Abstract
AIM
To determine mechanisms of action of the gasotransmitter hydrogen sulfide (H2S) on contractile activity in circular muscle of rat jejunum.
METHODS
Jejunal circular muscle strips were prepared to measure isometric contractions. Effects of sodium hydrosulfide (NaHS), a H2S donor, were evaluated on spontaneous contractile activity and after precontraction with bethanechol. L-cysteine was evaluated as an endogenous H2S donor. We evaluated extrinsic nerves, enteric nervous system, visceral afferent nerves, nitric oxide, K+ATP and K+Ca channels, and myosin light chain phosphatase on action of H2S using non-adrenergic/non-cholinergic conditions, tetrodotoxin, capsaicin, L-NG-nitro arginine (L-NNA), glibenclamide, apamin, and calyculin A, respectively, and electrical field stimulation (EFS).
RESULTS
NaHS dose-dependently and reversibly inhibited spontaneous and bethanechol-stimulated contractile activity (p<0.05). L-cysteine had a dose-dependent inhibitory effect. Non-adrenergic/non-cholinergic conditions, tetrodotoxin, capsaicin, L-NNA, or apamin had no effect on contractile inhibition by NaHS; in contrast, low dose glibenclamide and calyculin A prevented NaHS-induced inhibition. We could not demonstrate H2S release by EFS.
CONCLUSIONS
H2S inhibits contractile activity of jejunal circular muscle dose-dependently, in part by K+ATP channels and via myosin light chain phosphatase, but not via pathways mediated by the extrinsic or enteric nervous system, visceral afferent nerves, nitric oxide, or K+Ca channels.
Keywords: hydrogen sulfide, motility, contractile activity, jejunum circular smooth muscle
INTRODUCTION
It has become clear that not only classic neurotransmitters, such as acetylcholine or norepinephrine, but also small molecules of gas, such as nitric oxide (NO) and carbon monoxide (CO), are released from nerves within the gut and play an important physiologic role in signal transduction in the control of small intestinal motility. Hydrogen sulfide (H2S) appears to be one of the newest members of the “gasotransmitter” family [1,2]. Endogenous H2S is produced from the substrate L-cysteine by two enzymes, cystathionine beta (b) synthase (CBS) and cystathionine gamma (g) lyase (CSE). Not only H2S, but also other gasotransmitters like NO and CO are generated endogenously “on demand” and regulated enzymatically.
The mechanisms of action of H2S have been well delineated in vascular smooth muscle [3]. H2S opens ATP-sensitive potassium channels (K+ATP channels) leading to closing of voltage Ca2+ channels, hyperpolarization of the membrane potential, and subsequent vasorelaxation. Very few investigations, however, have explored the mechanisms of action of H2S in the modulation of small intestinal motility. Two early studies found that exogenous NaHS, an H2S donor, caused a dose-dependent inhibition of jejunal and ileal contractile activity [4-6], an effect that was independent of K+ATP channel activity [5]. Prior work in our laboratory showed that the enzymes that generate endogenous H2S, CBS and CSE, are expressed in the enteric nerves of the small intestine. H2S inhibited spontaneous and cholinergic-stimulated contractile activity in longitudinal muscle of rat jejunum and ileum [7,8], but surprisingly K+ATP channels appeared to mediate only a small part of the effect on contractile activity in the longitudinal muscle [7]. Moreover, the inhibitory effect of H2S did not appear to be mediated by the extrinsic or enteric nervous systems, primary visceral afferent nerve fibers, NO pathways, or K+ATP or K+Ca channels.
The aim of our current study was to determine the effects and mechanisms of action of H2S applied either exogenously and released endogenously on contractile activity specifically in the circular muscle of rat jejunum. Selective study of the different layers of gut smooth muscle (circular, longitudinal) and different regions (jejunum, ileum) have shown differing responses to similar stimuli [9-13]. Although both muscular layers of the small intestine are involved in motor function, contractile force and vectors are different and, thus, it would be important to understand the selective regulation of each muscle layer, especially if we can target the site and action of H2S for clinical applicability. By using specific pharmacologic inhibitors which block potential pathways of signal transduction, exogenously applied L-cysteine, the substrate for endogenous production of H2S, and using electric field stimulation (in attempt to release H2S from enteric nerves), we designed our experiments to investigate the effects of exogenous application and endogenous release of H2S. Our hypothesis was that H2S released “on demand” from enteric nerves acts as an endogenous inhibitor of contractile activity of the circular smooth muscle of rat jejunum by a direct effect on smooth muscle activity via opening of K+ATP channels.
MATERIALS AND METHODS
Preparation of Animals
Procedure and animal care were performed according to the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the Mayo Foundation in accordance with the guidelines of the National Institutes of Health and the Public Health Service Policy of the Human Use and Care of Laboratory Animals and was approved by the IACUC of the Mayo Clinic.
