Abstract
Locomotor systems are often controlled by specialized cephalic neurons and undergo modulation by sensory inputs. In many species, dedicated brain regions initiate and maintain behavior and set the duration and frequency of the locomotor episode. In the leech, removing the entire head brain enhances swimming, but the individual roles of its components, the supra- and subesophageal ganglia, in the control of locomotion are unknown. Here we describe the influence of these two structures and that of the tail brain on rhythmic swimming in isolated nerve cord preparations and in nearly-intact leeches suspended in an aqueous, “swim-enhancing” environment. We found that, in isolated preparations, swim episode duration and swim burst frequency are greatly increased when the supraesophageal ganglion is removed, but the subesophageal ganglion is intact. The prolonged swim durations observed with the anterior-most ganglion removed were abolished by removal of the tail ganglion. Experiments on the nearly intact leeches show that, in these preparations, the subesophageal ganglion acts to decrease cycle period but, unexpectedly, also decreases swim duration. These results suggest that the supraesophageal ganglion is the primary structure that constrains leech swimming; however, the control of swim duration in the leech is complex, especially in the intact animal.
Keywords: Hirudo, brain, swimming, motor control, sensory modulation
Introduction
It has been well documented that many invertebrate and some vertebrates can generate rhythmic behaviors in the absence of cephalic neural structures or “brains,” with the dynamics of these behaviors modified only slightly from those of the intact animal (Kien 1983; Brodfuehrer and Friesen 1986a; Thompson 1986a,b; Chrachri and Clarac 1990; Cohen 1992; Facciponte and Lange 1992; Marder et al. 2005; Kagaya and Takahata 2010; Puhl and Mesce 2010; Mullins et al., 2011b). These animals have thus been utilized to investigate the role of cephalic ganglia, together with sensory feedback, in controlling rhythmic behaviors. For example, the praying mantis and stick insect are both able to walk after isolation of the ventral nerve cord from the brain with only minor changes in their gait (Roeder 1937; Graham 1979), while locusts lacking descending outputs are capable of flight (Wilson 1961). The direction of the change in duration and frequency of the locomotor response following such manipulations is mixed. In the locust, removal of just the brain as well as the brain plus the subesophageal ganglia (SubEG) resulted in a decrease in the step frequency and duration of the walking bouts (Kien 1983). In contrast, whereas praying mantises with the SubEG destroyed were nearly motionless, those with just the supraesophageal ganglion (SupraEG) removed initiated movement more readily and walked for longer periods of time than control animals (Roeder 1937). In cockroaches, these two ganglia had different effects on locomotion depending on whether walking or flying was being examined (Ridgel and Ritzmann 2005; Gal and Libersat 2006). Finally, removing multiple anterior ganglia from the grasshopper results in continuous rhythmic oviposition digging (Thompson 1986a,b; da Silva and Lange 2011).
In the leech, the “head brain” comprises both the SubEG and SupraEG. Removal of the entire brain prevents coordinated crawling (Puhl and Mesce 2010) but enhances rhythmic swimming; swims are easier to initiate and have longer durations (Brodfuehrer and Friesen 1986a; Brodfuehrer et al. 1993). The head brain in the leech has usually been treated as a single entity, with relatively little effort directed toward understanding the respective roles of its ganglia in the control of locomotion. One study concluded that removal of the SubEG reduced activity levels in nearly-intact animals (Cornford et al. 2006); however, because the SupraEG was also disconnected from the midbody nerve cord in these experiments, individual functions of the two structures could not be confirmed. The most extensive set of behavioral experiments addressing this topic were performed by Erich Schlüter (1933), who reported that leeches with the SubEG but not the SupraEG intact increased the number of swims and decreased cycle periods as compared to intact leeches. However, his surgery necessarily eliminated rostral sensory input, which may have been the source of the observed effects, because leeches lacking this input exhibit enhanced swimming activity (Esch et al. 2002). Further, Schlüter was unable to compare the regional effects of the brain on fictive locomotion in isolated nerve cords to actual locomotion in nearly intact animals.
Here we describe our investigations of the individual roles of the SubEG and SupraEG, and also of the tail brain in the control of swimming locomotion in the medicinal leech. These experiments were conducted in isolated nerve cord preparations as well as in nearly intact animals placed in a “swim-enhancing” environment. We found that the duration of swim episodes and the frequency of swim bursts within episodes are strikingly increased by the removal of the SupraEG in isolated nerve cord preparations if the tail ganglion is attached. In contrast, all nearly intact, suspended leeches had extremely prolonged swim episodes with short cycle periods (high burst frequency); those with the SupraEG excised had the shortest cycle periods. Unexpectedly, the duration of swim episodes was slightly, but significantly, decreased by the presence of the SubEG. These results demonstrate that the SupraEG is the primary site for constraining leech locomotion in vitro; however, the influence of brain regions is more complex in the intact swimming animal.
Materials and Methods
Leech nervous system and terminology
The leech ventral nerve cord comprises 21 segmental (midbody) ganglia, flanked by head (H) and tail (T) brains (Fig. 1a). The head brain comprises supra- and subesophageal ganglia – SupraEG and SubEG, respectively (Fig. 1b). Midbody ganglia are identified by an M and a number, with the most rostral midbody ganglion labeled “M1.” Here, preparations comprising chains of ganglia (e.g., H - T or SubEG - T) are referred to as different “nerve-cord classes.”
Figure 1.
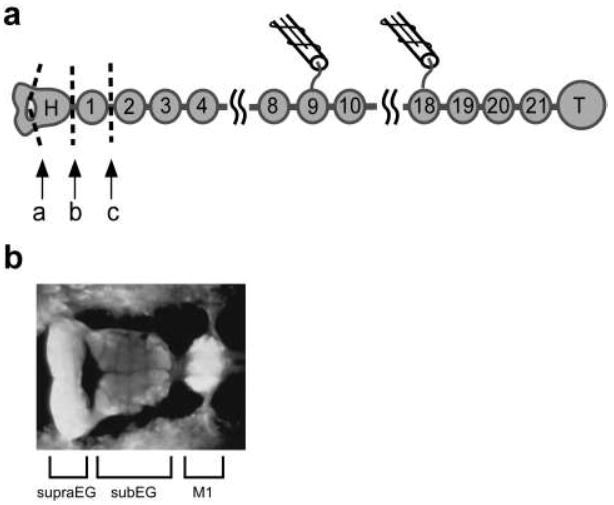
Leech central nervous system. a Schematic of isolated nervous system preparations. The CNS comprises the supraesophageal ganglion (SupraEG), the subesophageal ganglion (SubEG), a chain of midbody ganglia (M1-M21) and the large tail brain (T). The combined SupraEG and SubEG make up the head brain. Specific preparations include the entire CNS (H-T), the CNS with the SupraEG removed by cutting at ‘a’ (SubEG-T), the midbody ganglia and tail brain (M1-T) generated by cutting at ‘b’ and a further reduced version generated by cutting at ‘c’ (M2-T). Suction electrodes were placed on DP nerves for recording and stimulation. In nearly intact preparations, these cuts were made through a slit in the body wall. b Light micrograph of the ventral aspect of the head brain and M1
Preparations
Adult medicinal leeches, Hirudo verbana, were supplied by Niagara Medical Leeches (Cheyenne, WY) and Leeches USA (Westbury, NY). Leeches were kept in aquaria at 18-21°C on a 12-h light/12-h dark cycle or under ambient lighting. (Data presented in this paper were the result of a collaborative effort, therefore there are slight differences in the conditions and equipment for similar experiments.) Prior to surgery, leeches were anesthetized with 4°C leech saline containing (in mmol/L) 115 NaCl, 4 KCl, 1.8 CaCl2, 2 MgCl2, and 10 HEPES buffer (pH 7.4; Friesen 1981).
