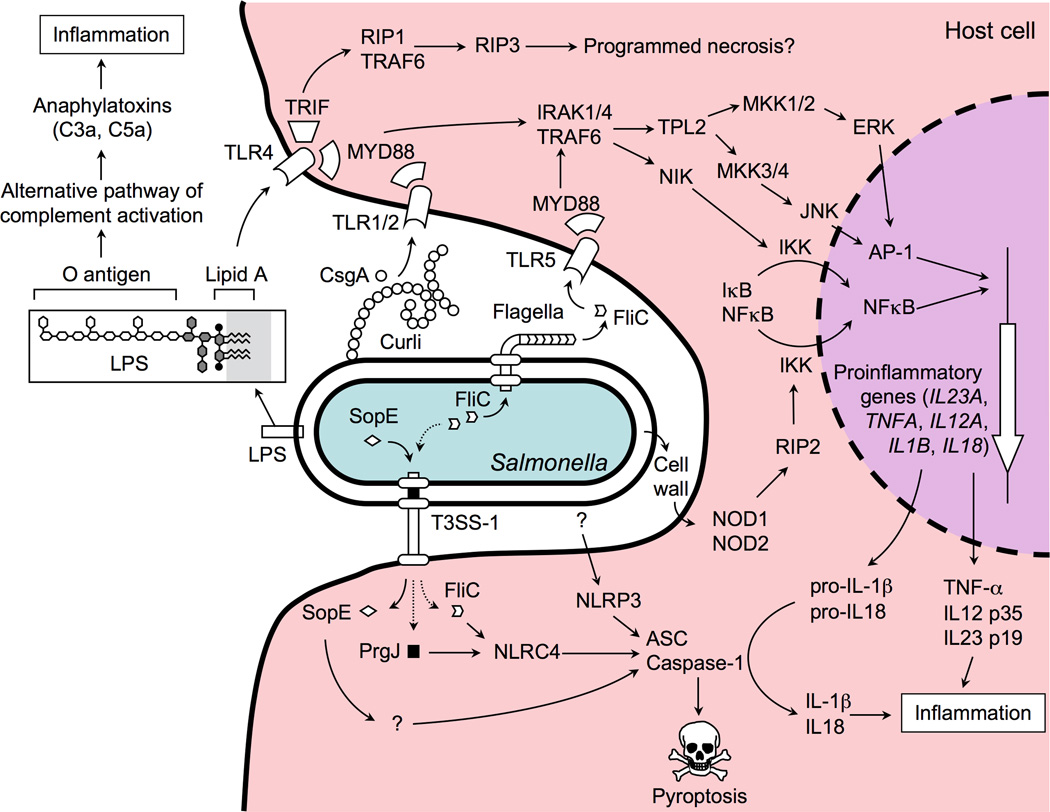
Figure 1. Detection of S. Typhimurium by the innate immune systems initiates inflammation.
The host senses the presence of S. Typhimurium in tissue by detecting PAMPs (e.g. LPS, curli, FliC) or patterns of pathogenesis (e.g. cytosolic access by the T3SS-1) through pathogen recognition receptors located in the cytosol (NOD1, NOD2, NLRC4, and NLRP3), the cell membrane (TLR1/TLR2, TLR4 and TLR5) or the humoral compartment (complement). Signaling through these pathogen recognition receptors results in the production of a pro-inflammatory cocktail (anaphylatoxins, IL-1β, IL-12, IL-18, IL-23, TNF-α and IFN-γ) that initiates the orchestration of antibacterial responses in tissue.
PATTERN RECOGNITION
The innate immune system detects the presence of S. Typhimurium in tissue by two distinct mechanisms, each involving a multitude of receptors. The first mechanism, termed pattern recognition, enables the host to distinguish self from bacteria by detecting conserved microbial structures, known as pathogen associated molecular patterns (PAMPs) [13], by humoral proteins, such as complement, or by host cell receptors, such as Toll-like receptors (TLRs).