Recording of Contractile Activity
Male Lewis rats (Harlan-Sprague-Dawley, Indianapolis, IN) weighing 275-350 g were anesthetized by inhalation of 2% isoflurane (Abbott Laboratories, North Chicago, IL) and maintained with intraperitoneal sodium pentobarbital (30-50 mg/kg; AmproPharmacy, Arcadia, CA). A segment of jejunum 10 cm distal to the duodenojejunal junction was harvested and kept in chilled, modified Krebs-Ringer's bicarbonate solution (concentrations in mmol/L: NaCl 116.4, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 23.8, calcium disodium edentate 0.26, and glucose 11.1) pre-oxygenated with 95% oxygen/5% carbon dioxide (Praxair, Burr Ridge, IL). After opening the intestinal segment along the mesenteric border, full-thickness muscle strips (1×8 mm; width × length) were cut in the direction of the circular muscle layer. By doing so, we could measure primarily the effect of circular muscle contraction; contraction of the longitudinal muscle in this experimental methodology should be negligible [8, 10]. We specifically did not remove the mucosa and submucosa purposely in order to maintain an intact, transmural anatomy and all enteric neural connections. Both ends of the muscle strip were tied with 5-0 silk and suspended vertically in 10-ml tissue chambers filled with modified Krebs-Ringer's bicarbonate solution kept at 37.5°C and bubbled continuously with 95% oxygen/5% carbon dioxide. One end of the muscle strip was connected to a fixed hook, while the other end was attached to a metal hook connected to a noncompliant force transducer (Kulite Semiconductors Products, Inc., Leonia, NJ); this experimental set up allowed us to measure the isometric force generated by the circular muscle of the strip. Contractile activity was monitored by an 8-channel recorder (Grass 7D polygraph; Grass Instrument Co, Quincy, MA) in real-time while being displayed in parallel and stored digitally on a personal computer using dedicated software (MP-100A-CE and AcqKnowledge; Biopac Systems, Inc., Goleta, CA); detailed computer analysis followed later. Our system has been well described previously [10-12] . At the conclusion of the experiment, each muscle strips was blotted on filter paper and weighed to standardize contractile data as per mg tissue weight.
Experimental Design
Muscle strips were equilibrated for 60-90 min with washout of the bath solution every 15 min to allow development of stable, spontaneous contractile activity. Thereafter, we determined the optimal length (Lo) of each muscle strip by incremental stretching at 5- to 10-min intervals to a length beyond which further stretching no longer increased either amplitude or frequency of spontaneous contractile activity; all subsequent experiments were performed at this Lo. Muscle strips not developing a stable, characteristic pattern of spontaneous contractile activity were excluded from the study. Each experimental condition was carried out in at least two muscle strips per rat with a minimum of 6 rats per condition.
Exogenous H2S dose-response
We used the well-established H2S donor NaHS. At pH of 7.4 and temperature of 37.5°C, 18.5% of NaHS exists as H2S in solution [14]. After preliminary experiments to determine dosage ranges, four different, escalating concentrations of NaHS (10-5, 10-4, 2×10-4, 5×10-4 M) yielding concentrations of H2S in solution of about 1.8, 18, 36, and 90 mM, respectively, were added to eight muscle strips per rat in 15 rats with washout of the bath solution between each dose. We used NaHS as the exogenous donor of H2S, because not only is it much easier technically to use, but it is also more reliable in attaining an accurate concentration of H2S in the bath solution than is possible by preparing a solution by bubbling of H2S through the bath. Based on our findings in longitudinal muscle [7,8] and others’ work showing a differential effect of H2S according to the contractile state of the muscle [5], the effect of NaHS (5×10-4 M) was also determined on muscle strips pre-contracted by application of NaHS 90 s after exposing the muscle strips to the muscarinic agonist bethanechol at 10-4 M; this dose of bethanechol increased the frequency and amplitude of contractions without the marked, non-phasic (non-physiologic), tonic component attained by pharmacologic doses [15]. Because L-cysteine is a substrate for endogenous enzymatic production of H2S [2], we hypothesized that exogenous application of L-cysteine would increase endogenous production of H2S, allowing us to determine if the H2S released would have some effect on contractile activity. The effect of L-cysteine was studied at three doses of 10-4, 10-3, and 10-2 M cumulatively.
Neural effects
Non-adrenergic and non-cholinergic (NANC) conditions were established by adding atropine (10-7 M), phentolamine (10-5 M), and propranolol (5×10-6 M) to the bath; These NANC conditions allowed us to investigate the role of adrenergic and cholinergic neurons in mediating the effect of NaHS as reported previously [7-9,12]. NaHS 5×10-4 M was administered 30 min after establishment of NANC conditions; because the muscarinic receptor antagonist atropine was used to establish NANC conditions, we did not study the effect of NaHS after pre-contraction with bethanechol. Thereafter, the effect of the global neural inhibitor tetrodotoxin (TTX; 10-6 M) was studied on baseline activity in the same two muscle strips as for the NANC conditions [8,12,15]. TTX inhibits voltage-gated, fast sodium channels in nerve cell membranes to prevent depolarization of the cell membrane, thereby preventing release of neurotransmitters from virtually all nerves within the muscle strip. After exposure of the muscle strips to TTX for 30 min, the effect of NaHS was evaluated at a dose of 5×10-4 M.
Visceral afferent nerves
We used capsaicin, an agonist of transient receptor potential vanilloid receptor 1 (TRPV-1), to investigate the role of visceral afferent nerves in mediating the effect of NaHS. When administered exogenously, capsaicin desensitizes primary afferent nerve fibers in vitro [16-20]; we confirmed the effectiveness of capsaicin in preliminary experiments by observing a tachyphylaxis, i.e. a lack of an immediate contractile response (which occurred on first exposure to capsaicin) to subsequent doses of capsaicin. After exposure to capsaicin at two separate doses of 10-5 and 10-4 M for 30 min, the effect of 5×10-4 M NaHS was studied.
Nitric Oxide
Next we used the NO synthase inhibitor L-NG-nitro arginine (L-NNA) to investigate any effect of the NO pathway and/or any interaction with NO in mediating the effect of NaHS. After exposure to L-NNA at two log doses (10-4 and 10-3 M) for 30 min, the effect of 5×10-4 M NaHS was studied. L-NNA inhibits endogenous production of NO at these doses [9].