Experiments were performed on isolated leech nerve cords and on nearly-intact leeches. Dissections and lesions were carried out in wax bottomed dishes filled with cold saline. Isolated preparations: The body wall was removed and the nervous system was either left intact (H-T preparation) or had the following ganglia removed: SupraEG (SubEG-T), H (M1-T) or H-M1 (M2-T) (Fig. 1a). In some experiments the SupraEG was physically and M20-T were functionally removed (SubEG-M19). The nerve cord was held in place by magnetic pins or minutien pins in a glass bottomed-dish covered with a thin layer of Sylgard. Preparations were superfused with normal saline or (to aid swim initiation in half of H-T preparations) saline containing 50 μM serotonin. Serotonin does not affect swim maintenance properties (Willard 1981). In some experiments, the SubEG-T preparation was generated following initial recordings from an H-T nerve cord. For preparations involving intracellular recordings, the sheath was removed from the ventral side of the appropriate ganglion. Nearly-intact (NI) preparations: For minimally dissected preparations, a small incision was made in the body wall and the nerve cord was severed at the locations marked “a,” “b” or “c” in Fig. 1a to generate SubEG-T NI (nearly intact), M1-T NI or M2-T NI preparations, respectively. To make sensory input comparable among the nerve-cord classes, the peripheral nerves to the head brain were cut in H-T NI and SubEG-T NI preparations. Sometimes the nerves to M1 were removed as well. In all preparations, the body wall of midbody segment 17 was denervated. Threads to suspend the leech were attached to either side of the denervated segment 17 and to the denervated rostral sucker.
Our experiments implicitly assume that any differences in the hormonal environment of the leech CNS in our preparations have minor functional consequences. This assumption is supported by decades of experimentation on a wide variety of leech preparations, which have generated similar records in isolated, semi-intact and nearly intact animals (Pearce and Friesen 1984; Kristan et al., 2005; Puhl and Mesce 2010).
Procedures and analysis
Isolated preparations
We compared swim durations and cycle periods in four isolated nerve-cord classes - H-T, SubEG-T, M1-T and M2-T. Swimming was initiated either by a train of 2-4 V, 5 ms, 20 Hz pulses applied to a DP nerve, depolarizing current injection into cell E21 or it arose spontaneously. For comparisons between isolate nerve cord classes, swim duration was characterized by our standard measure - the number of motor neuron impulse bursts per swim episode (BPE) recorded from the DP nerve. For each experiment we determined the duration of the longest swim in that experiment and the mean swim duration and then averaged these respective values for each nerve-cord class. Because a small number of the SubEG-T preparations had extremely long swims (e.g., > 4000 BPE), which, if included, would skew the average toward these large numbers, the maximum swim duration was cut off at 300 BPE (i.e., swims with more than 300 bursts were scored as 300 BPE). These truncated values were only used for the comparisons between the isolated nerve cord classes. These averages for isolated SubEG-T preparations are called “longest computed swim durations,” and are actually an underestimation. The same set of preparations were used for both types of swim duration analyses except that the one nearly-continuously swimming preparation was excluded from the analysis of the mean duration.
Swim durations recorded in SubEG-T preparations are reported in units of time (min) as well as BPE in order to make direct comparisons of swim duration in isolated and nearly-intact preparations. For the other nerve-cord classes, swim duration in minutes was estimated from BPE and cycle period; we considered these estimations appropriate as swim duration was many times longer in the nearly-intact than isolated H-T, M1-T and M2-T nerve-cord classes.
Cycle periods
To quantify changes in cycle period in isolated preparations, defined as the time interval between reference points in two bursts, we used the periods of cycles 3 and 4 in the first five recorded swims that were initiated by DP-nerve shock or occurred spontaneously. We examined DP-initiated and spontaneous swims separately because we found that in M2-T preparations spontaneous swims had greater cycle periods than those initiated by nerve shock (paired t-test, p = 0.033). Cycles 3 and 4 were used because cycles 1-2 are often erratic in isolated preparations and most swim episodes were at least 5 bursts. Spontaneous swims were included only if at least 5 s had elapsed since termination of the previous swim. Cycle periods were determined by exporting data to Matlab (The Mathworks, Natick, MA) and analyzed with our custom-designed Rhythm Analysis System (RAS; Hocker et al. 2000). Burst statistics were determined by using the middle spike in each burst as the phase reference point.
Block of tail ganglion input
We examined the effect of the tail ganglion on swim duration by eliminating all spontaneous and evoked activity in M20-T in SubEG-T preparations by creating a well filled with isotonic sucrose around the caudal nerve cord (M20-T) (see Brodfuehrer et al. 1993). This procedure allowed functional and reversible removal of caudal ganglia. Ganglia M20-M21 were included with the tail brain in the sucrose well because these caudal ganglia are close together; forming a well around the tail brain alone is technically impossible. Of the five experiments conducted in this fashion, one did not have long swims (>1 min) in the saline condition (SubEG-T) and hence was excluded from the analysis.
Nearly-intact preparations
Swim duration and cycle period were compared in the four nerve-cord classes in nearly intact (NI) preparations, H-T NI, SubEG-T NI, M1-T NI and M2-T NI. These animals were suspended in normal saline by threads in Plexiglas troughs either 2.8 or 6.2 cm wide. At rest the leeches were >1 cm below the surface of the saline. Each leech was observed for 30 min, swim duration was recorded with a stopwatch. If a leech stopped swimming, and did not spontaneously start again, it was prodded to initiate swimming after an interval of about 60 s. Cycle period data were obtained by recording the first several cycles of swim episodes occurring during the first 10 min of each experiment with a video camera (PixeLINK, Ottawa, ON) at a minimum of 30 fps. In some cases, capturing cycle periods from multiple swims required terminating swimming using mechanical touch; this occurred prior to the 30 min observation period. Four cycles within the first five seconds of the swims were used for our analysis. Cycle period was determined from video frames viewed with ImageJ (NIH).
In the first set of experiments, leeches were placed in a trough 2.8 cm wide; however, in some preparations (especially those with the head brain present) the leeches occasionally contacted the side of the trough, which could potentially affect swim parameters. Leeches tested in a wider trough (6.2 cm), in which the animals never contacted the side, had the same cycle period (p>0.50) but tended towards longer swim durations (p<0.15). Therefore, for the swim duration analysis on H-T and SubEG-T preparations, those data obtained from experiments in the narrow trough were discarded. All but two of twelve M2-T and M1-T preparations swam for the entire observation period in the narrow trough, so experiments from these preparations were not repeated. Cycle period analysis was performed on data from preparations in both troughs. Because our observation period ended at 30 min, we did not compare swim duration among the preparations; rather, the fraction of leeches of each nerve-cord class that swam for the entire observation period was calculated.
Electrophysiology
Swims in isolated preparations were monitored by extracellular suction electrode recordings from dorsal posterior (DP) nerves, which reveal the rhythmic bursting in the MN cell DE-3 (Kristan and Calabrese 1976). Swimming was initiated by electrical stimulation of a DP nerve in the caudal nerve cord.
Sharp microelectrodes for intracellular recording were manufactured with a P-87 Flaming Brown Micropipette Puller (Sutter Instruments, Novato, CA) and filled with 2.7 or 3.0 M KAc and 20 mM KCl; resistances were 30 – 60 MΩ. Neuroprobe 1600 amplifiers (AM Systems) were used to make intracellular recordings and current injection in bridge mode. Extracellular signals were amplified by preamplifiers and then, along with intracellular signals, were digitized with PowerLab and displayed with LabChart software (AD Instruments, Colorado Springs, CO) at a sampling rate of 4 kHz. Intracellular recordings were obtained from the somata of neurons identified by location, size and electrical and functional properties.