For example, the O-antigen of the S. Typhimurium lipopolysaccharide (LPS) is a PAMP detected by complement component 3 (C3), thereby initiating the alternative pathway of complement activation. The complement fragments C3a and C5a generated during this process are also known as the anaphylatoxins, due to their potency in inducing inflammatory responses [14]. Besides the O-antigen, S. Typhimurium LPS contains a lipid A moiety, which is a powerful agonist of TLR4 [15]. Curli, an amyloid fibril present in the extracellular matrix of S. Typhimurium biofilms, is the main TLR1/TLR2-ligand detected on intact bacterial cells [16,17]. Finally, motility of S. Typhimurium is mediated by flagella, whose major protein subunit, flagellin (FliC), is a PAMP stimulating TLR5 [18]. TLR4, TLR1/TLR2 and TLR5 engage a common adaptor protein, myeloid differentiation primary response gene 88 (MYD88), to initiate mitogen activated protein (MAP) kinase signal transduction pathways that induce expression of pro-inflammatory genes by activating two transcription factors, activator protein 1 (AP-1) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) (Figure 1).
DETECTION OF PATHOGEN-INDUCED PROCESSES
Although the name suggests a specific association with pathogens, all microbes produce PAMPs regardless of their pathogenic potential. Thus pattern recognition is not sufficient to distinguish a virulent pathogen, such as S. Typhimurium, from other microbes with lower disease-causing potential. To mount a response that is commensurate with the threat, the host uses a second mechanism for assessing the pathogenic potential of S. Typhimurium by detecting pathogen-induced processes [19].
One pathogen-induced process that marks S. Typhimurium for recognition by the host is the T3SS-1-dependent delivery of proteins into the host cell cytosol. T3SS-1-dependent cytosolic access is accompanied by the delivery of conserved components of the T3SS-1 needle complex (PrgJ) [20] and an inadvertent translocation of flagellin into host cells [21]. The presence of PrgJ and flagellin is detected by a nucleotide-binding oligomerization domain (NOD)-like receptor (NLR), termed NLRC4 (NLR family caspase-associated recruitment domain [CARD]-containing protein 4) (Figure 1) [20,22,23]. In turn, NLRC4 forms a complex with caspase-1 and apoptosis-associated speck-like protein containing a CARD (ASC), resulting in cleavage and activation of caspase-1. Upon activation, caspase-1 proteolytically cleaves the precursor forms of interleukin (IL)-1β and IL-18, into active mature peptides. Alternatively, S. Typhimurium can activate caspase-1 through NLRP3 (NLR family pyrin domain-containing protein 3) by an unknown mechanism [24]. A small fraction of S. Typhimurium clinical isolates is lysogenized with a bacteriophage carrying the sopE gene, which encodes a T3SS-1 effector protein [25]. Translocation of SopE into the host cell cytosol results in caspase-1 activation through an unknown mechanism [26]. Finally, another mechanism for detecting cytosolic access by S. Typhimurium is the activation of NOD1 and NOD2, two intracellular sensors of bacterial cell wall fragments [27–29]. NOD1 and NOD2 interact with protein kinase receptor interacting protein-2 (RIP2) to mediate NF-κB activation. Detection of pathogen-induced processes enables the host to escalate innate responses to levels that adequately address the risk posed by S. Typhimurium infection.
THE THREE BRANCHES OF AN ANTIBACTERIAL RESPONSE
Pattern recognition and the detection of pathogen-induced processes by epithelial cells, mononuclear cells (i.e. macrophages and dendritic cells) and complement results in the production of IL-1β, IL-12, IL-18, IL-23, tumor necrosis factor (TNF)-α, interferon (IFN)-γ and C5a (Figure 1), a cocktail that serves as a starting signal for the orchestration of anti-bacterial responses in tissue. The events set in motion by the release of this pro-inflammatory cocktail culminate in the orchestration of three host defense pathways, including macrophage activation, neutrophil recruitment and the epithelial release of antimicrobials into the intestinal lumen (Figure 2) [30].
Figure 2. The three branches of an antibacterial inflammatory response.
Detection of S. Typhimurium by mononuclear cells, epithelial cells and complement triggers a cytokine storm in host tissue, which culminates in the orchestration of three major antibacterial responses: macrophage activation, neutrophil recruitment and the epithelial release of antimicrobials.
In brief, IL-12 and IL-18 induce production of IFN-γ, a cytokine that greatly enhances the ability of macrophages to kill intracellular S. Typhimurium [31]. Neutrophil recruitment is orchestrated by several partially redundant mechanisms. IL-23 and IL-1β synergize in inducing the production of IL-17A by T helper (TH)17 cells, γδ T cells, and NKT cells [29,32–34]. In turn, IL-17A acts in concert with IL-1β and TNF-α to induce the release of CXC chemokines from epithelial cells [34,35]. CXC chemokines and C5a are potent chemoattractants for neutrophils, a cell type that is particularly effective in killing extracellular S. Typhimurium [36–38]. Finally, IL-23 contributes to the production of IL-22, a cytokine that stimulates epithelial cells to release antimicrobials, such as lipocalin-2, into the intestinal lumen [39].