K+ channel activity
We used glibenclamide at doses of 10-5 and 10-4 M to investigate involvement of K+ATP channels in mediating the effect of NaHS; glibenclamide blocks the K+ATP channel at these doses [4]. We used 10-6 and 5×10-6 M apamin to investigate involvement of K+Ca channels in the response to NaHS; apamin blocks K+Ca channels at these doses [4]. After exposure to either glibenclamide at two log doses of 10-5 and 10-4 M for 30 min or apamin at 10-6 and 5×10-6 M for 30 min, the effect of 5×10-4 M NaHS was studied.
Myosin light chain phosphatase
Based on a previous study that suggested that H2S acts via myosin light chain phosphatase activity [21], we used calyculin A at a dose of 10-6 M to investigate the involvement of myosin light chain phosphatase in the response to NaHS. calyculin A blocks myosin light chain phosphatase at this dose. [21,22] After exposure to 10-6 M calyculin A for 30 min, the effect of 5×10-4 M NaHS was studied.
Electrical field stimulation (EFS)
In six other muscle strips from 6 rats, the response to EFS was evaluated at 3 and 20 Hz using a constant voltage (20 V), pulse width (4 msec), and duration of stimulation (10 s) similar to our previous work [12]; each condition was evaluated in at least 2 separate muscle strips. We chose 3 Hz as an inhibitory EFS and 20 Hz as an excitatory EFS based on preliminary experiments. All EFS studies were performed under NANC conditions with atropine (10-7M), phentolamine (10-5 M), and propranolol (5×10-6 M) in the bath to block the more dominant adrenergic and cholinergic effects induced by EFS. Between each application of EFS, 10 min were allowed for spontaneous contractile activity to recover before the next EFS was applied; the bath solution was changed after each series of stimulations. First, we determined the response of spontaneous contractile activity to EFS under NANC conditions in all six muscle strips as control conditions. After completing these control conditions, we evaluated EFS separately after inhibiting of the endogenous H2S-producing enzymes CBS and CSE, and endogenous NO synthase to inhibit NO release, and after adding a competitive inhibitor of vasoactive intestinal peptide (VIP). First, we used the CBS inhibitor aminooxyacetic acid (AOAA) at a dose of 10-3 M based on prior experiments [7]. The effect of a 30 min exposure to AOAA on baseline contractile activity was evaluated for 15 min; thereafter, the response to EFS was studied in the presence of AOAA. In two other muscle strips, we used the CSE inhibitor DL-propargylglycine (PPG) at a dose of 2×10-3 M [23]. The effect of PPG on baseline contractile activity was evaluated for 15 min; thereafter, the response to EFS was studied in the presence of PPG. After washout of the bath solution, we evaluated the combination of AOAA (10-3 M) and PPG (2×103 M) for 30 min on spontaneous contractile activity and, thereafter, the response to EFS in the presence of both AOAA and PPG.
In two other muscle strips from 6 rats, we evaluated the effects of inhibiting the dominant NANC inhibitory neurotransmitters NO and VIP in attempt to reveal any more subtle effects of the release of endogenous H2S by EFS. First, we used 10-3 M L-NNA. After determining the response of baseline contractile activity to L-NNA exposure for 30 min, the response to EFS was studied in the presence of L-NNA. After washout of the bath solution, we evaluated the combination of L-NNA (10-3 M) and the VIP antagonist [D-p-Cl-Phe6,Leu17]-VIP (10-6 M) for 30 min on baseline contractile activity, after which the response to EFS was studied in the presence of L-NNA and the VIP antagonist. After washout of the bath solution, the effect of the combination of all four inhibitors/antagonists, L-NNA (10-3 M), VIP antagonist (10-6 M), AOAA (10-3 M), and PPG (2×10-3 M), on baseline contractile activity was studied after exposure for 30 min; thereafter EFS was studied in the presence of all four inhibitors.
Data Analysis
Phasic changes in force (total contractile activity) were measured as area under the contractile curve (AUC) and were analyzed by a data acquisition system (AcqKnowledge). We set the baseline tone before each intervention as zero when we calculated the AUC, which enabled us to analyze only the phasic contractile activity. We also measured and analyzed changes of mean amplitude, baseline tone, and frequency under each condition. Thereafter, the effects of each of the experimental conditions, NaHS, L-cysteine, NANC conditions, TTX, capsaicin, L-NNA, glibenclamide, apamin, and calyculin A, on spontaneous activity were measured for 5 min and compared to the baseline contractile activity for 5 min measured immediately before each drug was administered; this approach allowed us to control for any effects on baseline contractile activity by any of the drugs tested. In contrast, the effect of administration of AOAA and/or PPG and L-NNA alone or in combination with the VIP antagonist on spontaneous contractile activity was measured for 15 min after exposure of the muscle to these agents for 30 min and was compared to the 5 min immediately before administration of the antagonists. When muscle strips were pre-contracted with bethanechol, the subsequent response to NaHS was measured for 5 min and compared to the same duration of bethanechol-alone activity. For the dose responses to NaHS, the responses after pre-contraction, and the responses to the various inhibitors/antagonists, the values across the two muscle strips were meaned, and the mean responses across the 6 rats were calculated. Drug responses are given as % change from baseline contractile activity (defined as 0%), with positive values representing an increase and negative values a decrease in contractile activity.
The response to EFS was studied for the 10 s of EFS in all experiments; the “off contraction” that occurred immediately after termination of EFS was not evaluated. According to preliminary experiments, we used 3 Hz as net inhibitory frequencies and 20 Hz as a stimulatory EFS frequency [12,15]. Contractile activity was expressed as the percent of baseline contractile activity for an equally long interval (10 s) measured separately in each muscle strip during the 40 s immediately before EFS and corrected for a 10 second duration.