Statistical analysis
Statistical analyses were carried out with Prism5 (GraphPad, La Jolla, CA); all graphs also were generated with this program. Results are reported as means and standard error. All post-test comparisons were made with the Newman-Keuls method. Individual data points that deviated more than four standard deviations from the mean (outliers) were discarded.
Results
Supraesophageal ganglion is the source of inhibition to the swim system in the head brain
Early studies suggested that the inhibitory influence of the head brain on swimming is largely due to neurons in the supraesophageal ganglion (SupraEG; Schlüter 1933). To specifically examine the role of the SupraEG in determining swim duration, we measured swim durations in isolated preparations with the SupraEG selectively removed (Fig. 1). Previous studies reported swim durations comprising ~15 cell DE-3 motor neuron bursts per episode (BPE) in head brain through tail brain (H-T) preparations, and 20 BPE in midbody ganglion 2 through tail brain (M2-T) preparations (Brodfuehrer et al. 1993). Remarkably, in two subesophageal ganglion through tail brain (SubEG-T) preparations, we observed swimming that lasted over 30 min and generated more than 2000 continuous bursts; activity durations that we had never previously observed in any preparation of the isolated nerve cord. Indeed, one preparation swam continuously for an hour, approximately 5000 bursts, only terminating following an electrical shock to a DP nerve. In this example, after a brief interval of non-rhythmic DP nerve activity, swimming resumed (Fig. 2a). In other SubEG-T preparations, swim episodes were shorter, nevertheless extended swim durations (episodes lasting 1- 10 min with 70-800 BPE) often occurred.
Figure 2.
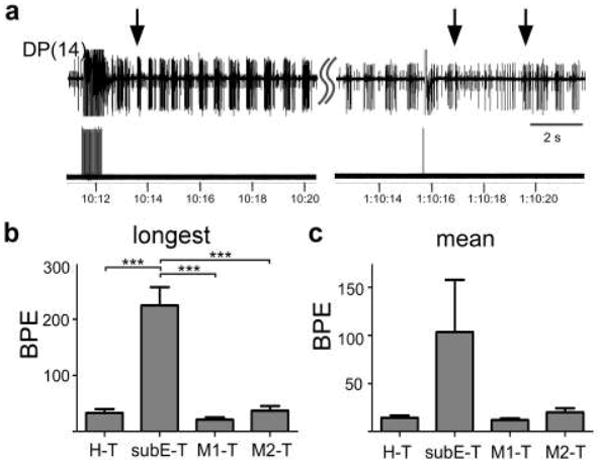
SubEG extends swim duration in isolated preparations. a Initiation (left arrow) and termination (middle arrow) of an hour-long swim episode evoked by stimulating a DP nerve (lower trace) in a SubEG-T preparation. A new swim episode began spontaneously soon after the first episode ended (right arrow). Upper trace is an extracellular recording from DP(14). The stimulus was applied to DP(17). b,c Measures of swim duration in the isolated preparations of the four nerve-cord classes. b Longest computed swim episodes. c Mean swim duration. Overall ANOVA is significant (p = 0.04). Bars are SE. Significance determined by one-way ANOVA; asterisks on figures refer to Newman-Keuls post-test results. *ρ < 0.05, ** ρ < 0.01, ***ρ < 0.001 (Table 1). BPE - burst per swim episode; BPE - burst per swim episode
The data outlined above suggest that the SupraEG inhibits swimming, while the SubEG facilities this behavior. To further examine and quantify the effect that specific neural regions have on swim duration, we compared SubEG-T preparation to two standard preparations used in studying swimming, H-T and M2-T. M2-T preparations are often used as swimming is easily evoked and has less erratic cycle periods than H-T preparations. We also examined M1-T preparations to determine if M1 contributed to the enhanced swimming observed in SubEG-T preparations; although one study has indicated that M1 is inhibitory, its effects with the tail brain attached were not tested (Brodfuehrer and Friesen 1986a). Because swim durations, even within single experiments, can be highly variable, both the longest computed swim duration as well as the mean swim duration were compared. The average longest computed swim for SubEG-T preparations was over 200 BPE (Fig. 2b; Table 1) and the longest swims in individual SubEG-T experiments ranged from 72 BPE to virtually continuous swimming (n = 7 leeches). In contrast, the average longest swims for H-T, M1-T and M2-T preparations were all under 40 BPE; all significantly shorter than in SubEG-T preparations (Fig. 2b; Table 1). The average longest swims in H-T, M1-T and M2-T preparations were not significantly different from each other. We also took the mean swim duration in each experiment; comparisons of this measure for the nerve-cord classes had similar trends as the longest computed swim comparisons, with a substantially higher SubEG-T average mean duration, 90.5 BPE, then the H-T (14.4 BPE), M1-T (16.8 BPE) and M2-T (20.0 BPE) preparations (Fig. 2c; Table 1). However, although a one-way ANOVA demonstrated overall significance between the nerve-cord classes (p = 0.04), no post-test comparisons were significant. This lack of significance can likely be ascribed to the large variance between the individual SubEG-T experiments; following the removal of the experiment with the largest mean SubEG-T swim duration (372.5 BPE) from the analysis, SubEG –T mean swim duration was significantly larger than all other nerve-cord classes (Newman-Keuls post test, p<0.001 for all SubEG-T comparisons).
Table 1.
Swim duration and cycle period for the four nerve-cord classes. ‘Longest computed’ averaged values (ave) from the longest computed swim in each experiment. ‘Mean’ – average of the mean swim duration in each experiment. Cycle periods are taken from swims initiated either spontaneously or by nerve-shock (evoked). ave – average values SE – standard error n – number of preparations
| H-T | SubEG-T | M1-T | M2-T | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration (BPE) | ave | SE | n | ave | SE | n | ave | SE | n | ave | SE | n |
| Longest computed | 33.0 | 7.0 | 10 | 224.6 | 32.6 | 7 | 21.0 | 4.4 | 4 | 37.1 | 8.3 | 8 |
| mean | 14.4 | 2.3 | 10 | 90.5 | 41.7 | 6 | 16.8 | 4.3 | 4 | 20.0 | 4.1 | 8 |
| CYCLE PERIOD (s) | ||||||||||||
| spontaneous | 0.69 | 0.03 | 10 | 0.55 | 0.04 | 6 | 0.84 | ---- | 2 | 0.85 | 0.04 | 7 |
| evoked | 0.76 | 0.04 | 13 | 0.56 | 0.04 | 7 | 0.83 | 0.07 | 4 | 0.76 | 0.12 | 8 |
There was large variability in the swim durations both within and between individual SubEG-T preparations. In some experiments, the longest swim was under a minute (~72 BPE), easily within the range of H-T, M2-T and M1-T preparations. In other SubEG-T preparations, only a few prolonged swims occurred, which were interspersed with many shorter swims. Most SubEG-T preparations exhibited both long and short swims. Despite this variability, we conclude that the SupraEG acts to restrict swimming and that with the SupraEG removed, the SubEG acts to extend the duration of suprathreshold excitation in the swim-maintenance system, and thereby extends swim duration.