In summary, detection of S. Typhimurium by the innate immune system activates an antibacterial response consisting of three branches, one directed against intracellular bacteria in tissue, one directed against extracellular bacteria in tissue and one directed against luminal bacteria (Figure 2) [30].
TAKING ONE FOR THE TEAM
The fraction of the S. Typhimurium population that enters the tissue is detectable by electron microscopy in mononuclear cells and neutrophils [40]. Resident tissue macrophages represent the preferred intracellular niche of S. Typhimurium that supports its growth [41]. However, activation of caspase-1 during S. Typhimurium infection triggers a form of pro-inflammatory macrophage cell death [42,43], termed pyroptosis [44] (Figure 1). Release from macrophages by pyroptosis renders S. Typhimurium vulnerable to phagocytosis by neutrophils, a cell type capable of killing S. Typhimurium, thereby helping to control bacterial numbers in tissue [38]. Alternatively, S. Typhimurium can trigger macrophage cell death through a T3SS-1-independent mechanism [45] that involves signaling through TLR4 and its adaptor protein TRIF (TIR-domain-containing adapter-inducing interferon-β) [46]. TRIF physically interacts with receptor-interacting protein (RIP)1 and RIP3 [47], forming a complex that can induce programmed necrosis [48]. Increasing production of IFN-γ during the transition from innate to adaptive immune responses enhances the ability of macrophages to kill intracellular S. Typhimurium [31]. Finally, production of specific antibodies by the adaptive immune response further enhances phagocyte-killing mechanisms [49]. As a result, S. Typhimurium is beginning to be cleared from intestinal tissues with the onset of adaptive immune responses.
In conclusion, the localization within tissue represents a dead end for S. Typhimurium, as the pathogen does not transmit to a susceptible host from this location, but instead is eventually killed by two branches of the host’s antibacterial response, macrophage activation and neutrophil recruitment (Figure 2). Interestingly, the fraction of the S. Typhimurium population that remains in the intestinal lumen is thriving in the inflamed gut and becomes a sizeable fraction of the intestinal microbiota [50–52]. It has therefore been proposed that induction of an inflammatory response by the invading fraction of the S. Typhimurium population, through self-destructive cooperation, empowers the luminal fraction of the S. Typhimurium population to bloom in the inflamed gut [53].
FOOD FROM THE FIRE
Interestingly, growth of S. Typhimurium is aided by the very inflammatory responses that are aimed at controlling luminal bacteria, including the epithelial release of antimicrobials and the epithelial transmigration of neutrophils into the intestinal lumen (Figure 2). For example, the antimicrobial protein lipocalin-2 is released into the intestinal lumen, where it binds enterobactin, a low molecular weight iron chelator (siderophore) produced by many enteric bacteria [39]. By sequestering enterobactin, lipocalin-2 exerts a bacteriostatic activity on bacteria that depend on this siderophore for iron acquisition [54]. In addition to enterobactin, S. Typhimurium produces a glycosylated derivative of enterobactin, termed salmochelin [55], that is no longer bound by lipocalin-2, thereby conferring resistance to this antimicrobial [56]. Salmochelin bestows a growth advantage upon S. Typhimurium in the lumen of the inflamed intestine [39], presumably because lipocalin-2 suppresses growth of competing microbes (Figure 3).