All data are expressed as mean±SEM. Analysis of variance (ANOVA) was used to analyze the effects of a dose-response to NaHS, while paired Student's t-tests were used to compare the effects of different drugs and EFS; when individual comparisons were made, we used the a Bonferroni correction to correct for the multiple comparisons.
Drugs
Apamin, AOAA, atropine sulfate, bethanechol chloride, capsaicin, L-cysteine, glibenclamide, L-NNA, phentolamine hydrochloride, PPG, DL-propranolol hydrochloride, NaHS, TTX, [D-p-Cl-Phe6,Leu17]-VIP were purchased from Sigma-Aldrich, St. Louis, MO and calyculin A from Tocris Bioscience, St. Louis, MO. For the stock solution, capsaicin, glibenclamide, and calyculin A were dissolved in dimethylsulfoxide (Sigma-Aldrich, St. Louis, MO). L-NNA and DL-propranolol hydrochloride were dissolved in 0.5 N hydrochloric acid (HCl), and 0.1 N HCl was used for further dilutions to 10-4 M of L-NNA; preliminary experiments showed that the concentration of dimethylsulfoxide and 0.5 N HCl used to dissolve these agents had no effect on spontaneous contractile activity or pH of the bath solution. All other drugs were dissolved in purified water.
RESULTS
Response to NaHS (Exogenous donor of H2S)
NaHS inhibited spontaneous activity in a dose-dependent manner (p<0.05) (Fig 1 and 2a). These effects of NaHS on spontaneous contractile activity occurred by inhibiting total contractile activity (area under the contractile curve) and by decreasing frequency of contractions; NaHS did not, however, change amplitude or baseline tone of spontaneous contractions until a concentration of 5×10-4M was reached (Table 1). In contrast, NaHS at doses of 10-4 M and 5×10-4 M did not inhibit any aspect of contractile activity after precontraction with 10-4 M bethanechol (Fig 2b); i.e. NaHS did not change the amplitude, baseline tone, or frequency after precontraction with bethanechol.
Figure 1.
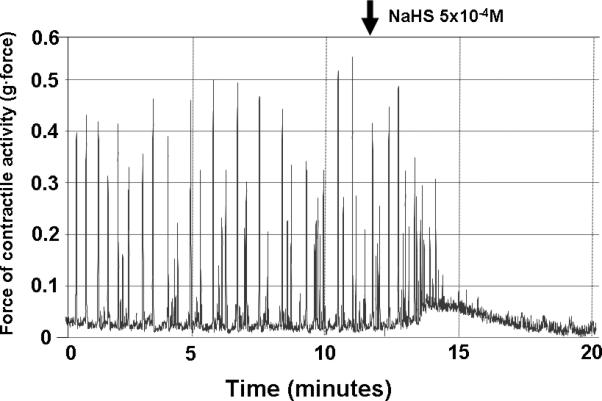
Effects of NaHS on spontaneous contractile activity. NaHS at 5×10-4M inhibited contractile activity by decreasing amplitude and frequency.
Figure 2.


Effect of NaHS on a) spontaneous and b) bethanechol-stimulated contractile activity. Spontaneous contractile activity measured by area under the contractile curve for 5 minutes was defined as 0%; therefore, negative values represent inhibitory effects on contractile activity. NaHS inhibited spontaneous contractile activity in a dose-dependent manner; *p<0.05 compared to spontaneous contractile activity (ANOVA). Doses of 10-4M and 5×10-4M of NaHS had no effect on contractile activity after pre-contraction.
TABLE 1.
Effect of NaHS on spontaneous contractile activity*
| 10-5 M | 10-4 M | 2×10-4 M | 5×10-4 M | |
|---|---|---|---|---|
| Total contractile activity (AUC)† | -9±3 | -14±2** | -28±7** | -44±3** |
| Amplitude | -2±1 | -4±1 | -7±2 | -12±1 |
| Baseline tone | -4±1 | -4±1 | -4±1 | -5±1 |
| Frequency | -5±2 | -10±2 | -26±2** | -58±3** |
Percent change from baseline contractile activity, baseline defined as zero; mean±SEM; n=15 rats
Area under the contractile curve
p<0.05 compared to baseline contractile activity defined as 0%
Effect of endogenous substrate of H2S
Administration of L-cysteine inhibited spontaneous contractile in a dose-dependent manner (p<0.05) (Fig 3) by decreasing amplitude, baseline tone, and frequency of spontaneous contractions (Table 3).
Figure 3.
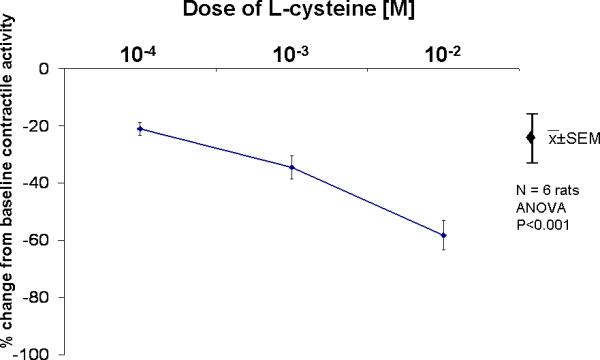
Effects of L-cysteine on spontaneous contractile activity. L-cysteine inhibited spontaneous contractile activity in a dose-dependent manner, *p<0.05 compared to spontaneous contractile activity (ANOVA).
TABLE 3.