Subesophageal ganglion provides excitation to decrease cycle periods
Cycle period in the isolated nerve cord is inversely related to the depolarization levels of the swim-gating neurons, cells 204 (Weeks and Kristan 1978; Debski and Friesen 1986). Because cell 204 is a crucial component of the swim maintenance system (Weeks and Kristan 1978; Friesen et al. 2011), cycle period can be considered a good measure of the excitation level in this system. We used this relationship to ask whether, in addition to extending swim duration, the maintenance system in SubEG-T preparations also has higher activity levels. To answer this question the periods of cycles 3 and 4 were compared in the four nerve-cord classes. We found that the cycle periods for both DP-initiated and spontaneous swims in isolated SubEG-T preparations were significantly shorter than in the other three nerve-cord classes (Fig. 3; Table 1). Figure 3a shows excerpts of representative spontaneous swims in SubEG-T and M2-T preparations. Clearly, cycle periods for spontaneous or evoked swims are shorter when the SubEG was included in the preparation and shortest when the SupraEG was removed. In the latter preparation, cycle periods approximated those observed in intact leeches. These results support the hypothesis that the SubEG, in the absence of the SupraEG, strongly drives the swim-maintenance system.
Figure 3.
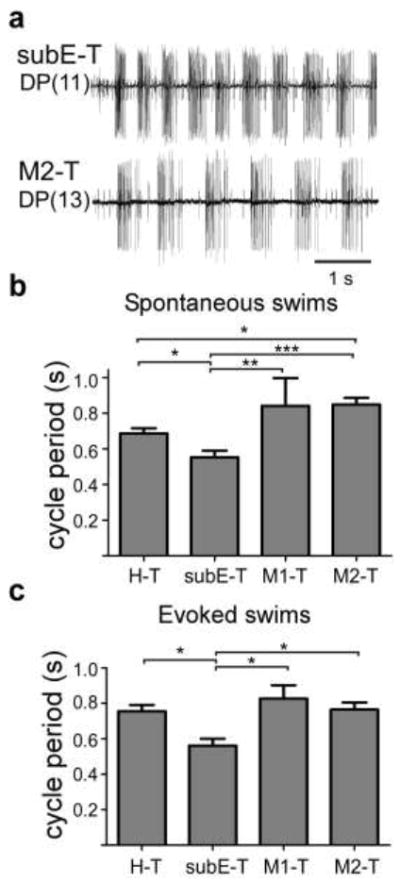
Cycle periods are shorter in isolated SubEG-T preparations. a Initial bursts of spontaneous swim episodes in SubEG-T (upper trace) and in M2-T (lower trace) preparations. b,c Averaged cycle periods in spontaneous (b) and evoked swims (c). One-way ANOVA with Newman-Keuls post-test. *ρ < 0.05, ** ρ < 0.01, ***ρ < 0.001
Importance of the tail ganglion for prolonged swimming
It was previously reported that removal of the tail ganglion shortens swim duration in preparations with the head brain removed (Brodfuehrer et al. 1993; Puhl and Mesce 2010; Friesen et al. 2011). We were interested in determining whether the presence of the tail ganglion was necessary for the occurrence of the extended swims in preparations with only the SupraEG removed. We examined this by functional and reversible removal of M20-T with an isotonic sucrose solution (Fig. 4a; n=4). Removal of the tail ganglion significantly shortened the longest swim duration (from 194.5 +/- 40.1 BPE to 23.3 +/- 6.0 BPE, p = 0.019 paired t-test) and the mean swim duration (from 78.1 +/- 16.9 to 11.4 +/- 1.1 BPE, p = 0.026). Importantly, extended swim durations did not occur in any preparations with the tail ganglion functionally detached; the longest observed swim in this condition was 39 BPE. Figure 4b shows multiple swims in a SubEG-T preparation (top three traces; top two traces are truncated episodes) and that application of isotonic sucrose to M20-T decreases swim duration significantly (Fig. 4b, traces 3-6). Washout of the sucrose restores the extended swim durations. Figure 4c shows the swim durations in one experiment (the example in Fig. 4b) with sucrose repeatedly applied and washed off M20-T.
Figure 4.
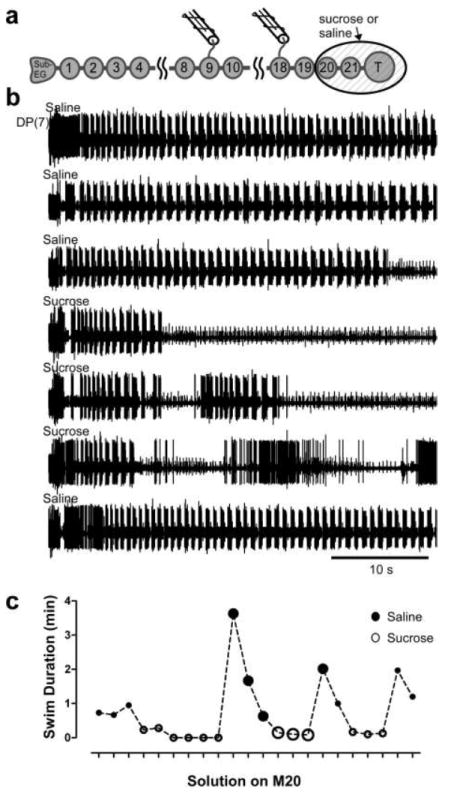
Tail ganglion is necessary for prolongation of swim duration by SubEG. a Schematic of isolated nervous system preparation with sucrose well. SupraEG was removed, and swimming was elicited under the condition of either with saline or sucrose around M20-T. The sucrose functionally, and reversibly, removes these ganglia from the preparation. b Sequential swim episodes elicited in preparations under the condition of either saline (large filled circles in c) or sucrose (large open circles in c) in the well surrounding M20-T in an isolated SubEG-T preparation. Extracellular recordings from DP(7). c Swim duration (min) in one experiment as a function of whether saline or sucrose was in the well surrounding M20-T. The large circles represent the evoked swims shown in b. Filled circles – saline in well; open circles – sucrose in well
Activity of identified neurons in SubEG-T preparations
After determining the influence of the SubEG on the swim-maintenance system, we examined the functions of several previously identified interneurons associated with generating or modulating swimming to test whether their properties were altered when the SupraEG is removed. One of these, a trigger neuron, cell Tr2 found in the SubEG, was initially identified as a swim-initiating neuron and later found to also be capable of terminating swim episodes (Brodfuehrer and Friesen 1986b; O Gara and Friesen 1995). Injections of depolarizing current into this cell in SubEG-T preparations revealed no differences from those reported earlier in H-T preparations (Fig. 5a). Similarly, we found that cell 208, a neuron that conveys inputs from cell 204 to swim-oscillator interneurons (Nusbaum et al. 1987) has its usual oscillatory activity during swimming (Fig. 5b).
Figure 5.