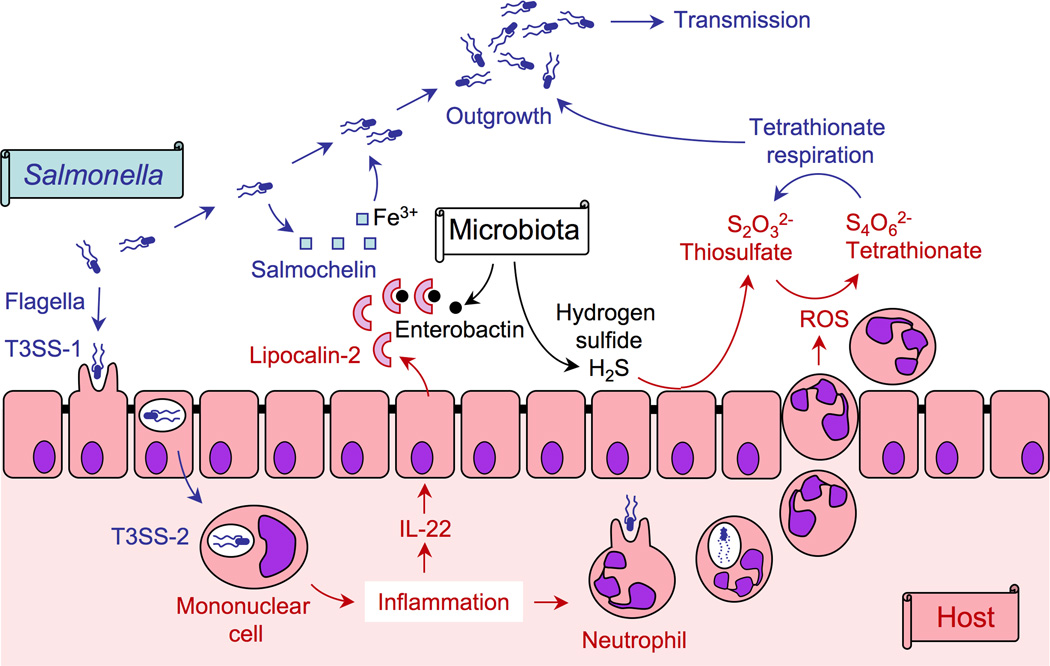
Figure 3. Salmonella, the host and its microbiota.
S. Typhimurium uses its virulence factors (flagella, T3SS-1 and T3SS-2) to invade the epithelium and survive in mononuclear cells. The ensuing inflammatory response results in the epithelial release of an antimicrobial (lipocalin-2) that sequesters iron chelators (enterobactin) produced by the microbiota, but not an iron chelator (salmochelin) produced by S. Typhimurium. ROS generated by neutrophils migrating into the intestinal lumen oxidize an endogenous sulfur compound (thiosulfate) to generate a respiratory electron acceptor (tetrathionate) that enables S. Typhimurium to edge out the fermenting microbiota, thereby enhancing transmission of the pathogen.
In healthy individuals, the gut lumen is thought to be fairly anaerobic, with traces of oxygen being readily consumed by the microbiota. The majority of the microbiota are strictly anaerobic bacteria belonging to the classes Bacteroidetes and Clostridiales that rely on fermentation of amino acids and complex polysaccharides. During infection with S. Typhimurium, migration of neutrophils into the intestinal lumen is associated with changes in this microbial community structure [57]. This ultimately results in the enrichment of the pathogen in the gut lumen and a relative depletion of Bacteroidetes and Clostridiales.
One fermentation end product generated by the microbiota is hydrogen sulfide (H2S), a cytotoxic compound that is converted to thiosulfate (S2O32−) by the colonic epithelium. During inflammation, neutrophils that transmigrate into the intestinal lumen release reactive oxygen species (ROS) in an attempt to kill bacteria. A by-product of releasing ROS is the oxidation of thiosulfate (S2O32−) to tetrathionate (S4O62−) [58]. In contrast to the fermenting microbiota, S. Typhimurium can use tetrathionate as a terminal electron acceptor to support its growth by anaerobic respiration, which is more efficient for energy production than fermentation [59]. In addition, S. Typhimurium might benefit from inflammation by gaining access to new nutrients because anaerobic tetrathionate respiration facilitates growth on poorly fermentable carbon sources. Collectively, growth by tetrathionate respiration enables S. Typhimurium to outgrow competing microbes, resulting in a marked increase in the relative abundance of the pathogen in intestinal contents [58]. In turn, enhanced growth in the intestinal lumen promotes transmission of S. Typhimurium by the fecal oral route [51] (Figure 3).
In summary, the benefit S. Typhimurium harnesses from intestinal inflammation is largely based on an improved access to nutrient sources. It has thus been speculated that S. Typhimurium virulence evolved predominantly as a means to access nutrient resources in its host [60].