Effect of L-cysteine on spontaneous contractile activity*
| 10-4 M† | 10-3 M† | 10-2 M† | |
|---|---|---|---|
| Area under the curve (AUC) | -21±2 | -35±4 | -58±5 |
| Amplitude | -4±1 | -8±1 | -13±1 |
| Baseline tone | -5±1 | -9±1 | -14±2 |
| Frequency | -11±3 | -13±3 | -18±4 |
Percent change from baseline contractile activity, baseline defined as zero; mean±SEM; n=6 rats.
p<0.05 compared to control.
Role of Neural Pathways
To investigate the involvement of neural pathways, 5×10-4 M NaHS was administered under NANC conditions and after pretreatment with TTX. Neither NANC conditions nor TTX altered baseline spontaneous contractile activity (data not shown); when muscle strips were exposed to 5×10-4 M NaHS, no significant changes in the NaHS-induced inhibitory effects on total contractile activity were noted under NANC conditions or after pretreatment with TTX (Table 2).
TABLE 2.
Effect of 5×10-4 M NaHS on spontaneous activity* in the absence and presence of inhibitors
| M NaHS alone | TTX | Capsaicin | L-NNA | Glibenclamide | Apamin | Calyculin A | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5×10-4 | NANC† | 10-6 M | 10-5 M | 10-4 M | 10-4 M | 10-3M | 10-5 M | 10-4 M | 10-6 M | 5×10-6 M | 10-6 M | |
| Total contractile activity (AUC)** | -44±3 | -33±9 | -46±13 | -26±11 | -31±10 | -24±6 | -35±12 | -15±7‡ | -38±11 | -40±11 | -46±11 | -12±12‡ |
| Amplitude | -12±1 | -11±2 | -12±2 | -10±4 | -11±3 | -9±1 | -10±4 | -2±1‡ | -9±3 | -10±2 | -13±3 | -2±3‡ |
| Baseline tone | -5±1 | 1±2‡ | -3±2 | -18±7‡ | 13±21 | -8±2 | -12±3‡ | -2±1 | -3±2 | -4±2 | -5±1 | 4±3‡ |
| Frequency | -58±3 | -52±11 | -51±19 | 59±29‡ | 21±22‡ | 1±19‡ | -4±19‡ | 50±19‡ | -57±17 | -33±30 | -49±27 | -65±20 |
Percent change from baseline contractile activity, baseline defined as zero; mean±SEM; n=6 rats
Atropine (10-7 M) and propranolol (5×10-6 M)
Area under the contractile curve
p<0.05 compared to control response (5x10-4 M NaHS alone) (ANOVA)
Role of Primary Neural Afferents
To investigate the involvement of primary afferent nerve fibers, primary afferent nerve fibers were desensitized with capsaicin (10-5 and 10-4 M). Desensitization of visceral afferent nerves had no effect on spontaneous contractile activity (data not shown); when muscle strips were exposed to 5×10-4 M NaHS, no change in the NaHS-induced inhibitory effect on total contractile activity was noted (Table 2).
Role of Nitrergic Pathways
To investigate any interaction between H2S and the release of NO, L-NNA was used to block NO production. Treatment with L-NNA had no effect on spontaneous contractile activity (data not shown); when muscle strips were exposed to 5×10-4 M NaHS, no change in the NaHS-induced inhibitory effect on total contractile activity was noted (Table 2).
Role of K+ channels
To investigate the involvement of K+ATP and K+Ca channels, glibenclamide and apamin were used. The lesser dose of 10-5 M glibenclamide had no effect on baseline spontaneous contractile activity (data not shown). After this pretreatment with 10-5 M glibenclamide, the inhibitory response to 5×10-4 M NaHS was inhibited markedly. In contrast, the greater dose of 10-4 M glibenclamide also had no effect on baseline spontaneous contractile activity (data not shown), but under these conditions, 5×10-4 M NaHS induced an inhibitory effect similar to control conditions (Table 2).
Treatment with apamin had no effect on spontaneous contractile activity (data not shown); thereafter, when the muscle strips were exposed to 5×10-4 M NaHS, no change in the NaHS-induced inhibitory effect on total contractile activity was noted (Table 2).
Role of Myosin Light Chain Phosphatase
To investigate the involvement of myosin light chain phosphatase, calyculin A was used. Calyculin A had no effect on baseline spontaneous contractile activity (data not shown). After pretreatment with calyculin A, 5×10-4 M NaHS no longer induced an inhibitory effect on spontaneous contractile (Table 2).
Response to EFS
Under NANC conditions, EFS at 3Hz decreased contractile activity for the entire 10 seconds; in the presence of inhibitors of endogenous H2S-producing enzymes (AOAA alone, PPG alone, or AOAA and PPG), there was no significant inhibition of the inhibitory effect of EFS at 3Hz. In contrast, the presence of L-NNA alone, the combination of L-NNA and the VIP antagonist, and the combination of all 4 inhibitors (L-NNA, VIP antagonist, AOAA and PPG), all inhibited partially the EFS-induced inhibitory effect (Fig 4a). EFS at 20Hz increased contractile activity for the entire 10 seconds under NANC conditions (p<0.05); In the presence of any combination of the inhibitors, there were no significant differences in the stimulatory effects of EFS compared to control conditions (Fig 4b).
Figure 4.