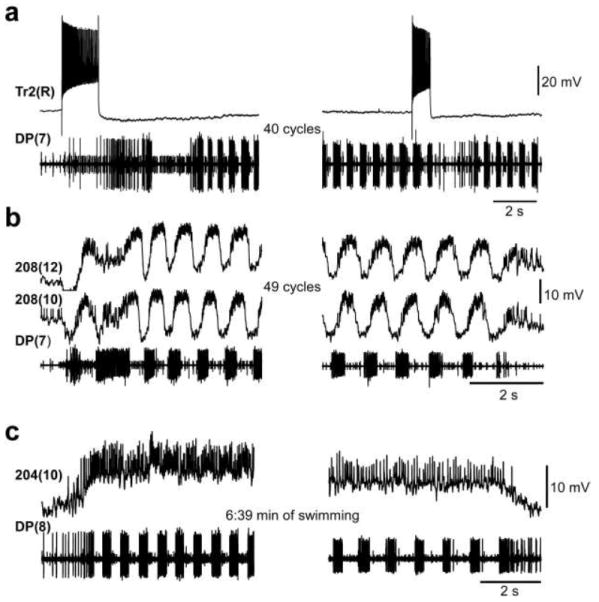
Interneuron activity in SubEG-T preparations. a Trigger neuron Tr2 depolarization triggers and subsequently terminates swimming. b Excitatory drive neuron, cell 208, is depolarized during swimming with superimposed large-amplitude membrane potential oscillations. c Swim-gating neuron, cell 204, is tonically depolarized during swimming. In each record, the lower trace is an extracellular DP nerve recording; the upper traces are intracellular penetrations of the neurons indicated. Except for the prolonged swimming, these records are similar to those recorded in H-T or M2-T preparations
Because cell 204 is the main driver of swimming activity, we also examined its activity in preparations lacking the SupraEG. Intracellular recordings of cell 204 potentials in SubEG-T preparations showed that its activity in this preparation is similar to that observed previously; namely, cell 204 depolarizes at swim-initiation and remains depolarized, with high impulse frequency until swim-termination, even during extended swim episodes (Fig. 5c). Because the characteristics of these interneurons was not substantially altered by removal of the SupraEG (Fig. 5c), we conclude that changes in swim maintenance in different nerve cord classes must arise from either relatively subtle changes in activity levels in these neurons or from other, perhaps unidentified, neurons.
SubEG has different effect on swim-maintenance parameters in nearly intact preparations
After determining that isolated preparations with an intact SubEG but with the SupraEG removed exhibited enhanced swimming activity, we tested whether the SupraEG had similar effects on swimming in nearly intact animals. Does sensory feedback change the contributions of regions of the head brain to swim maintenance? Nearly intact leeches (NI) with the following configurations of the nerve cord, H-T NI, SubEG-T NI, M1-T NI and M1-2 NI (see Fig. 1a), were suspended in a trough filled with saline (Fig. 6a) - a swim-enhancing aquatic environment (Esch et al. 2002). Swim durations in these preparations were recorded during a 30 min observation trial. Surprisingly, we found that a large majority of M2-T NI (7/8) and M1-T NI (3/4) preparations swam for the entire 30 min test interval, whereas only a minority of the SubEG-T (1/4) and H-T (2/5) preparations swam the entire time, suggesting that the SubEG might actually act to decrease swim duration. Because results were similar in preparations that included the SubEG (H-T, SubEG-T) and in those with the SubEG removed (M1-T, M2-T), the preparations were grouped into two categories: “SubEG(+)” and “SubEG(-)”, respectively. The fraction of preparations that swam for the entire 30 min trial in the SubEG(+) group (0.33) was significantly lower than that in SubEG(-) preparations (0.83; Fig. 6b; Fisher s exact test, p = 0.032). The average longest swim duration in the preparations that did not swim for the entire trial ranged from 5.8 – 15.6 min in three H-T NI preparations, but exceeded 20 min in SubEG-T NI preparations. The shorter swim durations in SubEG(+) preparations suggest that there might be neurons in the SubEG as well as the SupraEG that inhibit swimming through sensory feedback.
Figure 6.
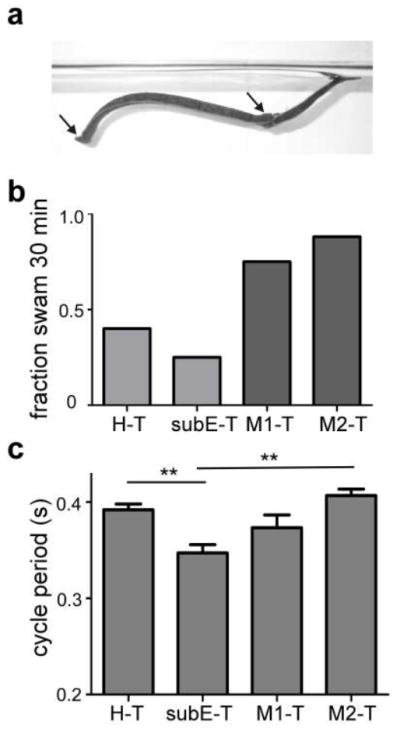
Swim maintenance in nearly-intact leeches. a Suspended swimming leech. The leech was suspended by threads (not visible) attached (at arrows) to the denervated rostral sucker (at left) and to either side of denervated segment M17. b Fraction of experiments that swam for the entire 30 min observation trial. Light gray indicates preparations with SubEG present (SubEG(+)), dark gray indicates preparations in which the SubEG removed (SubEG(-)). Fisher s exact test (SubEG(+) vs. SubEG(-)), ρ = 0.032. c Cycle periods in the four types of nearly intact preparations. One-way ANOVA, asterisks on figure indicate Newman-Keuls post-test. ** ρ < 0.01
Cycle periods in nearly intact animals were shorter than those observed in the isolated nerve cords for all preparations (p <0.01 for all comparisons between isolated and NI nerve-cord class counterparts, t-test; cf., Fig. 6c and Fig. 3b,c). Within the nearly-intact nerve-cord classes, the SubEG-T cycle periods were the smallest, with an average of 0.35 +/- 0.01 s (Fig. 6c) and were significantly smaller than those in H-T NI (0.39 +/- 0.01 s; n = 5) and M2-T NI (0.41 +/- 0.01 s; n = 3), but not M1-T (0.37 +/- 0.01 s; n = 3) preparations. Therefore, removal of the SupraEG, but not the SubEG, led to increased excitation in the swim-maintenance system.
The average longest duration swim of NI SubEG-T preparations was about twice as long as that of isolated SubEG-T preparations (25.9 vs. 10.6 min, p = 0.058); however, the longest swim durations in isolated H-T, M1-T and M2-T preparations (0.73, 0.42 and 0.81 min, respectively) were a small fraction of the longest swim durations in their nearly-intact counterparts (19.1, 25.2 and 28.3 min respectively). Interestingly, in nearly intact animals, the longest swim for each preparation was almost always the initial one; this phenomenon occurred in 75% of nearly-intact experiments. The duration of subsequent swims was usually much briefer. (In isolated preparations, short swim episodes were interspersed with the long ones.) The duration of second longest swim in all nearly intact leeches was on average only 25% of the duration of the longest swim, whereas this value was 70% in isolated nerve cords.
Comparisons between swimming in nearly intact leeches and isolated nerve cords demonstrate that sensory feedback has a strong and complex influence on the swim-maintenance system. In our swim-enhancing environment, the influence of nerve cord configuration on swimming is altered and reduced from that of the isolated nerve cord.
Discussion
Our overall aim was to clearly identify the individual contributions of the SupraEG and SubEG to the control of swimming in the medicinal leech and to examine the interaction of sensory input with these two structures. With swim duration and cycle period as measures of activity levels of the swim maintenance systems, our data demonstrate that the SupraEG is the prime source of inhibition for the swim system, whereas the overall effect of the SubEG is excitatory (Fig. 7). These results come with two caveats. First, the presence of the tail brain is necessary for observing extended swim durations in isolated preparations when the SupraEG is removed. Second, in semi-intact animals the removal of the SupraEG leads to reduced cycle periods, but the presence of the SubEG slightly shortens swim duration. Finally, sensory input in a swim-enhancing environment greatly excites the swim maintenance system.
Figure 7.