CONCLUSIONS
Research on S. Typhimurium pathogenesis has traditionally focused on how virulence factors enable the pathogen to overcome host defenses. However, recent insights into the interplay between S. Typhimurium, the host and its microbiota suggest that virulence factors enable the pathogen to elicit help from the host inflammatory response to gain an advantage in its growth competition with the resident microbiota (Figure 3). Thus, the deployment of virulence factors could be seen as an attempt to create a niche in the host in which S. Typhimurium can edge out competing microbes to ensure its transmission. Identification of the metabolic pathways that support growth of S. Typhimurium over other microbes in the inflamed gut has the potential to identify new targets for intervention, which represents a promising line for future investigation.
HIGHLIGHTS.
Non-typhoidal Salmonella serotypes trigger innate immune responses by using their virulence factors to invade the intestinal epithelium and survive in macrophages.
The innate immune surveillance system responds to the presence of non-typhoidal Salmonella serotypes in tissue with an inflammatory reaction that culminates in macrophage activation, neutrophil recruitment and the epithelial release of antimicrobials.
While inflammatory responses control the pathogen in tissue, neutrophil transepithelial migration and the epithelial release of antimicrobials provide a benefit during their competition with the resident microbiota in the lumen, thereby enhancing transmission of non-typhoidal Salmonella serotypes.
In conclusion, the deployment of virulence factors could be seen as an attempt to create a niche in the host in which non-typhoidal Salmonella serotypes can edge out competing microbes to ensure their transmission.
ACKNOWLEDGEMENTS
Work in A.J.B.’s laboratory is supported by Public Health Service Grants AI040124, AI044170, AI076246, AI088122 and AI096528. P.T. is supported by a scholarship from the Faculty of Medicine, Chiang Mai University, Thailand.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
Papers of special interest (*)
Papers of outstanding interest (**)
- 1.Harris JC, Dupont HL, Hornick RB. Fecal leukocytes in diarrheal illness. Ann Intern Med. 1972;76:697–703. doi: 10.7326/0003-4819-76-5-697. [DOI] [PubMed] [Google Scholar]
- 2.Tsolis RM, Xavier MN, Santos RL, Bäumler AJ. How to become a top model: impact of animal experimentation on human Salmonella disease research. Infect Immun. 2011;79:1806–1814. doi: 10.1128/IAI.01369-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsolis RM, Adams LG, Ficht TA, Bäumler AJ. Contribution of Salmonella typhimurium virulence factors to diarrheal disease in calves. Infect Immun. 1999;67:4879–4885. doi: 10.1128/iai.67.9.4879-4885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitt CK, Ikeda JS, Darnell SC, Watson PR, Bispham J, Wallis TS, Weinstein DL, Metcalf ES, O'Brien AD. Absence of all components of the flagellar export and synthesis machinery differentially alters virulence of Salmonella enterica serovar Typhimurium in models of typhoid fever, survival in macrophages, tissue culture invasiveness, and calf enterocolitis. Infect Immun. 2001;69:5619–5625. doi: 10.1128/IAI.69.9.5619-5625.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galan JE, Curtiss R., 3rd Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci U S A. 1989;86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khoramian-Falsafi T, Harayama S, Kutsukake K, Pechere JC. Effect of motility and chemotaxis on the invasion of Salmonella typhimurium into HeLa cells. Microb Pathog. 1990;9:47–53. doi: 10.1016/0882-4010(90)90039-s. [DOI] [PubMed] [Google Scholar]
- 7.Fu Y, Galan JE. The Salmonella typhimurium tyrosine phosphatase SptP is translocated into host cells and disrupts the actin cytoskeleton. Mol Microbiol. 1998;27:359–368. doi: 10.1046/j.1365-2958.1998.00684.x. [DOI] [PubMed] [Google Scholar]