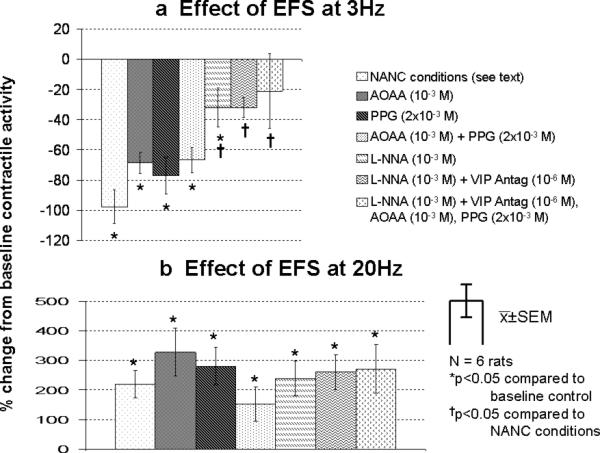
Effect of EFS at a) 3Hz and at b) 20Hz for the entire 10 s of EFS in the presence of inhibitors. The area under the contractile curve immediately before EFS was defined as 0%; positive values represent a stimulatory effect on contractile activity, while negative values represent an inhibitory effect. In the presence of these inhibitors, various significant difference of the effect of EFS compared to baseline control condition for the entire 10 s.
DISCUSSION
The aim of our study was to determine the effects and mechanisms of action of endogenously-released H2S on contractile activity in the circular muscle of the rat jejunum. We studied jejunal circular muscle as part of our ongoing, comprehensive approach to characterize inhibitory neurotransmitters in the small intestine, in hopes of targeting these mechanisms both to better understand the physiology of the sulfidergic pathways and also to potentially exploit these sulfidergic pathways in various forms of intestinal dysmotility. [8-11,13]. By using targeted inhibitors which block different potential pathways of signal transduction, we showed that H2S at physiologically-attained concentrations inhibited reversibly the spontaneous contractile activity in rat jejunal circular muscle. This effect was not mediated via the enteric nervous system, primary visceral afferent nerve fibers, production of NO, or K+Ca channels but rather in part via K+ATP channels and via myosin light chain phosphatase, unlike in the rat longitudinal muscle of jejunum [7] or ileum [8]. Our experiments with EFS attempted unsuccessfully to uncover an inhibitory effect of H2S released endogenously from intrinsic nerves by nerve stimulation.
To the best of our knowledge, there are few other reports that describe the effects of H 2S on contractile activity in the small intestine. Several reports have shown that exogenous H2S manifests an inhibitory effect on spontaneous contractile activity in the jejunal longitudinal muscle of mouse [4], as well as in pre-contracted ileal circular muscle of guinea pig [6]. There have been no reports, however, which study comprehensively the effect of H2S on jejunal circular smooth muscle. We demonstrated a dose-dependent, reversible inhibitory effect of NaHS on spontaneous contractile activity in the rat jejunal circular muscle.
Our experiments in jejunal circular muscle build on and expand similar prior comprehensive work in longitudinal muscle of the jejunum and ileum. The current work led to findings that differ considerably from our prior studies in the longitudinal muscle of the jejunum [7] and ileum [8]. In the jejunal circular muscle, inhibition of contractile activity occurs at a lesser dose, there was no inhibition of bethanechol-stimulated contractile activity, L-cysteine had a dose-dependent inhibitory effect, and both low-dose glibenclamide and calyculin A prevented the NaHS-induced inhibition of contractile activity. Each of these effects are distinctly different from the effects in longitudinal muscle of the jejunum and ileum—further supporting our basic tenet that different regions of the gut (jejunum, ileum) and different muscle layers (circular, longitudinal) are subject to different neural and/or pharmacologic control.
H2S has been shown consistently to inhibit contractile activity in the small intestine at doses within the range of physiologically-measurable concentrations, i.e. <200 μ M [7,8]. In the longitudinal muscle of the jejunum and ileum of the rat, inhibitory effects were noted at doses of NaHS of 10-3 M [7,8]. In our current experiment, a substantially lesser dose of NaHS (2×10-4M) effectively inhibited spontaneous contractile activity, suggesting that H2S exerts a much more potent effect in the jejunal circular muscle. Indeed, we [12] and others have shown that several regulatory agents display vastly different effects in the circular versus the longitudinal muscle, helping to explain the complexity of control of contractile activity of the gut. While longitudinal and circular muscle work together to propel intraluminal content distally, modulation of their contractile activity appear to occur by obviously different mechanisms.
The inhibitory effects in the circular muscle of exogenous L-cysteine, the primary substrate for H2S production, are consistent with the above observations. In our prior experiments with longitudinal muscle, exogenous L-cysteine had no demonstrable effect on contractile activity. In contrast, in jejunal circular muscle in our current experiments, L-cysteine induced a consistent, dose-dependent inhibition of circular muscle contractile activity, suggesting that by providing large amounts of substrate to drive H2S production by the endogenous, H2S-producing enzymes CBS and CSE, both of which we have shown to be present in the rat small bowel [7], endogenous synthesis of H2S under these basal conditions would be augmented and the effects of increasing amounts of H2S production would be evident. Our experiments are consistent with this hypothesis. Although L-cysteine can be hydrolyzed chemically to H2S by a nonspecific, non-enzymatic process, we saw no such inhibitory effects in longitudinal muscle exposed to similar concentrations of L-cysteine, making this possibility less attractive. In contrast, there may be a neural modulation of contractile activity by hydrogen sulfidergic pathways in the longitudinal muscle layer not present in the circular muscle layer.