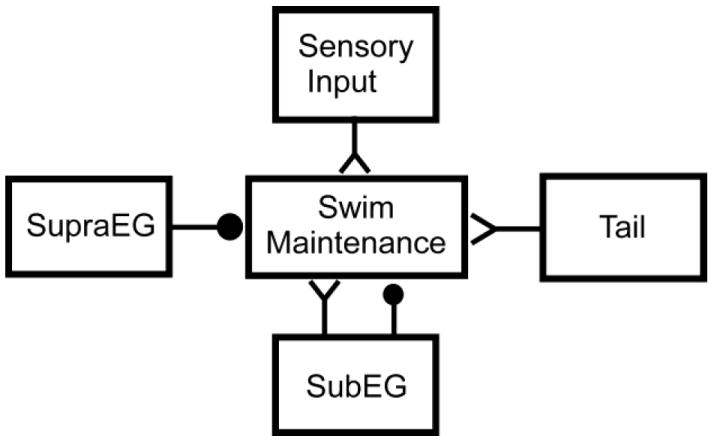
Model for role of leech CNS components in the control of swimming. The SupraEG inhibits the maintenance system. The SubEG provides overall excitation to the swim maintenance system, but contains some inhibitory actions arising from sensory feedback. Certain types of sensory feedback can provide massive excitation to the swim-maintenance system in the appropriate environment; other sensory feedback would inhibit swim-maintenance (not shown). ‘Y’ endings denote excitatory interactions; filled circles denote inhibition
Functional specialization of the brain
Although the leech head brain is less complex than those of vertebrates, our data as well as those of other studies show that the head brain, in its entirety, plays a specialized role in controlling behavior. For example, application of neuromodulators to the head brain of the leech has different effects on locomotor behavior than the application of these same substances to midbody ganglia (Crisp and Mesce 2003). Further, several neurons important for activation or termination of swimming and crawling have been identified in the head brain, but these are not present in the midbody ganglia (Brodfuehrer and Friesen 1986b; Brodfuehrer et al. 1995; Esch et al. 2002). The head brain is also necessary for coordinated crawling in the leech (Puhl and Mesce 2010) and mediates the decision of whether to swim or crawl (without the head brain, the decision is almost exclusively to swim). Our data show that the head brain itself also has functionally differentiated compartments with separate, even opposing functions in swim-maintenance.
Delineation of cephalic regions that control swimming
Our data demonstrate that the majority of the inhibition of the swim system previously ascribed broadly to the head brain originates more specifically in the SupraEG. When both are present, the inhibition from the SupraEG overrides or inhibits the excitation of the SubEG, as revealed by studies demonstrating that swimming is more difficult to initiate and has a shorter duration in H-T preparations than in M2-T preparations. Interestingly, we did not see differences in swim duration between the H-T and M2-T preparations, likely due to the great variability that occurred between experiments within each nerve-cord class; previous studies used the reversible “sucrose-knife” technique for comparisons between the two conditions, which removed the effect of experiment to experiment variability. We did, however, see significantly shorter cycle periods in M2-T than H-T preparations during spontaneous swimming, suggesting a dominating effect of the SubEG.
The interactions between the SupraEG and SubEG that lead to these effects are unclear. The SupraEG could act directly on the SubEG to suppress its swim-excitatory effects. Alternatively, the two structures could act independently on midbody ganglia, one providing inhibition, one excitation, to relevant swim-neurons in the midbody ganglia. Interestingly, the presence of prolonged swims in SubEG-T preparations requires the presence of the tail brain. It is already known that the removal of tail brain shortens swim duration in preparations with or without the head brain attached (Brodfuehrer et al. 1993; Friesen et al. 2011). However, here, removing the tail brain not only shortened, but completely eliminated the extended swims observed in SubEG-T preparations. Therefore, the combination of the SubEG and tail brain, removed from the inhibitory influence of the SupraEG, can lead to swims with durations that are greater than the simple sum of durations in preparations with only one of these structures (SubEG or tail brain) removed. We can speculate that neurons in these structures interact to recruit some critical number of swim-maintenance neurons, which then sustain the underlying excitation through supralinear summation of the individual swim-sustaining effects. In any event, it is clear that the regulation of swimming in the leech is very widely distributed, with interactions that span the entire CNS, from head to the tail brains.
Comparison to other species
The gross effects of the anterior ganglia on locomotion vary between species and behaviors. In cockroach flight, the SubEG is the inhibitory structure, while the SupraEG is excitatory - removing both of them also causes inhibition (Gal and Libersat 2006). However, the opposite is true in examining cockroach walking – here, like leech swimming, removal of the SupraEG lead to prolonged walking bouts, however removing the SubEG and SupraEG nearly eliminated walking altogether (Ridgel and Ritzmann 2005; Gal and Libersat 2006). In the praying mantis, removal of the protocerebral ganglion (brain) results in increased locomotion, but subsequent removal of the SubEG results in decreased locomotion (Roeder 1937). In the locust, any lesions of anterior ganglia reduced walking speed and duration (Kien 1983). Only in the reproductive behaviors of the cricket (Matsumoto and Sakai 2000) and grasshopper (Thompson 1986a,b; da Silva and Lange 2011) does removal of multiple anterior ganglia result in disinhibition, as removal of H-M1 disinhibits swimming in the leech (Brodfuehrer and Friesen, 1986a). In the cricket, the inhibition is caused by the brain and not the SubEG. (In many species, the SubEG is not considered a part of the brain). Importantly, only the experiments on the cricket were performed in isolated as well as nearly intact animals, so the effect of interaction of the anterior ganglia with sensory input is largely unknown in most invertebrates.
Inhibition by cephalic regions is a crucial part of vertebrate locomotion as well. In lampreys, brainstem locomotor regions appear to be under tonic GABA inhibition, and disinhibition of these areas is required to elicit swimming (Ménard et al. 2007; Ménard and Grillner 2008). In fact, inhibition by GABAergic pallidal neurons is a crucial component of most vertebrate locomotor programs (Grillner et al. 2005). Through direct neural connections or through neuromodulation, the leech SupraEG appears to provide tonic inhibition to the swim system; its removal appears to lead to disinhibition. Cephalic inhibition, therefore, is an important component of locomotor systems.
Swim-maintenance
In multiple species, the neurons or neuronal populations responsible for maintaining behavior over a period of time also control the cycle period of the behavior. (Kudo and Yamada 1987; Brodin et al. 1988; Böhm and Schilderger 1992; Cazalets et al. 1992; Di Prisco et al. 1997; Deliagina et al. 2000; Hedwig, 2000; Whelan et al. 2000; Dembrow et al. 2003; Paggett et al. 2004; Arshavsky et al. 2010; Mullins et al. 2011a,b). In this study, although we saw a consistent reduction in cycle period in isolated SubEG-T preparations compared to the other nerve-cord classes (within the parameters of our analysis, early in the swim), swim duration was quite variable both within and between SubEG-T preparations, indicating that unknown factors contribute to the duration of individual swims. Consequently, the SubEG appears to consistently increase the degree of excitation of the maintenance system early in the swim (decreased cycle periods), but inconsistently sustains this level of excitation to prolong swimming (variable swim durations). These data indicate that the control of swim duration may be more complex than the regulation of cycle period.