- 8.Raffatellu M, Wilson RP, Chessa D, Andrews-Polymenis H, Tran QT, Lawhon S, Khare S, Adams LG, Bäumler AJ. SipA, SopA, SopB, SopD and SopE2 contribute to Salmonella enterica serotype Typhimurium invasion of epithelial cells. Infect. Immun. 2005;73:146–154. doi: 10.1128/IAI.73.1.146-154.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S, Santos RL, Tsolis RM, Stender S, Hardt W-D, Bäumler AJ, Adams LG. SipA, SopA, SopB, SopD and SopE2 act in concert to induce diarrhea in calves infected with Salmonella enterica serotype Typhimurium. Infect. Immun. 2002;70:3843–3855. doi: 10.1128/IAI.70.7.3843-3855.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ochman H, Soncini FC, Solomon F, Groisman EA. Identification of a pathogenicity island for Salmonella survival in host cells. Proc. Natl. Acad. Sci. USA. 1996;93:7800–7804. doi: 10.1073/pnas.93.15.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santos RL, Raffatellu M, Bevins CL, Adams LG, Tukel C, Tsolis RM, Baumler AJ. Life in the inflamed intestine, Salmonella style. Trends Microbiol. 2009;17:498–506. doi: 10.1016/j.tim.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nunes JS, Lawhon SD, Rossetti CA, Khare S, Figueiredo JF, Gull T, Burghardt RC, Bäumler AJ, Tsolis RM, Andrews-Polymenis HL, et al. Morphologic and cytokine profile characterization of Salmonella enterica serovar typhimurium infection in calves with bovine leukocyte adhesion deficiency. Veterinary pathology. 2010;47:322–333. doi: 10.1177/0300985809358037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Haas PJ, van Strijp J. Anaphylatoxins: their role in bacterial infection and inflammation. Immunol Res. 2007;37:161–175. doi: 10.1007/BF02697367. [DOI] [PubMed] [Google Scholar]
- 15.Vazquez-Torres A, Vallance BA, Bergman MA, Finlay BB, Cookson BT, Jones-Carson J, Fang FC. Toll-like receptor 4 dependence of innate and adaptive immunity to Salmonella: importance of the Kupffer cell network. J Immunol. 2004;172:6202–6208. doi: 10.4049/jimmunol.172.10.6202. [DOI] [PubMed] [Google Scholar]
- 16.Tükel C, Nishimori JH, Wilson RP, Winter MG, Keestra AM, van Putten JP, Bäumler AJ. Toll-like receptors 1 and 2 cooperatively mediate immune responses to curli, a common amyloid from enterobacterial biofilms. Cellular microbiology. 2010;12:1495–1505. doi: 10.1111/j.1462-5822.2010.01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tükel Ç, Wilson RP, Nishimori JH, Pezeshki M, Chromy BA, Bäumler AJ. Responses to amyloids of microbial and host origin are mediated through Toll-like receptor 2. Cell Host Microbe. 2009;6:45–53. doi: 10.1016/j.chom.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–1885. doi: 10.4049/jimmunol.167.4.1882. [DOI] [PubMed] [Google Scholar]
- 19. Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell Host Microbe. 2009;6:10–21. doi: 10.1016/j.chom.2009.06.007.. This milestone review introduced the concept that the host innate surveillance system detects pathogen-induced processes to distinguish virulent pathogens from other microbes with lower disease-causing potential.
- 20.Miao EA, Mao DP, Yudkovsky N, Bonneau R, Lorang CG, Warren SE, Leaf IA, Aderem A. Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci U S A. 2010;107:3076–3080. doi: 10.1073/pnas.0913087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun YH, Rolan HG, Tsolis RM. Injection of flagellin into the host cell cytosol by Salmonella enterica serotype Typhimurium. J Biol Chem. 2007;282:33897–33901. doi: 10.1074/jbc.C700181200. [DOI] [PubMed] [Google Scholar]
- 22.Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 23.Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 24.Broz P, Newton K, Lamkanfi M, Mariathasan S, Dixit VM, Monack DM. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. The Journal of experimental medicine. 2010;207:1745–1755. doi: 10.1084/jem.20100257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirold S, Rabsch W, Rohde M, Stender S, Tschape H, Russmann H, Igwe E, Hardt WD. Isolation of a temperate bacteriophage encoding the type III effector protein SopE from an epidemic Salmonella typhimurium strain. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:9845–9850. doi: 10.1073/pnas.96.17.9845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller AJ, Hoffmann C, Galle M, Van Den Broeke A, Heikenwalder M, Falter L, Misselwitz B, Kremer M, Beyaert R, Hardt WD. The S. Typhimurium effector SopE induces caspase-1 activation in stromal cells to initiate gut inflammation. Cell Host Microbe. 2009;6:125–136. doi: 10.1016/j.chom.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Le Bourhis L, Magalhaes JG, Selvanantham T, Travassos LH, Geddes K, Fritz JH, Viala J, Tedin K, Girardin SE, Philpott DJ. Role of Nod1 in mucosal dendritic cells during Salmonella pathogenicity island 1-independent Salmonella enterica serovar Typhimurium infection. Infect Immun. 2009;77:4480–4486. doi: 10.1128/IAI.00519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geddes K, Rubino S, Streutker C, Cho JH, Magalhaes JG, Le Bourhis L, Selvanantham T, Girardin SE, Philpott DJ. Nod1 and Nod2 regulation of inflammation in the Salmonella colitis model. Infect Immun. 2010;78:5107–5115. doi: 10.1128/IAI.00759-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geddes K, Rubino SJ, Magalhaes JG, Streutker C, Le Bourhis L, Cho JH, Robertson SJ, Kim CJ, Kaul R, Philpott DJ, et al. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nature medicine. 2011 doi: 10.1038/nm.2391. [DOI] [PubMed] [Google Scholar]
- 30.Winter SE, Keestra AM, Tsolis RM, Bäumler AJ. The blessings and curses of intestinal inflammation. Cell Host Microbe. 2010;8:36–43. doi: 10.1016/j.chom.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mastroeni P. Immunity to systemic Salmonella infections. Curr Mol Med. 2002;2:393–406. doi: 10.2174/1566524023362492. [DOI] [PubMed] [Google Scholar]
- 32.Godinez I, Haneda T, Raffatellu M, George MD, Paixao TA, Rolan HG, Santos RL, Dandekar S, Tsolis RM, Bäumler AJ. T cells help to amplify inflammatory responses induced by Salmonella enterica serotype Typhimurium in the intestinal mucosa. Infect Immun. 2008;76:2008–2017. doi: 10.1128/IAI.01691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godinez I, Raffatellu M, Chu H, Paixao TA, Haneda T, Santos RL, Bevins CL, Tsolis RM, Bäumler AJ. Interleukin-23 orchestrates mucosal responses to Salmonella enterica serotype Typhimurium in the intestine. Infect Immun. 2009;77:387–398. doi: 10.1128/IAI.00933-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keestra AM, Godinez I, Xavier MN, Winter MG, Winter SE, Tsolis RM, Bäumler AJ. Early, MyD88-dependent induction of interleukin-17A expression during Salmonella colitis. Infect Immun. 2011 doi: 10.1128/IAI.00018-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang S, Adams LG, Nunes J, Khare S, Tsolis RM, Bäumler AJ. Secreted effector proteins of Salmonella enterica serotype Typhimurium elicit host-specific chemokine profiles in animal models of typhoid fever and enterocolitis. Infect Immun. 2003;71:4795–4803. doi: 10.1128/IAI.71.8.4795-4803.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conlan JW. Neutrophils prevent extracellular colonization of the liver microvasculature by Salmonella typhimurium. Infect Immun. 1996;64:1043–1047. doi: 10.1128/iai.64.3.1043-1047.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dejager L, Pinheiro I, Bogaert P, Huys L, Libert C. Role for neutrophils in host immune responses and genetic factors that modulate resistance to Salmonella enterica serovar typhimurium in the inbred mouse strain SPRET/Ei. Infection and immunity. 2010;78:3848–3860. doi: 10.1128/IAI.00044-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nature immunology. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raffatellu M, George MD, Akiyama Y, Hornsby MJ, Nuccio SP, Paixao TA, Butler BP, Chu H, Santos RL, Berger T, et al. Lipocalin-2 resistance confers an advantage to Salmonella enterica serotype Typhimurium for growth and survival in the inflamed intestine. Cell Host & Microbe. 2009;5:476–486. doi: 10.1016/j.chom.2009.03.011.. Experiments described in this article suggest that lipocalin-2 resistance enables S. Typhimurium to edge out competing microbes in the lumen of the inflamed intestine.