The mechanism of action of H2S in the gut has been very elusive. Our current experiments with neural antagonists failed to implicate neural pathways, either NANC nerves, visceral afferent nerves, or extrinsic nerves in the action of NaHS, similar to our other experiments in rat longitudinal muscle [7,8]. Similarly, although H2S appears to work synergistically with NO in vascular smooth muscle [24-26], neither our current work in jejunal circular muscle, our past work in jejunal and ileal longitudinal muscle, nor the work of others in jejunum and colon [4,27] were able to show involvement of nitrergic nerves in mediating the effects of NaHS. These observations suggest that NaHS exerts its inhibitory effect by a direction action of gut smooth muscle.
In the vascular system, H2S mediates its inhibitory effects by opening K+ATP channels to induce cellular hyperpolarization, closing of voltage-gated calcium channels, and muscular relaxation. Similarly, K+ATP channels appear to mediate the inhibitory effects of NaHS in rat colon [4,27]. In contrast, most prior experiments in the small bowel have shown that inhibition of K+ATP channels by glibenclamide had little or no effect on NaHS-induced inhibiton [4,7,8]. In jejunal circular muscle, however, low-dose glibenclamide (10-5 M), but not the greater dose (10-4 M), effectively prevented the inhibitory effects of NaHS, thereby suggesting that the HsS released by NaHS has a direct effect on circular muscle of the rat jejunum by opening K+ATP channels. Interestingly, K+Ca channels were not involved, because apamin had no effect. Again, this observation is consistent with differing modulatory mechanisms on contractile activity in different regions of the gut and muscular layers within the same region of the gut.
We also investigated the role of calyculin A, an inhibitor of myosin light-chain phosphatase. Prior work showed that calyculin A decreased the NaHS-induced relaxation of mouse gastric fundus [21]. Our results showed quite clearly that at least part of the inhibitory effect of NaHS was mediated also through myosin light-chain phosphatase activity, possibly in concert with the opening of K+ATP channels. Whether H2S acts directly on myosin light-chain phosphatase or more likely that this phosphatase acts downstream in one of the intracellular signaling pathways mediating the effects of K+ATP channels is unknown and will require further work to determine.
Finally, we attempted to explore the role of enteric neurons in modulating contractile activity by the release of H2S endogenously. As stated above, we have shown that the enzymes that synthesize H2S endogenously, CBS and CSE, are present in neurons with fibers that innervate the rat muscular layers of the small bowel [7]. As we and others have shown for NO and other NANC inhibitory neurotransmitters, we hoped to provide evidence that H2S was released during low-frequency EFS, a form of global excitation of all neurons within the muscle strip. Low-frequency EFS is well established to induce inhibition of contractile activity, but by non-selective release of many neurotransmitters. Despite blocking of the effects of the dominant neurotransmitters (acetylcholine, norepinephrine, NO, and VIP) using targeted inhibitors or antagonists, and individual inhibition of CBS and CSE by AOAA and PPG, we could not provide any indirect evidence of release of H2S. Though disappointing, especially because we have shown that both CBS and CSE are present within the small intestinal wall, the effects of endogenously-released H2S may have been masked by the concomitant release of the more dominant inhibitory neurotransmitters NO and/or VIP, which have very strong effects on muscle contractile activity. EFS non-selectively stimulates intrinsic nerves, whereas it remains possible and rather likely that inhibitory modulation of contractile activity occurs via selective activation of specific inhibitory nerves, either nitrergic, VIPergic, or hydrogen sulfidergic nerves, which then alter contractile activity via selective mechanisms..
In summary, our experiments implicate H2S as a physiologically-relevant, inhibitory gasotransmitter in the rat jejunal circular muscle. The different mechanisms by which H2S appears to exert its effects in the jejunal circular muscle compared to the jejunal and ileal longitudinal muscle further demonstrates the complexity of the control of motility both between various anatomic regions of the gut (stomach, small bowel, colon) and the various muscle layers in any individual region (circular or longitudinal muscle).
ACKNOWLEDGEMENTS
The authors want to thank Deborah I. Frank for her assistance in the preparation of this manuscript.
This work was supported in part by a grant from the National Institutes of Health DK39337-18 (Dr. Sarr).
Footnotes
This work was presented as a poster at the 52nd Annual Meeting of the Society for Surgery of Alimentary Tract in Chicago, IL on May 8, 2011
REFERENCES
- 1.Wang R. The gasotransmitter role of hydrogen sulfide. Antioxid Redox Signal. 2003;5:493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- 2.Kasparek MS, Linden DR, Kreis ME, Sarr MG. Gasotransmitters in the gastrointestinal tract. Surgery. 2008;143:455–459. doi: 10.1016/j.surg.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowicka E, Beltowski J. Hydrogen sulfide (H2S)-the third gas of interest for pharmacolgists. Pharmacol Rep. 2007;59:4–24. [PubMed] [Google Scholar]
- 4.Gallego D, Clave P, Donovan J, Rahmati R, Grundy D, Jimenez M, Beyak MJ. The gaseous mediator, hydrogen sulphide, inhibits in vitro motor patterns in the human, rat and mouse colon and jejunum. Neurogatroenterol Motil. 2008;20:1306–1316. doi: 10.1111/j.1365-2982.2008.01201.x. [DOI] [PubMed] [Google Scholar]