Sensory Feedback
When placed in deep water, leeches usually swim until they encounter an object (Kristan et al. 1974). Denervation of the head brain in otherwise intact leeches increases the likelihood of swimming (Esch et al. 2002). Therefore it is not surprising that all our leech preparations exhibited prolonged swimming when suspended in deep saline. The excitation provided to the maintenance system by sensory feedback in this environment reduced the large differences in swim maintenance parameters between the SubEG-T and the other isolated nerve-cord classes. However, the SubEG-T NI preparations did retain the shortest cycle periods of all the nerve-cord classes, indicating that the SubEG, even in nearly intact preparations, provides extra excitation to the maintenance system. Interestingly, sensory feedback had the strongest effects, in terms of prolonged activation of the maintenance system (swim duration) on those preparations in which the entire brain had been removed (M1-T and M2-T), results that are in stark contrast to those obtained in the isolated system. These data suggest first, that there are neurons in the head brain that individually, or as elements of intersegmental circuits, inhibit the maintenance system (Fig. 6). Although two interneurons, Tr2 (Brodfuehrer and Friesen 1986a; O Gara and Friesen 1995) and SIN1 (Brodfuehrer and Burns 1995), in the SubEG, can terminate swimming when stimulated, it is unlikely that their activity alone is sufficient to cause the increase in swim duration following removal of the SubEG ganglion. Second, the data show that the excitatory effects of sensory feedback on the maintenance system do not require the head brain; that is, sensory input can interact directly with neurons in midbody ganglia to sustain swimming. Given that both isolated and nearly intact preparations can generate swimming for many minutes, these data suggest that the termination of these long swim episodes is an active process rather than the result of cellular or synaptic fatigue, which we have previously proposed as a mechanism that limits swim duration to shorter swim episodes (Friesen et al. 2011). Impulse adaptation in cat motor neurons has recently been found to reverse during locomotion presumably due to some form of neuromodulation (Brownstone et al. 2011). Perhaps a similar phenomenon occurs among swim-maintenance interneurons, where fatigue mechanisms are reduced following removal of the SupraEG or in the presence of a sensory environment favorable to swimming.
Acknowledgments
This research was supported by grants from the National Science Foundation (IOS-0615631 to WOF and JH, and IOS-0113276 to PDB) and a National Institutes of Health NRSA fellowship to OJM (NIH NRSA GC11999).
Abbreviations
- BPE
bursts per swim episode
- CP
cycle period
- DP
dorsal posterior (nerve)
- H
head ganglion
- M#
midbody ganglion (numbered 1 through 21)
- MN
motoneuron
- NI
nearly intact
- SupraEG
supraesophageal ganglion
- SubEG
subesophageal ganglion
- T
tail ganglion
References
- Arshavsky YI, Deliagina TG, Orlovsky GN. In: The swimming circuit in the Pteropod Mollusc Clione. Shepherd GM, Grillner S, editors. Oxford University Press; 2010. pp. 474–479. [Google Scholar]
- Böhm H, Schildberger K. Brain neurones involved in the control of walking in the cricket Gryllus bimaculatus. J Exp Biol. 1992;166:113–130. [Google Scholar]
- Brodfuehrer PD, Burns A. Neuronal factors influencing the decision to swim in the medicinal leech. Neurobiol Learn Mem. 1995;63:192–199. doi: 10.1006/nlme.1995.1020. [DOI] [PubMed] [Google Scholar]
- Brodfuehrer PD, Parker HJ, Burns A, Berg M. Regulation of the segmental swim-generating system by a pair of identified interneurons in the leech head ganglion. J Neurophysiol. 1995;73:983–992. doi: 10.1152/jn.1995.73.3.983. [DOI] [PubMed] [Google Scholar]
- Brodfuehrer PD, Kogelnik AM, Friesen WO, Cohen AH. Effect of the tail ganglion on swimming activity in the leech. Behav Neural Biol. 1993;59:162–166. doi: 10.1016/0163-1047(93)90912-2. [DOI] [PubMed] [Google Scholar]
- Brodfuehrer PD, Friesen WO. Control of leech swimming activity by the cephalic ganglia. J Neurobiol. 1986a;17:697–705. doi: 10.1002/neu.480170612. [DOI] [PubMed] [Google Scholar]
- Brodfuehrer PD, Friesen WO. Initiation of swimming activity by trigger neurons in the leech subesophageal ganglion. I. Output connections of Tr1 and Tr2. J Comp Physiol A. 1986b;159:489–502. doi: 10.1007/BF00604169. [DOI] [PubMed] [Google Scholar]
- Brodin L, Buchanan JT, Hokfelt T, Grillner S, Rehfeld JF, Frey P, Verhofstad AA, Dockray GJ, Walsh JH. Immunohistochemical studies of cholecystokininlike peptides and their relation to 5-HT, CGRP, and bombesin immunoreactivities in the brainstem and spinal cord of lampreys. J Comp Neurol. 1988;271:1–18. doi: 10.1002/cne.902710103. [DOI] [PubMed] [Google Scholar]
- Brownstone RM, Krawitz S, Jordan LM. Reversal of the late phase of spike frequency adaptation in cat spinal motoneurons during fictive locomotion. J Neurophysiol. 2011;105:1045–1050. doi: 10.1152/jn.00411.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalets JR, Sqalli-Houssaini Y, Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. J Physiol. 1992;455:187–204. doi: 10.1113/jphysiol.1992.sp019296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrachri A, Clarac F. Fictive locomotion in the fourth thoracic ganglion of the crayfish, Procambarus clarkii. J Neurosci. 1990;10:707–719. doi: 10.1523/JNEUROSCI.10-03-00707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AH. The role of heterarchical control in the evolution of central pattern generators. Brain Behav Evol. 1992;40:112–124. doi: 10.1159/000113907. [DOI] [PubMed] [Google Scholar]
- Cornford A, Kristan WB, 3rd, Malnove S, Kristan WB, Jr, French KA. Functions of the subesophageal ganglion in the medicinal leech revealed by ablation of neuromeres in embryos. J Exp Biol. 2006;209:493–503. doi: 10.1242/jeb.02030. [DOI] [PubMed] [Google Scholar]
- Crisp KM, Mesce KA. To swim or not to swim: regional effects of serotonin, octopamine and amine mixtures in the medicinal leech. J Comp Physiol A. 2003;189:461–470. doi: 10.1007/s00359-003-0424-0. [DOI] [PubMed] [Google Scholar]
- da Silva R, Lange AB. Evidence of a central pattern generator regulating spermathecal muscle activity in Locusta migratoria and its coordination with oviposition. J Exp Biol. 2011;214:757–763. doi: 10.1242/jeb.049379. [DOI] [PubMed] [Google Scholar]
- Debski EA, Friesen WO. Role of central interneurons in habituation of swimming activity in the medicinal leech. J Neurophysiol. 1986;55:977–994. doi: 10.1152/jn.1986.55.5.977. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Fagerstedt P. Responses of reticulospinal neurons in intact lamprey to vestibular and visual inputs. J Neurophysiol. 2000;83:864–878. doi: 10.1152/jn.2000.83.2.864. [DOI] [PubMed] [Google Scholar]
- Dembrow NC, Jing J, Proekt A, Romero A, Vilim FS, Cropper EC, Weiss KR. A newly identified buccal interneuron initiates and modulates feeding motor programs in aplysia. J Neurophysiol. 2003;90:2190–2204. doi: 10.1152/jn.00173.2003. [DOI] [PubMed] [Google Scholar]
- Di Prisco GV, Pearlstein E, Robitaille R, Dubuc R. Role of sensory-evoked NMDA plateau potentials in the initiation of locomotion. Science. 1997;278:1122–5. doi: 10.1126/science.278.5340.1122. [DOI] [PubMed] [Google Scholar]