- 40.Santos RL, Zhang S, Tsolis RM, Bäumler AJ, Adams LG. Morphologic and molecular characterization of Salmonella typhimurium infection in neonatal calves. Vet. Pathol. 2002;39:200–215. doi: 10.1354/vp.39-2-200. [DOI] [PubMed] [Google Scholar]
- 41.Fields PI, Swanson RV, Haidaris CG, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monack DM, Raupach B, Hromockyj AE, Falkow S. Salmonella typhimurium invasion induces apoptosis in infected macrophages. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:9833–9838. doi: 10.1073/pnas.93.18.9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen LM, Kaniga K, Galan JE. Salmonella spp. are cytotoxic for cultured macrophages. Molecular microbiology. 1996;21:1101–1115. doi: 10.1046/j.1365-2958.1996.471410.x. [DOI] [PubMed] [Google Scholar]
- 44.Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends in microbiology. 2001;9:113–114. doi: 10.1016/s0966-842x(00)01936-3. [DOI] [PubMed] [Google Scholar]
- 45.Lindgren SW, Stojiljkovic I, Heffron F. Macrophage killing is an essential virulence mechanism of Salmonella typhimurium. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:4197–4201. doi: 10.1073/pnas.93.9.4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cook P, Totemeyer S, Stevenson C, Fitzgerald KA, Yamamoto M, Akira S, Maskell DJ, Bryant CE. Salmonella-induced SipB-independent cell death requires Toll-like receptor-4 signalling via the adapter proteins Tram and Trif. Immunology. 2007;122:222–229. doi: 10.1111/j.1365-2567.2007.02631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaiser WJ, Offermann MK. Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. Journal of immunology. 2005;174:4942–4952. doi: 10.4049/jimmunol.174.8.4942. [DOI] [PubMed] [Google Scholar]
- 48.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gondwe EN, Molyneux ME, Goodall M, Graham SM, Mastroeni P, Drayson MT, MacLennan CA. Importance of antibody and complement for oxidative burst and killing of invasive nontyphoidal Salmonella by blood cells in Africans. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3070–3075. doi: 10.1073/pnas.0910497107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J, et al. Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol. 2007;5:2177–2189. doi: 10.1371/journal.pbio.0050244.. This article provides conclusive evidence that inflammation promotes outgrowth of S. Typhimurium in the intestinal lumen.
- 51. Lawley TD, Bouley DM, Hoy YE, Gerke C, Relman DA, Monack DM. Host transmission of Salmonella enterica serovar Typhimurium is controlled by virulence factors and indigenous intestinal microbiota. Infect Immun. 2008;76:403–416. doi: 10.1128/IAI.01189-07.. This study provides rare experimental support for the idea that the ability to bloom in the intestinal lumen during gastroenteritis increases the ability of S. Typhimurium to be transmitted by the fecal oral route.
- 52.Barman M, Unold D, Shifley K, Amir E, Hung K, Bos N, Salzman N. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect Immun. 2008;76:907–915. doi: 10.1128/IAI.01432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ackermann M, Stecher B, Freed NE, Songhet P, Hardt WD, Doebeli M. Self-destructive cooperation mediated by phenotypic noise. Nature. 2008;454:987–990. doi: 10.1038/nature07067. [DOI] [PubMed] [Google Scholar]
- 54.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 55.Hantke K, Nicholson G, Rabsch W, Winkelmann G. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc Natl Acad Sci U S A. 2003;100:3677–3682. doi: 10.1073/pnas.0737682100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fischbach MA, Lin H, Zhou L, Yu Y, Abergel RJ, Liu DR, Raymond KN, Wanner BL, Strong RK, Walsh CT, et al. The pathogen-associated iroA gene cluster mediates bacterial evasion of lipocalin 2. Proc Natl Acad Sci U S A. 2006;103:16502–16507. doi: 10.1073/pnas.0604636103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sekirov I, Gill N, Jogova M, Tam N, Robertson M, de Llanos R, Li Y, Finlay BB. Salmonella SPI-1-mediated neutrophil recruitment during enteric colitis is associated with reduction and alteration in intestinal microbiota. Gut microbes. 2010;1:30–41. doi: 10.4161/gmic.1.1.10950.. This is the first study to demonstrate that neutrophil transepithelial migration alters the bacterial community structure in the intestine.
- 58. Winter SE, Thiennimitr P, Winter MG, Butler BP, Huseby DL, Crawford RW, Russell JM, Bevins CL, Adams LG, Tsolis RM, et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature. 2010;467:426–429. doi: 10.1038/nature09415.. This article introduces the concept that the host response feeds enteric pathogens, thereby empowering them to edge out beneficial microbes in the environment of the inflamed gut.
- 59.Hensel M, Hinsley AP, Nikolaus T, Sawers G, Berks BC. The genetic basis of tetrathionate respiration in Salmonella typhimurium. Mol Microbiol. 1999;32:275–287. doi: 10.1046/j.1365-2958.1999.01345.x. [DOI] [PubMed] [Google Scholar]
- 60.Rohmer L, Hocquet D, Miller SI. Are pathogenic bacteria just looking for food? Metabolism and microbial pathogenesis. Trends in microbiology. 2011 doi: 10.1016/j.tim.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]