- 5.Teague B, Asiedu S, Moore PK. The smooth muscle relaxant effect of hydrogen sulfide in vitro: evidence for a physiological role to control intestinal contractility. Br J Pharmachol. 2002;137:139–145. doi: 10.1038/sj.bjp.0704858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun. 1997;237:527–531. doi: 10.1006/bbrc.1997.6878. [DOI] [PubMed] [Google Scholar]
- 7.Kasparek MS, Linden DR, Farrugia G, Sarr MG. Hydrogen sulfide modulates contractile function in rat jejunum. J Surg Res. 2011 Apr 22; doi: 10.1016/j.jss.2011.03.069. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagao M, Linden DR, Duenes JA, Sarr MG. Mechanisms of action of the gasotransmitter hydrogen sulfide in modulating contractile activity of longitudinal muscle of rat ileum. J Gastrointest Surg. 2011;15:12–22. doi: 10.1007/s11605-010-1306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasparek MS, Fatima J, Iqbal CW, Duenes JA, Sarr MG. Role of VIP and substance P in NANC innervation in the longitudinal smooth muscle of the rat jejunum - influence of extrinsic denervation. J Surg Res. 2007;141:22–30. doi: 10.1016/j.jss.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Kasparek MS, Fatima J, Iqbal CW, Duenes JA, Sarr MG. Long-Term Effects of Extrinsic Denervation on VIP and Substance P Innervation in Circular Muscle of Rat Jejunum. J Gastrointestinal Surg. 2007;11:1339–1350. doi: 10.1007/s11605-007-0212-1. [DOI] [PubMed] [Google Scholar]
- 11.Kasparek MS, Fatima J, Iqbal CW, Duenes JA, Sarr MG. Effect of chronic, extrinsic denervation on functional NANC innervation with vasoactive intestinal polypeptide and substance P in longitudinal muscle of rat jejunum. Neurogatroenterol Motil. 2008;20:243–252. doi: 10.1111/j.1365-2982.2007.01021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasparek MS, Fatima J, Iqbal CW, Duenes JA, Sarr MG. Age-related changes in functional NANC innervation with VIP and substance P in the jejunum of Lewis rats. Auton Neurosci. 2009;151:127–134. doi: 10.1016/j.autneu.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kasparek MS, Fatima J, Iqbal CW, Sarr MG. Effects of extrinsic denervation on innervation with VIP and substance P in circular muscle of rat jejunum. Neurogatroenterol Motil. 2008;20:808–817. doi: 10.1111/j.1365-2982.2008.01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dombkowski RA, Russell MJ, Olson KR. Hydrogen sulfide as an endogenous regulator of vascular smooth muscle tone in trout. Am J Physiol Regul Integr Comp Physiol. 2004;286:678–685. doi: 10.1152/ajpregu.00419.2003. [DOI] [PubMed] [Google Scholar]
- 15.Ohtani N, Balsiger BM, Anding WJ, Duenes JA, Sarr MG. Small bowel transplantation induces adrenergic hypersensitivity in ileal longitudinal smooth muscle in rats. J Gastrointestinal Surg. 2000;4:77–85. doi: 10.1016/s1091-255x(00)80036-0. [DOI] [PubMed] [Google Scholar]
- 16.Schicho R, Krueger D, Zeller F, Von Weyhern CW, Frieling T, Kimura H, Ishii I, De Giorgio R, Campi B, Schemann M. Hydrogen sulfide is a novel prosecretory neuromodulator in the guinea-pig and human colon. Gastroenterology. 2006;131:1542–1552. doi: 10.1053/j.gastro.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 17.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- 18.Holzer P. Capsaicin as a tool for studying sensory neuron functions. Adv Exp Med Biol. 1991;298:3–16. doi: 10.1007/978-1-4899-0744-8_1. [DOI] [PubMed] [Google Scholar]
- 19.Patacchini R, Santicioli P, Giuliani S, Maggi CA. Hydrogen sulfide (H2S) stimulated capsaicin-sensitive primary afferent neurons in the rat urinary bladder. Br J Pharmachol. 2004;142:31–34. doi: 10.1038/sj.bjp.0705764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trevisani M, Patacchini R, Nicoletti P, Gatti R, Gazzieri D, Lissi N, Zagli G, Creminon C, Geppetti P, Harrison S. Hydrogen sulfide causes vanilloid receptor 1-mediated neurogenic inflammation in the airways. . Br J Pharmachol. 2005;145:1123–1131. doi: 10.1038/sj.bjp.0706277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhaese I, Lefebvre RA. Myosin light chain phosphatase activation is involved in the hydrogen sulfide-induced relaxation in mouse gastric fundus. Eur J Pharmacol. 2009;606:180–186. doi: 10.1016/j.ejphar.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 22.Dhaese I, Van Colen I, Lefebvre RA. Mechanisms of action of hydrogen sulfide in relaxation of mouse distal colonic smooth muscle. Eur J Pharmacol. 2009;628:179–186. doi: 10.1016/j.ejphar.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Linden DR, Sha L, Mazzone A, Stoltz GJ, Bernard CE, Furne JK, Farrugia G, Szurszewski JH. Production of the gaseous signal molecule hydrogen sulfide in mouse tissues. J neurochem. 2008;106:1577–1585. doi: 10.1111/j.1471-4159.2008.05502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali MY, Ping CY, Mok YY, Ling L, Whiteman M, Bhatia M, Moore P. Regulation of vascular nitric oxide in vitro and in vivo; a new role for endogenous hydrogen sulfide? Br J Pharmachol. 2006;149:625–634. doi: 10.1038/sj.bjp.0706906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fiorucci S, Distrutti E, Cirino G, Wallace JL. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology. 2006;131:259–271. doi: 10.1053/j.gastro.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 26.Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 27.Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Antonelli E, Roviezzo F, Morelli A, Cirino G, Wallace JL, Fiorucci S. Evidence that hydrogen sulfide exerts antinociceptive effects in the gastrointestinal tract by activating KATP channels. J Pharmacol Exp Ther. 2006;316:325–335. doi: 10.1124/jpet.105.091595. [DOI] [PubMed] [Google Scholar]