- Esch T, Mesce KA, Kristan WB., Jr Evidence for sequential decision making in the medicinal leech. J Neurosci. 2002;22:11045–11054. doi: 10.1523/JNEUROSCI.22-24-11045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facciponte G, Lange AB. Characterization of a novel central pattern generator located in the VIIth abdominal ganglion of Locusta. J Insect Physiol. 1992;38:1011–1022. [Google Scholar]
- Friesen WO, Mullins OJ, Xiao R, Hackett JT. Positive feedback loops sustain repeating bursts in neuronal circuits. J Biol Physics. 2011;37:317–345. doi: 10.1007/s10867-010-9210-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen WO. Physiology of water motion detection in the medicinal leech. J Exp Biol. 1981;92:255–275. doi: 10.1242/jeb.92.1.255. [DOI] [PubMed] [Google Scholar]
- Gal R, Libersat F. New vistas on the initiation and maintenance of insect motor behaviors revealed by specific lesions of the head ganglia. J Comp Physiol A. 2006;192:1003–1020. doi: 10.1007/s00359-006-0135-4. [DOI] [PubMed] [Google Scholar]
- Grillner S, Hellgren J, Menard A, Saitoh K, Wikstrom MA. Mechanisms for selection of basic motor programs--roles for the striatum and pallidum. Trends Neurosci. 2005;28:364–370. doi: 10.1016/j.tins.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Graham D. Effects of circum-oesophageal lesion on the behaviour of the stick insect Carausius morosus. II. Changes in walking coordination. Biol Cybern. 1979;32:147–152. [Google Scholar]
- Hedwig B. Control of cricket stridulation by a command neuron: efficacy depends on the behavioral state. J Neurophysiol. 2000;83:712–722. doi: 10.1152/jn.2000.83.2.712. [DOI] [PubMed] [Google Scholar]
- Hocker CG, Yu X, Friesen WO. Functionally heterogeneous segmental oscillators generate swimming in the medical leech. J Comp Physiol A. 2000;186:871–883. doi: 10.1007/s003590000140. [DOI] [PubMed] [Google Scholar]
- Kagaya K, Takahata M. Readiness discharge for spontaneous initiation of walking in crayfish. J Neurosci. 2010;30:1348–1362. doi: 10.1523/JNEUROSCI.4885-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kien J. The initiation and maintenance of walking in the locust: an alternative to the command concept. Proc R Soc Lond B. 1983;219:137–174. [Google Scholar]
- Kristan WB, Jr, Calabrese RL, Friesen WO. Neuronal control of leech behavior. Prog Neurobiol. 2005;76:279–327. doi: 10.1016/j.pneurobio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Kristan WB, Jr, Calabrese RL. Rhythmic swimming activity in neurones of the isolated nerve cord of the leech. J Exp Biol. 1976;65:643–68. doi: 10.1242/jeb.65.3.643. [DOI] [PubMed] [Google Scholar]
- Kristan WB, Jr, Stent GS, Ort CA. Neuronal control of swimming in the medicinal leech. I. Dynamics of the swimming rhythm. J Comp Physiol A. 1974;94:97–119. 97. [Google Scholar]
- Kudo N, Yamada T. N-methyl-D,L-aspartate-induced locomotor activity in a spinal cord-hindlimb muscles preparation of the newborn rat studied in vitro. Neurosci Lett. 1987;75:43–48. doi: 10.1016/0304-3940(87)90072-3. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D, Schulz DJ, Taylor AL. Invertebrate central pattern generation moves along. Curr Biol. 2005;15:R685–99. doi: 10.1016/j.cub.2005.08.022. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Sakai M. Brain control of mating behavior in the male cricket Gryllus bimaculatus DeGeer: brain neurons responsible for inhibition of copulation actions. J Insect Physiol. 2000;46:539–552. doi: 10.1016/s0022-1910(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Menard A, Grillner S. Diencephalic locomotor region in the lamprey--afferents and efferent control. J Neurophysiol. 2008;100:1343–1353. doi: 10.1152/jn.01128.2007. [DOI] [PubMed] [Google Scholar]
- Menard A, Auclair F, Bourcier-Lucas C, Grillner S, Dubuc R. Descending GABAergic projections to the mesencephalic locomotor region in the lamprey Petromyzon marinus. J Comp Neurol. 2007;501:260–273. doi: 10.1002/cne.21258. [DOI] [PubMed] [Google Scholar]
- Mullins OJ, Hackett JT, Friesen WO. Local-distributed integration by a novel neuron ensures rapid initiation of animal locomotion. J Neurophysiol. 2011a;105:130–144. doi: 10.1152/jn.00507.2010. [DOI] [PubMed] [Google Scholar]
- Mullins OJ, Hackett JT, Buchanan JT, Friesen WO. Neuronal control of swimming behavior: comparison of vertebrate and invertebrate model systems. Prog Neurobiol. 2011b;93:244–269. doi: 10.1016/j.pneurobio.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP, Friesen WO, Kristan WB, Jr, Pearce RA. Neural mechanisms generating the leech swimming rhythm: swim-initiator neurons excite the network of swim oscillator neurons. J Comp Physiol A. 1987;161:355–66. doi: 10.1007/BF00603961. [DOI] [PubMed] [Google Scholar]
- O’Gara BA, Friesen WO. Termination of leech swimming activity by a previously identified swim trigger neuron. J Comp Physiol A. 1995;177:627–36. doi: 10.1007/BF00207191. [DOI] [PubMed] [Google Scholar]
- Paggett KC, Jackson AW, McClellan AD. Organization of higher-order brain areas that initiate locomotor activity in larval lamprey. Neuroscience. 2004;125:25–33. doi: 10.1016/j.neuroscience.2004.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce RA, Friesen WO. Intersegmental coordination of leech swimming: comparison of in situ and isolated nerve cord activity with body wall movement. Brain Res. 1984;299:363–366. doi: 10.1016/0006-8993(84)90720-0. [DOI] [PubMed] [Google Scholar]
- Puhl JG, Mesce KA. Keeping it together: mechanisms of intersegmental coordination for a flexible locomotor behavior. J Neurosci. 2010;30:2373–2383. doi: 10.1523/JNEUROSCI.5765-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgel AL, Ritzmann RE. Effects of neck and circumoesophageal connective lesions on posture and locomotion in the cockroach. J Comp Physiol A. 2005;191:559–573. doi: 10.1007/s00359-005-0621-0. [DOI] [PubMed] [Google Scholar]
- Roeder KD. The control of tonus and locomotor activity in the praying mantis (Mantis Religiosa L) J Exp Zoo. 1937;76:353–374. [Google Scholar]
- Schlüter E. Die Bedeutung des Centralnervensystems von Hirudo medicinalis für Locomotion and Raumorientierung. Z wiss Zool. 1933;143:538–593. [Google Scholar]
- Thompson KJ. Oviposition digging in the grasshopper. I. Functional anatomy and the motor programme. J Exp Biol. 1986a;122:387–411. doi: 10.1242/jeb.122.1.387. [DOI] [PubMed] [Google Scholar]
- Thompson KJ. Oviposition digging in the grasshopper. II. Descending neural control. J Exp Biol. 1986b;122:413–425. doi: 10.1242/jeb.122.1.413. [DOI] [PubMed] [Google Scholar]
- Weeks JC, Kristan WB., Jr Initiation, maintenance and modulation of swimming in the medicinal leech by the activity of a single neurone. J Exp Biol. 1978;77:71–88. [Google Scholar]
- Whelan P, Bonnot A, O’Donovan MJ. Properties of rhythmic activity generated by the isolated spinal cord of the neonatal mouse. J Neurophysiol. 2000;84:2821–2833. doi: 10.1152/jn.2000.84.6.2821. [DOI] [PubMed] [Google Scholar]
- Willard AL. Effects of serotonin on the generation of the motor program for swimming by the medicinal leech. J Neurosci. 1981;1:936–944. doi: 10.1523/JNEUROSCI.01-09-00936.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DM. The central nervous control of flight in a locust. J Exp Biol. 1961;38:471–490. [Google Scholar]


